Barium sulphate catalyzed dehydration of lactic acid to acrylic acid†
Jiansheng
Peng
a,
Xinli
Li
a,
Congming
Tang
*a and
Wei
Bai
b
aChemical Synthesis and Pollution Control Key Laboratory of Sichuan Province, China West Normal University, Nanchong, Sichuan 637002, P.R. China. E-mail: tcmtang2001@163.com
bChengdu Institute of Organic Chemistry, Chinese Academy of Sciences, Chengdu, Sichuan 610041, P.R. China
First published on 23rd October 2013
Abstract
Dehydration of lactic acid was performed over various metal sulphates. BaSO4 was found to show an efficient activity for dehydration of lactic acid to acrylic acid due to the moderate acidity on its surface. Under the optimal conditions, 99.8% lactic acid conversion and 74.0% acrylic acid selectivity were achieved over the BaSO4 catalyst.
Preparation of bulk chemicals such as acrylic acid,1 propionic acid,2 ethanol,3,4 pyruvic acid5 and ethylene glycol6 from biomass has become an increasingly important subject in the modern industrial society.7 Acrylic acid and acrylates are widely used for the synthesis of polymers, dispersants, adhesives and coatings.8 Acrylic acid from lactic acid (LA) is viewed as a potential route due to the excessive depletion of fossil resources like petroleum, coal, and natural gas for the rapid development of the chemical industry.2,9–13 The modified NaY catalysts were demonstrated to show a high efficiency for dehydration of LA to acrylic acid.1,14–17 It is well known that the dehydration of LA is catalyzed by acid sites. Acidity is a key factor in the process of dehydration of lactic acid. Strong acidity is a great disadvantage for catalytic dehydration of LA, while it favors decarbonylation and/or decarboxylation of LA to acetaldehyde.15,18 In contrast, a catalyst with no acid sites or with basicity will be inefficient for catalytic dehydration of LA. Thus to adjust the acidity strength of a catalyst to an appropriate degree is a crucial step for efficient catalytic dehydration. A case in point is that several modifiers are used to change the surface acidity of NaY zeolites to achieve a high catalytic performance.14,19 But these modifiers16,17 are easily lost from the surface of the catalyst under an atmosphere of water vapor because they have an excellent solubility in water, resulting in deactivation of the catalyst with time on stream. An insoluble salt in water with an appropriate acidity may be the ideal type of catalyst for dehydration of lactic acid at high temperature. The Ca3(PO4)2–Ca2P2O7 (50
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50 wt%) catalyst has been demonstrated to efficiently catalyze dehydration of methyl lactate.20 The CaSO4–CuSO4 composite catalysts have a high activity for dehydration of lactic acid or lactates.21,22 More recently, Ghantani et al.23 reported that calcium hydroxyapatite catalyzed the dehydration of LA to acrylic acid, achieving 100% conversion of LA and 60% selectivity toward acrylic acid under the optimal reaction conditions. To the best of our knowledge, the highest yield (68%) of acrylic acid for the dehydration of lactic acid was obtained for the CaSO4–Na2SO4 composite catalysts.24 However, the knowledge of the relationship between acidity of catalysts and the activity for dehydration of lactic acid is still quite limited. Herein, we present a study on acidity of metal sulphates and catalytic activity for dehydration of LA to acrylic acid. Furthermore, other parameters such as calcination temperature, reaction temperature, lactic acid concentration and reaction time are also discussed.
50 wt%) catalyst has been demonstrated to efficiently catalyze dehydration of methyl lactate.20 The CaSO4–CuSO4 composite catalysts have a high activity for dehydration of lactic acid or lactates.21,22 More recently, Ghantani et al.23 reported that calcium hydroxyapatite catalyzed the dehydration of LA to acrylic acid, achieving 100% conversion of LA and 60% selectivity toward acrylic acid under the optimal reaction conditions. To the best of our knowledge, the highest yield (68%) of acrylic acid for the dehydration of lactic acid was obtained for the CaSO4–Na2SO4 composite catalysts.24 However, the knowledge of the relationship between acidity of catalysts and the activity for dehydration of lactic acid is still quite limited. Herein, we present a study on acidity of metal sulphates and catalytic activity for dehydration of LA to acrylic acid. Furthermore, other parameters such as calcination temperature, reaction temperature, lactic acid concentration and reaction time are also discussed.
Initially, acidity of metal sulphates and catalytic activity for dehydration of lactic acid to acrylic acid were investigated and the results are given in Table 1. The data of the BET surface area of metal sulphates are shown in the ESI (Table S1†). Conversion of LA drastically changed depending on the type of metal sulphate. LA was almost fully converted in the process of catalytic reaction using Al2SO4, NiSO4, CaSO4 and BaSO4. As for ZnSO4 and Na2SO4, the conversion of LA was less than 50%. Unlike the tendency of LA conversion to change depending on the type of metal sulphate, acrylic acid selectivity increased from Al2(SO4)3 with only 9.4% to CaSO4 with 68.6%. Conversely, selectivity toward acetaldehyde decreased from Al2SO4 with 79.0% to BaSO4 with only 20.3%. The formation of other products such as propionic acid, 2,3-pentanedione and acetic acid was also influenced by the type of metal sulphate. In order to further understand the factors which affect the conversion of LA to acrylic acid, the acidity strength and density of all catalysts were tested using the Hammett indicator method. The results are given in Table 2. It is clearly seen that the order of acidity strength for metal sulphates is Al3+ > Zn2+ > Ni2+ > Mg2+ > Ba2+ > Ca2+ > Na+. Although the conversion of lactic acid fluctuated with the acidity strength of the catalyst, the selectivity toward acrylic acid decreased with an increase in the definite range of acidity strength of catalysts and the selectivity toward acetaldehyde increased with an increase in the definite range of acidity strength of catalysts. Strong acidity favored decarbonylation or decarboxylation of LA to form acetaldehyde. Among all metal sulphates, Al2(SO4)3 exhibits the strongest acidity, and serious decarbonylation reaction occurs to form acetaldehyde with a high selectivity of 79.0%. Similar results were reported by Katryniok et al.18 In tested metal sulphates, BaSO4, CaSO4 and MgSO4 have a moderate acidity, and more than 60% selectivity of acrylic acid has been achieved over these catalysts. Compared with MgSO4, both BaSO4 and CaSO4 cannot make dimethyl yellow with a pKa of +3.3 to display acidic colour, suggesting that the acidity strength of BaSO4 and CaSO4 is weaker than that of MgSO4. Higher selectivity of acrylic acid was obtained over the BaSO4 and CaSO4 catalysts. It seems as if it is an advantage for dehydration of LA to acrylic acid when the surface acidity of the catalyst becomes weaker. This prompted us to investigate a catalyst with weaker acidity. Na2SO4 was chosen and it cannot make methyl red with a pKa of +4.8 to display acidic colour, indicating that its H0 is above +4.8 and the acidity is the weakest. From the results obtained for Na2SO4 including 30.0% conversion of lactic acid as well as 27.0% selectivity of acrylic acid, we can conclude that the rare acidity on the surface of the catalyst is a disadvantage for dehydration of LA. Therefore, the H0 characterizing the acidity strength of the catalyst lies between +3.3 and +4.8, favoring the conversion of LA to form acrylic acid.
| Catalystc | LA conv. [%] | Sel.b [%] | ||||
|---|---|---|---|---|---|---|
| AA | AD | PA | PD | ACA | ||
| a Conditions: calcination temperature 500 °C, reaction temperature 400 °C, catalyst: 0.50–0.60 g, particle size: 20–40 meshes, carrier gas N2: 1 mL min−1, feed flow rate: 1 mL h−1, LA feedstock: 20 wt% in water. b LA: lactic acid, AA: acrylic acid, AD: acetaldehyde, PA: propionic acid, PD: 2,3-pentanedione, ACA: acetic acid. c Carbon recovery is above 96% except for the Na2SO4 catalyst (85%). | ||||||
| Al2(SO4)3 | 100 | 9.4 | 79.0 | 5.5 | 0.7 | 1.9 |
| NiSO4 | 99.8 | 19.4 | 54.4 | 9.3 | 1.3 | 2.6 |
| ZnSO4 | 45.0 | 26.4 | 55.1 | 12.5 | 1.5 | 3.6 |
| MgSO4 | 85.0 | 61.7 | 29.0 | 6.2 | 1.2 | 1.6 |
| BaSO4 | 99.7 | 65.7 | 20.3 | 9.4 | 1.7 | 1.9 |
| CaSO4 | 99.8 | 68.6 | 20.5 | 7.6 | 0.9 | 1.6 |
| Na2SO4 | 30.0 | 27.0 | 23.0 | 24.1 | 1.3 | 3.3 |
| Catalystb | Acidity density of catalyst [mmol n-butylamine per g catalyst] | |||
|---|---|---|---|---|
| +4.8(a) | +4.0(b) | +3.3(c) | +1.5(d) | |
| 4.0 < H0 < 4.8 | 3.3 < H0 < 4.0 | 1.5 < H0 < 3.3 | H 0 < 1.5 | |
| a Hammett indicators, a: methyl red, b: 4-phenylazo-1-naphthylamine, c: dimethyl yellow, d: 4-(phenylazo)diphenylamine; “—”: non-acidic colour for the indicator. b All catalysts were calcinated at 500 °C for 6 h. | ||||
| Al2(SO4)3 | 0.0063 | 0.0271 | 0.0065 | 0.0025 |
| NiSO4 | 0.0006 | 0.0224 | 0.0056 | — |
| ZnSO4 | 0.0180 | 0.0130 | 0.0100 | — |
| MgSO4 | 0.0036 | 0.0016 | 0.0032 | — |
| BaSO4 | 0.0042 | 0.0114 | — | — |
| CaSO4 | 0.0101 | 0.0079 | — | — |
| Na2SO4 | — | — | — | — |
In a preliminary experiment, the stability of BaSO4 and CaSO4 was firstly evaluated (seen in Fig. S6 and S7†). Although CaSO4 has an excellent initial activity, the catalytic performance drastically decreased with an increase of reaction time. A possible reason is that the hydrothermal stability of CaSO4 is poor. Compared with CaSO4, the BaSO4 catalyst displays an excellent stability for catalytic activity due to the excellent hydrothermal stability of BaSO4. In addition, it has no strongly acid sites for causing side reactions (e.g. coke deposition) and has only moderately acid sites favoring dehydration of lactic acid to acrylic acid. Thereafter, we focused on the catalytic performance of the BaSO4 catalyst.
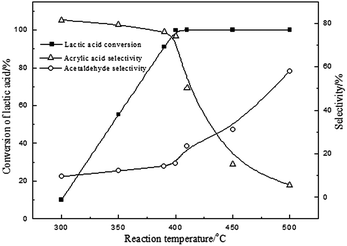
Due to the moderate acidity on the surface of BaSO4 with the range of H0 between +3.3 and +4.8, it exhibited an efficient activity. It is generally believed that calcination can improve the acidity and the stability in the process of vapour phase catalytic reaction.20,23 The influence of calcination temperature of BaSO4 on dehydration of LA was investigated and the results are shown in Table 3. It is clearly seen that LA conversion increases with the increase of calcination temperature up to 700 °C, and it slightly decreases with further increase to 900 °C. Interestingly, the surface acidity density of catalysts with different acidity distributions also increases with the enhancement of calcination temperature (seen in Table 3). But it started to decrease at 900 °C. This result further demonstrated that an increase of the acid amount of the catalyst with +3.3 < H0 < +4.8 favored the conversion of LA to acrylic acid. Besides, the BaSO4 catalysts with different calcination temperatures were also characterized by FTIR spectra and powder X-ray diffraction, and the results are shown in ESI (Fig. S2 and S3†). It is clearly seen that all characteristic peaks remain unanimous with each other, but the peak intensity is different for different calcination temperatures, indicating a change of catalytic performance. Other characterizations such as SEM and TG analysis are also given in ESI (Fig. S4 and S5†). Thus the optimal calcination temperature should be around 700 °C. At a fixed calcination temperature of 700 °C, the effect of the reaction temperature on the dehydration of LA was further investigated and the results are given in Fig. 1. From the results presented in Fig. 1, this reaction is very sensitive to temperature. LA conversion increased linearly in the range of 300–400 °C and LA was almost fully converted when the reaction temperature was above 400 °C. The selectivity to acrylic acid decreases with an increase in the reaction temperature. For example, acrylic acid selectivity is higher than 70% when the reaction temperature is lower than 400 °C, while it decreases to 5.6% at 500 °C. In contrast, the selectivity of acetaldehyde increased with an increase of the reaction temperature, indicating that high temperature favors the decarbonylation or decarboxylation to form acetaldehyde. Considering the conversion of LA and acrylic acid selectivity, the optimal reaction temperature should be about 400 °C. In addition, the effect of water is shown in Fig. 2. Conversion of lactic acid as well as selectivity to acrylic acid were slightly affected when the concentration of lactic acid was lower than 20 wt%. However, conversion of lactic acid as well as selectivity to acrylic acid decreased with an increase of lactic acid concentration. These results suggested that higher yield for acrylic acid is achieved at low concentration of lactic acid. High concentration of LA favored coke on the surface of the catalyst, resulting in a decrease of catalytic active sites (decrease of LA conversion). 2,3-Pentanedione selectivity increased with an increase of lactic acid concentration. It is known that the formation of 2,3-pentanedione is via a Claisen condensation reaction of two lactate moieties followed by decarboxylation and dehydration steps. Thus, an increase of the lactic acid concentration favors its condensation reaction to form 2,3-pentanedione. As is well known to all, changes of crystal water for the catalysts with crystal water influenced the acid strength. However, as for the BaSO4 catalyst, it has no crystal water. Therefore, the effect of water on the acid strength of the BaSO4 catalyst is very limited. Subsequently, the effect of time on stream on the catalytic performance was also investigated, and the results are shown in Fig. S7.†
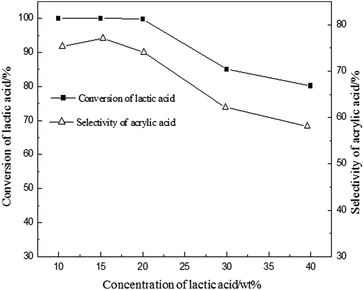 | ||
| Fig. 2 Effect of lactic acid concentration. Conditions: catalyst: 0.50–0.60 g, particle size: 20–40 meshes, carrier gas N2: 1 mL min−1, feed flow rate: 1 mL h−1, reaction temperature: 400 °C. | ||
| Calcination temp. [°C] | LA conv.b [%] | AA sel.c [%] | Acidity distributiond [mmol n-butylamine per g catalyst] | |
|---|---|---|---|---|
| 4.0 < H0 < 4.8 | 3.3 < H0 < 4.0 | |||
| a Conditions: reaction temperature 400 °C, BaSO4 catalyst: 0.57 g, particle size: 20–40 meshes, carrier gas N2: 1 mL min−1, feed flow rate: 1 mL h−1, LA feedstock: 20 wt% in water. b LA: lactic acid. c AA: acrylic acid. d Acidity distribution of the catalyst under different calcination temperatures. | ||||
| No | 67.5 | 77.0 | 0.0038 | 0.0032 |
| 300 | 68.0 | 75.1 | 0.0039 | 0.0101 |
| 500 | 99.7 | 65.7 | 0.0042 | 0.0114 |
| 700 | 99.8 | 74.0 | 0.0066 | 0.0163 |
| 900 | 95.0 | 65.8 | 0.0033 | 0.0095 |
Conclusions
In this work, we have presented a study on acidity of metal sulphates and the activity for dehydration of LA to acrylic acid. The catalyst with a moderate acidity strength of +3.3 < H0 < +4.8 exhibits an efficient activity. It is in agreement with the basic principles of heterogeneous catalysis that larger amounts of active sites (moderately acid sites) increase the catalytic activity (conversion of LA). LA conversion and product distribution are sensitive to reaction temperature. Low reaction temperature favors the dehydration of LA to form acrylic acid, while high temperature favors decarbonylation/decarboxylation of LA to form acetaldehyde. BaSO4 was found to have an excellent activity for dehydration of LA to acrylic acid. Under the optimal reaction conditions, 99.8% LA conversion and 74.0% acrylic acid selectivity were achieved over the BaSO4 catalyst.Acknowledgements
This work was supported by the Scientific Research Fund of Chemical Synthesis and Pollution Control Key Laboratory of Sichuan Province with project number of CSPC2012-7, the Scientific Research Fund of China West Normal University with project numbers of 10B004 and 12B019 and the Nanchong Key Technology R&D Program of Sichuan, China with project number of 11A0051.Notes and references
- J. F. Zhang, Y. L. Zhao, M. Pan, X. Z. Feng, W. J. Ji and C. T. Au, ACS Catal., 2011, 1, 32–41 CrossRef CAS.
- T. J. Korstanje, H. Kleijn, J. Jastrzebski and R. Gebbink, Green Chem., 2013, 15, 982–988 RSC.
- N. Rocha, M. A. Barros, J. Fischer, U. Coutinho and V. L. Cardoso, Renewable Energy, 2013, 57, 432–435 CrossRef CAS PubMed.
- Y. Liu, X. Z. Yuan, H. J. Huang, X. L. Wang, H. Wang and G. M. Zeng, Fuel Process. Technol., 2013, 112, 93–99 CrossRef CAS PubMed.
- S. Lomate, T. Bonnotte, S. Paul, F. Dumeignil and B. Katryniok, J. Mol. Catal. A: Chem., 2013, 377, 123–128 CrossRef CAS PubMed.
- N. Ji, T. Zhang, M. Y. Zheng, A. Q. Wang, H. Wang, X. D. Wang and J. G. G. Chen, Angew. Chem., Int. Ed., 2008, 47, 8510–8513 CrossRef CAS PubMed.
- D. Esposito and M. Antonietti, ChemSusChem, 2013, 6, 989–992 CrossRef CAS PubMed.
- C. M. Tang, Y. Zeng, X. G. Yang, Y. C. Lei and G. Y. Wang, J. Mol. Catal. A: Chem., 2009, 314, 15–20 CrossRef CAS PubMed.
- C. Gao, C. Q. Ma and P. Xu, Biotechnol. Adv., 2011, 29, 930–939 CrossRef CAS PubMed.
- M. S. Holm, S. Saravanamurugan and E. Taarning, Science, 2010, 328, 602–605 CrossRef CAS PubMed.
- C. M. Tang, Y. Zeng, P. Cao, X. G. Yang and G. Y. Wang, Catal. Lett., 2009, 129, 189–193 CrossRef CAS.
- C. T. Lira and P. J. McCrackin, Ind. Eng. Chem. Res., 1993, 32, 2608–2613 CrossRef CAS.
- G. C. Gunter, R. H. Langford, J. E. Jackson and D. J. Miller, Ind. Eng. Chem. Res., 1995, 34, 974–980 CrossRef CAS.
- J. Yan, D. Yu, H. Li, P. Sun and H. Huang, J. Rare Earths, 2010, 28, 803–806 CrossRef CAS.
- P. Sun, D. H. Yu, Z. C. Tang, H. Li and H. Huang, Ind. Eng. Chem. Res., 2010, 49, 9082–9087 CrossRef CAS.
- P. Sun, D. H. Yu, K. M. Fu, M. Y. Gu, Y. Wang, H. Huang and H. H. Ying, Catal. Commun., 2009, 10, 1345–1349 CrossRef CAS PubMed.
- H. J. Wang, D. H. Yu, P. Sun, J. Yan, Y. Wang and H. Huang, Catal. Commun., 2008, 9, 1799–1803 CrossRef CAS PubMed.
- B. Katryniok, S. Paul and F. Dumeignil, Green Chem., 2010, 12, 1910 RSC.
- J. Yan, D. Yu, P. Sun and H. Huang, Chin. J. Catal., 2011, 32, 405–411 CrossRef CAS.
- J. H. Hong, J.-M. Lee, H. Kim, Y. K. Hwang, J.-S. Chang, S. B. Halligudi and Y.-H. Han, Appl. Catal., A, 2011, 396, 194–200 CrossRef CAS PubMed.
- J. F. Zhang, J. P. Lin, X. B. Xu and P. L. Cen, Chin. J. Chem. Eng., 2008, 16, 263–269 CrossRef CAS.
- J. F. Zhang, J. P. Lin and P. L. Cen, Can. J. Chem. Eng., 2008, 86, 1047–1053 CrossRef CAS.
- V. C. Ghantani, S. T. Lomate, M. K. Dongare and S. B. Umbarkar, Green Chem., 2013, 15, 1211–1217 RSC.
- R. E. Holmen, US Patent, 2859240, 1958 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c3gc42028k |
| This journal is © The Royal Society of Chemistry 2014 |