Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Long-chain aliphatic polyesters from plant oils for injection molding, film extrusion and electrospinning†
Florian
Stempfle‡
a,
Benjamin S.
Ritter‡
bc,
Rolf
Mülhaupt
*bc and
Stefan
Mecking
*a
aChair of Chemical Materials Science, Department of Chemistry, University of Konstanz, Universitätsstraße 10, D-78457 Konstanz, Germany. E-mail: stefan.mecking@uni-konstanz.de; Fax: +49 7531 88-5152; Tel: +49 7531 88-2593
bFreiburg Materials Research Center (FMF), Stefan-Meier-Straße 21, D-79104 Freiburg, Germany. E-mail: rolf.muelhaupt@makro.uni-freiburg.de; Fax: +49 761 203-6319; Tel: +49 761 203-6273
cInstitute for Macromolecular Chemistry of the University of Freiburg, Stefan-Meier-Straße 31, D-79104 Freiburg, Germany
First published on 17th March 2014
Abstract
The polycondensation of long-chain α,ω-diesters with long-chain α,ω-diols, prepared by means of catalytic conversion of plant oils, affords linear aliphatic polyesters. They contain both long crystallizable polyethylene-like hydrocarbon segments and ester moieties in the backbone. In a convenient catalytic one-step process a high-purity polycondensation grade dimethyl-1,19-nonadecanedioate monomer is obtained directly from the technical grade methyl ester of high oleic sunflower oil. Likewise, dimethyl-1,23-tricosanedioate is derived from methyl erucate. The successful scale-up renders both intermediates available on a 100 g scale. Injection molded parts of polyester-19.19 and -23.23 with a number average molecular mass of Mn = 3 × 104 g mol−1 possess an elongation at break of >600% and a Young's modulus of 400 MPa. Electrospinning produces non-woven meshes. The polyesters prepared even enable film extrusion and represent new blend components for a variety of thermoplastics including polyethylene.
Introduction
Dicarboxylic acids derived from plant oils are attractive intermediates for the synthesis of polymer materials.1 Similar to polyethylene, the incorporation of long methylene sequences enables polymer crystallization and renders polyesters hydrophobic. Long-chain aliphatic polyesters that incorporate the full length of the plant oil methylene sequences possess high melting points (Tm > 100 °C) and crystallization temperatures, suitable for thermoplastic processing. This differs from shorter chain analogs. Here, the incorporation of aromatic comonomers such as terephthalic acid is required in order to improve dimensional stability, heat distortion temperature and mechanical properties of aliphatic polyesters.2More recently, entirely chemical catalytic conversions of plant oils have complemented the existing approach of biotechnological ω-oxidation as a route to long-chain aliphatic monomers.3,4
Self-metathesis5–9 generates (after subsequent double bond hydrogenation) a long-chain α,ω-diacid ester along with a stoichiometric amount of linear hydrocarbon as the main products (Scheme 1). In this context, the implementation of a commercial-scale refining of palm oil to chemicals by metathesis with 1-butene is notable.10,11 Due to the specifics of the process and of olefin metathesis in general, a significant amount of self-metathesis will occur as a side reaction to yield 1,18-octadecene dioate.12
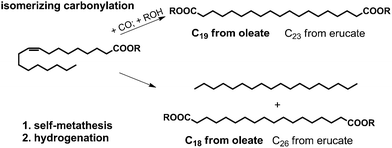 | ||
| Scheme 1 Main products of the isomerizing carbonylation and self-metathesis, respectively, of unsaturated fatty acid esters. | ||
A different route has recently been found with isomerizing alkoxycarbonylation (Scheme 1).13–15 Other than metathesis, the long-chain methylene sequence of the fatty acid feedstock is fully incorporated into the α,ω-diacid ester product. This is a result of the highly kinetically controlled outcome of the isomerizing carbonylation reaction vs. the equilibrium product distribution from metathesis.16
Polycondensation reactions are well established and used widely in industry for the synthesis of, amongst others, polyesters. Nonetheless, obtaining sufficient molecular weight for achieving entanglement and mechanical strength is not trivial for a given set of monomers. Typically, polyester molecular weights are in the range of 104 to 5 × 104 g mol−1 in order to prevent embrittlement and premature mechanical failure on straining. Molecular weights of polyesters or polyamides prepared by step-growth polycondensation are highly sensitive to the presence of impurities and require a high purity and accurate stoichiometry of difunctional monomers. Obtaining a material with useful mechanical properties from a new type of monomer is always precarious, and there is no generic protocol. For example, most A2 + B2 syntheses of polyester materials employ a diol which is volatile to a relevant extent under polycondensation conditions such that it can be used in excess in the initial reaction mixture. This does not apply to the polyesters studied here. Due to these and other general limitations only little is known about the materials properties of such entirely long-chain aliphatic polyesters based on symmetric α,ω-functionalized monomers. We now report on the mechanical properties and processing including blending of recently reported14,15 entirely aliphatic semicrystalline polyesters based on linear long-chain dicarboxylic acids and diols.
Results and discussion
Monomer and polymer synthesis
As an access to long-chain linear aliphatic diesters and diols, we utilized isomerizing alkoxycarbonylation. High oleic sunflower oil methyl ester (Dakolub MB9001) or technical grade methyl erucate, respectively, was used as a starting material. A well-defined catalyst precursor, [1,2-bis{(di-tert-butylphosphino)methyl}benzene palladium ditriflate],15 was employed. It is significantly more efficient in comparison with catalysts obtained by mixing a palladium source and the diphosphine in situ, which require a five-fold excess of the diphosphine.17,18 As a prerequisite for the studies of polyesters reported here, this monomer synthesis was investigated on an enhanced scale in a 1 L pressure reactor (Table 1). No adverse effects on productivity, selectivity or product purity vs. smaller scale syntheses15 were observed. Upon crystallization from methanol, used as the reaction medium, the linear C19 and C23 diesters were obtained on a 100 g scale in >99% purity (Fig. 1). The residual filtrate predominantly consists of (isomerized) starting material and in principle can be reused again. Even the α,β-unsaturated carbonyl compounds which represent the most stable isomer can be reacted to the desired linear products.13 | ||
| Fig. 1 Photograph of dimethyl 1,19-nonadecanedioate as obtained from entry 1, Table 1. | ||
| Entry | Substrate | Conversionb | Selectivityb | Isolated yield of pure product |
|---|---|---|---|---|
| a Conditions: 430 mmol substrate, 0.86 mmol [(dtbpx)Pd(OTf)2] (dtpbx = 1,2-bis{(di-tert-butylphosphino)methyl}benzene), 550 mL MeOH, 20 bar CO, 90 °C, 120 h. b Determined by GC analysis of the crude reaction mixture. | ||||
| 1 | Methyl oleate | 89% | 89% | 115 g (76%) |
| 2 | Methyl erucate | 72% | 84% | 96 g (54%) |
Long-chain α,ω-diol monomers were generated by reduction of the aforementioned diesters, stoichiometrically with LiAlH4 or by catalytic hydrogenation with Saudan's19 ruthenium catalyst. Previous work by our laboratory demonstrated that polycondensation of stoichiometric amounts of these difunctional A2 + B2 monomers catalyzed by titanium alkoxides afforded the corresponding long-chain aliphatic polyesters, namely poly[1,19-nonadecadiyl-1,19-nonadecanedioate] (PE-19.19) and poly[1,23-tricosadiyl-1,23-tricosanedioate] (PE-23.23).14 In order to elucidate the polyesters’ materials properties, increased quantities of polymer were required. Thus polymerizations were typically performed on a scale of 20 mmol of either monomer, affording between 10 and 15 g of polyester (Table 2).§ Optimized reaction conditions essentially comprised 0.04 mol% of Ti(OBu)4 and stirring for 16 hours at 200 °C and 0.01 mbar with a helicon stirrer (cf. Experimental section). Polymerization under these conditions and on this scale yielded polyesters with a number average molecular weight of Mn 3.0 × 104 g mol−1 as determined by both 1H-NMR quantification of end-groups and high temperature size exclusion chromatography (SEC) relative to polyethylene standards. In view of the largely polyethylene-like character of the polymer chains, SEC vs. polyethylene standards appears most appropriate in comparison with other common standards.
T
m![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) a/°C a/°C |
T
c![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) a/°C a/°C |
M
n,NMR![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) b/g mol−1 b/g mol−1 |
M
n,GPC![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) c/g mol−1 c/g mol−1 |
M
w/Mn![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) c c |
|
|---|---|---|---|---|---|
| a Determined by DSC with a heating/cooling rate of 10 K min−1. b Determined by end-group analysis from 1H NMR spectroscopy. c GPC at 160 °C in trichlorobenzene versus polyethylene standards. | |||||
| PE-19.19 | 102 | 83 | 2.9 × 104 | 3.0 × 104 | 2.6 |
| PE-23.23 | 107 | 86 | 3.9 × 104 | 3.9 × 104 | 2.4 |
In view of melt processing, required amongst others for the preparation of samples for tensile testing (vide infra), it is worth noting that the polyesters prepared display a simple rheological behavior (Fig. 2 and ESI†). The storage modulus (G′) and loss modulus (G′′) can be superimposed only by horizontal shifts along the frequency axis.20 This shows that no undesired chemical or physical changes occur in the melt, such as changes of molecular weight by degradation, further reactions of end-groups, or crosslinking.
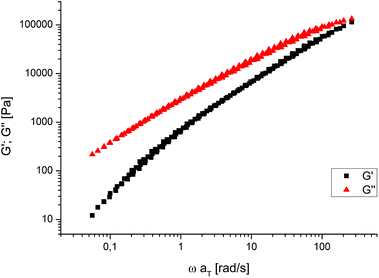 | ||
| Fig. 2 Master curves of G′ and G′′ for PE-19.19 (from measurements at 130, 140, 150, 160 and 170 °C). Shifting temperature: 150 °C. | ||
Tensile properties
Concerning the mechanical properties of long-chain A2B2-type polyesters, Cho et al. investigated polyester-30,30 from polycondensation of diethyl-1,30-tricontanedioate and tricontane-1,30-diol.21 However, an elongation at break of only 5% was found, indicating that the molecular weight of the material was likely insufficient to enable effective chain entanglement.For tensile testing of PE-19.19 and -23.23 generated by the above protocol, dumbbell test specimens were obtained by injection molding using a mini-compounder (Experimental section and ESI†).
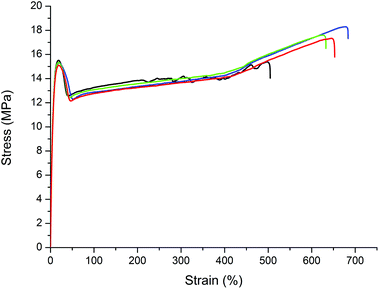
Following a linear and nonlinear viscoelasticity regime (I), a neck region with strain softening (II) and plastic flow (III), strain hardening (IV) is also observed as concluded from the characteristic changes of slope in the stress–strain curves (Fig. 3 and ESI†).22 That is, the polyesters prepared clearly show typical properties of a polymeric material rather than a brittle wax-like behavior.
Both polyesters show Young's moduli around 400 MPa and an elongation at break of more than 600% (Table 3). In detail, PE-23.23 displays a slightly higher modulus vs.PE-19.19, as expected due to the higher crystallinity14 arising from the longer methylene sequences. These tensile properties are similar to those of polyesters [O(CH2)nC(O)]x generated by ring opening chain growth polymerization of naturally occurring pentadecalactone (n = 15)23–25 or AB-polycondensation of 14-hydroxytetradecane carboxylic acid from enzymatic ω-hydroxylation of myristic acid (n = 14).26 By comparison, high density polyethylene (HDPE) from linear insertion chain growth typically displays a Young's modulus of ca. 1 GPa.27
| Young's modulus [MPa] | Yield stress [MPa] | Yield strain [%] | Tensile stress at break [MPa] | Tensile strain at break [%] | |
|---|---|---|---|---|---|
| a True stress at break calculated after cross-section area correction. | |||||
| PE-19.19 | 408 | 15.3 | 18.3 | 15.9 | 619 |
| PE-23.23 | 436 | 16.7 | 19.7 | 17.0 | 678 |
Shore D hardness increases with increasing chain length of the repeat units’ hydrocarbon segments from 54 for PE-19.19 to 56 for PE-23.23. This approaches the hardness of 60 to 70 reported for HDPE.28
Major application areas for conventional polyethylene particularly in the food sector are packaging materials. For this application, low density polyethylene is blow molded or extruded to produce films. As shown in Fig. 4, both PE-19.19 and PE-23.23 were successfully extruded by means of a twin-screw mini-extruder to produce films. This is quite remarkable as film breaking during extrusion is a frequently encountered problem. Apparently, the combination of melt stability (viscosity) and solidification behaviour is sufficient here. With an average film thickness of 60 microns, they showed little opacity (Fig. 4). Hence, applications such as bio-based packaging films and bags appear feasible.
Applications of thermoplastics in such diverse areas as clothing textiles and filters require non-wovens. To this end, a further preliminary insight into processing beyond the injection molding of samples for tensile testing was provided by electrospinning. Spinning from a pre-heated 7.5 wt% solution which was stabilized with 1 wt% (±)-α-tocopherol in 1,1,2,2-tetrachloroethane¶ yielded non-woven meshes of polyester-19,19 fibers with average diameters on the order of a few microns (Fig. 5).
Dynamic mechanical analysis (DMA)
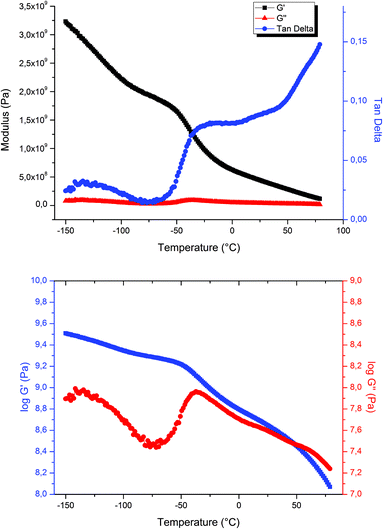
As a result of the high degree of crystallinity (χ) of about 70% for PE-19.19 and 75% for PE-23.23 as determined by wide-angle X-ray scattering,14 even upon rapid cooling or heating in DSC no glass transitions could be observed reliably due to the small portion of amorphous polymer subject to vitrification or softening, respectively. Dynamic mechanical analyses (DMA, at a frequency of 1 Hz) were more conclusive to this end. For PE-19.19 from the loss modulus vs. temperature a Tg of −37 °C was observed (Fig. 6). The longer-chain aliphatic polyester PE-23.23 exhibits a slightly higher glass-transition temperature of −27 °C (cf. ESI†). This difference can be ascribed to the enhanced crystallinity due to the longer methylene sequences and the resulting stronger restriction in mobility.29![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ||
||
Besides the relaxation due to the glass transition, both polymers show a second relaxation phenomenon at around −130 °C, which can be ascribed to the motion of the methylene sequences of the polyester main chain.30,31
Polyester melt compounding
Polymer blends offer the opportunity to expand the property profiles of polymeric materials. In fact, when appropriate compatibility is assured, polymer blends exhibit new properties which substantially deviate from the properties expected from just taking into account the properties and the mixing ratio of the individual blend components.32 Moreover, blends with commodity polymers are attractive with respect to improving cost efficiency.Blending of the long-chain polyesters (PE-19.19 and PE-23.23) with various types of thermoplastics was studied. For this purpose 5 and 15 wt%, respectively, of these polyesters were melt-compounded for 3 min at 150 rpm in a twin-screw mini-extruder with 1 wt% of (±)-α-tocopherol added as a stabilizer. DMA test specimens were produced by means of subsequent injection molding. Thermal properties were determined by means of both DMA and DSC measurements. As hydrocarbon blend components we investigated linear low density polyethylene (LLDPE, Affinity 8200G) and high density polyethylene (HDPE, Hostalen GC 7260), as well as a thermoplastic elastomer (SEBS, Kraton G1643 MS). According to the thermal and mechanical properties (see ESI†) all blends had a rather poor compatibility of both components. This was also observed for blends with a biodegradable aliphatic–aromatic polyester (Ecoflex F Blend C1200, BASF). Long-chain polyesters were successfully melt-compounded with talcum as a filler. Talcum is well established as a filler of engineering thermoplastics, especially in automotive applications.33,34 Talcum efficiently reinforces PE-19.19 und PE-23.23 (Table 4). The addition of 25 wt% talcum doubles the storage modulus. On increasing the talcum content to 45 wt%, a three-fold increase of the storage modulus was achieved with respect to the bulk polyester.
| Filler content/wt% |
T
m![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) a/°C a/°C |
T
c![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) a/°C a/°C |
G′ (RT)b/MPa | G′′ (RT)b/MPa | |
|---|---|---|---|---|---|
| a Determined by DSC with a heating/cooling rate of 15 K min−1. b Determined by DMA (1 Hz, 0.1% deformation). | |||||
| PE-19.19 | 0 | 102 | 83 | 1100 | 84 |
| PE-19.19 | 25 | 108 | 87 | 1510 | 130 |
| PE-19.19 | 45 | 107 | 88 | 1908 | 180 |
| PE-23.23 | 0 | 107 | 86 | 880 | 60 |
| PE-23.23 | 25 | 108 | 91 | 1950 | 150 |
| PE-23.23 | 45 | 109 | 95 | 2830 | 200 |
Summary and conclusions
These findings show that useful and attractive materials properties can be achieved for aliphatic polyesters generated by polycondensation of long-chain aliphatic diacids with long-chain diols. This is relevant, amongst others, as melting and crystallization temperatures of these new materials are advantageously high in comparison with existing shorter chain aliphatic polyesters. At the same time, the required monomers are accessible from plant oils in one or two steps only via self-metathesis or isomerizing alkoxycarbonylation as two complementary approaches. Here, we also demonstrate that polymerization grade long-chain α,ω-diacid ester monomers can be obtained conveniently on a 100 g scale in polycondensation grade purity using standard laboratory equipment by isomerizing alkoxycarbonylation.We have demonstrated that long-chain all-aliphatic polyesters meet the demands of injection molding, film extrusion and even electrospinning to produce non-woven meshes. High Young's moduli and elongation at break are attainable. Polyesters-19.19 and -23.23 were phase-separated from selected polymer matrices, while filling with talcum enhances the storage modulus significantly. This offers prospects for novel bio-based packaging materials, non-wovens and moldings.
Experimental section
Materials and general considerations
Methanol was distilled from magnesium turnings and iodine prior to use. Toluene was distilled from sodium and THF from sodium/benzophenone. All other solvents were used in technical grade as received. Carbon monoxide (3.7) and hydrogen (5.0) were supplied by Air Liquide. [Pd(dtbpx)(OTf)2] was prepared according to a reported procedure.15 High oleic sunflower oil methyl ester (92.5% of methyl oleate) supplied by Dako AG and methyl erucate (>90%) from TCI were degassed prior to use. Sodium methoxide (95%), titanium(IV) butoxide (≥97%) and (±)-α-tocopherol (synthetic, ≥96%) were purchased from Sigma Aldrich.NMR spectra were recorded on a Varian Inova 400. 1H and 13C chemical shifts were referenced to the solvent signals. High-temperature NMR measurements of polymers were performed in 1,1,2,2-tetrachloroethane-d2 (Euroisotop) at 130 °C. DSC analyses were performed on a Netzsch Phoenix 204 F1 instrument with a heating and cooling rate, respectively, of 10 K min−1. Data reported are from second heating cycles. GPC analyses of polymers were carried out in 1,2,4-trichlorobenzene at 160 °C at a flow rate of 1 mL min−1 on a Polymer Laboratories 220 instrument equipped with Olexis columns with differential refractive index, viscosity, and light-scattering (15° and 90°) detectors. Data reported were determined directly against linear PE standards. Gas chromatography was carried out on a PerkinElmer (PE) Clarus 500 instrument with an autosampler and FID detection on a PerkinElmer Elite-5 (5% diphenyl–95% dimethylpolysiloxane) Series capillary columns (length: 30 m, inner diameter: 0.25 mm, film thickness: 0.25 mm), using helium as a carrier gas at a flow rate of 1.5 mL min−1. The injector temperature was 300 °C. After injection the oven was kept isothermal at 90 °C for 1 min, heated at 30 K min−1 to 280 °C, and kept isothermal at 280 °C for 8 min.
Monomer synthesis
Alkoxycarbonylations of unsaturated long-chain fatty acid esters were carried out in a mechanically stirred 1.1 L stainless steel pressure reactor, equipped with a heating/cooling jacket supplied with a thermostat controlled by a thermocouple dipping into the reaction mixture. Prior to alkoxycarbonylation experiments the reactor was evacuated and purged with nitrogen several times.Catalytic reduction of the α,ω-diesters was performed analogously to a previously reported procedure.15 In brief, reduction (50 bar H2, 100 °C) was performed here on a scale of ca. 12 g of diester in THF solvent to yield, after recrystallization, pure diol in ca. 85% yield.
Polycondensation
Polyesters were prepared in a 100 mL two-necked Schlenk tube equipped with an overhead stirrer. Efficient mixing of the highly viscous polymer melt was achieved by a helical agitator. Under a static argon atmosphere the monomers (20.0 mmol of dimethyl 1,19-nonadecanedioate or diethyl-1,23-tricosanedioate, respectively, and 20.0 mmol of the corresponding α,ω-diol) were filled into the reaction vessel and melted by heating to 120 °C. A 0.6 mL aliquot of a 0.028 M titanium(IV) butoxide solution in toluene was injected, and the temperature was raised to 200 °C over the course of 8 h. Finally, the polymer melt was stirred for about 16 h at this temperature under reduced pressure (0.01 mbar).Tensile testing
Dogbone-shaped sample bars for tensile testing (75 × 12.5 × 2 mm3; ISO 527-2, type 5A) were prepared via melt compounding at 180 °C and 150 rpm for 3 min, using a DSM Xplore Micro 5cc Twin Screw Compounder and a DSM Xplore Micro 4cc Injection Molding Machine. In order to prevent oxidative degradation the polymers were stabilized with 1 wt% of (±)-α-tocopherol. For producing the polyester films a DSM Xplore Film device was used.After preconditioning the samples overnight at 23 °C tensile tests were performed on a Zwick Z005 instrument according to ISO 527 (crosshead speed 50 mm min−1). The Zwick test Xpert software version 11.0 was used to collect and analyze the results. Young's modulus, yield stress, yield strain, tensile stress at break and tensile strain at break were obtained by averaging the data from several test specimens.
Shore D Hardness
Measuring Shore D Hardness (according to DIN 535054) was performed with a Zwick 3150 H04 instrument at room temperature. For determination of hardness 12.5 × 12.5 × 4 mm test specimens were used. Values given are the average of six independent measurements.Dynamic mechanical analysis (DMA)
Dynamic-mechanical analyses (DMA) were recorded on melt compounded rectangular specimens (length × width × thickness = 25 × 6 × 2 mm3) using a Triton Technology TTDMA instrument equipped with single cantilever geometry. Measurements were performed from −150 °C to 80 °C at a heating rate of 3 °C min−1 and a frequency of 1 Hz. The Triton Technology DMA software was used to acquire and process the data. Glass transition temperatures (Tg) were determined from the temperature position of the maximum in loss modulus (G′′).Dynamic-mechanical analyses on the polymer compounds were performed using a TA Instruments Q 800 with single cantilever geometry. Measurements were performed from −60 °C to 10 °C under the melting point of the compounds investigated at a heating rate of 3 °C min−1, a deformation of 0.1% and a frequency of 1 Hz.
Rheological studies
Rheological measurements were carried out with an Advanced Rheometric Expansion System (ARES) from Rheometric Scientific. Test specimens (25 mm diameter) were prepared with a Dr Collin vacuum press 200 PV at 180 °C, and a pressure of 30 bar was sustained for 30 minutes. Polymers were stabilized with 1 wt% of (±)-α-tocopherol. Specimens were analyzed using a plate/plate geometry (25 mm diameter). The frequency was varied between 0.1 and 100 rad s−1. The temperature was raised from 110 to 180 °C during the measurement.Shifting of isothermal curves was performed only in a horizontal dimension, leading to bT = 1. The Carreau–Yasuda equation was used to extrapolate data to obtain the zero shear viscosity (cf. ESI†).
Acknowledgements
Financing by the Stiftung Baden-Württemberg (Programm Umwelttechnologieforschung, U14) is gratefully acknowledged. We thank Dako AG for donation of Dakolub MB9001. We also thank Prof. Dr Christian Friedrich for fruitful discussions and Dr Ralf Thomann for acquiring SEM images. Assistance by Markus Heiny is acknowledged.Notes and references
- U. Biermann, U. Bornscheuer, M. A. R. Meier, J. O. Metzger and H. J. Schäfer, Angew. Chem., Int. Ed., 2011, 50, 3854–3871 CrossRef CAS PubMed
.
-
M. Yamamoto, U. Witt, G. Skupin, D. Beimborn and R.-J. Müller, in Biopolymers, ed. A. Steinbüchel and Y. Doi, Wiley-VCH, Weinheim, 2002, vol. 4, pp. 299–311 Search PubMed
.
- S. Picataggio, T. Rohrer, K. Deanda, D. Lanning, R. Reynolds, J. Mielenz and L. D. Eirich, Nat. Biotechnol., 1992, 10, 894–898 CrossRef CAS PubMed
.
- W. Lu, J. E. Ness, W. Xie, X. Zhang, J. Minshull and R. A. Gross, J. Am. Chem. Soc., 2010, 132, 15451–15455 CrossRef CAS PubMed
.
- M. B. Dinger and J. C. Mol, Adv. Synth. Catal., 2002, 344, 671–677 CrossRef CAS
.
- A. K. Chatterjee, T.-L. Choi, D. P. Sanders and R. H. Grubbs, J. Am. Chem. Soc., 2003, 125, 11360–11370 CrossRef CAS PubMed
.
- H. L. Ngo, K. Jones and T. A. Foglia, J. Am. Oil Chem. Soc., 2006, 83, 629–634 CrossRef CAS PubMed
.
- C. Vilela, A. J. D. Silvestre and M. A. R. Meier, Macromol. Chem. Phys., 2012, 213, 2220–2227 CrossRef CAS
.
- F. Stempfle, P. Ortmann and S. Mecking, Macromol. Rapid Commun., 2013, 34, 47–50 CrossRef CAS PubMed
.
-
S. A. Cohen, M. L. Luetkens, C. Balakrishnan and R. Snyder (Elevance Renewable Sciences), WO2011/046872A2, 2011 Search PubMed
.
- Press release by Elevance Renewable Sciences on July 18, 2013.
- S. Chikkali and S. Mecking, Angew. Chem., Int. Ed., 2012, 51, 5802–5808 CrossRef CAS PubMed
.
- C. Jiménez-Rodriguez, G. R. Eastham and D. J. Cole-Hamilton, Inorg. Chem. Commun., 2005, 8, 878–881 CrossRef PubMed
.
- D. Quinzler and S. Mecking, Angew. Chem., Int. Ed., 2010, 49, 4306–4308 CrossRef CAS PubMed
.
- F. Stempfle, D. Quinzler, I. Heckler and S. Mecking, Macromolecules, 2011, 44, 4159–4166 CrossRef CAS
.
- P. Roesle, C. J. Dürr, H. M. Möller, L. Cavallo, L. Caporaso and S. Mecking, J. Am. Chem. Soc., 2012, 134, 17696–17703 CrossRef PubMed
.
- G. Walther, J. Deutsch, A. Martin, F.-E. Baumann, D. Fridag, R. Franke and A. Köckritz, ChemSusChem, 2011, 4, 1052–1054 CrossRef CAS PubMed
.
- M. R. L. Furst, R. Le Goff, D. Quinzler, S. Mecking, C. H. Botting and D. J. Cole-Hamilton, Green Chem., 2012, 14, 472–477 RSC
.
- L. A. Saudan, C. M. Saudan, C. Debieux and P. Wyss, Angew. Chem., Int. Ed., 2007, 46, 7473–7476 CrossRef CAS PubMed
.
-
P. C. Hiemenz and T. P. Lodge, Polymer Chemistry, CRC Press, Boca Raton, FL, 2nd edn, 2007 Search PubMed
.
- I. Cho and K. Lee, Macromol. Chem. Phys., 1997, 198, 861–869 CrossRef CAS
.
-
I. M. Ward and J. Sweeney, Mechanical properties of solid polymers, Wiley, Chichester, 3rd edn, 2012 Search PubMed
.
- J. Cai, C. Liu, M. Cai, J. Zhu, F. Zuo, B. S. Hsiao and R. A. Gross, Polymer, 2010, 51, 1088–1099 CrossRef CAS PubMed
.
- I. van der Meulen, E. Gubbels, S. Huijser, R. Sablong, C. E. Koning, A. Heise and R. Duchateau, Macromolecules, 2011, 44, 4301–4305 CrossRef CAS
.
- For fibers cf. M. de Geus, I. van der Meulen, B. Goderis, K. van Hecke, M. Dorschu, H. van der Werff, C. E. Koning and A. Heise, Polym. Chem., 2010, 1, 525–533 RSC
.
- C. Liu, F. Liu, J. Cai, W. Xie, T. E. Long, S. R. Turner, A. Lyons and R. A. Gross, Biomacromolecules, 2011, 12, 3291–3298 CrossRef CAS PubMed
.
-
K. S. Whiteley, T. G. Heggs, H. Koch, R. L. Mawer and W. Immel, Polyolefins, in Ullmann's Encyclopedia of Industrial Chemistry, ed. W. Gerhartz and B. Elvers, Wiley-VCH, Weinheim, 6th edn, 2005 Search PubMed
.
- For example, Hostalen GC 7260 (LyondellBasell) according to the suppliers data sheet exhibits a shore D hardness of 61.
- B. M. Grieveson, Polymer, 1960, 1, 499–512 CrossRef CAS
.
- M. Ito, M. Kubo, A. Tsuruta and K. Tanaka, J. Polym. Sci., Polym. Phys. Ed., 1978, 16, 1435–1446 CrossRef CAS
.
- M. Letizia Focarete, M. Scandola, A. Kumar and R. A. Gross, J. Polym. Sci., Part B: Polym. Phys., 2001, 39, 1721–1729 CrossRef
.
- L. Yu, K. Dean and L. Li, Prog. Polym. Sci., 2006, 31, 576–602 CrossRef CAS PubMed
.
-
M. Terada and T. Sugimoto (Nissan Motor Co., Ltd; Mitsubishi Petrochemical Co., Ltd), GB2246358 A, 1992 Search PubMed
.
-
H. Baann (Borealis A/S), WO1996/9622328A1, 1996 Search PubMed
.
Footnotes |
| † Electronic supplementary information (ESI) available: Stress–strain curves, DMA traces, and rheology master curve of polyester-23.23, image of test bar for tensile testing before and after strain experiment, additional rheological data. See DOI: 10.1039/c4gc00114a |
| ‡ Both authors contributed equally to this work. |
| § For polyester-19.19 prepared on a larger scale of 50 mmol, essentially identical molecular weights and tensile properties were observed. |
| ¶ Electrospinning from more environmentally benign non-chlorinated solvent such as decaline may be possible. However, this requires higher temperatures to ensure solubility of the polymer, not accessible with the electrospinning setup available. |
| || For poly(ω-hydroxyl tetradecanoic acid) (PE-14) a Tg of −30 °C has been reported,26 and for poly(ω-pentadecalactone) (PE-15) a Tg of −27 °C.31 |
| ** For NMR spectroscopic data cf.15 |
| This journal is © The Royal Society of Chemistry 2014 |