Emerging pollutants in the Esmeraldas watershed in Ecuador: discharge and attenuation of emerging organic pollutants along the San Pedro–Guayllabamba–Esmeraldas rivers†
A.
Voloshenko-Rossin
a,
G.
Gasser
a,
K.
Cohen
a,
J.
Gun
ab,
L.
Cumbal-Flores
c,
W.
Parra-Morales
d,
F.
Sarabia
e,
F.
Ojeda
e and
O.
Lev
*a
aThe Casali Institute and the Institute of Chemistry, The Hebrew University of Jerusalem, Jerusalem, 91904, Israel. E-mail: ovadia@huji.ac.il
bDepartment of Environmental Health, The Hadassa Academic College, Jerusalem, 96970, Israel
cCenter of Nanoscience and Nanotechnology, Universidad de las Fuerzas Armadas, Sangolquí, Ecuador
dFacultad de Ciencias Químicas, Universidad Central del Ecuador, Quito, Ecuador
eEmpresa Pública Metropolitana de Agua Potable y Saneamiento, Quito, Ecuador
First published on 17th October 2014
Abstract
Water quality characteristics and emerging organic pollutants were sampled along the San Pedro–Guayllabamba–Esmeraldas River and its main water pollution streams in the summer of 2013. The annual flow rate of the stream is 22![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 Mm3 y−1 and it collects the wastewater of Quito-Ecuador in the Andes and supplies drinking water to the city of Esmeraldas near the Pacific Ocean. The most persistent emerging pollutants were carbamazepine and acesulfame, which were found to be stable along the San Pedro–Guayllabamba–Esmeraldas River, whereas the concentration of most other organic emerging pollutants, such as caffeine, sulfamethoxazole, venlafaxine, O-desmethylvenlafaxine, and steroidal estrogens, was degraded to a large extent along the 300 km flow. The mass rate of the sum of cocaine and benzoylecgonine, its metabolite, was increased along the stream, which may be attributed to coca plantations and wild coca trees. This raises the possibility of using river monitoring as an indirect way to learn about changes in coca plantations in their watersheds. Several organic emerging pollutants, such as venlafaxine, carbamazepine, sulphamethoxazole, and benzoylecgonine, survived even the filtration treatment at the Esmeraldas drinking water system, though all except for benzoylecgonine are found below 20 ng L−1, and are therefore not likely to cause adverse health effects. The research provides a way to compare drug consumption in a major Latin American city (Quito) and shows that the consumption of most sampled drugs (carbamazepine, venlafaxine, O-desmethylvenlafaxine, sulphamethoxazole, ethinylestradiol) was below their average consumption level in Europe, Israel, and North America.
000 Mm3 y−1 and it collects the wastewater of Quito-Ecuador in the Andes and supplies drinking water to the city of Esmeraldas near the Pacific Ocean. The most persistent emerging pollutants were carbamazepine and acesulfame, which were found to be stable along the San Pedro–Guayllabamba–Esmeraldas River, whereas the concentration of most other organic emerging pollutants, such as caffeine, sulfamethoxazole, venlafaxine, O-desmethylvenlafaxine, and steroidal estrogens, was degraded to a large extent along the 300 km flow. The mass rate of the sum of cocaine and benzoylecgonine, its metabolite, was increased along the stream, which may be attributed to coca plantations and wild coca trees. This raises the possibility of using river monitoring as an indirect way to learn about changes in coca plantations in their watersheds. Several organic emerging pollutants, such as venlafaxine, carbamazepine, sulphamethoxazole, and benzoylecgonine, survived even the filtration treatment at the Esmeraldas drinking water system, though all except for benzoylecgonine are found below 20 ng L−1, and are therefore not likely to cause adverse health effects. The research provides a way to compare drug consumption in a major Latin American city (Quito) and shows that the consumption of most sampled drugs (carbamazepine, venlafaxine, O-desmethylvenlafaxine, sulphamethoxazole, ethinylestradiol) was below their average consumption level in Europe, Israel, and North America.
Environmental impactEmerging contaminants' origin and attenuation were studied along the San Pedro–Guayllabamba–Esmeraldas River, one of Ecuador's most important rivers, which starts at the Andes and discharges to the Pacific Ocean, where it supplies the drinking water to Esmeraldas city and nearby communities. The research illuminates the similarity and the differences between this Andean stream and the better-researched streams in North America and Europe. The concentration and per capita consumption of most studied emerging contaminants were lower compared to developed countries. Carbamazepine and acesulfame were found at high concentrations and behaved as conservative compounds and thus could be used as wastewater tracers. Benzoylecgonine and cocaine concentrations increased along the river, while the level of other pollutants decreased, which is interpreted to result from coca plants. |
Introduction
The abundance of emerging pollutants in wastewater and surface water, as well as ground water, received much attention in recent years.1–6 The compounds of this vaguely defined set of contaminants are often toxic to humans and exhibit ecotoxicity at very low concentrations.7–12 Some are being used as domestic pollution markers and for pollution source tracking due to their low degradation rates and high abundance in and specificity to domestic wastes.13–19 Some emerging contaminants are good indicators for dysfunctioning water treatment systems. Abundance of some emerging contaminants in wastewater provides an objective means to probe socioeconomics of the source population and the relationship between diet and culture, without using questionnaires and surveys of the studied population. This is particularly informative for illicit and pharmaceutical drug usage.20–23 Several reviews on the occurrence of emerging pollutants in the environment have recently been published, which provide a thorough account of the abundance of emerging pollutants in west Europe, Australia, North America,2,3,24,25 and in China26 and other regions of the world.27–29 While Europe and North America are rather well covered by comprehensive surveys, relatively little was reported on other regions of the world and particularly on Latin America. Our literature survey of emerging organic contaminants in South and Central America revealed very few dedicated research articles on emerging contaminants.27,30–37 Even those are mostly devoted to the occurrence of emerging contaminants in wastewater in Brazil and Mexico and for the removal of emerging contaminants from wastewater by different techniques. Fewer research projects addressed the pollution of rivers and surface water by emerging organic pollutants. Presumably, it is assumed that the high flow rates and high temperatures in the tropics dilute the pollutants and renders them undetectable and useless as pollution markers and consumption probes. This is unfortunate, since the developing countries are challenged more than others with polluted water sources and should rely more than others on sensitive markers to identify minute domestic pollution of their drinking water sources. Likewise, diet and drug uptake depend on socioeconomics and culture and those are bound to vary in different regions of the world. It is unclear whether the markers that are so often used to detect domestic pollution in Europe and North America are sufficiently abundant in Latin America, and whether they are at all suitable pollution markers outside the industrialised countries. Moreover, whereas in the industrialised countries the treatment systems are well developed and provide adequate protection for drinking water supplies, and this is not guaranteed for Latin America, which again underscores the need for emerging contaminant surveys in economically strained regions of the world.This research addressed the largest water stream in the Esmeraldas watershed of Ecuador. Fig. 1 shows a map of the watersheds of Ecuador. The Esmeraldas stream is one of the largest in Ecuador, though the Ecuadorian Amazonas sources are larger.38 The Esmeraldas watershed is unique in receiving domestic wastes of the Metropolitan District of Quito and also supplying drinking water to a large city, Esmeraldas, the Atacames shore resort, as well as a few other small towns near the Pacific coast. The extent to which the Esmeraldas and the Guayllabamba Rivers affect drinking water aquifers supplying water to nearby villages is left unresearched, but the consequences of the research may teach on the importance of future investigations. The main stream that is addressed in this research starts at the (Río) San Pedro River in the region that Alexander von Humboldt named the Avenue of Volcanoes (a corridor of volcanoes that includes the Corazón, Rumiñahui, Illinizas peaks, Viudita, Pasochoa, Sincholagua, and the Cotopaxi, which measures 5897 m in altitude). The San Pedro River discharges to the Guayllabamba River, which unites with the Blanco River to form the Esmeraldas River. The last is discharged to the Pacific near Esmeraldas City.
The Esmeraldas watershed covers over 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 km2, and the flow rate to the Pacific Ocean, 22
000 km2, and the flow rate to the Pacific Ocean, 22![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 Mm3 per year, is one of the largest in Ecuador due to the high average precipitation rate in the watershed, approximately 2000 mm per year.38 The wastewater of the Metropolitan District of Quito, approximately 152 Mm3 per year, was recently quantified by a detailed survey by EPMAPS, Quito's water and sanitation company. The data were taken from the characterisation report of the Quito's Rivers Pollution Control Program which monitored representative wastewater discharges in the city of Quito.39 This flow was discharged by 1.46 million residents. However, the San Pedro stream also disposes the wastewater of all 2.3 million residents of the Pichincha province (according to the 2010 census), which also eventually reaches the San Pedro and Guayllabamba Rivers. The total wastewater flow constitutes approximately 1% of the average discharge of the Esmeraldas River to the Pacific. The effect of this discharge was never studied and thus, the San Pedro–Guayllabamba–Esmeraldas stream may serve as a test case, which combines academic significance as a major tropical stream and a practical importance as a drinking water source.
000 Mm3 per year, is one of the largest in Ecuador due to the high average precipitation rate in the watershed, approximately 2000 mm per year.38 The wastewater of the Metropolitan District of Quito, approximately 152 Mm3 per year, was recently quantified by a detailed survey by EPMAPS, Quito's water and sanitation company. The data were taken from the characterisation report of the Quito's Rivers Pollution Control Program which monitored representative wastewater discharges in the city of Quito.39 This flow was discharged by 1.46 million residents. However, the San Pedro stream also disposes the wastewater of all 2.3 million residents of the Pichincha province (according to the 2010 census), which also eventually reaches the San Pedro and Guayllabamba Rivers. The total wastewater flow constitutes approximately 1% of the average discharge of the Esmeraldas River to the Pacific. The effect of this discharge was never studied and thus, the San Pedro–Guayllabamba–Esmeraldas stream may serve as a test case, which combines academic significance as a major tropical stream and a practical importance as a drinking water source.
This research was conducted to quantify important organic micropollutants in an Andean stream, to compare the abundance of these pollutants in Quito wastes to the more intensely researched European and North American ones, to elucidate the more persistent and abundant organic pollutants that can be used as tracers for domestic wastewater of the Metropolitan District of Quito, and to use them for quantification of the impact of the pollution in the Sierra on the water source of the coastal town of Esmeraldas, 250 km downstream and 3 km lower in altitude.
The target emerging pollutants included acesulfame (ACS) and carbamazepine (CBZ) and its metabolites, which have proven to be excellent quantitative tracers of domestic wastewater,13,14,16–18 the widely researched antibiotic drug sulphamethoxazole (SMX),40 the psychiatric drug fluoxetine (Prozac, FLX), which is widely used and is ecotoxic at the concentration range found in wastewater treatment plant effluents,41,42 and venlafaxine (VNF) and its main metabolite O-desmethylvenlafaxine (O-DMV),43,44 which are widely used serotonin–norepinephrine reuptake inhibitor (SNRI) antidepressants. The steroid estrogens, 17β-estradiol (E2), estrone (E1), and 17α-ethinylestradiol (EE2), which are abundant at low concentrations in wastewater, exhibit a very low ecotoxicity threshold, and affect fish fertility at exceedingly low concentrations,45–47 were also researched. Fishing in the Guayllabamba and the Esmeraldas Rivers is an important industry and tourist activity. EE2 is a synthetic steroidal hormone used in birth control pills, and it is discharged to the environment exclusively from domestic wastes. Since Ecuador does not manufacture birth control pills, EE2 can be considered as a specific domestic wastewater tracer; in addition, we included in our target contaminant list cocaine (benzoylmethylecgonine, COC) and its metabolite benzoylecgonine (BE). The illicit drugs were also included because Ecuador decriminalised the possession of a small amount of cocaine one day prior to our first sampling date. Most of Latin America has enacted a similar policy. It was hoped that the current investigation would help set a reference baseline for long-term cocaine usage trends following the act of decriminalisation.
Experimental section
Materials
All reference compounds were of high purity (>98%). Carbamazepine (CBZ), acridine (ACIN), acridone (ACON), 10-hydroxy-10,11-dihydrocarbamazepine (10OH-CBZ), 10,11-dihydro-10,11-dihydroxy carbamazepine (CBZ-DiOH), carbamazepine 10,11-epoxide (CBZ-E), caffeine, sulphamethoxazole (SMX), venlafaxine (VNF), fluoxetine (FLX), cocaine (COC), benzoylecgonine (BE), caffeine 13C3, and sulphamethoxazole-d4 (SMX-d4) were purchased from Sigma-Aldrich. Acesulfame K (the ACS salt) was purchased from Fluka. Acesulfame-d4 (ACS-d4), O-desmethylvenlafaxine (O-DMV), and venlafaxine-d6 (VNF-d6) were obtained from Toronto Research Chemicals Inc. (Canada). Carbamazepine-d10 (CBZ-d10) was purchased from C/D/N Isotopes Ltd, Pointe-Claire, Canada. 17β-estradiol (E2), estrone (E1), and ethinylestradiol (EE2) ELISA kits were purchased from Tokiwa Chemical Industries Ltd, Tokyo.Ammonium acetate (purity >98%) and chloride standards such as NaCl were purchased from Merck. Individual stock solutions were prepared by dissolving the compounds in HPLC-grade methanol. Acetonitrile (ACN), formic acid, and hydrochloric acid (37%) were supplied by Sigma-Aldrich. Purity of all organic solvents was higher than 99.8%. The nitrogen used for drying the solid-phase cartridges and for evaporation of solvents was of 99.995% purity from Maxima, Israel. Ultrapure water was provided by using a Millipore laboratory water purification system.
Sampling and storage procedures
Three sampling campaigns were carried out in summer time, starting on 30 June, 19 August, and 16 October, each for three to four days. Sampling trips were scheduled after dry intervals of several days in order to avoid dilution. Automatic composite sampling was carried out at each location using a Teledyne ISCO 6712 Full-Size Portable Sampler.According to the recommendation of the ‘Standard Methods for the examination of water & wastewater’52–54 the samples intended for analysis of micropollutants, DOC, TDN, and major cations by ICP-OES were acidified with concentrated HCl to pH < 2. Chloride and nitrate samples were collected separately and stored without the addition of HCl. Samples for DOC, TDN, nitrate, chlorate, sulphate, and major cations were filtered through a 0.45 μm filter.
Pretreatment of samples
The samples were transferred under refrigeration to the laboratory and stored in a refrigerator (4 ± 2 °C). Extraction was conducted within 120 h of sampling. Extracts were stored for less than 1 week in dark beakers in a refrigerator.Analytical methods
An analytical methodology for screening and confirming the presence of ACS, CBZ, ACIN, ACON, 10OH-CBZ, CBZ-DiOH, CBZ-E, caffeine, SMX, VNF, O-DMV, FLX, COC, and BE in water samples was developed using the Agilent G6410A triple quadrupole mass spectrometer (QQQ) with electrospray ionisation ion source (ESI). The method was developed following the guidelines in EPA Method 1694 (ref. 48) for elution of acid and basic compounds from solutions containing less than 1% solids. The maximal total suspended solids, TSS, of the current studies was 0.2 g L−1. A known amount of labelled compounds CBZ-d10, VNF-d6, caffeine 13C3, and SMX-d4 was added to the samples. The compounds were then extracted by Solid Phase Extraction (SPE) with Oasis HLB 60 mg cartridges (Waters, Milford, MA) using 1000 mL of each samples from points 1, 2, 7–11 and 200 mL of the more polluted samples (points 3–6). Analytes were subsequently eluted with methanol and formic acid solutions, and the mixed extracts were concentrated to a final volume of 5 mL by nitrogen flow. ACS was extracted by SPE using styrol–divinylbenzene cartridges (J.T. Baker SDB) according to a reported method49 and quantification was done with respect to ACS-d4.We used an Agilent 1200 high performance liquid chromatograph. The main characteristics of the LC/MS/MS method are presented in Table 1. Analyte separation was conducted with an Agilent ZORBAX Eclipse Plus C18 (2.1 mm ID, 100 mm length, and 3.5 μm particle size). Column temperature was set at 25 °C. The mobile phase consisted of 10% ACN, 90% H2O, and 0.1% formic acid. The eluent composition was as follows: initial conditions, 10% ACN fed at 0.2 mL min−1 for 5 minutes. At t = 6 min, the flow rate was increased to 0.3 mL min−1, and the composition was ramped to 60% ACN, maintaining a flow rate of 0.3 mL min−1 until t = 24 min. Finally, the composition of the eluent was changed gradually to 100% ACN at t = 30 min. Injection volume was 15 μL. Quantifications were carried out by isotope labelled internal standards for CBZ, CAF, VNF, and SMX and by multipoint calibrations for all other analytes. Limits of quantification (LOQs) calculated at 10 times the background level are shown in Table 1, along with the recovery at 1000 ng L−1 for points 1, 2, 7–11 (LOQs for points 3–6 were 10 times larger). The linearity of the response of three orders of magnitude was demonstrated (R2 > 0.99) for all the pharmaceuticals studied.
| Compound | Precursor ion | Product ions, quantifier and (qualifier) | Fragmentation voltage (V) | Collision energy (eV) | Electrospray ionization (ESI) | Mean recovery (±standard deviation) | LOQa (ng L−1) |
|---|---|---|---|---|---|---|---|
| a LOQ values refer to the analysis of points 1, 2, and 7–11. | |||||||
| ACS | 162 | 82, (78) | 70 | 10, 30 | Negative | 72.1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ± ±![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9.1 9.1 |
10 |
| CBZ | 237 | 194, (192), (179) | 120 | 15, 10, 35 | Positive | 86.2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ± ±![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 14.2 14.2 |
1 |
| ACIN | 180 | 178, (151) | 130 | 40, 55 | Positive | 75.4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ± ±![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 18.3 18.3 |
1 |
| ACON | 196 | 167, (139) | 115 | 35, 40 | Positive | 83.2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ± ±![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 11.2 11.2 |
1 |
| 10OH-CBZ | 255 | 194, (192) | 110 | 15, 25 | Positive | 79.3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ± ±![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10.9 10.9 |
1 |
| CBZ-DiOH | 271 | 236, (180) | 110 | 10, 30 | Positive | 71.4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ± ±![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10.8 10.8 |
5 |
| CBZ-E | 253 | 180, (211) | 115 | 10, 15 | Positive | 81.2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ± ±![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 13.7 13.7 |
1 |
| Caffeine | 194.9 | 137.7, (109.8) | 110 | 20, 25 | Positive | 82.1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ± ±![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10.2 10.2 |
2 |
| SMX | 254 | 156, (92) | 110 | 10, 15 | Positive | 76.1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ± ±![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9.7 9.7 |
1 |
| VNF | 278 | 58, (121) | 110 | 15, 35 | Positive | 88.2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ± ±![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10.2 10.2 |
0.1 |
| O-DMV | 264 | 58, (107) | 105 | 15, 30 | Positive | 86.4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ± ±![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9.7 9.7 |
0.1 |
| FLX | 310 | 148 | 85 | 0 | Positive | 74.6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ± ±![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 17.3 17.3 |
10 |
| COC | 304 | 182.2, (82.1) | 120 | 15, 15 | Positive | 81.1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ± ±![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 17.2 17.2 |
1 |
| BE | 290 | 167.5, (104.6) | 120 | 15, 30 | Positive | 10 | |
| CBZ-d10 | 247 | 204, (202) | 120 | 15, 10 | Positive | — | |
| ACS-d4 | 166 | 85.8, (77.7) | 70 | 10, 30 | Negative | — | |
| VNF-d6 | 284 | 58, (120.5), (265.5) | 110 | 15, 35, 15 | Positive | — | |
| Caffeine 13C3 | 197.9 | 139.7, (111.8) | 110 | 20, 25 | Positive | — | |
| SMX-d4 | 258 | 159.7, (96) | 110 | 10, 25 | Positive | — | |
The same LC/MS/MS with negative mode electrospray ionisation source and the Agilent ZORBAX Eclipse Plus C18 (2.1 × 100 mm, 3.5 μm) column were used for ACS analysis. The LOQ of ACS was 10 ng L−1 (Table 1). The column temperature for ACS determination was set at 25 °C. The mobile phase consisted of 40% methanol and 60% H2O with 5 mM ammonium acetate, pH 5.5. The eluent composition is as follows: initial conditions, 40% methanol fed at 0.2 mL min−1 for 2 minutes. Finally, the composition of the eluent was changed gradually to 100% methanol at t = 10 min. The injection volume was 15 μL. Quantification of ACS was done with respect to ACS-d4.
The total coliform counts and BOD5 were performed by EMAAP-Quito laboratory following Standard Methods.50
Dissolved organic carbon (DOC) and total dissolved nitrogen (TDN) analyses were conducted using a SHIMADZU TOC-VCPH instrument.
ELISA kits were used to measure estrone (E1), 17-β estradiol (E2), and 17-α ethinylestradiol (EE2) according to a reported method.51 All kits were validated for water analysis by the manufacturer, Tokiwa Chemical Industries Ltd.
Chloride, nitrate, and sulphate were determined by using an ion chromatograph (Dionex ICS5000). A sample, typically 0.1 mL, was introduced into an ion chromatograph. Sodium, potassium, calcium, and magnesium were analysed by using an inductively coupled plasma-optical emission spectrometer (ICP-OES Varian 720).
The study sites
The San Pedro–Guayllabamba–Esmeraldas stream was sampled in the summer of 2013 in seven locations along the water flow by three sampling campaigns, each lasting three to four days. Additionally, four pollution sources were sampled on the same sampling trips. The geographic locations of the sampling sites are presented in Fig. 1, and the pertinent characteristics and flow rates are presented in Table 2. The San Pedro River from El Chaupi (point 1) down to Amaguaña (point 2) is rural, with grazing fields and many small villages. From Amaguaña, the area becomes densely populated with some agricultural industries, such as slaughterhouses and agricultural packing facilities. Along 70 km from Amaguaña down to point 7, the San Pedro and Guayllabamba Rivers receive numerous untreated wastewater streams of the Quito District. Three such streams were sampled in this campaign: The Machángara S (point 3) stream in the Machángara River collects much of the wastewater of southern Quito. Machángara N (point 4) channels most of the wastewater of the northern neighbourhoods of Quito. An additional point assigned as Cumbayá is located in the San Pedro River itself (point 5). However, upstream to this point the San Pedro stream is diverted to a hydroelectric power generation system, and therefore this stream represents only local runoffs and wastewater streams that are mixed with the main stream of the San Pedro River after the hydroelectric station.| # | Name | Location | Altitudeb (m) | Distancea (km) | Number of sampling trips | Annual flow rate (Mm3 per year) | Summer flow rate (L s−1) |
|---|---|---|---|---|---|---|---|
| a Distances are from El Chaupi. b Altitude above sea level. | |||||||
| 1 | El Chaupi | San-Pedro River | 3340 | 0 | 2 | 0.19 | |
| 2 | Amaguaña | San-Pedro River | 2560 | 50 | 2 | 1.75 | |
| 3 | Machángara S | Southern Machángara River | 2760 | Side stream | 3 | 16 | 0.44 |
| 4 | Machángara N | Northern Machángara River | 2600 | Side stream | 2 | 44 | 1.24 |
| Wastes | Total wastewater feed | 3000–2000 | Side streams | — | 239 | 6.67 | |
| 5 | Cumbayá | San-Pedro River | 2250 | 85 | 1 | 0.019 | |
| 6 | Monjas | Monjas River | 1900 | Side stream | 1 | 0.095 | |
| 7 | Guayllabamba (bridge) | Guayllabamba River | 1800 | 120 | 2 | 9.52 | |
| 8 | Guayllabamba | Guayllabamba River | 1450 | 135 | 3 | 28.6 | |
| 9 | Las Golondrinas | Guayllabamba River | 200 | 210 | 2 | 8800 | 126 |
| 10 | Rio Esmeraldas before treatment | Esmeraldas River | 50 | 300 | 2 | 22![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
314 |
| 11 | San Mateo-after treatment | San Mateo–Esmeraldas River | 50 | 300 | 2 | — | — |
A survey carried out by Quito-EPMAPS, the water and sanitation company of the Metropolitan District of Quito, quantified the wastewater flow rates of the discharged wastewater streams to the San Pedro River.39 The yearly average and the summer average flow rates are presented in Table 2. In total, 152 Mm3 per year of wastewater of 1.46 million residents is discharged to the San Pedro River. The summer flow rates are on the average 12% lower than the annual average. However, since most of the wastewater of the 2.3 million residents of the Pichincha Province eventually reaches the San Pedro–Guayllabamba River, we estimate that the wastewater total annual flow is 239 Mm3 per year and the water quality characteristics of the Machángara and Monjas Rivers are representative of the wastewater quality of the whole province. We sampled an additional point in the San Pedro River, which is denoted as Guayllabamba (bridge) in Table 2 (point 7), and then, two additional points close to the beginning and the end of the Guayllabamba River were sampled as well (denoted as Guayllabamba (8) and Las Golondrinas (9) in Table 2). The Esmeraldas River was sampled at the inlet to the San Mateo drinking water treatment system (point 10). Water samples were also collected after filtration but before disinfection (point 11). The treatment at San Mateo consists of alum flocculation, sedimentation, and deep bed sand filtration. The water is supplied to some 350![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 residents of the city of Esmeraldas and to nearby neighbourhoods.
000 residents of the city of Esmeraldas and to nearby neighbourhoods.
Results and discussion
Quito's polluting streams
Tables 3, 4, and S1† show the water quality parameters at each of the study points that are shown on the map in Fig. 1. Table 3 shows the aggregate parameters, Table 4 shows the concentrations of the emerging contaminants and their metabolites, and Table S1 in the ESI† shows inorganic water quality parameters.| DOC | TDN | Log coliforms, (units per 100 mL) | BOD5 | COD | pH | Turbidity [NTU] | Conductivity [mS] | DO [mg L−1] | |
|---|---|---|---|---|---|---|---|---|---|
| a b/t, before treatment. b a/t, after treatment. | |||||||||
| El Chaupi | 3.7 (0.0) | 9.2 (11.8) | 2.5 (0.4) | 18.5 (4.9) | 50.5 (7.8) | 7.7 (0.0) | 23.5 (2.1) | 0.2 (0.0) | 6.4 (0.1) |
| Amaguaña | 3.4 (0.2) | 2.2 (0.4) | 5.8 (1.6) | 33.5 (16.3) | 66.5 (36.1) | 8.4 (0.1) | 33.5 (2.1) | 0.6 (0.0) | 6.8 (0.1) |
| Machángara S | 27.2 (0.9) | 26.3 (1.1) | 7.7 (2.1) | 179.5 (30.4) | 394.0 (4.2) | 7.9 (0.1) | 417.0 (60.8) | 0.7 (0.0) | 5.3 (0.4) |
| Machángara N | 68.4 | 31.3 | 8.8 | 251.0 | 514.0 | 7.6 | 265.0 | 0.7 | 1.1 |
| Cumbayá San Pedro | 3.7 | 3.7 | 6.2 | 27.0 | 55.0 | 9.0 | 84.0 | 0.8 | 5.8 |
| Monjas | 15.6 | 26.3 | 5.6 | 184.0 | 562.0 | 8.1 | 999.0 | 700.0 | 5.0 |
| Guayllabamba (bridge) | 8.0 | 8.9 | 7.2 | 27.0 | 71.0 | 8.3 | 245.0 | 0.5 | 5.3 |
| Guayllabamba | 6.8 (0.7) | 8.3 (1.4) | 3.9 (0.8) | 38.5 (20.5) | 128.5 (91.2) | 8.0 (0.1) | 117.6 (134.9) | 0.6 (0.0) | 7.0 (0.6) |
| Las Golondrinas | 2.6 (0.2) | 3.7 (2.8) | 2.9 (1.6) | 24.0 (7.1) | 63.0 (35.4) | 8.0 (0.1) | 259.5 (23.3) | 109.1 (154.0) | 7.1 (0.2) |
| Esmeraldas b/ta | 1.4 | 0.8 | 1.7 | 4.0 | 14.0 | 7.7 | — | 0.2 | 6.6 |
| Esmeraldas a/tb | 1.2 | 0.0 | 1.2 | 7.4 | — | 0.2 | 6.0 | ||
| Units | El Chaupi | Amaguania | Machangra S | Machangra N | Cumbaya San Pedro | Monjas | Guayllabamba (bridge) | Guayllabamba | Las Golondrinas | Esmeraldas b/t | Esmeraldas a/t | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ACS | μg L−1 | ND | 0.9 (0.1) | 26.2 (3.7) | 22.2 (2.5) | 4.3 | 31.0 | 14.5 (1.9) | 7.9 (1.3) | 1.8 (0.0) | 0.7 (0.1) | 0.7 (0.3) |
| CBZ | μg L−1 | ND | 11.6 (0.6) | 598.0 (200.0) | 830.0 (435.6) | 100.0 | 670.0 | 355.0 (53.0) | 195.5 (27.6) | 39.5 (13.4) | 11.5 (7.8) | 16.0 (4.2) |
| ACIN | μg L−1 | ND | ND | 5.3 (6.1) | 12.5 (17.7) | ND | ND | 12.0 (8.5) | ND | ND | ND | ND |
| ACON | μg L−1 | ND | 2.0 (2.8) | 26.7 (7.6) | 7.5 (3.5) | 3.0 | 3.0 | 14.0 (7.1) | 3.0 (1.4) | 3.0 (1.4) | ND | ND |
| 10-OH-CBZ | μg L−1 | ND | 5.5 (7.8) | 109.3 (62.6) | 99.5 (20.5) | 20.0 | 212.0 | 169.0 (36.1) | 32.0 (9.9) | 12.2 (2.5) | 4.1 (4.1) | 7.5 (6.4) |
| CBZ-DiOH | μg L−1 | ND | ND | 1.7 (2.9) | 5.0 (7.1) | ND | ND | ND | ND | ND | ND | ND |
| CBZ-E | μg L−1 | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND |
| Sum of CBZ metabolites | μg L−1 | ND | 7.5 | 143.0 | 124.5 | 23.0 | 215.0 | 195.0 | 35.0 | 15.2 | 4.1 | 7.5 |
| CAF | μg L−1 | 2.0 (2.8) | 92.3 (105.7) | 4444.3 (5309.1) | 5597.0 (5077.0) | 87.0 | 550.0 | 752.0 (175.4) | 240.0 (297.0) | 17.5 (10.6) | 7.0 (9.9) | 4.0 (5.7) |
| SMX | μg L−1 | ND | 6.0 (8.5) | 309.0 (184.9) | 201.0 (210.7) | 27.0 | 250.0 | 61.0 (20.5) | 26.5 (2.1) | 6.0 (1.4) | 6.3 (6.7) | 6.0 (5.7) |
| VNF | μg L−1 | ND | ND | 25.0 (11.4) | 12.5 (17.7) | 12.5 | 400.0 | 55.0 (31.8) | 36.0 (22.6) | 12.0 (0.0) | 2.0 (2.8) | 4.0 (5.7) |
| O-DMV | μg L−1 | ND | 6.0 (8.5) | 75.0 (35.4) | 42.5 (24.7) | 68.0 | 590.0 | 128.0 (50.9) | 62.3 (81.7) | 26.5 (30.4) | 5.5 (7.8) | 7.5 (10.6) |
| FLX | μg L−1 | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND |
| COC | μg L−1 | ND | 10.5 (13.4) | 415.3 (113.2) | 560.0 (84.9) | 6.3 | 100.0 | 222.0 (55.2) | 45.5 (0.7) | 5.5 (3.5) | ND | ND |
| BE | μg L−1 | ND | ND | 917.3 (192.6) | 452.0 (370.5) | 58.0 | 1065.0 | 383.0 (44.5) | 154.5 (37.5) | 54.0 (25.5) | 37.5 (34.6) | 33.5 (4.9) |
| E2 | μg L−1 | ND | ND | 1.0 (1.2) | 0.3 (0.2) | 1.4 | 2.2 | 0.3 | 0.4 | ND | 0.1 | ND |
| E1 | μg L−1 | ND | 0.4 | 7.4 (6.5) | 11.4 (10.1) | ND | ND | 2.0 (0.0) | 0.6 | ND | 0.5 (0.1) | 0.3 (0.1) |
| EE2 | μg L−1 | ND | ND | ND | 0.3 (0.2) | ND | ND | 0.3 | ND | ND | ND | ND |
The concentrations of four wastewater streams that contaminate the San Pedro River were measured. The aggregate water quality parameters at Machángara S (point 3) and Machángara N (point 4), which carry the wastewater of the southern and northern neighbourhoods of Quito, and two local streams at the Monjas River (point 6) and at Cumbayá (point 5) are presented as bars in the various frames in Fig. 2. The average BOD5 in Machángara N (4) and Machángara S (3) is 200 mg L−1, which is within the 190 ± 80 mg L−1 range that is characteristic of untreated wastewater.55 The organic loading is only 23 mg BOD5 pc per year, somewhat low compared to the 18 to 45 mg BOD5 pc per year based on the average reported organic loading in various countries.55
 | ||
| Fig. 2 Aggregate water quality parameters in various pollution streams discharging to the San Pedro River. (Table 3 presents the average and standard deviations of the analyses). | ||
The other characteristic parameters of the wastewater streams (points 3–8), such as the COD to BOD5 ratio (which is close to 2), the ratio between TDN and DOC, the high total coliform counts, and the lower DO at Machángara N, which is the only point where the water was sampled from a close conduit, agree well with the parametric ranges of domestic wastewater. The DOC to BOD5 ratio is somewhat lower than expected, since BOD5 analysis was performed on unfiltered samples, whereas DOC represents dissolved matter only.
Fig. 3 shows the average levels of the emerging pollutants in the polluting streams. It is interesting to compare the concentrations and loadings per capita of the emerging pollutants in Quito wastewater streams with reported values in other parts of the world. In all cases, we calculated the pollutant loading levels per capita based on the observed concentrations in the large wastewater streams, Machángara S and Machángara N, giving a larger weight, proportional to its flow rate (as depicted in Table 2) to Machángara N.
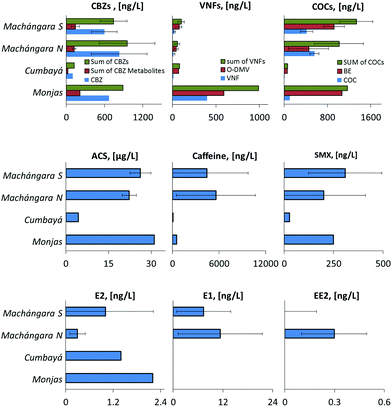 | ||
| Fig. 3 Emerging pollutants in various pollution streams discharging to the San Pedro River. Table 3 presents the average and standard deviations of the analyses. | ||
The level of the acesulfame K sweetener ingredient, ACS, in the wastewater of the Quito District reflects a loading of 2460 ± 300 mg pc per year, which is similar to the level of 3040 ± 1040, 3300 ± 230, 1460 ± 365, and 4020 ± 1600 mg pc per year reported in Germany,19 Israel,19,56 Canada,13 and Switzerland,57 respectively.
Among the pharmaceutical compounds, the most abundant was caffeine, with a level of 5300 ± 5100 ng L−1. Caffeine is frequently found in very large concentrations in the influents of wastewater treatment plants; for example, recently Deblonde et al., 2011 (ref. 58) reviewed 44 publications and found a level of 56![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 ± 52
000 ± 52![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 ng L−1 in the influents of wastewater treatment plants, although the removal percentage in the wastewater treatment plants was very high, around 97%. The level of caffeine in surface water is also very large and it spans a large range; for example, Kolpin et al. reported a range of 14 to 6000 ng L−1 in a comprehensive survey of US streams.59 The wide range of reported concentrations and the fast degradation rates limit the usefulness of caffeine as a wastewater marker.60
000 ng L−1 in the influents of wastewater treatment plants, although the removal percentage in the wastewater treatment plants was very high, around 97%. The level of caffeine in surface water is also very large and it spans a large range; for example, Kolpin et al. reported a range of 14 to 6000 ng L−1 in a comprehensive survey of US streams.59 The wide range of reported concentrations and the fast degradation rates limit the usefulness of caffeine as a wastewater marker.60
CBZ sales in Europe (and Israel) are reported to be in the range of 610 to 980 mg pc per year. Assuming 26% excretion in sewage (according to Oosterhuis et al., 2013 (ref. 61)), this range gives 158 to 255 mg pc per year loading in the untreated sewer.61 For the Quito District, we received values of 365 and 95 mg pc per year for the consumed and excreted CBZ, respectively. The CBZ level is somewhat higher in the northern parts of Quito, which on the average is richer but overall its per capita consumption is significantly lower than in Europe.
The level of SMX consumption in Europe is 390 ± 230 mg pc per year,62 which after taking an estimated 22.5% excretion gives a discharge level of 90 ± 55 mg pc per year (22.5% corresponds to the average of the excretion level range given by Carballa et al., 2008 (ref. 62)). The excretion of SMX in Quito is 21 ± 21 mg pc per year, which is much lower than the reported Israeli and European levels.
There is less data regarding VNF and its main metabolite O-DMV in the untreated wastes. In Spain, the level of VNF in wastewater influents was reported by Gracia-Lor et al.63 to be 43 mg pc per year based on VNF alone. In Israel, the level of discharge based on the sum of VNF and O-DMV is 88 mg pc per year.44 The discharge of O-DMV and VNF in Quito is 7.1 ± 4.7 mg pc per year, which is much lower than the reported values.
FLX was always below the detection level. This is not surprising in view of findings in other places of the world that this compound is highly degradable,59 and in view of the low usage of other drugs in the District of Quito.
The estrogenic activity of municipal wastewater is attributed mainly to estrone (E1), 17β-estradiol (E2), and 17α-ethinylestradiol (EE2). The reported range of estrogens in effluents is exceedingly large, spanning a range of 0 to 670 ng L−1 for E1 (ref. 64), 0 to 161 ng L−1 for E2 (ref. 63), and 0 to 100 ng L−1 for EE2.65 The exceedingly large variability stems from their fast degradation rate, the cleavage of glucuronic, sulphate, and other post-translation conjugates, and in the case of EE2 also from the inter-societal variability in the use of birth control pills. Additionally, chemical interferences spread the data even further, even when the analysis is carried out by MS-MS or ELISA. A detailed human excretion model predicts66 excretion levels of only 50, 12, and 3.2 ng L−1 for E1, E2, and EE2, respectively (based on the influent flow of 100 m3 per year), which adds confusion when these values are compared to the wide range of reported levels in wastewater influents. For the Quito District we received levels of 10 ± 9.2, 0.5 ± 0.5, and 0.25 ± 0.2 for E1, E2, and EE, respectively (<ND is taken as 0.1 in this averaging). The low level of E2 can be attributed to transformation of E2 to E1 during the flow in the Machángara River prior to our sampling point. Indeed, the average E2 removal in water treatment plants is rather large, 81%.66 The low level of EE2 is attributed to low usage of birth control pills, which is explained by the large Catholic population in Ecuador.
Cocaine (COC) and its main metabolite, BE, were also measured in the wastewater of the Quito District. The concentration of COC and BE were 415 ± 113 and 917 ± 192 in Machángara S and 560 ± 84.9 and 450 ± 370 ng L−1 in Machángara N. These levels correspond to a daily disposal to the waste water of 0.13 ± 0.04 and 0.28 ± 0.06 mg pc per day of COC and BE in Machángara S and 0.16 ± 0.02 and 0.13 ± 0.11 of cocaine and benzoylecgonine in Machángara N. It is possible to compute the daily usage of cocaine based on an excretion ratio of 38% and 36% for the cocaine and benzoylecgonine, respectively.22 Using these levels, we obtain a daily uptake of 1.1 mg pc per day and 0.77 mg pc per day for the southern and northern regions of Quito, respectively. These levels are not significantly different from each other (given the rather large standard deviations involved). These levels are within the concentration range that is usually found in developed countries. Thomas et al.22 compared illicit drug usage in 19 European countries and reported usage levels ranging between 0.002 and 2 mg pc per day. The average reported usage in half of the surveyed cities in Europe was larger than 1 mg pc per day.
The level of cocaine and its metabolite in the wastewater of Quito seemed to be surprisingly low in view of the decriminalisation of the possession of cocaine in Ecuador that took effect one day after our sampling campaign, and in view of the notorious neighbourhoods of Ecuador, Colombia, and Peru (Fig. 1). Additionally, coca tea, which is also called te' de coca in Ecuador, is a popular and legal herbal drink in Quito. Quito is located 2800 m above sea level, and coca tea is believed to prevent altitude sickness, and therefore is popular among Quito tourists.67,68 Coca tea is made of coca leaves and a single cup contains some 4 mg of coca alkaloids, approximately 20% of a line of cocaine.69 We observed a large difference between BE to COC ratios in the southern (2.3) and northern (0.84) regions of Quito. The abnormally low ratio in the north might be explained by the different sources of cocaine (i.e., sniffed vs. drunk), which are metabolised differently, and this postulate deserves more thorough dedicated research.
In summary, the concentrations of most water quality parameters in the wastewater of the Quito District are within their range in raw wastewater in developed countries; however, the per capita loadings of VNF, O-DMV, SMX, and CBZ and, to some extent, also of EE2 are considerably lower than in the developed countries. It is tempting to conclude, based on these differences, that pharmaceutical drugs are used at lower per capita levels in Ecuador compared to developed countries, but more research is needed to assess this preliminary conclusion.
Water quality trends along the San Pedro–Guayllabamba–Esmeraldas River
The DOC and BOD5 are the most informative organic load parameters, and they complement each other in this study. BOD5 characterises better high organic loads, such as wastewater sources, whereas DOC is a much better quantifier for uncontaminated surface water and drinking water. Indeed, BOD5 and DOC describe very well the evolution of pollution in the water stream. Organic loading is rather low in the rather pristine stream at points 1 and 2, upstream of the highly populated area, and then the stream is contaminated until it leaves the District of Quito. Downstream of the urbanised region, the loading is gradually reduced by dilution (Table 2) and degradation. However, the organic loading remains rather high due to the high speed and turbulent flow. The concentration trends of all the aggregate parameters, except for DO, follow the BOD and DOC trends (Fig. 4), that is, they all start at moderate levels in El Chaupi and Amaguaña and reach the maximal level after the District of Quito (point 7), and then dilute gradually along the Guayllabamba and Esmeraldas Rivers. However, the values of organic, microbial, and inorganic loadings in El Chaupi are not small; BOD5 is close to 20 and COD is close to 40 mg L−1, since the surface streams are highly turbulent and run in fertile agricultural soil. The average high total coliform count (103 counts per mL) reflects grazing activity in the area.Emerging pollutants follow a similar trend (Fig. 5); the stream becomes increasingly polluted when it passes near the District of Quito, and then the pollution is gradually attenuated down the Esmeraldas River until it reaches the San Mateo drinking water treatment plant. The emerging pollutants are much more specific to domestic wastes than aggregate water quality parameters, and thus their concentration is below the detection limit at El Chaupi, which confirms that the organic loading (DOC and BOD5) is not of domestic origin. Similar to the steeper increase rate (compared to the organic loading), the attenuation of the emerging pollutants is also steeper after the stream leaves the District of Quito. The steep decrease of the individual pollutants is attributed to the specificity to domestic wastes. After its emergence from the District of Quito, the stream passes through a much less populated area, which discharges relatively little domestic waste and a lot of agricultural inputs; thus, dilution by a surface stream reduced the level of the emerging contaminants more than it decreased the aggregate organic parameter levels.
We analysed the metabolites of four contaminants and these followed different patterns. The ratios of O-DMV to VNF, BE to COC, and E1 to E2 are increased along the Guayllabamba–Esmeraldas stream, since the metabolites, O-DMV, BE, and E1, are more stable than the parent contaminants. Further downstream, EE2 completely disappears as well. CBZ behaved differently; most CBZ metabolites (ACIN, ACON, and CBZ-DiOH), were abundant in the wastewater of Quito and disappeared downstream. Cytochrome p450 catalyses conversion of carbamazepine in the human body to give carbamazepine-10,11-epoxide, but this can be hydrolysed either in the human body or after excretion to the more stable dihydroxy metabolites and to acridine and acridone.68,70 Several reports on the degradation of CBZ by fungi and formation of the abovementioned metabolites were published,71,72 showing that the metabolism can continue after excretion. In our case, the intermediate carbamazepine-10,11-epoxide (CBZ-E) was always below the detection limit, showing that sufficient time elapsed before the streams reached our sampling points to allow complete degradation of the CBZ-E. 10 hydroxy-10,11-dihydrocarbamazepine was more stable than all other metabolites and remained at considerable levels along the whole stream, and it was found even after treatment in the San Mateo water treatment facility.
There was very good correspondence between practically all water quality parameters, different aggregate pollutants, and emerging pollutants vs. the organic loading. In fact, the correlation coefficients, R, of all the aggregate attributes or emerging contaminants (except for DO) and BOD5 were larger than 0.45. Except for 4 parameters (VNF [0.44], O-DMV [0.47], and E1 and EE2 [0.46, 0.5]), the correlation coefficients between the various compounds and BOD5 were larger than 0.64. The correlation table among the different parameters is provided in Table S2.† The best predictive variables that showed correlation coefficients larger than 0.85 for 11 out of 17 parameters were BOD5 and CBZ. ACS and TDN were also good predictors, though only 8 compounds of the 17 obeyed the abovementioned criterion.
The major inorganic ions that are presented in Table S1† revealed very low correlation with the organic loading. In fact, even boron, which is often regarded as a wastewater tracer,73 had very low correlation with BOD5. Apparently, the Quito population relies less than Europeans on washing machine detergents, and therefore boron cannot serve as a domestic waste tracer in Ecuador.
It was illuminating to study the attenuation pattern of the emerging contaminants from Guayllabamba (bridge) (point 7) down to the San Mateo treatment system. In this case, although the correlation coefficients were still high, the plots of the different emerging contaminants vs. CBZ (which is always plotted on the abscissa of Fig. 6) deviated from linearity, and were usually convex (except for VNF, which showed poor regression coefficients and large standard deviations, as shown in Table 4). The only exception was the linear regression of CBZ and ACS, which showed very good linearity with a coefficient of determination, R2 = 0.97 (Fig. 6).
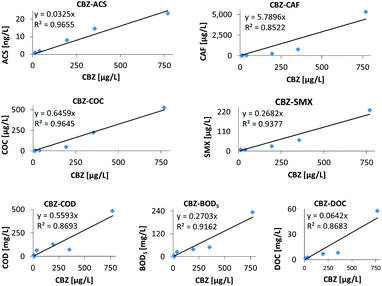 | ||
| Fig. 6 Linear relationships between carbamazepine and various water quality parameters downstream to the District of Quito (the points in each graph correspond from left to right to sampling points 11, 10, 9, 8, and 7 and the average level in the Quito wastes as given in Table 1). | ||
Fig. 7 shows the changes in the mass rate of the different pollutants at the four sampling points after leaving the District of Quito (points 7 to 10), the corresponding value for point 11, after the drinking water treatment at San Mateo (which was treated as if the whole Esmeraldas stream passed through the treatment, for the sake of clarity) and the corresponding mass rate of the parameters at the combined effluents of Pichincha Province (the row marked ‘wastes’ in Table 2). The mass rates were calculated by multiplication of the relevant concentrations by the average summer flow rate (Table 2). Unlike the concentration trends, the mass rate dependence on location allows distinction between degradation and mere dilution. The mass rate-location dependence of the various emerging pollutants is qualitatively different for different pollutants. Caffeine is strongly degraded along the stream, and so are the three estrogens (not shown), and cocaine (Fig. 7). ACS and CBZ behave as conservative compounds, showing insignificant degradation compared to the precision of our measurements. There is some increase of the mass rate of these compounds between point 8 (Guayllabamba) and point 9 (Las Golondrinas), which is attributed to the wastewater contribution of villages and small towns that discharge their wastes along the 85 km between these two points. The precision levels of SMX and the sum of VNF and O-DMV do not allow us to draw conclusions based on our measurements.
The sum of the levels of COC and BE is interesting, because it is increased along the Esmeraldas River, showing that more COC and BE were added than degraded in the Esmeraldas River. BE and COC are not refractive and they are likely to be degraded along the Esmeraldas River; additionally, the small population in the area cannot account for such an increase in the sum of COC and BE (denoted in the figure as COCs), and therefore we postulate that wild coca trees, which are part of the naturally occurring flora of the region, and maybe even small coca plantations are the reason for this mass increase. Climatic conditions along the Esmeraldas River and lower regions of the Guayllabamba are favourable for coca cultivation. One of the major coca plantation areas is in the southern regions of Colombia near the border of Ecuador, that is, near the Esmeraldas watershed that designates the Esmeraldas province as a “coca cultivation risk area” (see Map 4 in ref. 74). If our postulate is right, then it has far-reaching implications, since monitoring of rivers in Latin America could provide a very good way to spot illegal coca plantations, which despite the decriminalisation process in Ecuador, remain a challenge for law enforcement agencies. Coca plantations move rapidly and are difficult to spot, and thus abnormal concentrations of BE and COC in water streams can help pinpoint the location of illegal plantations.
We describe the mass rate on a yearly basis, despite the fact that our measurements were conducted only in the summer time, since unlike the observed concentrations, which are bound to decrease by dilution in the winter time, the mass rate is not seasonal. The consumption of most drugs is not seasonal, and, in fact, the degradation rate is likely to be even lower in winter time.
Level of emerging contaminants in the Guayllabamba River and in the San Mateo water treatment plant
The conventional water treatment in San Mateo is not sufficient to reduce the level of organics to the levels expected of well-maintained drinking water, and the DOC remains larger than 1 mg L−1 even after filtration. The very low concentrations of emerging pollutants in San Mateo does not allow accurate evaluation of the degree of pollutant removal, though the fact that the water was treated by alum is apparent, since the sulphate level is increased after the treatment (Table S1†). Unfortunately, the treatment is not optimal and very low removal of total coliform counts is achieved (last two rows of Table 2).One of the objectives of this research was to find out whether the city of Quito affects the water quality in the Esmeraldas River and the water source of the Esmeraldas City. The qualitative answer to this question is positive; most of the emerging pollutants that we have researched were found even after the conventional drinking water treatment of the San Mateo plant. CBZ, ACS, SMX, O-DMV, and BE were found, albeit at low levels, in the San Mateo plant in Esmeraldas City, and as expected, survived to a large extent the coagulation–sedimentation–filtration treatment. The levels were not large, and far below human risk levels,75 but they show that degradation along the 250 km stream is not sufficient to eliminate Quito's pollution. Under such conditions, it is likely that other contaminants and their metabolites also survive the 250 km distance and reach the drinking water plant of the city of Esmeraldas.
It is interesting to note, however, that villages that rely on the Guayllabamba River for direct or indirect water consumption of drinking water or for fishing activities may be affected by Quito wastes. At least in the summer time, the level of some of the contaminants, such as carbamazepine and its metabolites (>50 ng L−1) and BE (>50 ng L−1) in Las Golondrinas is significant, though in a recent survey Bruce et al.75 provided examples of US drinking water containing up to 250 ng L−1 of CBZ. The ecotoxicity of SMX and VNF is only in the microgram per litre range.76,77 The situation is more severe at the beginning of the Guayllabamba River, where the level of the contaminants is two to three times larger, and their variety is considerably larger.
As for fish toxicity, we could not find aquatic ecotoxicity data for O-DMV, but VNF and FLX exhibited some mild ecotoxic effects in the ng L−1 range. The C-start performance (a measure of escape capability) of larvae was adversely affected even at this low level of antidepressants.78 The sum of O-DMV and VNF in Las Golondrinas is 38 ng L−1, and even at the Esmeraldas it is 7 ng L−1.
Recently, the European Unity proposed environmental quality standards for EE2 (at 0.035 ng L−1) and E2 (at 0.4 ng L−1). This directive was later amended,79 but it reflects the fish ecotoxicity concerns over EE2 and E2. The level of E2 in Guayllabamba exceeds the proposed European guidelines, although it did not exceed the predicted no effect concentrations (PNEC) of 1 ng L−1 E2 equivalent total estrogens of the Environmental Agency of the United Kingdom (2002).80
Concluding remarks
This research delineates emerging contaminants' origin and attenuation along the Machangara–San Pedro–Guayllabamba–Esmeraldas River, one of Ecuador's most important rivers, which starts at the Andes and discharges to the Pacific Ocean, where it supplies drinking water to Esmeraldas City. Quantification of the impact of the wastewater discharge practice in the Metropolitan District of Quito on the Esmeraldas River shows that although such influence certainly exists and several emerging pollutants persist and even survive the filtration system of Esmeraldas City, they are all at sufficiently low levels and are not likely to adversely affect the health of the water consumers.The research illuminates the similarity and the differences between this Andean stream and the better-researched streams in North America and Europe. Carbamazepine and acesulfame behaved as conservative compounds and could be used as wastewater tracers, whereas the other surveyed drugs were disposed at lower loadings per capita and decayed along the stream. Cocaine and benzoylecgonine mass rates increased along the Esmeraldas River, which points to an additional non-domestic source from wild or cultivated coca trees in the watershed. Further research is needed to clarify whether the benzoylecgonine level in rivers can be used as a tracer for illegal plantations in the watershed.
Acknowledgements
OL and JG thank the Prometeo Project of SENESCYT. AV gratefully acknowledges the financial support of Teva Ltd. We thank Christian Xavier Paredes Illanes for his valuable help in the sampling trips. We thankfully acknowledge the help and participation of EPMAPS which were essential for this study.References
- C. G. Daughton and T. A. Ternes, Environ. Health Perspect., 1999, 107, 907–938 CrossRef CAS.
- B. Halling-Sorensen, S. N. Nielsen, P. F. Lanzky, F. Ingerslev, H. C. H. Lutzhoft and S. E. Jorgensen, Chemosphere, 1998, 36, 357–394 CrossRef CAS.
- T. Heberer, Toxicol. Lett., 2002, 131, 5–17 CrossRef CAS.
- R. P. Schwarzenbach, B. I. Escher, K. Fenner, T. B. Hofstetter, C. A. Johnson, U. von Gunten and B. Wehrli, Science, 2006, 313, 1072–1077 CrossRef CAS PubMed.
- S. D. Richardson, Anal. Chem., 2007, 79, 4295–4323 CrossRef CAS PubMed.
- D. W. Kolpin, E. T. Furlong, M. T. Meyer, E. M. Thurman, S. D. Zaugg, L. B. Barber and H. T. Buxton, Environ. Sci. Technol., 2002, 36, 1202–1211 CrossRef CAS.
- S. R. Ahmed, Toxicology, 2000, 150, 191–206 CrossRef CAS.
- F. M. Hekster, R. W. P. M. Laane and P. de Voogt, Rev. Environ. Contam. Toxicol., 2003, 179, 99–121 CAS.
- M. D. Hernando, M. Mezcua, A. R. Fernandez-Alba and D. Barcelo, Talanta, 2006, 69, 334–342 CrossRef CAS PubMed.
- J. P. Sumpter and A. C. Johnson, Environ. Sci. Technol., 2005, 39, 4321–4332 CrossRef CAS.
- J. W. Bickham, S. Sandhu, P. D. N. Hebert, L. Chikhi and R. Athwal, Mutat. Res., Rev. Mutat. Res., 2000, 463, 33–51 CrossRef CAS.
- L. S. Shore and A. Pruden, in Hormones and Pharmaceuticals Generated by Concentrated Animal Feeding Operations : Transport in Water and Soil, Springer-Verlag, Dordrecht, New York, 1 edn, 2009 Search PubMed.
- J. Spoelstra, S. L. Schiff and S. J. Brown, PLoS One, 2013, 8, e82706 Search PubMed.
- W. D. Robertson, D. R. Van Stempvoort, D. K. Solomon, J. Homewood, S. J. Brown, J. Spoelstra and S. L. Schiff, J. Hydrol., 2013, 477, 43–54 CrossRef CAS PubMed.
- A. Daneshvar, K. Aboulfadl, L. Viglino, R. Broseus, S. Sauve, A. S. Madoux-Humery, G. A. Weyhenmeyer and M. Prevost, Chemosphere, 2012, 88, 131–139 CrossRef CAS PubMed.
- G. Gasser, M. Rona, A. Voloshenko, R. Shelkov, N. Tal, I. Pankratov, S. Elhanany and O. Lev, Environ. Sci. Technol., 2010, 44, 3919–3925 CrossRef CAS PubMed.
- G. Gasser, M. Rona, A. Voloshenko, R. Shelkov, O. Lev, S. Elhanany, F. T. Lange, M. Scheurer and I. Pankratov, Desalination, 2011, 273, 398–404 CrossRef CAS PubMed.
- J. Oppenheimer, A. Eaton, M. Badruzzaman, A. W. Haghani and J. G. Jacangelo, Water Res., 2011, 45, 4019–4027 CrossRef CAS PubMed.
- M. Scheurer, F. R. Storck, C. Graf, H. J. Brauch, W. Ruck, O. Lev and F. T. Lange, J. Environ. Monit., 2011, 13, 966–973 RSC.
- J. Bones, K. V. Thomas and B. Paull, J. Environ. Monit., 2007, 9, 701–707 RSC.
- E. Zuccato, C. Chiabrando, S. Castiglioni, R. Bagnati and R. Fanelli, Environ. Health Perspect., 2008, 116, 1027–1032 CrossRef CAS PubMed.
- K. V. Thomas, L. Bijlsma, S. Castiglioni, A. Covaci, E. Emke, R. Grabic, F. Hernandez, S. Karolak, B. Kasprzyk-Hordern, R. H. Lindberg, M. L. de Alda, A. Meierjohann, C. Ort, Y. Pico, J. B. Quintana, M. Reid, J. Rieckermann, S. Terzic, A. L. N. van Nuijs and P. de Voogt, Sci. Total Environ., 2012, 432, 432–439 CrossRef CAS PubMed.
- Y. Vystavna, F. Huneau, V. Grynenko, Y. Vergeles, H. Celle-Jeanton, N. Tapie, H. Budzinski and P. Le Coustumer, Water, Air, Soil Pollut., 2012, 223, 2111–2124 CrossRef CAS.
- N. Nakada, T. Tanishima, H. Shinohara, K. Kiri and H. Takada, Water Res., 2006, 40, 3297–3303 CrossRef CAS PubMed.
- A. L. Spongberg and J. D. Witter, Sci. Total Environ., 2008, 397, 148–157 CrossRef CAS PubMed.
- B. J. Richardson, P. K. S. Larn and M. Martin, Mar. Pollut. Bull., 2005, 50, 913–920 CrossRef CAS PubMed.
- T. E. Felix-Cariedo, J. C. Duran-Alvarez and B. Jimenez-Cisneros, Sci. Total Environ., 2013, 454, 109–118 CrossRef PubMed.
- Z. Moldovan, Chemosphere, 2006, 64, 1808–1817 CrossRef CAS PubMed.
- Y. Xu, F. Luo, A. Pal, K. Y. H. Gin and M. Reinhard, Chemosphere, 2011, 83, 963–969 CrossRef CAS PubMed.
- J. Japenga, W. J. Wagenaar, W. Salomons, L. D. Lacerda, S. R. Patchineelam and C. M. Leitao, Sci. Total Environ., 1988, 75, 249–259 CrossRef CAS.
- A. Chavez, C. Maya, R. Gibson and B. Jimenez, Environ. Pollut., 2011, 159, 1354–1362 CrossRef CAS PubMed.
- F. B. Queiroz, E. M. F. Brandt, S. F. Aquino, C. A. L. Chernicharo and R. J. C. F. Afonso, Water Sci. Technol., 2012, 66, 2562–2569 CrossRef CAS PubMed.
- A. E. Navarro, M. E. Hernandez, J. M. Bayona, L. Morales and P. Ruiz, Int. J. Environ. Anal. Chem., 2011, 91, 680–692 CrossRef CAS.
- A. Aguilar, B. Jimenez, J. E. Becerril and L. P. Castro, Water Sci. Technol., 2008, 57, 927–933 CrossRef CAS PubMed.
- A. Melo-Guimaraes, F. J. Torner-Morales, J. C. Duran-Alvarez and B. E. Jimenez-Cisneros, Water Sci. Technol., 2013, 67, 877–885 CrossRef CAS PubMed.
- E. M. P. Brandt, F. B. de Queiroz, R. J. C. F. Afonso, S. F. Aquino and C. A. L. Chernicharo, J. Environ. Manage., 2013, 128, 718–726 CrossRef CAS PubMed.
- Y. Elorriaga, D. J. Marino, P. Carriquiriborde and A. E. Ronco, Bull. Environ. Contam. Toxicol., 2013, 90, 397–400 CrossRef CAS PubMed.
- J. O. Buckalew, L. Scott, M. James and P. Reed, in Cuerpo de Ingeníeros de los Estados Unidos de America Distrito de Mobile y Centro de Ingeniería Topográphica, 1998, pp. 1–49 Search PubMed.
- Unidad de Control de la Contaminación del Agua, Programa de Saneamiento Ambiental, EMAAP-Q, Plan de Descontaminación de los Ríos de Quito: Caracterización de las descargas de aguas residuales de la ciudad de Quito, EMAAP-Q, Quito-Ecuador, 2008, ch. 5, p. 355 Search PubMed.
- M. J. Benotti, R. A. Trenholm, B. J. Vanderford, J. C. Holady, B. D. Stanford and S. A. Snyder, Environ. Sci. Technol., 2009, 43, 597–603 CrossRef CAS.
- B. W. Brooks, C. M. Foran, S. M. Richards, J. Weston, P. K. Turner, J. K. Stanley, K. R. Solomon, M. Slattery and T. W. La Point, Toxicol. Lett., 2003, 142, 169–183 CrossRef CAS.
- K. Fent, A. A. Weston and D. Caminada, Aquat. Toxicol., 2006, 76, 122–159 CrossRef CAS PubMed.
- C. R.-G. Paola and W. Puettmann, Environ. Sci. Pollut. Res., 2012, 19, 689–699 CrossRef PubMed.
- G. Gasser, I. Pankratov, S. Elhanany, P. Werner, J. Gun, F. Gelman and O. Lev, Chemosphere, 2012, 88, 98–105 CrossRef CAS PubMed.
- C. Baronti, R. Curini, G. D'Ascenzo, A. Di Corcia, A. Gentili and R. Samperi, Environ. Sci. Technol., 2000, 34, 5059–5066 CrossRef CAS.
- K. L. Thorpe, R. I. Cummings, T. H. Hutchinson, M. Scholze, G. Brighty, J. P. Sumpter and C. R. Tyler, Environ. Sci. Technol., 2003, 37, 1142–1149 CrossRef CAS.
- T. Limpiyakorn, S. Homklin and S. K. Ong, Crit. Rev. Environ. Sci. Technol., 2011, 41, 1231–1270 CrossRef CAS.
- USEPA, Method 1694, 2007 Search PubMed.
- M. Scheurer, H. J. Brauch and F. T. Lange, Anal. Bioanal. Chem., 2009, 394, 1585–1594 CrossRef CAS PubMed.
- A. D. Eaton, L. S. Clesceri, E. W. Rice, A. E. Greenberg and M. A. H. Franson, in Standard Methods for the Examination of Water and Wastewater: Centennial Edition, American Public Health Association, Washington, D.C., 21 edn, 2005 Search PubMed.
- K. Barel-Cohen, L. S. Shore, M. Shemesh, A. Wenzel, J. Mueller and N. Kronfeld-Schor, J. Environ. Manage., 2006, 78, 16–23 CrossRef CAS PubMed.
- Standard Methods for the Examination of Water and Wastewater, Metals, 3010B, ed. A. E. Greenberg, L. S. Clesceri and A. D. Eaton, Washington, DC, 1992 Search PubMed.
- APHA Method 4500-NO2: Standard Methods for the Examination of Water and Wastewater, ed. A. E. Greenberg, L. S. Clesceri and A. D. Eaton, Washington, DC, 1992 Search PubMed.
- Standard Methods for the Examination of Water and Wastewater, Total Organic Carbon, 5310B, ed. A. E. Greenberg, L. S. Clesceri and A. D. Eaton, Washington, DC, 1992 Search PubMed.
- G. Tchobanoglous, F. L. Burton and H. D. Stensel, in Wastewater Engineering Treatment and Reuse, Tata Mc Grow Hill Publishing company, New Delhi, 4 edn, 2003 Search PubMed.
- G. Gasser, I. Pankratov, S. Elhanany, H. Glazman and O. Lev, Water Resour. Res., 2014, 50, 4269–4282 CrossRef.
- I. J. Buerge, H. R. Buser, M. Kahle, M. D. Muller and T. Poiger, Environ. Sci. Technol., 2009, 43, 4381–4385 CrossRef CAS.
- T. Deblonde, C. Cossu-Leguille and P. Hartemann, Int. J. Hyg. Environ. Health, 2011, 214, 442–448 CrossRef CAS PubMed.
- D. W. Kolpin, E. T. Furlong, M. T. Meyer, E. M. Thurman, S. D. Zaugg, L. B. Barber and H. T. Buxton, Environ. Sci. Technol., 2002, 36, 1202–1211 CrossRef CAS.
- M. Rona, G. Gasser, I. Negev, I. Pankratov, S. Elhanany, O. Lev and H. Gvirtzman, Water Resour. Res., 2014, 50, 1–22 CrossRef.
- M. Oosterhuis, F. Sacher and T. L. ter Laak, Sci. Total Environ., 2013, 442, 380–388 CrossRef CAS PubMed.
- M. Carballa, F. Omil and J. M. Lema, Chemosphere, 2008, 72, 1118–1123 CrossRef CAS PubMed.
- E. Gracia-Lor, J. V. Sancho, R. Serrano and F. Hernandez, Chemosphere, 2012, 87, 453–462 CrossRef CAS PubMed.
- Z. H. Liu, Y. Kanjo and S. Mizutani, Sci. Total Environ., 2009, 407, 731–748 CrossRef CAS PubMed.
- R. Hannah, V. J. D'Aco, P. D. Anderson, M. E. Buzby, D. J. Caldwell, V. L. Cunningham, J. F. Ericson, A. C. Johnson, N. J. Parke, J. H. Samuelian and J. P. Sumpter, Environ. Toxicol. Chem., 2009, 28, 2725–2732 CAS.
- A. C. Johnson and R. J. Williams, Environ. Sci. Technol., 2004, 38, 3649–3658 CrossRef CAS.
- A. M. Luks, S. E. McIntosh, C. K. Grissom, P. S. Auerbach, G. W. Rodway, R. B. Schoene, K. Zafren and P. H. Hackett, Wild. Environ. Med., 2010, 21, 146–155 CrossRef PubMed.
- K. Lertratanangkoon and M. G. Horning, Drug Metab. Dispos., 1982, 10, 1–10 CAS.
- A. J. Jenkins, T. Llosa, I. Montoya and E. J. Cone, Forensic Sci. Int., 1996, 77, 179–189 CrossRef CAS.
- B. M. Kerr, K. E. Thummel, C. J. Wurden, S. M. Klein, D. L. Kroetz, F. J. Gonzalez and R. H. Levy, Biochem. Pharmacol., 1994, 47, 1969–1979 CrossRef CAS.
- N. Golan-Rozen, B. Chefetz, J. Ben-Ari, J. Geva and Y. Hadar, Environ. Sci. Technol., 2011, 45, 6800–6805 CrossRef CAS PubMed.
- S. I. Kang, S. Y. Kang and H. G. Hur, Appl. Microbiol. Biotechnol., 2008, 79, 663–669 CrossRef CAS PubMed.
- A. Vengosh, K. G. Heumann, S. Juraske and R. Kasher, Environ. Sci. Technol., 1994, 28, 1968–1974 CrossRef CAS PubMed.
- UNODC, in Coca Cultivation in the Andean Region, a Survey of Bolivia, Colombia and Peru, 2007 Search PubMed.
- G. M. Bruce, R. C. Pleus and S. A. Snyder, Environ. Sci. Technol., 2010, 44, 5619–5626 CrossRef CAS PubMed.
- L. H. Yang, G. G. Ying, H. C. Su, J. L. Stauber, M. S. Adams and M. T. Binet, Environ. Toxicol. Chem., 2008, 27, 1201–1208 CrossRef CAS PubMed.
- Y. Kim, K. Choi, J. Y. Jung, S. Park, P. G. Kim and J. Park, Environ. Int., 2007, 33, 370–375 CrossRef CAS PubMed.
- M. M. Painter, M. A. Buerkley, M. L. Julius, A. M. Vajda, D. O. Norris, L. B. Barber, E. T. Furlong, M. M. Schultz and H. L. Schoenfuss, Environ. Toxicol. Chem., 2009, 28, 2677–2684 CrossRef CAS PubMed.
- A. C. Johnson, E. Dumont, R. J. Williams, R. Oldenkamp, I. Cisowska and J. P. Sumpter, Environ. Sci. Technol., 2013, 47, 12297–12304 CrossRef CAS PubMed.
- P. Matthiessen, D. Arnold, A. C. Johnson, T. J. Pepper, T. G. Pottinger and K. G. T. Pulman, Sci. Total Environ., 2006, 367, 616–630 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c4em00394b |
| This journal is © The Royal Society of Chemistry 2015 |




