Effects of sugarcane waste-products on Cd and Zn fractionation and their uptake by sugarcane (Saccharum officinarum L.)
Pensiri
Akkajit
ab,
Thomas
DeSutter
c and
Chantra
Tongcumpou
*ad
aCenter of Excellence on Hazardous Substance Management (HSM), Chulalongkorn University, Bangkok, Thailand 10330. E-mail: pensiri.a@hotmail.com; tchantra@chula.ac.th
bInternational Postgraduate Programs in Environmental Management, Chulalongkorn University, Phathumwan, Bangkok, Thailand 10330. E-mail: pensiri.a@hotmail.com
cDepartment of Soil Science, North Dakota State University, 214 Walster Hall, P.O. Box 7680, Fargo, ND 58108-6050, USA. E-mail: thomas.desutter@ndsu.edu
dEnvironmental Research Institute, Chulalongkorn University, Phathumwan, Bangkok, Thailand 10330. E-mail: tchantra@chula.ac.th; Fax: +66 2218 8210; Tel: +66 2218 8138
First published on 12th November 2013
Abstract
The effects of three sugarcane waste-products from an ethanol production plant on the fractionation of Cd and Zn in high Cd and Zn contaminated soil and metal accumulation in sugarcane (Saccharum officinarum L.) were studied, using the BCR sequential extraction and aqua regia extraction procedures. A pot experiment was performed for 4 months with four treatments: no-amendments (control), boiler ash (3% w/w), filter cake (3% w/w) and a combination of boiler ash and vinasse (1.5% + 1.5%, w/w). The results showed that all treatments reduced the most bioavailable concentrations of Cd and Zn (BCR1 + 2) in soils (4.0–9.6% and 5.5–6.3%, respectively) and metal uptake (μg) in the aboveground part of the sugarcane (up to 62% and 54% for Cd and Zn, respectively) as compared to the control. No visual symptoms of metal toxicity and no positive effect on the biomass production of sugarcane were observed. Both Cd and Zn were accumulated mainly in the underground parts of the sugarcane (root > shoot ≥ underground sett > leaf; and root > underground sett > shoot > leaf, respectively) and the translocation factors were below 1, indicating low metal uptake. The results suggested that even though sugarcane waste-products insignificantly promote sugarcane growth, they can be used in agriculture due to the low metal accumulation in sugarcane and the reduction in metal bioavailability in the soil.
Environmental impactWaste utilization of sugarcane waste-products generated from ethanol production processes as soil amendments on agricultural land for metal stabilization and agricultural purposes provides benefits in terms of waste recycling, in situ metal stabilization, and agricultural productivity. An understanding of sugarcane waste-products as soil amendments and soil constituents would be useful for developing management practices within this particular area of waste management, along with the protection of the environment from heavy metal translocation. Therefore, in-depth investigation and long term studies are necessary to determine the validity and feasibility of these waste-products before applying them to real field sites to ensure the experimental results. |
Introduction
Sugarcane (Saccharum officinarum L.) plantations for ethanol production have been promoted recently and are widely found in the north, northeast and central regions of Thailand. Sugarcane can adapt well to a wide range of growing conditions in the tropical and subtropical climates of Thailand with high biomass production.1 Sugarcane cultivation, specifically for ethanol production, was proposed as an alternative crop in the Mae Sot district, Tak province, with the purpose of reducing risks associated with exposure to Cd through food chain transfer.2 Waste utilization from ethanol production processes is one of the sustainable opportunities to help utilize materials effectively, reduce waste disposal, and add value to these byproducts. Boiler ash (BA), filter cake (FC), and vinasses (VN) are three waste-products produced from ethanol processes.Based on the above considerations, the use of waste-products as a soil amendment might be an alternative suitable option for the reclamation of contaminated soils through reducing metal bioavailability by in situ stabilization and improving soil quality. Numerous waste-products have been widely studied and used as soil amendments and immobilizing agents.3–5 The effects of sugarcane waste-products as a soil amendment on the Cd and Zn bioavailability in soils were studied previously with 3% (w/w) amendment application in a pot experiment.2 The results showed a reduction in the exchangeable fraction of Cd (BCR1) in the amended soils (2.3% to 4.7% and 9.4% to 39.9% in low and high Cd contaminated soils, respectively), as compared to the non-amended soils. In addition, the effects of sugarcane waste-products on sugarcane growth and its metal uptake in low Cd and Zn contaminated soil (less than 5 mg Cd per kg soil) were further studied.6 The results showed a positive effect on biomass production, however, no significant reduction of metal accumulation in the plants was observed.
In the present study, we extended the previous work by growing sugarcane in high Cd and Zn contaminated soils (less than 20 mg Cd per kg soil). We evaluated the usefulness of sugarcane waste-products as soil amendments in high Cd and Zn contaminated soil for promoting sugarcane growth, and Cd and Zn in situ stabilization was evaluated by studying the effects of sugarcane waste-products on the bioavailability of the Cd and Zn in the soil and metal uptake by sugarcane. This knowledge must lead to the development of a guideline for using sugarcane waste-products as soil amendments for soil and fertilizer management and for reducing heavy metal contamination in agricultural soils. According to this the following studies were carried out: (1) to investigate the effects of sugarcane waste-products (BA, FC, and VN) from an ethanol production plant as soil amendments on sugarcane growth and for reducing Cd and Zn bioavailability in high Cd and Zn contaminated soil, and (2) to determine the translocation and distribution of Cd and Zn within sugarcane at different growth stages over a time period of 4 months.
Materials and methods
Soils, sugarcane and amendments
Agricultural soils contaminated with high Cd and Zn (<20 mg Cd per kg soil), sugarcane waste-products (BA, FC and VN) from an ethanol production plant, and sugarcane plant samples (var. LK92-11) were collected from the Mae Sot District, Tak Province, Thailand. The soils and amendments were air-dried and ground to pass through a 2 mm mesh sieve prior to use in the pot experiment. There are four treatments: high Cd and Zn contaminated soil (1) without amendment (Ctrl), (2) with 3% BA (30 g BA per kg soil) (BA), (3) with 3% FC (30 g FC per kg soil) (FC), and (4) with 1.5% BA and 1.5% VN (15 g BA + 15 g VN per kg soil) (BAV). No fertilizer addition was used in this study. The experimental pots were arranged in a randomized complete block design and kept outdoors under natural sunlight at the Institute II building of Chulalongkorn University, Bangkok, Thailand (13.7383° N, 100.5324° E).The pot experiment was conducted for a period of 4 months. Soil and sugarcane samples were sacrificed and collected monthly on the same sampling dates (from T1 to T4 where T1, T2, T3 and T4 represent the 1, 2, 3 and 4 month growth stages of sugarcane, respectively). In order to constrain the analytical uncertainty of the results, the plants were grown in three different containers for each treatment during the whole experiment, and individual plants were taken from each container at each harvesting period. There were 4 batches of experiments in accordance with the four-month harvesting period. This resulted in a total of 48 experimental pots (3 replications, 4 treatments, and 4 collecting times). Each batch of plants from each container was analyzed independently and the chemical results of the three batches were averaged for each harvesting time.
The whole sugarcane plants were separated into four different parts, including roots, underground setts, shoots and leaves. The aboveground part of the sugarcane represents the leaves and shoots; while the underground part is the summation of the underground setts and roots. Shoot height (cm) was measured from the top of the underground sett to the tip of the longest leaf, and the shoot diameter (cm) was measured in the middle third of the sugarcane stalk. The roots, underground setts, shoots and leaves were oven-dried at 60 °C for 72 h, ground in an agate mortar and their dry weights (g per plant) were recorded.
The pH of the soil and amendments were determined for ratios of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 and 1
2 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 solid
5 solid![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) deionized water, respectively. Organic matter (OM), total nitrogen (Total.N), available phosphorous (Avail.P), and exchangeable potassium (Exc.K) were determined using the Walkley–Black method, the Kjeldahl Method, the BrayII method and 1M NH4-acetate extraction, respectively. In this study, total digestion according to the Standard US EPA Method 3052 (1996)7 and the three-step BCR sequential extraction proposed by the Standards, Measurements and Testing programme of the European Union (SM&T)8 including the residual fraction (BCR4) were performed for the soil analysis to determine the total and bioavailable Cd and Zn concentrations, respectively. The roots and underground setts of the sugarcane were determined using aqua regia extraction (3
deionized water, respectively. Organic matter (OM), total nitrogen (Total.N), available phosphorous (Avail.P), and exchangeable potassium (Exc.K) were determined using the Walkley–Black method, the Kjeldahl Method, the BrayII method and 1M NH4-acetate extraction, respectively. In this study, total digestion according to the Standard US EPA Method 3052 (1996)7 and the three-step BCR sequential extraction proposed by the Standards, Measurements and Testing programme of the European Union (SM&T)8 including the residual fraction (BCR4) were performed for the soil analysis to determine the total and bioavailable Cd and Zn concentrations, respectively. The roots and underground setts of the sugarcane were determined using aqua regia extraction (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio of 12 ml HCl and 4 ml HNO3); whereas the shoots and leaves were determined using a mixture of hydrogen peroxide (H2O2) and nitric acid (HNO3) (1
1 ratio of 12 ml HCl and 4 ml HNO3); whereas the shoots and leaves were determined using a mixture of hydrogen peroxide (H2O2) and nitric acid (HNO3) (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 v/v). The Cd and Zn concentrations in the soil and sugarcane were analyzed using graphite furnace atomic absorption spectrometry (GFAAS) and flame atomic absorption spectrometry (FAAS) (AAS-ZEEnit 700 Analytik), respectively. The accuracy of the analytical procedures for total digestion was verified using a soil certified reference material (CRM025-050, RTC) with four replicates. The percent recovery of the sum of the extracted Cd and Zn in all BCR fractions (BCR1, BCR2, and BCR3) plus the residual fraction (BCR4) was compared with the pseudo-total concentration (from aqua regia digestion) for an internal check.
3 v/v). The Cd and Zn concentrations in the soil and sugarcane were analyzed using graphite furnace atomic absorption spectrometry (GFAAS) and flame atomic absorption spectrometry (FAAS) (AAS-ZEEnit 700 Analytik), respectively. The accuracy of the analytical procedures for total digestion was verified using a soil certified reference material (CRM025-050, RTC) with four replicates. The percent recovery of the sum of the extracted Cd and Zn in all BCR fractions (BCR1, BCR2, and BCR3) plus the residual fraction (BCR4) was compared with the pseudo-total concentration (from aqua regia digestion) for an internal check.
The data were expressed as means with standard deviations (mean ± SD). The statistical analysis was based on one-way analysis of variance (ANOVA) for the comparison of statistical significances between treatments using the SPSS (Statistical Product and Service Solutions) version 17.0 software. The Duncan test at *p < 0.05 was used.
Results and discussion
Soil and amendment properties
The initial characteristics of the studied soil and the amendments used in this study are given in Table 1. The contaminated soil was slightly acidic (pH 6.6), nutrient poor (0.09% Total.N, 11 and 74 mg kg−1 of Avail.P and Exc.K, respectively) and low in organic matter content (2.58%). The soil contains high levels of Cd (19 mg kg−1) and Zn (914 mg kg−1). In comparison, BA is alkaline (pH 8.85) and FC and VN are slightly acidic (pH 6.02 and 6.58, respectively). These sugarcane wastes-products (BA, FC, and VN) contained very high organic matter contents (6.20%, 17.4%, and 15.2%), Avail.P (6964 mg kg−1, 1041 mg kg−1, and 4003 mg kg−1) and Exc.K (3325 mg kg−1, 5350 mg kg−1 and 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 663 mg kg−1), respectively as compared to the soil. The incorporation of sugarcane waste-products at 3% (w/w), however, did not cause an increase in the metal concentration in the soils (16.0–18.8 and 756–821 mg kg−1 for Cd and Zn, respectively).
663 mg kg−1), respectively as compared to the soil. The incorporation of sugarcane waste-products at 3% (w/w), however, did not cause an increase in the metal concentration in the soils (16.0–18.8 and 756–821 mg kg−1 for Cd and Zn, respectively).
| pH | OM (%) | Total.N (%) | Avail.P | Exc.K | Cd | Zn | |
|---|---|---|---|---|---|---|---|
| (mg kg−1) | |||||||
a BA = boiler ash; FC = filter cake; BAV = boiler ash and vinasse (50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50). 50).
|
|||||||
| Soil | 6.60 | 2.58 | 0.09 | 11 | 74 | 19 | 914 |
| BA | 8.85 | 6.20 | 0.12 | 6964 | 3325 | 0.01 | 60 |
| FC | 6.02 | 17.4 | 1.39 | 1041 | 5350 | 0.54 | 163 |
| BAV | 6.58 | 15.2 | 0.31 | 4003 | 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 663 663 |
0.44 | 117 |
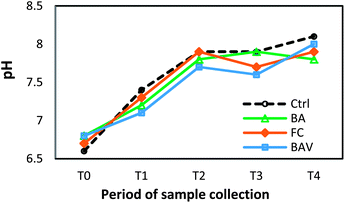
As soil pH is one of the most important factors affecting metal speciation and transformation, the soil pH was determined at every sampling period at each sugarcane growth stage (from T0 to T4) (Fig. 1). It was shown that the pH of the treated soils significantly increased (*p < 0.05) with the residence time from the first sampling (T0), with the highest pH occurring at the end of incubation period T4 (+1.0 to +1.5 pH unit increase). Cui et al.9 showed a similar pattern of pH change, with an initial increase followed by a decrease, and then the pH remained constant after incubating soils for a period of 6 months with Indian mustard. The initial increase in pH may be due to the alkaline amendments, which have a high K content that could contribute to an excess of Ca. Organic matter in the amendment materials used in this study may also be released and modify soil pH, depending on the buffering capacity of the soil, the composition of the organic matter and the soil physico-chemical properties.
Sugarcane growth parameters
The biomass production, expressed in dry weight (g per plant), of the sugarcane grown in different treated soils was measured at the end of the pot experiment (4 months) (Table 2). Without any soil amendment (Ctrl), the sugarcane could grow well in this high Cd contaminated soil as shoot, leaf, and root biomass (g per plant) remained very high. However, high metal loading in this high metal contaminated soil may have an influence on sugarcane growth, as illustrated by the lack of improvement in biomass production when amendments were added. The addition of 3% (w/w) BA and FC (BA and FC treatment) resulted in a negative growth response (not significant at *p < 0.05) when compared to the control (Ctrl). However, the highest biomass production was found in plants grown in BAV (but not significant), which were taller and had a larger number of leaves (data not shown) and showed a 23% increase in the leaf dry matter yield (Table 2). The elevated biomass production in BAV reflected an improvement in soil fertility and the supply of nutrients, especially K in the vinasse, that would promote and facilitate the plant growth. However their potential harmful effect on metal accumulation due to high biomass production should be carefully considered. No toxic symptoms were observed during the harvesting time. When the sugarcane was harvested 120 days after planting, most of the stalks (height) were generally more than 1.3 m long.| Treatment | Mean biomass dry weight (g per plant) ± SD | Height (cm) | Diameter (cm) | ||
|---|---|---|---|---|---|
| Leaf | Shoot | Root | |||
| a Mean values ± SD denoted by the same letter in a column indicates no significant difference according to the Duncan test at *p < 0.05. | |||||
| Ctrl | 26.9 ± 3.6a | 16.9 ± 4.5a | 11.8 ± 4.4a | 172 ± 9.6a | 1.93 ± 0.21a |
| BA | 11.9 ± 10.1a | 13.4 ± 3.8a | 5.80 ± 2.3a | 134 ± 51a | 1.80 ± 0.14a |
| FC | 13.1 ± 16.5a | 11.2 ± 10.5a | 4.28 ± 5.2a | 122 ± 39a | 1.23 ± 0.81a |
| BAV | 33.1 ± 8.3a | 16.2 ± 2.8a | 11.9 ± 6.2a | 166 ± 3.8a | 2.20 ± 0.10a |
Cd and Zn accumulation in sugarcane
The Cd and Zn concentrations (mg kg−1) in the different parts of the sugarcane at the plant growth stage of 4 months were determined (Table 3) to be in the following order: root > shoot ≥ underground sett > leaf, and root > underground sett > shoot > leaf, respectively. Many plant samples growing in polluted soils show high concentrations mainly in the roots due to the soil–plant transfer process.10 Regarding the total metal concentrations in the whole sugarcane samples (Table 3), Cd and Zn accumulation in sugarcane (0.5684–0.8737 and 75.1–117 mg kg−1 for Cd and Zn, respectively) is below the maximum concentration normally found in plants (2.0 and 400 mg kg−1, Cd and Zn, respectively).10 Additionally, the transfer factor (TF), which is the ratio of metal concentration (mg kg−1) in the sugarcane to the total metal concentration (mg kg−1) in the soil, was determined and the TF values of Cd and Zn were below 1 even in the control treatments (0.04–0.07 and 0.14–0.23, respectively), indicating low metal uptake and the effectiveness of sugarcane waste-products for in situ immobilization.| Cd concentration (mg kg−1) in sugarcane | ||||
|---|---|---|---|---|
| Ctrl | BA | FC | BAV | |
| Leaf | 0.3976b | 0.0273a | 0.1678ab | 0.3762b |
| Shoot | 0.9349a | 0.7009a | 0.8761a | 0.7813a |
| Sett | 0.8121a | 0.8087a | 0.7278a | 0.7488a |
| Root | 1.7464ab | 2.2681b | 0.8121a | 0.8323a |
| Total-sugarcane* | 0.8362a | 0.8737a | 0.5684a | 0.5975a |
| Total-soil** | 12.9a | 12.7a | 12.9a | 13.4a |
| TF | 0.06 | 0.07 | 0.04 | 0.04 |
| Zn concentration (mg kg−1) in sugarcane | ||||
|---|---|---|---|---|
| Ctrl | BA | FC | BAV | |
| a * = total concentration in sugarcane; ** = total concentration in soil. Values denoted by the same letter in a row indicates no significant difference according to the Duncan test at *p < 0.05. | ||||
| Leaf | 30.4a | 27.0a | 26.5a | 26.2a |
| Shoot | 104ab | 57.2a | 120b | 91ab |
| Sett | 140a | 134a | 135a | 77a |
| Root | 222a | 251a | 257a | 168a |
| Total-sugarcane* | 95.1a | 117a | 89.3a | 75.1a |
| Total-soil** | 520b | 499a | 523b | 527b |
| TF | 0.18 | 0.23 | 0.17 | 0.14 |
In addition, Cd and Zn accumulation (μg) in the aboveground and underground parts were determined (Table 4). It was found that the metal uptake of these two metals changed over time; they accumulated more in the underground parts in the first two month (T1 and T2) and then moved up to the aboveground parts at the end of harvesting period (T4). As expected, the total Cd and Zn accumulations (μg) were highest at the end of the pot experiment (T4) due to plant growth and metal accumulation. The metal increase in sugarcane was proposed to result from uptake by plants during the growth period.
| Cd | Metal uptake (μg) | |||||||
|---|---|---|---|---|---|---|---|---|
| T1 | T2 | T3 | T4 | |||||
| Aboveground | Underground | Aboveground | Underground | Aboveground | Underground | Aboveground | Underground | |
| Ctrl | 0.752 | 4.07 | 3.64 | 4.04 | 13.5 | 8.73 | 26.8 | 24.9 |
| BA | 0.299 | 5.49 | 2.56 | 7.36* | 13.2 | 7.54 | 10.2* | 18.9 |
| FC | 0.018 | 4.33 | 8.38 | 5.23 | 17.1 | 9.65 | 13.7* | 7.29* |
| BAV | 0.553 | 11.6* | 7.84 | 6.14 | 23.6 | 9.76 | 25.2 | 15.4* |
| Zn | T1 | T2 | T3 | T4 | ||||
|---|---|---|---|---|---|---|---|---|
| a Note: * = The mean value is significantly different compared to the Ctrl, according to the Duncan test at *p < 0.05. | ||||||||
| Ctrl | 66 | 650 | 388 | 1171 | 1767 | 1723 | 2489 | 3324 |
| BA | 27 | 841 | 366 | 824 | 1939 | 1503 | 1156* | 2661 |
| FC | 42 | 702 | 1016 | 1189 | 2142 | 1468 | 1645 | 1766 |
| BAV | 58 | 1757 | 1142 | 1719 | 2216 | 1380 | 2298 | 2797 |
Moreover, the results showed that the addition of 3% (w/w) BA or FC was effective in reducing Cd uptake in the aboveground part of the sugarcane at the end of the pot experiment, showing a 62.7% and 53.7% reduction, respectively, compared to the Ctrl (Table 4). However, no significant difference in Zn uptake (*p < 0.05) was observed in this study, except with the BA treatment.
The effect of amendments on bioavailability of Cd and Zn in soils
Among the chemical forms, the exchangeable (BCR1) and reducible (BCR2) fractions of the BCR sequential extraction can be used to determine the environmental risk associated with plant uptake, as they represent the most mobile and bioavailable fractions11 and could be used to evaluate the effect of the incorporated amendments on metal transformation and immobilization. Table 5 shows the metal concentration (mg kg−1) of the treated soils in these fractions (BCR1 + 2).| Treatment | BCR1 + 2 Cd concentration (mg kg−1) | ||||
|---|---|---|---|---|---|
| T0 | T1 | T2 | T3 | T4 | |
| Ctrl | 26.8 ± 2.2b | 14.3 ± 1.3a | 4.96 ± 0.8a | 5.32 ± 0.3b | 3.54 ± 0.1a |
| BA | 16.5 ± 5.5ab | 11.7 ± 1.4a | 4.80 ± 0.4a | 4.64 ± 0.2a | 3.30 ± 0.2a |
| FC | 24.3 ± 2.2ab | 13.9 ± 1.2a | 4.61 ± 0.2a | 4.69 ± 0.3a | 3.40 ± 0.3a |
| BAV | 19.2 ± 5.3a | 14.3 ± 1.3a | 5.27 ± 0.5a | 4.78 ± 0.3ab | 3.20 ± 0.4a |
| Treatment | BCR1 + 2 Zn concentration (mg kg−1) | ||||
|---|---|---|---|---|---|
| T0 | T1 | T2 | T3 | T4 | |
| a Mean values ± SD denoted by the same letter in a column indicates no significant difference according to the Duncan test at *p < 0.05. | |||||
| Ctrl | 647 ± 67b | 404 ± 38b | 64 ± 2.1ab | 182 ± 6.0a | 128 ± 6.6a |
| BA | 223 ± 169a | 333 ± 10a | 63 ± 1.1a | 180 ± 6.1a | 120 ± 2.4a |
| FC | 526 ± 20b | 402 ± 43b | 63 ± 0.8a | 174 ± 6.4a | 121 ± 8.6a |
| BAV | 468 ± 145b | 415 ± 27b | 66 ± 1.4b | 182 ± 4.4a | 120 ± 6.9a |
According to the results, reductions in the most bioavailable Cd and Zn concentrations (BCR1 + 2) (mg kg−1) at the end of the experiment (T4) were observed with BA, FC and BAV (not significant difference), and ranged from 4.0–9.6% and 5.5–6.3%, respectively. Two hypotheses can explain this phenomenon. Firstly, the direct effect of amendment addition of raising the pH of the treated soils throughout the experiment (Fig. 1). The precipitation of metal hydroxides and carbonates may cause metals to be less mobile in this study. Secondly, the binding of metals to the added amendments, which are known to be rich in metal binding sites, could occur. The results from this study clearly revealed that the pH increase, caused by the addition of amendments, had a major role in reducing the metal bioavailability in soils and thus its uptake by sugarcanes.
Transformation of Cd and Zn
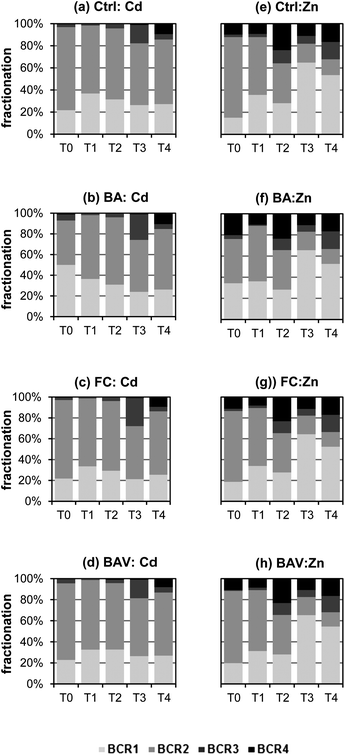
The reduction of the most mobile fraction of Cd and Zn (BCR1 + 2) shows an evolution with time towards a form of lower bioavailability. The effect of organic matter on metal mobility and speciation in terms of the quantitative organic matter bound fraction (BCR3) was considered in this study to explain the influence of organic matter in metal complex formation. According to the results, the percentage distribution of Cd and Zn in the oxidizable fraction (%BCR3), the relative concentration of BCR3 with the sum of all fractions, increased significantly as time increases (Fig. 2). The increment of the soil organic matter content of the treated soils encouraged the association of stable metal–organic complexes, which could diminish the solubility of the metal ions in the soil. The decrease of the bioavailable pool of Cd in the treated soils (BCR1 + 2) in this study revealed the potential use of sugarcane waste-products for metal stabilization and this could be attributed to metal complexation by organic matter. The finding of this study is in agreement with Mohamed et al.12 who evaluated the influence of rice straw, green manure and pig manure on the retention of Cd and found that the addition of organic amendments significantly reduced the soluble Cd, with increases in the organic-bound fraction.Conclusions
Overall, the addition of single boiler ash (BA) and filter cake (FC) at 3% (w/w) to highly Cd and Zn contaminated soil contributed to the reduction of Cd uptake in the aboveground part of sugarcane and the bioavailability of Cd (BCR1 + 2) in soil, while little effect on speciation of Zn was seen. However, no positive effect on plant growth or metal toxicity was visually seen in this study. This suggests the alternative use and reutilization of sugarcane waste-products in agricultural land, especially in high Cd and Zn contaminated sites. Nevertheless, the long term effect of repeated application of these materials still requires in-depth investigation.Acknowledgements
The authors acknowledge the Center of Excellence on Hazardous Substance Management (HSM) and the 90th Year Chulalongkorn Scholarship by Graduate School, Chulalongkorn University for financial support, and Mae Sot Clean Energy Company Limited, Tak Province, Thailand for providing the sugarcane waste-products.References
- T. Silalertruksa and S. H. Gheewala, Energy, 2009, 34, 1933–1946 CrossRef CAS PubMed.
- A. Pensiri, D. Thomas and C. Tongcumpou, J. Soils Sediments, 2013, 13, 1057–1068 CrossRef.
- R. A. Gilbert, D. R. Morris, C. R. Rainbolt, J. M. McCray, R. E. Perdomo, B. Eiland, G. Powell and G. Montes, Agron. J., 2008, 100, 845–854 CrossRef CAS.
- S. Lee, J. Lee, Y. J. Choi and J. Kim, Chemosphere, 2009, 77, 1069–1075 CrossRef CAS PubMed.
- P. Janoš, J. Vávrová, L. Herzogová and V. Pilařová, Geoderma, 2010, 159, 335–341 CrossRef PubMed.
- A. Pensiri, D. Thomas and C. Tongcumpou, Environ. Sci.: Processes Impacts, 2013, 15, 947–954 Search PubMed.
- USEPA, Method 3052: Microwave assisted acid digestion of siliceous and organically based matrices, US Environmental Protection Agency, Washington, DC, 1996 Search PubMed.
- Ph. Quevauviller, Methodologies in soil and sediment fractionation studies: single and sequential extraction procedures, The Royal Society of Chemistry, Cornwall, UK, 2002 Search PubMed.
- Y. Cui, J. Fu and X. Chen, J. Environ. Sci., 2011, 23, 624–632 CrossRef CAS.
- G. S. Sara, G. C. David, Q. N. M. Angeles and B. S. M. Milagros, Water, Air, Soil Pollut., 2012, 223, 559–572 CrossRef.
- A. Pensiri and C. Tongcumpou, Geoderma, 2010, 156, 126–132 CrossRef PubMed.
- I. Mohamed, B. Ahamadou, M. Li, C. Gong, P. Cai, W. Liang and Q. Huang, J. Soils Sediments, 2010, 10, 973–982 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2014 |