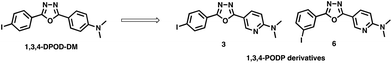
Novel radioiodinated 1,3,4-oxadiazole derivatives with improved in vivo properties for SPECT imaging of β-amyloid plaques†
Hiroyuki
Watanabe
a,
Masahiro
Ono
*a,
Hiroyuki
Kimura
a,
Kenji
Matsumura
a,
Masashi
Yoshimura
a,
Shimpei
Iikuni
a,
Yoko
Okamoto
b,
Masafumi
Ihara
c,
Ryosuke
Takahashi
d and
Hideo
Saji
a
aDepartment of Patho-Functional Bioanalysis, Graduate School of Pharmaceutical Sciences, Kyoto University, 46-29 Yoshida Shimoadachi-cho, Sakyo-ku, Kyoto 606-8501, Japan. E-mail: ono@pharm.kyoto-u.ac.jp; Fax: +81-75-753-4568; Tel: +81-75-753-4608
bDepartment of Pathology, National Cerebral and Cardiovascular Center, 5-7-1 Fujishiro-dai, Suita, Osaka 565-8565, Japan
cDepartment of Stroke and Cerebrovascular Diseases, National Cerebral and Cardiovascular Center, 5-7-1 Fujishiro-dai, Suita, Osaka 565-8565, Japan
dDepartment of Neurology, Graduate School of Medicine, Kyoto University, 54 Shogoin Kawahara-cho, Sakyo-ku, Kyoto 606-8507, Japan
First published on 10th December 2013
Abstract
We designed and synthesized a novel series of radioiodinated 3-(5-phenyl-1,3,4-oxadiazol-2-yl)pyridine (1,3,4-PODP) derivatives for imaging β-amyloid (Aβ) plaques in Alzheimer's disease (AD) brains using single photon emission computed tomography (SPECT). In binding experiments in vitro, 1,3,4-PODP derivatives (3 and 6) displayed high affinity for Aβ(1-42) aggregates (25 and 14 nM, respectively). In experiments in vivo, [123/125I]3 and [123/125I]6 displayed good initial uptake into and rapid washout from the brain in normal mice, and clearly labeled Aβ plaques in Tg2576 mice. Furthermore, specific labeling of Aβ plaques was observed in in vitro autoradiography of postmortem AD brain sections. These results suggested that 1,3,4-PODP derivatives may be useful SPECT probes for detecting Aβ plaques in AD brains.
Introduction
As the world's population ages, the number of patients with Alzheimer's disease (AD) is expected to increase rapidly.1 However, there is no definitive diagnosis method for patients with AD. Since the formation and deposition of β-amyloid (Aβ) plaques is an early event and a hallmark of AD, the imaging of Aβ plaques in vivo may lead to presymptomatic diagnosis.2–4 Recently, several imaging technologies, including positron emission tomography (PET),5–14 single photon emission computed tomography (SPECT),15–23 magnetic resonance imaging,24,25 and optical imaging,26,27 have been applied for imaging Aβ plaques. Among them, because PET and SPECT are superior in terms of quantitative capability, these modalities are attractive tools for early diagnosis of AD. Recently, [18F]AV-45, which is a PET probe for imaging Aβ plaques, was approved by the U.S. Food and Drug Administration (FDA). Furthermore, several PET probes, such as [18F]BAY94-9172,9,10 [18F]GE067,11,12 and [18F]AZD4694,6,7 are currently in phase 2–3 clinical trials. Conversely, [123I]IMPY is the only probe for SPECT to be tested in humans.16 Because of in vivo metabolism instability, however, [123I]IMPY was not able to distinguish between AD and a cognitively normal brain. Compared to PET, SPECT is more valuable in terms of routine diagnostic use. Therefore, the development of more useful Aβ imaging probes for SPECT has been strongly desired. Recently, many SPECT probes, including benzimidazole,18 styrylpyridine,15 1,4-diphenyltriazole,22 and aurone19 derivatives, have been reported for their utility as Aβ imaging probes in in vitro and in vivo experiments, but no reports have shown the results of clinical tests.Recently, we have reported radioiodinated 2,5-diphenyl-1,3,4-oxadiazole (1,3,4-DPOD) as a novel scaffold for in vivo imaging of Aβ plaques in the brain.28 In particular, 4-(5-(iodophenyl)-1,3,4-oxadiazol-2-yl)-N,N-dimethylbenzenamine (1,3,4-DPOD-DM) showed excellent affinity to Aβ aggregates and high initial uptake of the brain in normal mice. However, slow washout from the brain made it unsuitable for imaging in vivo. Therefore, additional structural modification of 1,3,4-DPOD-DM was needed to apply to in vivo imaging of Aβ plaques.
Some groups have reported that favorable in vivo pharmacokinetics of an Aβ imaging probe was achieved by converting a phenyl group into a pyridine group, that is, decreasing the lipophilicity of the probes.13,14,29 Furthermore, this conversion did not affect their binding affinity to Aβ aggregates. Based on these previous findings to improve in vivo pharmacokinetics of 1,3,4-DPOD-DM, we planned to develop novel 1,3,4-oxadiazole derivatives with less lipophilicity by replacing one of the phenyl groups of 1,3,4-DPOD-DM with one pyridine group (Fig. 1). In addition, we designed p- and m-iodinated derivatives to evaluate their stability against deiodination metabolism. In the present study, we designed and synthesized radioiodinated 3-(5-phenyl-1,3,4-oxadiazol-2-yl)pyridine (1,3,4-PODP) derivatives and evaluated their potential as SPECT probes for imaging Aβ plaques in vivo.
Results and discussion
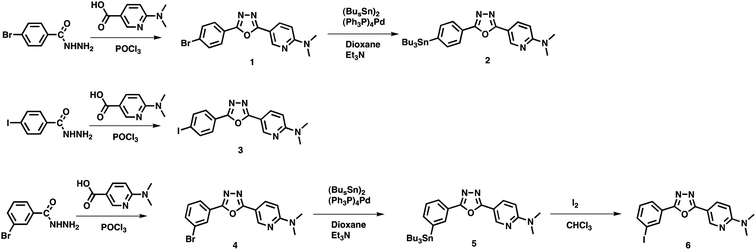
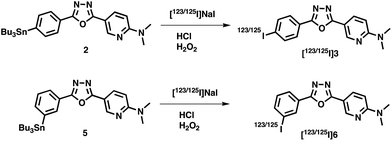
The synthesis of 1,3,4-PODP derivatives is outlined in Scheme 1. Compounds 1, 3, and 4 were prepared by 6-(N,N-dimethylamino)nicotinic acid and p-bromobenzhydrazide, p-iodobenzhydrazide, and m-bromobenzhydrazide in the presence of POCl3 (4–34% yields). Tributyltin derivatives (2 and 5) were prepared from the corresponding bromo compounds (1 and 4) using a halogen to tributyltin exchange reaction catalyzed by Pd(0) in yields of 60 and 28%. 5 was readily reacted with iodine in chloroform at room temperature to give an iodo derivative 6. The desired radioiodinated ligands [123/125I]3 and [123/125I]6 were successfully prepared from the corresponding tributyltin derivatives through a standard iododestannylation reaction, using hydrogen peroxide as the oxidant (Scheme 2). It was anticipated that a non-carrier-added preparation would result in a final product bearing the theoretical specific activity similar to that of 123I (87.7 × 102 TBq mmol−1) or 125I (81.4 TBq mmol−1). The radiochemical identities of radioiodinated ligands were verified by co-injection with non-radioactive compounds from HPLC profiles. [123/125I]3 and [123/125I]6 were each obtained in a radiochemical yield of 23–30% with a radiochemical purity of >99% after HPLC.First, we evaluated the stability of 1,3,4-PODP derivatives in mouse plasma. Almost all radioactivity derived from [125I]3 and [125I]6 existed as an intact form, suggesting that these compounds are stable in mouse plasma (Fig. S1†).
In vitro binding experiments to quantify the affinity of 1,3,4-PODP derivatives for Aβ(1-42) aggregates were carried out in solution with [125I]IMPY as the ligand. These compounds inhibited the binding of [125I]IMPY to Aβ(1-42) aggregates in a dose-dependent manner. The Ki values estimated for 3 and 6 were 24.8 and 13.6 nM, respectively (Table 1). These Ki values of 1,3,4-PODP were higher than those of 1,3,4-DPOD-DM (3.91 nM) and IMPY (3.05 nM), but 3 and 6 still had sufficient binding affinity for the in vivo imaging of Aβ aggregates in the brain.
Next, we determined log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P values to compare the lipophilicity of 1,3,4-oxadiazole derivatives to predict their pharmacokinetics in the brain (Table 2). As expected, because a pyridyl group is introduced into the PODP scaffold instead of a phenyl group into the DPOD scaffold, the log
P values to compare the lipophilicity of 1,3,4-oxadiazole derivatives to predict their pharmacokinetics in the brain (Table 2). As expected, because a pyridyl group is introduced into the PODP scaffold instead of a phenyl group into the DPOD scaffold, the log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P values of 1,3,4-PODP (log
P values of 1,3,4-PODP (log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P = 2.05 and 2.06 for 3 and 6, respectively) were lower than that of 1,3,4-DPOD-DM (2.43).28 Our previous paper reported that 1,3,4-DPOD-DM showed slow washout from the brain, resulting in high background. Since the slow washout of 1,3,4-DPOD-DM from the brain may be attributable to its high lipophilicity, 3 and 6 with lower lipophilicity were expected to show more favorable pharmacokinetics in the brain than the DPOD derivatives.
P = 2.05 and 2.06 for 3 and 6, respectively) were lower than that of 1,3,4-DPOD-DM (2.43).28 Our previous paper reported that 1,3,4-DPOD-DM showed slow washout from the brain, resulting in high background. Since the slow washout of 1,3,4-DPOD-DM from the brain may be attributable to its high lipophilicity, 3 and 6 with lower lipophilicity were expected to show more favorable pharmacokinetics in the brain than the DPOD derivatives.
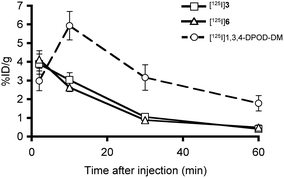
To evaluate the uptake into the brain of [125I]3 and [125I]6, biodistribution experiments were performed in normal mice. [125I]3 and [125I]6 displayed high initial uptake (3.88 and 4.12% ID g−1) at 2 min post-injection. In addition, they displayed rapid clearance from the normal brain (0.40 and 0.48% ID g−1) at 60 min post-injection. Compared to the brain2min/brain60min ratios of 1,3,4-DPOD-DM (1.7),28 values of [125I]3 and [125I]6 (9.7 and 8.6) were improved markedly, reflecting their log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P values (Fig. 2). Since no Aβ plaques exist in the normal mouse brain, the radioactivity derived from the probes should wash out rapidly. These results suggested that desirable pharmacokinetics of [125I]3 and [125I]6 were achieved by converting a phenyl group in 1,3,4-DPOD-DM to a pyridyl group. As shown in Fig. S1,† both [125I]3 and [125I]6 were stable in mouse plasma in vitro. However, these compounds showed high radioactivity accumulation in the stomach, possibly due to deiodination in vivo (Table S1†). The difference in the stability between in vitro and in vivo may be attributable to radiometabolites produced by the in vivo metabolism in organs such as the liver and kidney. In addition, the substitution position of iodine in the 1,3,4-PODP may not be affected by in vivo stability against deiodination metabolism.
P values (Fig. 2). Since no Aβ plaques exist in the normal mouse brain, the radioactivity derived from the probes should wash out rapidly. These results suggested that desirable pharmacokinetics of [125I]3 and [125I]6 were achieved by converting a phenyl group in 1,3,4-DPOD-DM to a pyridyl group. As shown in Fig. S1,† both [125I]3 and [125I]6 were stable in mouse plasma in vitro. However, these compounds showed high radioactivity accumulation in the stomach, possibly due to deiodination in vivo (Table S1†). The difference in the stability between in vitro and in vivo may be attributable to radiometabolites produced by the in vivo metabolism in organs such as the liver and kidney. In addition, the substitution position of iodine in the 1,3,4-PODP may not be affected by in vivo stability against deiodination metabolism.
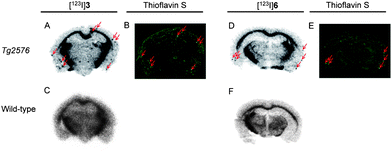
To test the labeling of Aβ plaques in vivo, we carried out ex vivo autoradiography in Tg2576 mice, which have been widely used to evaluate the specific binding of Aβ plaques,5,21,26 and wild-type mice as controls. Autoradiography using [123I]3 and [123I]6 showed labeling of Aβ plaques in the Tg2576 mouse brain (Fig. 3A and D), while the wild-type mouse brain showed no such labeling (Fig. 3C and F). Aβ plaques were confirmed to be present by co-staining the sections with thioflavin S, a dye commonly used to stain Aβ plaques (Fig. 3B and E). These results suggested that these probes penetrated the blood–brain barrier and selectively labeled the Aβ plaques in the brain, as reflected by the in vitro binding assays and biodistribution experiments. Many radioiodinated Aβ imaging probes have been reported, but few probes have been evaluated by ex vivo autoradiography using AD model mice except for imidazopyridine20,23 and benzimidazole18 derivatives. The successful results in the ex vivo autoradiographic study of 1,3,4-PODP should make it a novel attractive scaffold for imaging Aβ plaques with SPECT.
Furthermore, we carried out SPECT imaging experiments with [123I]6 to evaluate its feasibility of imaging Aβ plaques in vivo. In transversal images, the accumulation of radioactivity in the brain of the Tg2576 mouse was higher than that of the wild-type mouse (Fig. S2†). Although detailed analyses are needed in the future, this preliminary result suggests that [123I]6 may be a potential SPECT probe for imaging Aβ plaques.
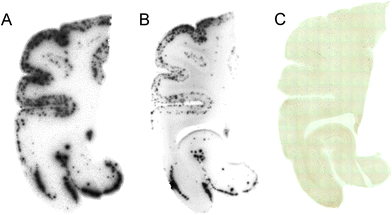
Furthermore, we investigated the affinity of [125I]3 and [125I]6 for Aβ plaques by in vitro autoradiography in human AD brain sections. The autoradiogram of [125I]3 and [125I]6 showed high levels of radioactivity in the brain sections (Fig. 4A and B). We confirmed that hot spots of [125I]3 and [125I]6 corresponded to those of in vitro immunohistochemical staining (Fig. 4C). These results indicate that [125I]3 and [125I]6 have the potential to detect Aβ plaques in the human AD brain.
Conclusion
In summary, we successfully designed and synthesized novel radioiodinated 1,3,4-PODP derivatives. These ligands showed high affinity for Aβ aggregates in vitro and for Aβ plaques in sections of autopsied AD brains. Compared to 1,3,4-DPOD-DM, they displayed good penetration of and fast wash out from the brain in biodistribution experiments using normal mice. In addition, ex vivo autoradiography of brain sections from Tg2576 mice after the injection of 1,3,4-PODP derivatives showed specific binding of Aβ plaques. Taken together, the present results suggested that radioiodinated 1,3,4-PODP derivatives may be useful probes for imaging Aβ plaques in the AD brain with SPECT.Acknowledgements
This research was supported by a grant from the Japan Society for the Promotion of Science (JSPS) through the “Funding Program for Next Generation World-Leading Researchers (NEXT Program)”, initiated by the Council for Science and Technology Policy (CSTP), and JSPS Research Fellowships for Young Scientists.References
- R. Brookmeyer, E. Johnson, K. Ziegler-Graham and H. M. Arrighi, Alzheimer's Dementia, 2007, 3, 186 CrossRef PubMed.
- W. E. Klunk, Neurobiol. Aging, 1998, 19, 145 CrossRef CAS.
- D. J. Selkoe, Physiol. Rev., 2001, 81, 741 CAS.
- C. R. Jack Jr, D. S. Knopman, W. J. Jagust, L. M. Shaw, P. S. Aisen, M. W. Weiner, R. C. Petersen and J. Q. Trojanowski, Lancet Neurol., 2010, 9, 119 CrossRef.
- M. Cui, M. Ono, H. Kimura, M. Ueda, Y. Nakamoto, K. Togashi, Y. Okamoto, M. Ihara, R. Takahashi, B. Liu and H. Saji, J. Med. Chem., 2012, 55, 9136 CrossRef CAS PubMed.
- A. Jureus, B. M. Swahn, J. Sandell, F. Jeppsson, A. E. Johnson, P. Johnstrom, J. A. Neelissen, D. Sunnemark, L. Farde and S. P. Svensson, J. Neurochem., 2010, 114, 784 CrossRef CAS PubMed.
- Z. Cselenyi, M. E. Jonhagen, A. Forsberg, C. Halldin, P. Julin, M. Schou, P. Johnstrom, K. Varnas, S. Svensson and L. Farde, J. Nucl. Med., 2012, 53, 415 CrossRef CAS PubMed.
- C. M. Clark, J. A. Schneider, B. J. Bedell, T. G. Beach, W. B. Bilker, M. A. Mintun, M. J. Pontecorvo, F. Hefti, A. P. Carpenter, M. L. Flitter, M. J. Krautkramer, H. F. Kung, R. E. Coleman, P. M. Doraiswamy, A. S. Fleisher, M. N. Sabbagh, C. H. Sadowsky, E. P. Reiman, S. P. Zehntner and D. M. Skovronsky, JAMA, J. Am. Med. Assoc., 2011, 305, 275 CrossRef CAS PubMed.
- C. C. Rowe, U. Ackerman, W. Browne, R. Mulligan, K. L. Pike, G. O'Keefe, H. Tochon-Danguy, G. Chan, S. U. Berlangieri, G. Jones, K. L. Dickinson-Rowe, H. P. Kung, W. Zhang, M. P. Kung, D. Skovronsky, T. Dyrks, G. Holl, S. Krause, M. Friebe, L. Lehman, S. Lindemann, L. M. Dinkelborg, C. L. Masters and V. L. Villemagne, Lancet Neurol., 2008, 7, 129 CrossRef CAS.
- W. Zhang, S. Oya, M. P. Kung, C. Hou, D. L. Maier and H. F. Kung, Nucl. Med. Biol., 2005, 32, 799 CrossRef CAS PubMed.
- M. Koole, D. M. Lewis, C. Buckley, N. Nelissen, M. Vandenbulcke, D. J. Brooks, R. Vandenberghe and K. Van Laere, J. Nucl. Med., 2009, 50, 818 CrossRef CAS PubMed.
- R. Vandenberghe, K. Van Laere, A. Ivanoiu, E. Salmon, C. Bastin, E. Triau, S. Hasselbalch, I. Law, A. Andersen, A. Korner, L. Minthon, G. Garraux, N. Nelissen, G. Bormans, C. Buckley, R. Owenius, L. Thurfjell, G. Farrar and D. J. Brooks, Ann. Neurol., 2010, 68, 319 CrossRef PubMed.
- M. Ono, Y. Cheng, H. Kimura, M. Cui, S. Kagawa, R. Nishii and H. Saji, J. Med. Chem., 2011, 54, 2971 CrossRef CAS PubMed.
- M. Cui, X. Wang, P. Yu, J. Zhang, Z. Li, X. Zhang, Y. Yang, M. Ono, H. Jia, H. Saji and B. Liu, J. Med. Chem., 2012, 55, 9283 CrossRef CAS PubMed.
- W. Qu, M. P. Kung, C. Hou, T. E. Benedum and H. F. Kung, J. Med. Chem., 2007, 50, 2157 CrossRef CAS PubMed.
- A. B. Newberg, N. A. Wintering, K. Plossl, J. Hochold, M. G. Stabin, M. Watson, D. Skovronsky, C. M. Clark, M. P. Kung and H. F. Kung, J. Nucl. Med., 2006, 47, 748 CAS.
- M. P. Kung, C. Hou, Z. P. Zhuang, B. Zhang, D. Skovronsky, J. Q. Trojanowski, V. M. Lee and H. F. Kung, Brain Res., 2002, 956, 202 CrossRef CAS.
- M. Cui, M. Ono, H. Kimura, H. Kawashima, B. L. Liu and H. Saji, Nucl. Med. Biol., 2011, 38, 313 CrossRef CAS PubMed.
- Y. Maya, M. Ono, H. Watanabe, M. Haratake, H. Saji and M. Nakayama, Bioconjugate Chem., 2009, 20, 95 CrossRef CAS PubMed.
- M. P. Kung, C. Hou, Z. P. Zhuang, A. J. Cross, D. L. Maier and H. F. Kung, Eur. J. Nucl. Med. Mol. Imaging, 2004, 31, 1136 CrossRef CAS PubMed.
- Z. P. Zhuang, M. P. Kung, A. Wilson, C. W. Lee, K. Plossl, C. Hou, D. M. Holtzman and H. F. Kung, J. Med. Chem., 2003, 46, 237 CrossRef CAS PubMed.
- W. Qu, M. P. Kung, C. Hou, S. Oya and H. F. Kung, J. Med. Chem., 2007, 50, 3380 CrossRef CAS PubMed.
- B. Yousefi, A. Manook, B. von Reutern, M. Schwaiger, A. Drzezga, H. Wester and G. Henriksen, MedChemComm, 2012, 3, 775 RSC.
- D. Yanagisawa, T. Amatsubo, S. Morikawa, H. Taguchi, M. Urushitani, N. Shirai, K. Hirao, A. Shiino, T. Inubushi and I. Tooyama, Neuroscience, 2011, 184, 120 CrossRef CAS PubMed.
- T. Amatsubo, D. Yanagisawa, S. Morikawa, H. Taguchi and I. Tooyama, Magn. Reson. Med. Sci., 2010, 9, 95 CrossRef CAS.
- C. Ran, X. Xu, S. B. Raymond, B. J. Ferrara, K. Neal, B. J. Bacskai, Z. Medarova and A. Moore, J. Am. Chem. Soc., 2009, 131, 15257 CrossRef CAS PubMed.
- M. Hintersteiner, A. Enz, P. Frey, A. L. Jaton, W. Kinzy, R. Kneuer, U. Neumann, M. Rudin, M. Staufenbiel, M. Stoeckli, K. H. Wiederhold and H. U. Gremlich, Nat. Biotechnol., 2005, 23, 577 CrossRef CAS PubMed.
- H. Watanabe, M. Ono, R. Ikeoka, M. Haratake, H. Saji and M. Nakayama, Bioorg. Med. Chem., 2009, 17, 6402 CrossRef CAS PubMed.
- A. E. Johnson, F. Jeppsson, J. Sandell, D. Wensbo, J. A. Neelissen, A. Jureus, P. Strom, H. Norman, L. Farde and S. P. Svensson, J. Neurochem., 2009, 108, 1177 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c3md00189j |
| This journal is © The Royal Society of Chemistry 2014 |