Biological effects and bioaccumulation of pharmaceutically active compounds in crucian carp caged near the outfall of a sewage treatment plant†
Jianchao
Liu
,
Guanghua
Lu
*,
Zhenghua
Zhang
,
Yijun
Bao
,
Fuli
Liu
,
Donghai
Wu
and
Yonghua
Wang
Key Laboratory for Integrated Regulation and Resources Development on Shallow Lakes, China Ministry of Education, College of Environment, Hohai University, 1 Xikang Road, 210098 Nanjing, China. E-mail: ghlu@hhu.edu.cn; Fax: +86-25-83787330; Tel: +86-25-83787894
First published on 22nd October 2014
Abstract
Pharmaceutically active compounds (PhACs) have been universally detected in rivers, lakes and coastal waters that are affected by effluents from sewage treatment plants (STPs). In this study, the biological effects and bioaccumulation of PhACs were assessed in crucian carp (Carassius auratus) caged in an effluent-receiving river for 21 days. Compared with control fish in the laboratory and at a reference site, a significant reduction in hepatosomatic index (HSI) and increase in the biotransformation enzymes ethoxyresorufin O-deethylase (EROD) and glutathione S-transferase (GST) activities were observed in the fish that was caged downstream from the STP outfall. In general, the total concentrations of PhACs detected in fish tissues were in the order as follows: liver > brain > gill > muscle > bile. The bioaccumulation factors (BAFs) for PhACs were between 195 and 2782 in the major storage tissue liver. The corresponding results for both risk quotient (RQ) and enhanced integrated biomarker response (EIBR) based on laboratory and field studies, respectively, indicated that environmental risk for adverse effects to aquatic organisms were clearly higher at the downstream of the STP outfall than at the upstream.
Environmental impactPharmaceutically active compounds (PhACs) have been universally detected in rivers, lakes and coastal waters affected by effluents from sewage treatment plants. In this work, we (1) investigated the occurrence and bioaccumulation of eight PhACs in fish caged in an effluent-receiving river in Nanjing, China; (2) determined biomarker responses of hepatosomatic index (HSI), biotransformation phase I enzyme ethoxyresorufin O-deethylase (EROD) and phase II enzyme glutathione S-transferase (GST) activities in the liver of fish; and (3) assessed the potential ecotoxicological effects of the contaminants using both risk quotient (RQ) and enhanced integrated biomarker response (EIBR) based on laboratory and field studies, respectively. The article could help to implement management strategies for pollution control of PhACs and be useful for policy-makers for upgrading sewage treatment plants. |
1. Introduction
Pharmaceutically active compounds (PhACs) detected in the environment are classified as emerging pollutants.1 From 2003 to 2011, the production of PhAC ingredients in China has doubled, and in 2011, approximately two million tons of PhACs were produced.2 Due to the continuous and increasing consumption and their incomplete elimination in sewage treatment plants (STPs), PhACs are ubiquitous compounds in rivers, lakes and coastal waters.3,4 Commonly, the concentrations of PhACs are below the concentration levels of μg L−1. However, PhACs are manufactured with the intent of providing beneficial effects for human/animal health, which are not necessarily the same for organisms subjected to a continual lifecycle exposure; thus, concern about their possible risks to aquatic ecosystems has been raised.5A number of studies have reported the fate and occurrence of PhACs in STP effluents and receiving waters. However, reports regarding the distribution of PhACs in the tissues of fish in the natural environment are lacking. This information is important with respect to toxicokinetics, allocation, and potential contaminant-associated effect as a function of tissue/organ.6 It has been reported that PhACs, such as diclofenac, naproxen, ibuprofen, carbamazepine, erythromycin and 17α-ethinylestradiol can be detected in the bile and muscle of wild fish linked to STPs, with high variance for bioaccumulation factors (BAFs).1,7,8 However, the wild fish are not confined to a fixed local environment, which could not reflect the actual environmental effects of STP effluents. Active biomonitoring (ABM) involves the transplantation of organisms that are collected from an unstressed, unpolluted population to selected polluted sites. One distinct advantage, compared with passive biomonitoring, is that of a well-defined exposure time and location. The chemical and biological consequences of this translocation, which usually involves caging the organisms, can then be monitored in space and time to assess the effects of exposure on selected endpoints.9 Our group had previously utilized ABM to evaluate the complex pollution in the northern section of Taihu Lake, China.10 ABM was also used to detect the pharmaceutical residues in fish downstream of STPs.11,12 ABM has been extensively used in environmental health assessments, and it exhibits better results than passive biomonitoring because organisms already present in situ may have adapted to the pollutants.
The objectives of the present study were to investigate the occurrence and bioaccumulation of eight PhACs in fish caged in an effluent-receiving river in Nanjing, China. The biomarker responses of hepatosomatic index (HSI), biotransformation phase I enzyme ethoxyresorufin O-deethylase (EROD) and phase II enzyme glutathione S-transferase (GST) activities of the fish were determined. In addition, both risk quotient (RQ) and enhanced integrated biomarker response (EIBR) based on laboratory and field studies, respectively, were applied to indirectly/directly estimate the potential ecotoxicological effects of the contaminants. The eight PhACs investigated in this study include antibiotics roxithromycin (ROX) and erythromycin (ERY), antifungal ketoconazole (KCZ), anti-inflammatories ibuprofen (IBU) and diclofenac (DIC), β-blocker propranolol (PRO), anti-epileptic carbamazepine (CBZ) and steroid hormone 17α-ethinylestradiol (EE2).
2. Materials and methods
2.1. Materials and fish exposure
Healthy crucian carp (Carassius auratus) (12.2 ± 1.2 cm; 27.2 ± 2.8 g; n = 100) were acclimatized for two weeks in dechlorinated municipal water prior to the test. The standards and acclimation conditions are provided in the ESI.†Crucian carp were transferred from the laboratory and caged in the New Qinhuai river watershed (Nanjing, China) adjacent to the Jiangning STP (32°00′11.04′′N; 118°51′25.14′′E) at one upstream and three downstream sites for 21 days in December, 2013. This STP serves 450![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 inhabitants, and the treatment process involves anoxic/anaerobic/aerobic (first-stage project) and oxidation processes (second-stage project). New Qinhuai river sampling sites and STPs location are indicated in Fig. 1. One reference site (S1) is located 0.5 km upstream of the Jiangning STP effluent outfall, but 7 riverine km downstream of the effluent outfall from the Chengnan STP. The three downstream stations (i.e., S2, S3 and S4) were 0.1, 1.5 and 3.1 km below the Jiangning STP effluent outfall, respectively (Fig. 1). Fish controls were cultured in dechlorinated municipal water in a laboratory because various pollutants are present in the natural river and a field negative control site for comparison was not available. At each field site, two non-toxic columnar polyethylene cages (diameter 55 cm and height 55 cm) were deployed, holding 15 fishes each. The cages were mesh-shaped with 1 cm holes. Each cage was fixed in place by three wooden pegs at approximately 20 cm below the water surface. Ten fish (i.e., three fish tested for enzyme assays and seven fish for chemical analysis) were collected and delivered to the laboratory at each station after 7, 14 and 21 days of exposure, respectively. In addition, triplicate water samples were collected at each ABM station, using standard depth- and width-integrated compositing techniques, and used for chemical analysis.
000 inhabitants, and the treatment process involves anoxic/anaerobic/aerobic (first-stage project) and oxidation processes (second-stage project). New Qinhuai river sampling sites and STPs location are indicated in Fig. 1. One reference site (S1) is located 0.5 km upstream of the Jiangning STP effluent outfall, but 7 riverine km downstream of the effluent outfall from the Chengnan STP. The three downstream stations (i.e., S2, S3 and S4) were 0.1, 1.5 and 3.1 km below the Jiangning STP effluent outfall, respectively (Fig. 1). Fish controls were cultured in dechlorinated municipal water in a laboratory because various pollutants are present in the natural river and a field negative control site for comparison was not available. At each field site, two non-toxic columnar polyethylene cages (diameter 55 cm and height 55 cm) were deployed, holding 15 fishes each. The cages were mesh-shaped with 1 cm holes. Each cage was fixed in place by three wooden pegs at approximately 20 cm below the water surface. Ten fish (i.e., three fish tested for enzyme assays and seven fish for chemical analysis) were collected and delivered to the laboratory at each station after 7, 14 and 21 days of exposure, respectively. In addition, triplicate water samples were collected at each ABM station, using standard depth- and width-integrated compositing techniques, and used for chemical analysis.
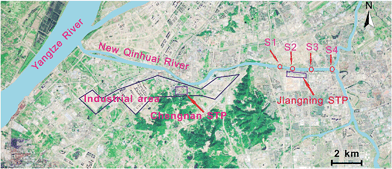 | ||
| Fig. 1 New Qinhuai River sampling sites (indicated with S1, S2, S3 and S4) and STP locations in the study areas. | ||
After transporting to the laboratory, the fish were anaesthetized with MS222 (100 mg L−1) and measured for length and weight. Then, the fish were sacrificed by cervical transection. Liver, brain, gill, muscle and bile tissues were immediately excised and weighed. All the tissues were washed with 0.15 M KCl, blotted with filter paper and immediately stored in liquid nitrogen.
2.2. Biomarker assays HSI, EROD and GST
Standardized protocols were used for the measurement of biomarkers in the liver of caged fish: Hepatosomatic index (HSI), EROD, GST and protein concentrations were calculated according to the previous studies.13–16 Detailed protocols are available in the ESI†.2.3. Sample extraction
Water samples were extracted and cleaned using the traditional solid-phase extraction (SPE) method applying Oasis HLB 6 cm3 (500 mg, Waters, USA) cartridges. Tissue samples were extracted by pressurized liquid extraction (PLE) using a Dionex ASE 350 system (Thermo Fisher, Germering, Germany) and concentrated in a Büchi R200 (Labortechnik, Flawail, Switzerland) rotary evaporator. Then, the concentrated solution was defatted using the freezing-lipid technique. Finally, the extracts were reconstituted with 1 mL of acetonitrile, and 50 μL of the 1 mg L−1 mixture containing the internal standards were added. Additional experimental details are provided in the ESI†.2.4. Instrumental analysis
Target compounds were analyzed in water and the tissues of caged fish were analyzed by conventional procedures based on ultrahigh performance liquid chromatography-tandem triple quadrupole mass spectroscopy (LC/MS/MS) equipped with an electrospray ionization source (Agilent Technologies). The limits of quantification (LOQ) of each PhACs were 0.27–0.65 ng L−1 and 0.4–1.2 ng g−1 for the water and fish tissues samples, respectively. The average recoveries for these spiked PhACs ranged from 83.7% to 105.2% for the water samples and from 62.4% to 83.6% for the fish samples. The concentrations of PhACs were reported as ng L−1 for the water samples and ng g−1 (wet weight) for the fish samples. Additional details on the analytical procedures used are given in the ESI†.2.5. Calculation of ecotoxicological risk
Both RQ and EIBR based on laboratory and field studies, respectively, were applied to indirectly/directly estimate the ecotoxicological risk of contaminants in the study area. The RQ model is a ratio between the measured environmental concentration (MEC) and their predicted non-effect concentration (PNEC) estimated from laboratory study. Based on the EMA guidelines17 and approaches proposed by Backhaus and Faust,18 the RQs of single compounds and their mixtures were calculated using the aquatic toxicity data for the detected PhACs and the most sensitive aquatic species. To address the biomarkers as a whole, EIBR was calculated to evaluate the toxicity at different sampling sites with the weighing of 1 for molecular (EROD and GST) and 3 for physiological (HSI) biomarkers, respectively.19 Detailed protocols are available in the ESI†.2.6. Statistical analysis
All the data were tested for normality and homogeneity of variances. The results were expressed as the mean ± standard deviation (SD). Differences among the groups were tested by one-way analysis of variance (ANOVA) using SPSS 17.0 with exposure location and time interval as factors. All the differences were considered significant with P < 0.05.3. Results and discussion
3.1. Occurrence of PhACs in the surface water of effluent-receiving river
Five of the eight PhACs were detected in the effluent-receiving river in Nanjing, with a detection rate of 100%. The concentrations of dissolved PhACs are listed in Table 1. The mean concentrations of five detected PhACs (ROX, ERY, PRO, CBZ and DIC) ranged from 0.58 to 40.39 ng L−1 at all the sampling sites, whereas those of KCZ, IBU and EE2 were not detected. The anti-inflammatory DIC showed the highest mean concentration of 40.39 ng L−1, which was comparable with levels measured in other rivers in China.20,21 Duan et al. confirmed that STP has limited efficiency in removing DIC, with the removal rate of <53%; thus, elevated concentrations of this chemical were observed in the aquatic systems.22 According to the European Community Directive 2013/39/EU, DIC shall be included for the first time in a “watch list” of emerging aquatic pollutions that could one day be placed on the priority list.23 In addition, several other compounds that were frequently detected in the surface water studies because of inefficient metabolism in humans and low removal efficiency during wastewater treatment were also detected in the present study; these compounds include CBZ, ROX and ERY, with the highest concentrations of 3.83, 33.61 and 10.22 ng L−1, respectively.24,25| Sites | Time | ROX | ERY | PRO | CBZ | DIC | SUM |
|---|---|---|---|---|---|---|---|
| S1 | 7 days | 8.23 ± 1.87 | 4.12 ± 0.54 | 0.21 ± 0.13 | 1.03 ± 0.34 | 10.54 ± 3.41 | 24.13 |
| 14 days | 10.41 ± 1.92 | 8.13 ± 2.10 | 0.69 ± 0.21 | 2.33 ± 1.23 | 30.85 ± 6.64 | 52.41 | |
| 21 days | 7.38 ± 1.19 | 8.47 ± 1.40 | 0.84 ± 0.54 | 2.65 ± 0.98 | 13.67 ± 4.13 | 33.01 | |
| Mean | 8.67 ± 1.56 | 6.91 ± 2.42 | 0.58 ± 0.33 | 2.00 ± 0.86 | 18.35 ± 10.94 | 36.32 | |
| S2 | 7 days | 20.45 ± 3.90 | 7.90 ± 1.40 | 1.29 ± 0.65 | 2.11 ± 0.84 | 34.10 ± 8.52 | 65.85 |
| 14 days | 26.76 ± 5.12 | 8.64 ± 2.09 | 0.63 ± 0.18 | 2.97 ± 0.42 | 49.15 ± 10.42 | 88.15 | |
| 21 days | 33.61 ± 4.18 | 10.22 ± 3.01 | 0.67 ± 0.20 | 3.83 ± 0.56 | 37.93 ± 7.45 | 86.26 | |
| Mean | 26.94 ± 6.58 | 8.92 ± 1.19 | 0.86 ± 0.37 | 2.97 ± 0.86 | 40.39 ± 7.82 | 80.09 | |
| S3 | 7 days | 18.69 ± 4.09 | 7.93 ± 1.23 | 0.69 ± 0.43 | 2.11 ± 0.69 | 30.41 ± 4.51 | 59.83 |
| 14 days | 20.70 ± 2.19 | 9.37 ± 2.13 | 0.61 ± 0.51 | 2.12 ± 0.81 | 48.72 ± 9.02 | 81.52 | |
| 21 days | 28.91 ± 3.43 | 8.31 ± 5.39 | 0.69 ± 0.36 | 1.86 ± 0.41 | 32.34 ± 6.59 | 72.11 | |
| Mean | 22.77 ± 5.41 | 8.54 ± 0.75 | 0.66 ± 0.05 | 2.03 ± 0.15 | 37.15 ± 10.06 | 71.15 | |
| S4 | 7 days | 13.55 ± 9.42 | 7.71 ± 2.67 | 0.68 ± 0.38 | 2.06 ± 0.27 | 23.00 ± 7.17 | 47.00 |
| 14 days | 17.44 ± 2.78 | 7.74 ± 2.21 | 0.73 ± 0.57 | 2.35 ± 0.65 | 42.04 ± 10.48 | 70.30 | |
| 21 days | 19.57 ± 3.75 | 5.62 ± 3.98 | 0.69 ± 0.25 | 1.46 ± 0.72 | 28.78 ± 8.41 | 56.12 | |
| Mean | 16.85 ± 3.05 | 7.02 ± 1.22 | 0.70 ± 0.03 | 1.96 ± 0.45 | 31.27 ± 9.76 | 57.81 |
Clearly, PhAC concentrations in the samples from downstream were higher than those from upstream in the river. For example, the mean concentration of ROX was found to be 8.67 ng L−1 at the S1 site (upstream 0.5 km), which had increased to 26.94 ng L−1 at the S2 site (downstream 0.1 km), suggesting a substantial input of this compound through STP effluent. Subsequently, ROX decreased to 16.85 ng L−1 at the S4 site (downstream 3.1 km), which is a clear sign of reduction. The same trend is observed for the other pharmaceuticals in the present and a previous study,22 which further demonstrates that effluents from STP are the primary contributor of PhAC pollution in urban rivers. This suggested that the levels of PhACs in river water depend not only on the loads from STP but on the river flow, biodegradation, photodegradation and adsorption on sediments, which acted upon the pharmaceuticals after their release.26
3.2. Bioaccumulation of PhACs
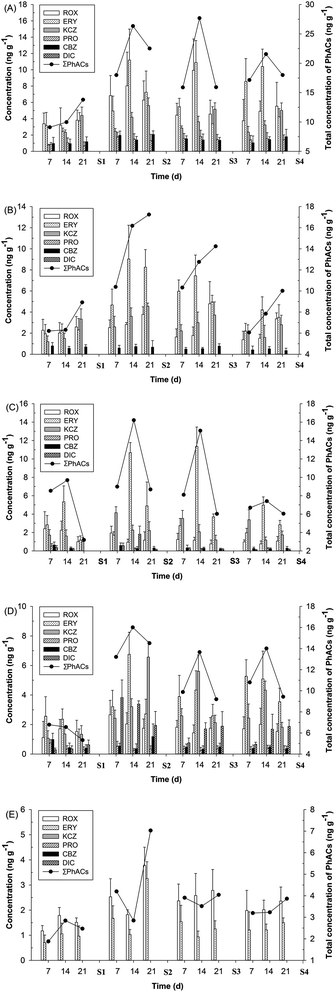
Six of the eight PhACs were detected in fish tissue samples. The concentrations of PhACs in the tissues of fish from four ABM sites are shown in Fig. 2. The compounds detected in the highest concentrations were ROX (9.94 ng g−1), PRO (1.84 ng g−1) and CBZ (2.07 ng g−1) in liver, ERY (11.35 ng g−1) in gills and KCZ (6.57 ng g−1) and DIC (3.83 ng g−1) in muscles. However, KCZ that was not detected in water samples was frequently detected in all of the fish samples. This implies that some compounds, such as KCZ, were not completely absent, and may experience a discontinuous presence (or below the limit of detection) in the river water.Unlike ROX, PRO and CBZ, the highest concentration of KCZ was found in fish muscle, which was in agreement with our previous study, where KCZ was only found in the muscle of wild fish collected from the downstream river of STPs.27 Moreover, the relatively high concentrations of KCZ were also found in the liver and brain, which exhibited an obvious time dependence. These results indicate that KCZ has a high potential for bioaccumulation in crucian carp. The previous studies have shown that KCZ acted as a potent inhibitor of CYP1A and CYP3A enzyme activities in fish.28,29 The inhibition of CYP enzyme activities (i.e., CYP1A and CYP3A) is linked to adverse outcomes in fish, such as the bioaccumulation of contaminants.28 No data were available in the previous literature for the bioaccumulation of KCZ in fish.
Overall, the higher total concentrations (9.10–27.68 ng g−1) of PhACs (ΣPhACs) were found to be in the liver, which is one of major targeted organs for accumulation, biotransformation and excretion of contaminants in fish, followed by the brain, gill, muscle and bile. After biotransformation in the liver, the metabolites and parent compounds are excreted to the small intestine via bile. However, the feeding status of the fish has a pronounced effect on the concentration of chemicals and metabolites in the bile. Following feeding, the fish empties the gall bladder into the intestine, and consequently the bile volume and the content of foreign chemicals will be low with no obvious time dependence.30 For the brain, which is a lipid-rich target tissue similar to the liver, appreciable levels (6.06–19.18 ng g−1) of ΣPhACs were observed. Notably, the accumulation of ΣPhACs in the brain steadily increased until the end of exposure (21 days), and the increasing trend was obvious with the decrease of concentrations of ΣPhACs in the surface water. However, brain/liver concentration ratios of ΣPhACs were 0.62 on average, indicating that PhACs are hindered from crossing the blood–brain barrier. Alternatively, these results indicate that brain tissue mainly consists of relatively polar lipids, which constitute unfavorable sites for PhACs accumulation compared with the triacylglycerol-rich composition of the liver.6 Moreover, gills are the first organs in contact with polluted water and suspended sediment particles; therefore, they can be the significant sites of interaction with contaminants. The concentrations of ΣPhACs in gill and muscle are similar, with the highest concentrations of 16.21 and 16.01 ng g−1, respectively, which were consistent with the concentrations of steroids (i.e., estrone, 17β-estradiol, EE2 and estriol) measured in wild fish from Dianchi Lake, China.31
Bioaccumulation is a process in which chemical substances are absorbed by aquatic organisms from all environmental sources.31 Based on the mean concentrations of PhACs in surface water and in the fish tissues at each sample site, the BAFs of five PhACs (ROX, ERY, PRO, CBZ and DIC) were calculated in different fish tissues, as shown in Table 2. BAFs for these compounds were below 1500 with the exception of PRO, which suggests a low bioaccumulation potential. The chemicals are defined as being “bioaccumulative” if the BAF is greater than 5000 in aquatic organisms, and as being “potentially bioaccumulative” if the BAF is in the range of 2000–5000 in aquatic organisms.32 In the present study, PRO exhibited the highest BAFs (2782), suggesting that the potential for bioaccumulation of PRO exists. The BAFs in the liver for ROX, ERY, PRO and CBZ are in the range of 195 to 2782. It is evident that the liver is a steadily accumulative organ for PhACs and a more suitable matrix for monitoring these compounds in aquatic environments. The previous studies have shown that pharmaceuticals at a certain environmental concentration interfere with the biological functions of organ/tissue in fish.33–35 However, no previous data prove that organ/tissue concentrations are at a level that has an impact on the biological functions of the organ/tissue. The knowledge of organ/tissue concentrations of the compounds in question would be of significant value in biological effect studies.
| Tissues | Sites | Time | ROX | ERY | PRO | CBZ | DIC |
|---|---|---|---|---|---|---|---|
| Liver | S1 | 7 days | 390 | 457 | 1425 | 482 | |
| 14 days | 368 | 356 | 1796 | 473 | |||
| 21 days | 440 | 539 | 1159 | 589 | |||
| S2 | 7 days | 254 | 556 | 1836 | 661 | ||
| 14 days | 298 | 1255 | 1575 | 471 | |||
| 21 days | 233 | 812 | 1370 | 698 | |||
| S3 | 7 days | 195 | 641 | 2400 | 757 | ||
| 14 days | 437 | 1275 | 2782 | 687 | |||
| 21 days | 201 | 396 | 2190 | 669 | |||
| S4 | 7 days | 223 | 1221 | 2017 | 535 | ||
| 14 days | 291 | 1482 | 2223 | 738 | |||
| 21 days | 328 | 601 | 2037 | 917 | |||
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||||||
| Brain | S1 | 7 days | 263 | 281 | 396 | ||
| 14 days | 233 | 323 | 284 | ||||
| 21 days | 298 | 333 | 349 | ||||
| S2 | 7 days | 94 | 972 | 197 | |||
| 14 days | 179 | 1125 | 246 | ||||
| 21 days | 140 | 589 | 225 | ||||
| S3 | 7 days | 72 | 700 | 240 | |||
| 14 days | 78 | 872 | 261 | ||||
| 21 days | 210 | 581 | 379 | ||||
| S4 | 7 days | 82 | 315 | 212 | |||
| 14 days | 90 | 600 | 260 | ||||
| 21 days | 199 | 499 | 178 | ||||
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||||||
| Gill | S1 | 7 days | 279 | 412 | 926 | 320 | 20 |
| 14 days | 259 | 772 | 151 | 10 | |||
| 21 days | 116 | 171 | |||||
| S2 | 7 days | 72 | 192 | 198 | 13 | ||
| 14 days | 35 | 1198 | 237 | 89 | 42 | ||
| 21 days | 43 | 548 | 106 | 2 | |||
| S3 | 7 days | 55 | 322 | 165 | 6 | ||
| 14 days | 51 | 1329 | 281 | 146 | |||
| 21 days | 34 | 434 | 104 | 4 | |||
| S4 | 7 days | 58 | 282 | 139 | 3 | ||
| 14 days | 46 | 710 | 333 | 121 | |||
| 21 days | 63 | 405 | 148 | 3 | |||
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||||||
| Muscle | S1 | 7 days | 129 | 371 | 1292 | 494 | 16 |
| 14 days | 197 | 342 | 591 | 219 | 19 | ||
| 21 days | 176 | 164 | 485 | 203 | 34 | ||
| S2 | 7 days | 99 | 360 | 556 | 184 | 88 | |
| 14 days | 76 | 758 | 266 | 124 | 78 | ||
| 21 days | 65 | 305 | 355 | 399 | 45 | ||
| S3 | 7 days | 80 | 456 | 539 | 232 | 20 | |
| 14 days | 63 | 507 | 366 | 170 | 46 | ||
| 21 days | 77 | 303 | 536 | 240 | 51 | ||
| S4 | 7 days | 102 | 751 | 451 | 201 | 21 | |
| 14 days | 120 | 725 | 569 | 251 | 54 | ||
| 21 days | 91 | 505 | 410 | 193 | 60 | ||
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||||||
| Bile | S1 | 7 days | 135 | 102 | |||
| 14 days | 206 | 153 | |||||
| 21 days | 174 | 139 | |||||
| S2 | 7 days | 94 | 188 | ||||
| 14 days | 68 | 116 | |||||
| 21 days | 140 | 365 | |||||
| S3 | 7 days | 104 | 180 | ||||
| 14 days | 113 | 110 | |||||
| 21 days | 122 | 147 | |||||
| S4 | 7 days | 118 | 172 | ||||
| 14 days | 120 | 173 | |||||
| 21 days | 140 | 214 | |||||
3.3. Biomarker responses
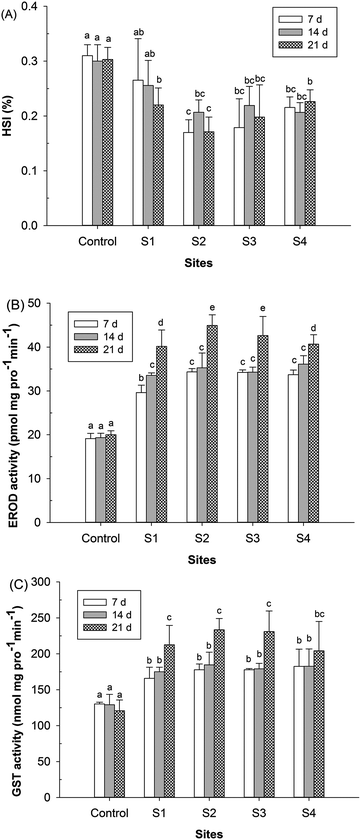
The results of the measured biomarker responses (HSI, EROD and GST), based on the male fish, are presented in Fig. 3. The biomarker responses in the control fish did not significantly alter during the experimental period. Tissue somatic indices (HSI) are the general measurements of the overall condition of the fish or the growth status of liver tissue. As shown in Fig. 3A, the HSI values of fishes at all of the monitoring sites (S1–S4) were clearly lower than those of the fish control throughout the exposure period, with the exception of the S1 site at days 7 and 14 (P < 0.05). Compared to the reference location S1, HSI was significantly decreased only at the site S2 at days 7 and 21 (P < 0.05). In a previous field study, decreased HSI was observed when fish were caged at the wastewater outlet of an oil refinery for 21 days, but the differences were not statistically significant.16 Previous laboratory studies have shown that certain pharmaceuticals (i.e., EE2 and IBU) did not result in significant effects on HSI,36 but HSI was significantly decreased by alachlor37 and verapamil.38 In fact, a decrease in HSI can be attributed to many factors, such as physiological development, food availability and parasites that can influence HSI.39Liver EROD and GST activities were obviously induced at all of the monitoring sites during the exposure period (P < 0.05), and the induction rate was higher at day 21 than at days 7 and 14 (Fig. 3B and C). The critical toxicity of any specific xenobiotic that is metabolized through both routes I and II depends on the capacity of the phase II enzymes to conjugate the reactive metabolites produced by phase I biotransformation reactions, in which EROD and GST activities play an important role.14 In the present study, EROD and GST activities exhibited a similar pattern, i.e., a positive correlation (Pearson coefficients >0.87; P < 0.05) was found between the induction of EROD and the increase of GST. Moreover, compared to the reference site S1, the induction rate of EROD was higher than that of GST in effluent-exposed fish caged downstream of the STP. The elevated levels of EROD and GST may result from the bioaccumulation of PhACs in fish from the STP effluents, which is known to occur for ROX, ERY and DIC based on the data from the laboratory studies.33,34,40 Generally, the maximum induction/inhibition rates of the three biomarkers (HSI, EROD and GST) were observed downstream of 0.1 km (S2), which clearly indicates that STP effluents induce biological effects in crucian carp, suggesting that crucian carp are among a group of the sensitive species to PhACs in effluent-exposed studies.
3.4. Environmental implication
Because some of the PhACs widely occurred in the effluent-receiving river, albeit at low levels, they may pose an environmental risk to aquatic organisms. The potential environmental risks of the detected PhACs were assessed on the basis of the RQ. In this study, the acute and chronic toxicity data of the detected PhACs on non-target organisms were collected from the literature, which are shown in Table S3.† The PNEC values for the most sensitive aquatic species were calculated based on the toxicity data and are shown in Table S4.† To better elucidate the risk levels, the values of the RQ were classified into three risk levels: 0.01–0.1, low risk; 0.1–1, medium risk; and >1, high risk.41 The RQ for each detected PhACs and their mixture was calculated, as shown in Fig. 4A. It was found that at least five PhACs posed low risk to aquatic organisms in the area of the study. DIC posed a high risk to the relevant sensitive aquatic organism (Oncorhynchus mykiss). Fig. 4A also provides an intuitive view of the total ecological risk at all the points. The total RQ of PhACs (ΣRQ) in all the regions ranged from 2.28 to 5.08. Furthermore, the S2 site showed the highest RQ value, which was more than two times higher than that for the reference location S1. The EIBR values were calculated by integrating three biomarkers and their weights (Fig. 4B). The EIBR revealed the same impact status as RQ from a biological aspect. Relatively high EIBR values during the entire exposure period were observed at sampling site S2, whereas the lowest EIBR values were exhibited at S1 and S4.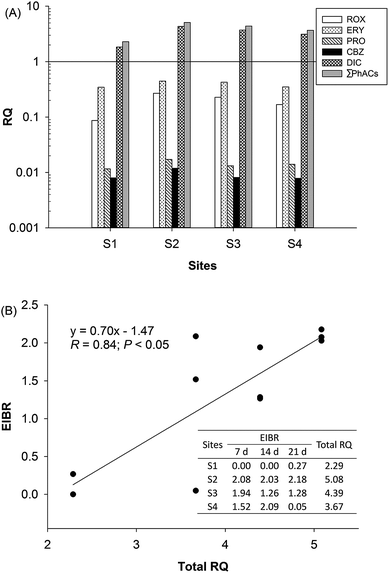 | ||
| Fig. 4 The RQ values (A) of single and total PhACs and the correlation (B) between the total RQ and EIBR values at different sampling sites. | ||
Both RQ and EIBR, based on the laboratory and field studies, respectively, have their place in the environmental risk assessment. EIBR presents an opportunity for a more holistic way of assessing the effects of environmental samples and wastes on ecosystems than RQ, which uses the detection of only a few classes of contaminants. However, the RQ could provide an assessment of the total toxicity contribution of the investigated PhACs that were included in the analytical survey, although it does not reflect the overall toxicity of the river near a STP. To quantitatively analyze the relationships between the two approaches, linear regression analysis was conducted (Fig. 4B). The result showed positive correlations between the ΣRQ of PhACs and EIBR values, with a correlation coefficient R value of 0.84 (P < 0.05). Because the EIBR and RQ are indicators of ecological risk, it appears as though the high risk of contamination in the effluent-receiving river is the result of the high contribution of STP effluents. Consistent with the previous studies, our results revealed that the pharmaceuticals- and metals-based RQ indexes and integrated biomarker response indexes showed clear positive correlations.42,43 This suggests that both the RQ and EIBR approaches are efficient instruments to provide an ecologically relevant assessment of the ecotoxicological risk and can facilitate the decision-making process in controlling the contamination at the downstream of STP.
4. Conclusions
In this study, five PhACs were universally detected in the effluent-receiving river in moderate concentrations. The concentrations of the detected PhACs in the liver, brain, gills and muscle of fish were highly variable. Generally, the highest concentrations of ΣPhACs in fish were observed in the liver, followed by the brain, gill, muscle and bile. However, KCZ exhibited a high bioaccumulation potential in muscle with the highest concentration of 6.57 ng g−1. Our results indicate that the uptake and bioaccumulation of contaminants, including PhACs from STP effluents, result in the inhibition of liver growth and an enhanced synthesis of the biotransformation enzymes EROD and GST. Potential adverse effects were found at all of the sampling points based on the RQ indexes; DIC and ERY accounted for the majority of the total RQ and were regarded as priority pollutants. A significant positive linear correlation between RQ and EIBR exhibited the mutual consistency of ecotoxicological assessment approaches based on the laboratory and field studies. The results from both RQ and EIBR highlight the fact that aquatic organisms are suffering from adverse health effects in the effluent-receiving river.Acknowledgements
This study was supported by the National Natural Science Foundation of China (Grant 51279061), the Innovation Program of Graduate Students in Jiangsu Province (Grant CXZZ13_0269) and the Fundamental Research Funds for the Central Universities (Grant 2014B02414).References
- J. M. Brozinski, M. Lahti, A. Meierjohann, A. Oikari and L. Kronberg, Environ. Sci. Technol., 2012, 47, 342–348 CrossRef PubMed.
- J. L. Liu and M. H. Wong, Environ. Int., 2013, 59, 208–224 CrossRef CAS PubMed.
- Q. Bu, B. Wang, J. Huang, S. Deng and G. Yu, J. Hazard. Mater., 2013, 262, 189–211 CrossRef CAS PubMed.
- R. Moreno-González, S. Rodríguez-Mozaz, M. Gros, E. Pérez-Cánovas, D. Barceló and V. M. León, Sci. Total Environ., 2014, 490, 59–72 CrossRef PubMed.
- M. González-Pleiter, S. Gonzalo, I. Rodea-Palomares, F. Leganés, R. Rosal and K. Boltes, et al. , Water Res., 2013, 47, 2050–2064 CrossRef PubMed.
- Y. Zhang, J. P. Wu, X. J. Luo, J. Wang, S. J. Chen and B. X. Mai, Environ. Pollut., 2011, 159, 3647–3652 CrossRef CAS PubMed.
- L. Gao, Y. Shi, W. Li, J. Liu and Y. Cai, J. Environ. Monit., 2012, 14, 1247–1254 CAS.
- B. Huang, B. Wang, D. Ren, W. Jin, J. Liu and J. Peng, et al. , Environ. Int., 2013, 59, 262–273 CrossRef CAS PubMed.
- V. Wepener, J. van Vuren, F. Chatiza, Z. Mbizi, L. Slabbert and B. Masola, Phys. Chem. Earth, 2005, 30, 751–761 CrossRef PubMed.
- C. Wang, G. Lu, W. Peifang, H. Wu, P. Qi and Y. Liang, Environ. Sci. Technol., 2011, 45, 3746–3752 CrossRef CAS PubMed.
- P. Rostkowski, J. Horwood, J. A. Shears, A. Lange, F. O. Oladapo and H. T. Besselink, et al. , Environ. Sci. Technol., 2011, 45, 10660–10667 CrossRef CAS PubMed.
- O. P. Togunde, K. D. Oakes, M. R. Servos and J. Pawliszyn, Environ. Sci. Technol., 2012, 46, 5302–5309 CrossRef CAS PubMed.
- M. M. Bradford, Anal. Biochem., 1976, 72, 248–254 CrossRef CAS.
- M. Frasco and L. Guilhermino, Fish Physiol. Biochem., 2002, 26, 149–156 CrossRef CAS.
- G. Lu, C. Wang and Z. Zhu, Bull. Environ. Contam. Toxicol., 2009, 82, 194–199 CrossRef CAS PubMed.
- L. Traven, V. Mićović, D. V. Lušić and T. Smital, Environ. Monit. Assess., 2013, 185, 9009–9018 CrossRef CAS PubMed.
- EMA (European Medicines Agency), Guideline on the Environmental Risk Assessment of Medicinal Products for Human Use, EMEA/CHMP/SWP/4447/00, 2006 Search PubMed.
- T. Backhaus and M. Faust, Environ. Sci. Technol., 2012, 46, 2564–2573 CrossRef CAS PubMed.
- C. Liu, V. W. Chang and K. Y. Gin, Environ. Toxicol. Chem., 2013, 32, 2226–2233 CrossRef CAS PubMed.
- L. Wang, G. G. Ying, J. L. Zhao, X. B. Yang, F. Chen and R. Tao, et al. , Sci. Total Environ., 2010, 408, 3139–3147 CrossRef CAS PubMed.
- J. L. Zhao, G. G. Ying, Y. S. Liu, F. Chen, J. F. Yang and L. Wang, et al. , Environ. Toxicol. Chem., 2010, 29, 1377–1384 CAS.
- Y. P. Duan, X. Z. Meng, Z. H. Wen, R. H. Ke and L. Chen, Sci. Total Environ., 2013, 447, 267–273 CrossRef CAS PubMed.
- EC (European Community), Directive 2013/39/EU of the European Parliament and of the Council of 12 August 2013 amending Directives 2000/60/EC and 2008/105/EC as regards priority substances in the field of water policy, 2013 Search PubMed.
- L. Jiang, X. Hu, D. Yin, H. Zhang and Z. Yu, Chemosphere, 2011, 82, 822–828 CrossRef CAS PubMed.
- S. L. Klosterhaus, R. Grace, M. C. Hamilton and D. Yee, Environ. Int., 2013, 54, 92–99 CrossRef CAS PubMed.
- N. Collado, S. Rodriguez-Mozaz, M. Gros, A. Rubirola, D. Barceló and J. Comas, et al. , Environ. Pollut., 2014, 185, 202–212 CrossRef CAS PubMed.
- J. Liu, G. Lu, Z. Xie, Z. Zhang and Z. Yan, Sci. Total Environ., Search PubMed submitted.
- M. C. Celander, Aquat. Toxicol., 2011, 105, 72–77 CrossRef CAS PubMed.
- Z. Yan, G. Lu, D. Wu, Q. Ye and Z. Xie, Aquat. Toxicol., 2013, 132–133, 19–25 CrossRef CAS PubMed.
- J. M. Kallio, M. Lahti, A. Oikari and L. Kronberg, Environ. Sci. Technol., 2010, 44, 7213–7219 CrossRef CAS PubMed.
- J. Liu, R. Wang, B. Huang, C. Lin, Y. Wang and X. Pan, Environ. Pollut., 2011, 159, 2815–2822 CrossRef CAS PubMed.
- J. A. Arnot and F. A. Gobas, Environ. Rev., 2006, 14, 257–297 CrossRef CAS.
- J. Liu, G. Lu, J. Ding, Z. Zhang and Y. Wang, Sci. Total Environ., 2014, 490, 914–920 CrossRef CAS PubMed.
- J. Liu, G. Lu, Y. Wang, Z. Yan, X. Yang and J. Ding, et al. , Chemosphere, 2014, 99, 102–108 CrossRef CAS PubMed.
- A. C. Mehinto, E. M. Hill and C. R. Tyler, Environ. Sci. Technol., 2010, 44, 2176–2182 CrossRef CAS PubMed.
- K. Ji, X. Liu, S. Lee, S. Kang, Y. Kho and J. P. Giesy, et al. , J. Hazard. Mater., 2013, 254–255, 242–251 CrossRef CAS PubMed.
- X. Yi, H. Liu, Y. Lu, J. Tao, H. Ding and M. Zhang, et al. , Bull. Environ. Contam. Toxicol., 2007, 79, 283–287 CrossRef CAS PubMed.
- Z. H. Li, J. Velisek, V. Zlabek, R. Grabic, J. Machova and J. Kolarova, et al. , J. Hazard. Mater., 2011, 185, 870–880 CrossRef CAS PubMed.
- W. Sanchez, B. Piccini, J. M. Ditche and J. M. Porcher, Environ. Int., 2008, 34, 791–798 CrossRef PubMed.
- S. Stepanova, E. Praskova, L. Chromcova, L. Plhalova, M. Prokes and J. Blahova, et al. , Environ. Toxicol. Pharmacol., 2013, 35, 454–460 CrossRef CAS PubMed.
- M. D. Hernando, M. Mezcua, A. Fernández-Alba and D. Barceló, Talanta, 2006, 69, 334–342 CrossRef CAS PubMed.
- J. L. Liu, Y. Yang, F. Liu and L. L. Zhang, Ecotoxicology, 2014, 23, 538–552 CrossRef CAS PubMed.
- Z. Yan, X. Yang, G. Lu, J. Liu, Z. Xie and D. Wu, Sci. Total Environ., 2014, 470, 171–179 CrossRef PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c4em00472h |
| This journal is © The Royal Society of Chemistry 2015 |