Progress on understanding spatial and temporal variability of PM2.5 and its components in the Detroit Exposure and Aerosol Research Study (DEARS)†
Carvin
Stevens
*a,
Ron
Williams
b and
Paul
Jones
b
aU.S. Environmental Protection Agency, Research Triangle Park, NC 27711, USA. E-mail: stevens.carvin@epa.gov; Fax: +1-919-541-0905; Tel: +1-919-541-1515
bU.S. EPA, Research Triangle Park, 107 T.W. Alexander Drive, NC 27711, USA. E-mail: williams.ronald@epa.gov; jones.paul-a@epa.gov
First published on 7th November 2013
Abstract
The Detroit Exposure and Aerosol Research Study (DEARS) measured personal exposures, ambient, residential indoor and residential outdoor concentrations of select PM2.5 aerosol components (SO4, NO3, Fe, Si, Ca, K, Mn, Pb, Zn, EC and OC) over a three year period (2004–2007). These events represented approximately 190 calendar days of monitoring which was performed in seven residential neighborhoods throughout Wayne County, MI. The selection of neighborhoods and participants for study inclusion was based upon an a priori hypothesis that each neighborhood represented a potentially distinct air quality scenario being influenced by both regional as well as local pollution sources. Daily (24 h integrated) measurement data were used to evaluate the spatial and temporal PM2.5 compositional variability of the personal, indoor and outdoor spatial settings as they related to a central ambient monitoring site (Allen Park). Many of the PM2.5 components were observed to have spatially different outdoor mass concentrations in matched neighborhood by neighborhood comparisons, with sulfate, OC, and NO3 being noted exceptions. Coefficient of divergence (COD) comparisons involving outdoor measures for Ca, Si, Fe, Zn, Pb, and EC revealed significant spatial variability. While concentrations of most components were lower indoors as compared to outdoor measures, K and Si indoor concentrations often reflected aerosol enrichment (indoor/outdoor ratios ≥ 1.2). Even when personal exposures were adjusted for day to day changes in ambient concentrations, certain components (Ca, Fe, Mn, Zn, among others) revealed a high degree of location-specific spatial variability suggesting the influences of personal activities and/or local source influences on total personal PM2.5 exposures. As a whole, findings indicate that reliance on a central ambient monitor as a surrogate for total personal and potentially even residential outdoor estimates of PM2.5 aerosol composition may provide an undesirable degree of exposure uncertainty for health-based risk estimates. The focus of this paper is on the spatial variability and uncertainty in using a central monitoring site to estimate exposures. Additional information concerning the DEARS can be found at http://www.epa.gov/DEARS/.
Environmental impactParticulate matter (PM) composition and size vary widely with both space and time. The variability in PM characteristics and sources are believed to influence human health risks. The quantitative relationships between concentrations of particulate matter (PM) and gaseous copollutants measured at stationary outdoor air monitoring sites and the contributions of these concentrations to actual personal exposures remains a focus in human health risk assessment. The results of this paper will have a significant impact in advancing the knowledge of environmental processes and impacts associated with exposure science. |
1 Introduction
Understanding the uncertainty of using a central (ambient) community monitoring site to estimate concentrations of air pollutant exposures for a given population or geographical area is of research interest to exposure scientists, environmental epidemiologists, and others involved in establishing health-based risk assessments (Dominici et al. 2006,1 Brook et al. 2011 (ref. 2)). Both spatial as well as temporal variability issues may be influencing factors on the reliability of using a central monitoring site as an adequate surrogate of a subject population's exposure. While the need to establish such potential measurement errors have been raised (Zeger et al. 2000;3 Navidi et al. 1994;4 Lipfert et al. 1997 (ref. 5)), few research studies have been specifically designed to examine this issue. The focus of this paper is on the spatial variability and uncertainty in using a central monitoring site to estimate exposures. The correlation of the central monitoring site to specific micro-environments (indoor, outdoor) and human exposures are the basis for these evaluations. The compositional components that are correlated are sulfates (SO4), nitrates (NO3), organic carbon (OC), elemental carbon (EC) and some of the crustal materials (Fe, Si, Ca, K, Mn, Pb, Zn).One such effort to obtain sufficient data to examine exposure measurement uncertainty in a given geographical area has recently been completed. The U.S. EPA conducted an intensive 3 year human observational exposure study entitled, the Detroit Exposure and Aerosol Research Study (DEARS). The study was conducted in Wayne County, MI from 2004 to 2007. The DEARS was designed to investigate the sources of different pollutants impacting households across a large metropolitan area and to determine the spatial and temporal variability of a wide range of pollutant species at the personal (P), residential indoor (I), and residential outdoor (O), settings. The DEARS involved 24 h-integrated (daily) monitoring associated with 142 participants and involving six selected neighborhoods. These six neighborhoods or enumeration monitoring areas (EMAs) were selected a priori as potentially being impacted by a wide range of both regional and local air quality sources. Summer and winter sampling schemes for each participant consisted of 5 days of monitoring each season (Tuesday–Saturday). In addition, daily pollutant measurements were taken at a centrally-located ambient monitoring site (A) at Allen Park, MI. Data from a total of three summer and three winter seasons were collected (Williams et al. 2009;6 EPA 2012 (ref. 7)). DEARS investigated the intra-urban variability in air pollution source impacts using receptor and statistical modeling of daily speciated PM2.5 and VOC measurements collected at residential outdoor locations across Wayne County, MI (Duvall et al. 2012;8 Bereznicki et al. 2012;9 George et al. 2010 (ref. 10)). Spatial relationships between coarse particulate matter in the DEARS were reported by Thornburg et al. 2010.11
Particulate matter (PM) represented one of the primary pollutants of interest in the DEARS with many reported PM pollutant sources present in the Detroit area. Wayne County, MI is consistently reported as one of the most polluted counties in the U.S. and the most polluted in Michigan as reported by the EPA's Toxic Release Inventory (TRI). DEARS research observed negligible PM total mass (coarse) concentration spatially in residential outdoor measurements across the Detroit urban air shed (Rodes et al. 2010 (ref. 12)). Spatial factors, such as distance from a highway, topography, land surface roughness, and the presence of other pollution sources affect the pollutant concentration and composition. Time-related factors, such as local meteorology (wind speed and direction, stability of the atmosphere boundary layer, precipitation, etc.), as well as traffic intensity may play a role in pollutant dispersion, and as a result in human exposure (Martuzeviciusa et al. 2004 (ref. 13)). George et al. 2010 (ref. 10) reported the spatiality influence of meteorology in neighborhood-based PM2.5 mass concentrations associated with the DEARS.
Local PM2.5 sources in Wayne County include industrial and residential combustion processes, motor vehicle emissions, residential and prescribed burning among a variety of others that contribute to the local air quality (Duvall et al. 2012;8 Bereznicki et al. 2012 (ref. 9)). PM2.5 is formed from combustion processes and chemical reactions in the atmosphere and contains a wide variety of primary components. The major components of PM2.5 are sulfates, nitrates, elemental/organic carbon (EC/OC), metals, and crustal elements. Some of these components have been reported to be associated with some negative health outcomes. Ostro et al. 2008 (ref. 14) found that cardiovascular mortality has been associated with PM2.5 and several of its species including EC, OC, nitrates, sulfates, potassium, copper and iron. EC/OC has been associated with respiratory and cardiovascular health effects (Gauderman et al. 2004;15 Peters et al. 2000 (ref. 16)). Sulfate (SO4) has advantages over other PM2.5 components for retrospective epidemiology because extensive epidemiological literature and large databases for sulfates exist as compared to studies of the other components. The association of mortality with SO4 is inconsistent. In a review of toxicologic studies, Schlesinger et al. (2003)17 suggested that SO4 is benign. In vivo studies PM2.5, Seagrave et al. (2006)18 found that lung toxicity and inflammation correlated with vehicular pollution but not secondary particles, including SO4. However, vehicular emissions are consistently associated with cardiac or other end points as reported by Grahame et al. (2007).19 Cavallari et al. 2008 (ref. 20) reports that the metal components of PM2.5 may be toxic and responsible for lung inflammation and cardiac arrhythmias, and Valko et al. 2006 (ref. 21) reported that metal-induced toxicity and carcinogenicity are caused by oxidative stress.
While specific PM mass components have been associated with health outcomes, little is known about the spatial and temporal variability of the mass concentrations of these components across a metropolitan area. Understanding such variability is critical in assessing the exposure measurement uncertainty or even the exposure misclassification errors in using a central community monitor to represent a given epidemiological study population (Zeger et al. 2000 (ref. 3)). Significant sources of the trace metal and the crustal components of PM2.5 in metropolitan settings may exist and could exhibit substantial spatial and temporal variability within such settings (Oglesby et al. 2000;22 Lau et al. 2009 (ref. 23)). It has been suggested that in such cases, centrally located community monitors might not be an adequate surrogate for residential concentrations and personal exposures to air pollutants (Kousa et al. 2002;24 Violante et al. 2006 (ref. 25)). Examination of spatial and temporal variations in the concentration and composition of PM has the potential to provide important insights into particle sources and atmospheric processes that influence particle formation (Olofson et al. 1994;26 Motallebi et al. 2003 (ref. 27)). Investigations involving the seasonal and annual variability of the components of PM2.5 would allow for the examination of the influence of the atmospheric contribution of a heavily industrialized urban center and the particulate matter composition (Ledoux et al. 2006 (ref. 28)).
To examine some of the issues discussed above relating to spatial and temporal variability of the major PM2.5 mass components, we will report daily (24 h) levels of personal, residential indoor and residential outdoor, as well as community-level concentrations of these components from the DEARS. A variety of statistical approaches are used in this assessment and extensive use of descriptive statistics, mixed models, and coefficient of divergence analyses provides the basis for summary findings.
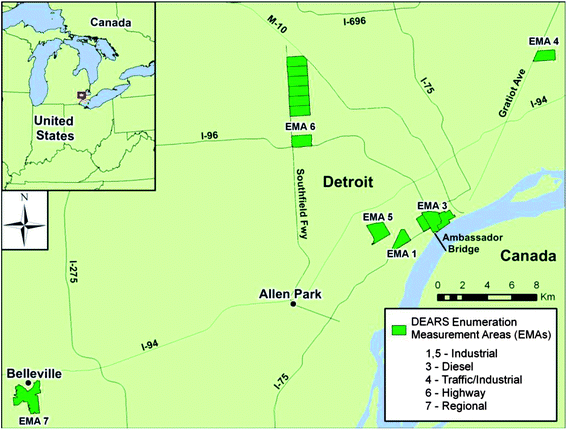
Detroit is located in Wayne County, MI and EMAs selected for the sampling in DEARS are located within the county (Fig. 1). Williams et al. 2009 (ref. 6) have described in great detail each EMA and their selection as part of the overall DEARS study design. In addition, preliminary investigations concerning potential industrial sources impacting the various EMAs have been reported (Duvall et al. 2012;8 Bereznicki et al. (ref. 9)). A selection of the EMAs was based on the proximity to point and line sources (local freeways or interstate highways) that were expected to impact these areas (ESI Table 1†). The mobile sources are represented as a distance either less than or greater than 300 m from the roadway. The 300 m distance cut-off for roadway proximity is based on the hypothesis that concentrations of some mobile source-related pollutants (VOCs) decrease significantly at distances beyond 300 m from the source. Findings in the DEARS have supported this element of the study design (Barzyk et al. 2009 (ref. 29)).
EMA 1 represents the Zug Island area, a heavily industrialized island in the city of River Rouge near the southern city limits of Detroit. A major source of pollution in this area is from the steel manufacturing process. The Ambassador Bridge is believed to be a major PM source located in EMA 3. The bridge joins the US to Ontario, Canada and is North America's most active international Border crossing. EMA 4 represents a mixture of both industrial as well as potential near-road impacts. Dearborn (EMA 5) is the center of the Detroit automotive industry. The EMAs includes six automotive factories on 600 acres (2.4 km2) of land, as well as steelmaking operations in the south end of Dearborn. A major source of air pollution in EMA 6 was hypothesized as the Southfield Freeway. The Michigan Department of Transportation (MDOT) surveys in 2010 showed that the highest traffic levels along the freeway were the 159![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 400 vehicles daily between Schoolcraft Road and Grand River Avenue in Detroit; the lowest counts were the 20
400 vehicles daily between Schoolcraft Road and Grand River Avenue in Detroit; the lowest counts were the 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 400 vehicles per day between the I-94 and Van Born Road interchanges (MDOT 2010 (ref. 30)). Belleville (EMA 7) was considered a priori to be a background site impacted almost entirely by regional air quality. The central (ambient) monitoring site at Allen Park was collocated with one operated by the State of Michigan as part of their state comprehensive air monitoring network. This site has historically been used for compliance in demonstrating attainment with the National Ambient Air Quality Standards (NAAQS). Additional information concerning the DEARS can be found at http://www.epa.gov/DEARS/.
400 vehicles per day between the I-94 and Van Born Road interchanges (MDOT 2010 (ref. 30)). Belleville (EMA 7) was considered a priori to be a background site impacted almost entirely by regional air quality. The central (ambient) monitoring site at Allen Park was collocated with one operated by the State of Michigan as part of their state comprehensive air monitoring network. This site has historically been used for compliance in demonstrating attainment with the National Ambient Air Quality Standards (NAAQS). Additional information concerning the DEARS can be found at http://www.epa.gov/DEARS/.
2 Methods & materials
2.1 Study design
Williams et al. 2009 (ref. 6) report on the design and field implementation for the DEARS. Personal samples were collected using active (2 lpm) PM2.5 personal monitors (PEMs) placed on sampling vests worn by the participants. The residential samples, collected using the personal monitoring device, were collected outside of the homes, and the community (ambient) based monitoring took place at Allen Park, MI. EMAs were selected based on proximity to known or suspected point and line sources. Participants were monitored for 5 consecutive (24 h) days in each of two consecutive seasons (summer, winter) from 2004–2007. Selection criteria for participants were that they must be (1) non-smokers, (2) living in a non-smoking household, (3) ambulatory, (4) expected to live in the same dwelling for the next 9 months, (5) living in a detached home, (6) 18 years of age or older, and (7) able to comprehend either English or Spanish instructions. There were no health restrictions on enrollment other than being ambulatory. Likewise, there were no enrollment restrictions on occupation, socioeconomic status, sex, or ethnicity. Residences were selected using randomized sampling in a geographically focused pattern recruitment of participants (Phillips et al. 2010 (ref. 31)).2.2 Sample collection and analyses
The measurements and analyses methods are generally described in Williams et al. 2009 (ref. 6) and are referenced in the ESI Table 2.†2.3 Data analysis
Descriptive statistics and distributions of PM2.5 components were tabulated by season and EMAs. Descriptive analyses included the use of several measures of centrality (e.g., means and medians) and measures of dispersion (e.g., standard deviations and range of distributions) to characterize the distribution of the PM2.5 components. Multivariate analysis included the use of mixed models to account for potential serial correlations between the repeated measurements. Multivariate analysis was performed using general linear models to examine the effect of seasonal variability on selected personal and outdoor elemental components. More specifically, we used the MIXED procedure in SAS (version 9.1) to account for potential serial correlations among repeated measures for each subject. The model was defined as:| Yij = β0 + β1X1ij + β2X2ij + εij |
The MIXED procedure requires a covariance structure to be specified in the model. We used the information criteria to produce the MIXED procedures as a tool in selecting a covariance for the model. We examined two covariance structures: compound symmetry (exchangeable) and autoregressive AR (1). After examining the two covariance structures, we chose the exchangeable covariance structure based on the Akaike Information Criteria (AIC) statistic. The AIC statistics associated with exchangeable covariance structure was smaller (Littell et al.32) than the one associated with AR (1), and therefore the exchangeable covariance was used in the analyses. The exchangeable covariance structure indicated that correlations of the repeated measures were relatively constant. Within the mixed model, we also generated least square means for both seasons for selected components. In additional to examining potential differences between the two seasons, the least square means provided a magnitude of the difference within-group means adjusted for other factors in the model. A statistical difference between variables being compared was reported when p-values were ≤0.05.
The coefficient of divergence (COD) between EMAs assessed spatiality of the PM2.5 components (Pinto et al. 2004 (ref. 33)). In this study, we examined 6 EMAs and the central site, resulting in 21 pair-wise spatial comparisons. A COD of 0 indicates complete homogeneity and a value of 1 indicates maximum differences. COD values between 0 and 0.2 are representative of good agreement between matched pairs. On the contrary, values greater than 0.2 to 1 are indicative of pairs that do not agree well and are non-representative of one another. Enrichment factors presented in the paper are the mass concentration ratios calculated using the matched daily average means to estimate the relationships of indoor to outdoor or personal to indoor mass concentration relationships.
3 Results and discussion
The mean statistical summary of the primary PM2.5 mass components measured at the central (ambient) monitoring site for the summer and winter seasons in the DEARS observed that the total daily PM2.5 mass concentrations ranged from 2.8 to 66.4 μg m−3 over the course of the full study. Using the accepted conversion factors for transforming elemental sulfur to sulfate (SO4 = S × 4.125), the results indicate that the total PM2.5 mass is composed of ∼36% SO4 in the summer. By contrast, NO3 was the major mass contributor observed during the winter seasons (∼29%). NO3 exhibited the greatest seasonal difference in mass concentration than any other component with the mean winter concentration being more than 4 times that of the summer (ESI Table 3†). Mean OC concentrations revealed little variability by season (∼1%). When original OC data was converted to its usual form for mass reporting (1.4 × OC), it contributed significantly to the total PM2.5 mass (∼23% in winter). Ca, Fe, K, Mn, Pb, Si, and Zn contributed significantly less mass to the total PM2.5 composition regardless of season. Mean mass concentrations for these elements was typically ≤200 ng m−3. Even so, on some occasions they were observed to be significantly elevated on a daily basis (e.g., Fe maximum = 7130 ng m−3). Descriptive statistical data for personal, indoor, outdoor and the central site are found in the ESI Tables 3–9.†Graphical representations (Charts 1 and 2) of the average concentrations of the data for the personal, indoor, outdoor and ambient concentrations of each component as they relate spatially for summer and winter show that the highest mass concentrations of PM2.5 varies significantly within EMA 4. The results for summer and winter show EMA 5 has the highest concentrations and greatest variance for the metals or crustal materials (Ca, Fe, K, Mn, Pb, Zn & Si) during summer and winter. EMA 5 (Dearborn) is a heavily industrialized area, and the most abundant metal was Fe contributions, averaging 921 ng m−3, were found there during the winter. Fe is the most abundant of the metals and is primarily associated with the soil and crustal elements of PM2.5. Duvall et al. 2011,8 related the impact of a variety of steel manufacturing and mixed industries in the DEARS as a source of the observed Fe concentrations, especially those associated with EMA 1 and 5.
 | ||
| Chart 1 Component mean concentrations (ng m−3) in each Enumeration Monitoring Area (EMA) personal, indoor, outdoor and central site (summer). | ||
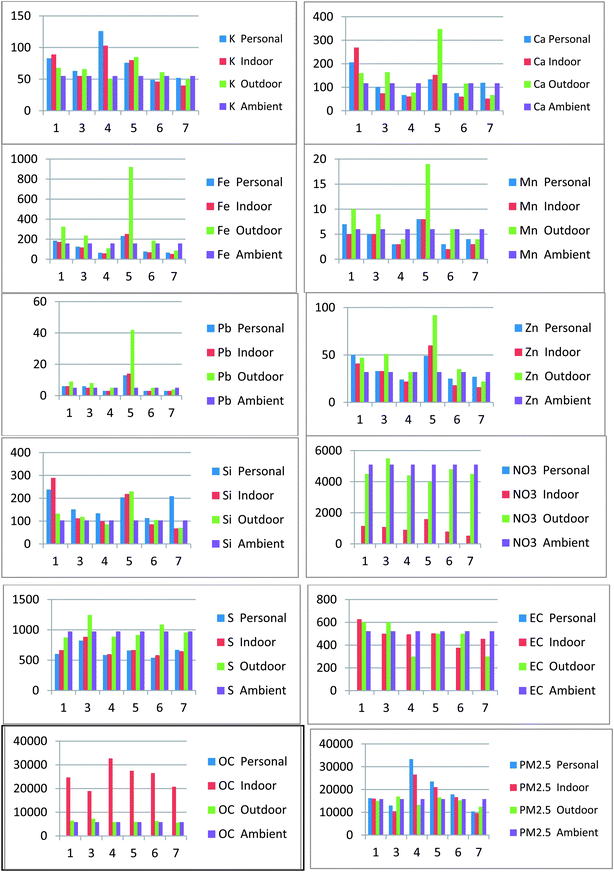 | ||
| Chart 2 Component mean concentrations (ng m−3) in each Enumeration Monitoring Area (EMA) personal, indoor, outdoor and central site (winter). | ||
In general, total PM2.5 mass across the EMAs was dominated by contributions from SO4, OC and NO3. Winter data reveal that mean total PM2.5 mass concentrations were highest in EMAs 3 and 5 (16.9 and 16.6 μg m−3, respectively) and EMAs 1 and 5 were highest in the summer (ESI Tables 4 and 5†). OC is the most abundant component for the outdoor residential spatial setting with the highest concentrations observed in the summer. These findings are consistent with reported findings that organic compounds of biogenic and anthropogenic origin often represent a large fraction, up to 40%, of total PM mass (Chow et al. 1993;34 Chow et al. 1994 (ref. 35)). SO4 is the second most abundant component in summer across the EMAs with NO3 being the second most abundant in winter. OC, SO4 and NO3 are considered secondary or regional components of PM2.5. The graphical representations for the seasonal variations are shown in Chart 3.
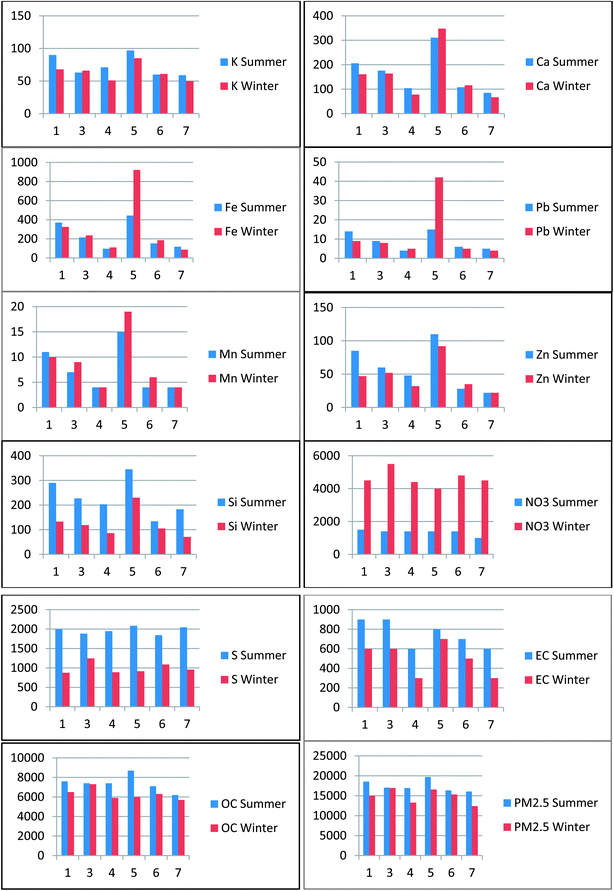 | ||
| Chart 3 Residential outdoor mean concentrations (ng m−3) in each Enumeration Monitoring Areas (summer vs. winter). | ||
Mixed model results indicate the impact of residential outdoor spatial PM2.5 component variability across the DEARS EMAs by seasons (Table 1). Day to day variability of ambient-based concentrations was accounted for in the modeling approach. The presence of a p-value ≤ 0.05 for any of the components for a given season is indicative that some significant degree of spatiality exists. Such a value indicates that at least one of the EMAs had observed mean mass concentrations statistically different than the overall modeled mean. Rodes et al. 2010 (ref. 12) had identified some preliminary findings indicating that some minor PM2.5 total mass heterogeneity existed across the DEARS on a daily basis (on the order of 1–2 μg m−3). The current findings validate that conclusion and provide for an EMA basis for such an observation. Using periodic sampling outdoor measures of S revealed low spatial variability. NO3 and OC exhibited low spatial variability during the winter seasons. Again, as regional pollutants, such a finding of general homogeneity is not unexpected. What is surprising is the consistent pattern of some degree of heterogeneity that exists for the elemental components as a whole. This finding indicates that local sources of the various elemental components exist and are impacting the air quality in one or more of the EMAs being compared. It further suggests that attempts to use a common ambient monitor to reflect neighborhood outdoor mass concentrations of select PM components of health interest (e.g., Fe, Zn, Mn) in epidemiological risk assessments may unknowingly introduce a high degree of exposure error. Considering that some of the DEARS EMAs were relatively close to one another (≤5 km distance) and that some degree of overall spatiality was still observed for many of the elements, proximity of an ambient monitor to a target population (nearby location), may not be a sufficient decision parameter alone in conducting research of that nature.
| Component | Season | Enumeration Monitoring Areas (EMAs) | p-Value | |||||
|---|---|---|---|---|---|---|---|---|
| 1 | 3 | 4 | 5 | 6 | 7 | |||
| a Conversions used in the manuscript to quantify SO4: SO4 = S × 4.125. b Conversions used in the table OC = OC × 1.4. | ||||||||
| Calcium | Summer | 208 | 171 | 75 | 300 | 111 | 98 | <0.01 |
| Winter | 163 | 165 | 77 | 338 | 115 | 73 | <0.01 | |
| Iron | Summer | 376 | 207 | 109 | 444 | 153 | 126 | <0.01 |
| Winter | 331 | 230 | 115 | 902 | 183 | 98 | <0.01 | |
| Potassium | Summer | 91 | 70 | 62 | 97 | 60 | 59 | <0.01 |
| Winter | 74 | 61 | 53 | 86 | 58 | 52 | <0.01 | |
| Manganese | Summer | 11 | 7 | 5 | 15 | 4 | 5 | <0.01 |
| Winter | 10 | 9 | 4 | 19 | 6 | 4 | <0.01 | |
| Lead | Summer | 12 | 8 | 6 | 15 | 6 | 5 | <0.01 |
| Winter | 9 | 7 | 5 | 42 | 5 | 4 | <0.01 | |
| Zinc | Summer | 83 | 61 | 29 | 107 | 28 | 22 | <0.01 |
| Winter | 49 | 48 | 31 | 94 | 34 | 25 | <0.01 | |
| Silicon | Summer | 288 | 247 | 201 | 344 | 195 | 205 | 0.003 |
| Winter | 132 | 119 | 86 | 229 | 105 | 67 | <0.01 | |
| Nitrates | Summer | 1487 | 1335 | 1294 | 1425 | 1342 | 1057 | <0.01 |
| Winter | 4982 | 5085 | 4841 | 4834 | 5041 | 4178 | 0.11 | |
| Sulfur | Summer | 1999 | 1850 | 1945 | 2086 | 1846 | 1875 | 0.95 |
| Winter | 889 | 1239 | 883 | 926 | 1077 | 1146 | 0.76 | |
| EC | Summer | 915 | 945 | 586 | 835 | 719 | 542 | <0.01 |
| Winter | 587 | 598 | 349 | 591 | 459 | 326 | <0.01 | |
| OC | Summer | 7657 | 7407 | 7304 | 8488 | 7153 | 6071 | <0.01 |
| Winter | 6680 | 6537 | 5836 | 6381 | 6460 | 5605 | 0.15 | |
| PM2.5 | Summer | 18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 611 611 |
17![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 146 146 |
15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 248 248 |
19![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 610 610 |
16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 242 242 |
15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 003 003 |
<0.01 |
| Winter | 16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200 200 |
16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 305 305 |
13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 403 403 |
18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 727 727 |
15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 017 017 |
12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 075 075 |
<0.01 | |
We further identify the inter-EMA comparability of the PM2.5 mass components (Table 2). Coefficient of divergence (COD) statistic is provided for the combined summer and winter measures. These measures provide a clear perspective on how residential outdoor measures in any one EMA compared to date-matched measures in all other EMAs and the central site. While there is not consensus of an exact COD value that constitutes statistical significance, literature indicates values >0.2 are indicative of the pairings that are somewhat not representative of each other (Thornburg et al. 2010 (ref. 11)). Using such a threshold indicator of heterogeneity, the regional nature of NO3, S (SO4), and OC is clearly established where most of the pairing are approximately 0.2 or less. These components EC, typically associated with automotive and other similar fossil fuel combustion sources, exhibited greater spatial variability as many of the pairings exhibited CODs > 0.30. This observed wide-spread heterogeneity strengthens the earlier statistical finding associated with EC in Table 1. K on the other hand, exhibits significantly less spatial variability with a majority of the pairings having COD values ≤ 0.25. K can be considered to have low spatial and temporal variability when the individual EMAs are correlated using continuous central site monitoring over all seasons. K has been considered a possible regional source but its origin has not been accounted for in the Detroit area (Duvall et al. 2012 (ref. 8)). Residential outdoor concentration pairings for Ca, Si, Mn, Zn, and Pb were routinely different across most of the EMA pairings (low spatial and temporal variability). EMA 7, the regional background site, often exhibited a concentration difference relative to the more metropolitan based EMAs (1, 3, 4, 5, and 6) with respect to elemental components. This is not surprising considering the lack of industrial and other identifiable sources in that location.
| Component | 1 vs. 3 | 1 vs. 4 | 1 vs. 5 | 1 vs. 6 | 1 vs. 7 | 1 vs. 9 | 3 vs. 4 | 3 vs. 5 | 3 vs. 6 | 3 vs. 7 | 3 vs. 9 | 4 vs. 5 | 4 vs. 6 | 4 vs. 7 | 4 vs. 9 | 5 vs. 6 | 5 vs. 7 | 5 vs. 9 | 6 vs. 7 | 6 vs. 9 | 7 vs. 9 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| a (Values greater than 0.2 are indicative of pairs that are non-representative of one another. The central site is represented as Enumeration Monitoring Areas (EMA) 9). Conversions used in the manuscript to quantify SO4: SO4 = S × 4.125, conversions used in the table OC = OC × 1.4. | |||||||||||||||||||||
| NO3 | 0.14 | 0.14 | 0.21 | 0.17 | 0.22 | 0.21 | 0.15 | 0.16 | 0.14 | 0.22 | 0.16 | 0.21 | 0.14 | 0.19 | 0.22 | 0.19 | 0.22 | 0.14 | 0.22 | 0.22 | 0.21 |
| S | 0.06 | 0.12 | 0.07 | 0.13 | 0.12 | 0.10 | 0.09 | 0.05 | 0.12 | 0.12 | 0.08 | 0.11 | 0.10 | 0.13 | 0.13 | 0.12 | 0.11 | 0.12 | 0.08 | 0.11 | 0.13 |
| K | 0.20 | 0.27 | 0.21 | 0.25 | 0.26 | 0.24 | 0.20 | 0.25 | 0.21 | 0.26 | 0.21 | 0.33 | 0.20 | 0.22 | 0.21 | 0.31 | 0.32 | 0.21 | 0.31 | 0.18 | 0.20 |
| Ca | 0.23 | 0.46 | 0.28 | 0.37 | 0.48 | 0.33 | 0.40 | 0.36 | 0.33 | 0.48 | 0.21 | 0.60 | 0.27 | 0.26 | 0.31 | 0.51 | 0.62 | 0.33 | 0.47 | 0.29 | 0.26 |
| Si | 0.40 | 0.44 | 0.29 | 0.70 | 0.52 | 0.48 | 0.73 | 0.38 | 0.36 | 0.52 | 0.44 | 0.48 | 0.44 | 0.55 | 0.46 | 0.47 | 0.53 | 0.36 | 0.46 | 0.86 | 0.41 |
| Mn | 0.35 | 0.50 | 0.34 | 0.44 | 0.53 | 0.43 | 0.40 | 0.43 | 0.37 | 0.54 | 0.38 | 0.57 | 0.41 | 0.51 | 0.44 | 0.57 | 0.64 | 0.37 | 0.54 | 0.40 | 0.34 |
| Fe | 0.35 | 0.51 | 0.39 | 0.39 | 0.56 | 0.42 | 0.33 | 0.43 | 0.27 | 0.56 | 0.31 | 0.61 | 0.28 | 0.33 | 0.36 | 0.51 | 0.65 | 0.27 | 0.51 | 0.37 | 0.29 |
| Zn | 0.32 | 0.48 | 0.38 | 0.50 | 0.52 | 0.44 | 0.37 | 0.42 | 0.40 | 0.52 | 0.37 | 0.54 | 0.39 | 0.43 | 0.41 | 0.57 | 0.62 | 0.40 | 0.51 | 0.35 | 0.37 |
| Pb | 0.40 | 0.52 | 0.42 | 0.46 | 0.86 | 0.52 | 0.48 | 0.44 | 0.99 | 0.86 | 0.67 | 0.54 | 0.37 | 0.78 | 0.55 | 0.54 | 0.63 | 0.99 | 0.54 | 0.75 | 0.93 |
| EC | 0.21 | 0.37 | 0.29 | 0.30 | 0.42 | 0.26 | 0.32 | 0.30 | 0.28 | 0.42 | 0.22 | 0.33 | 0.24 | 0.33 | 0.31 | 0.28 | 0.42 | 0.28 | 0.28 | 0.42 | 0.27 |
| OC | 0.15 | 0.16 | 0.14 | 0.15 | 0.21 | 0.16 | 0.14 | 0.14 | 0.15 | 0.21 | 0.15 | 0.15 | 0.15 | 0.51 | 0.16 | 0.15 | 0.21 | 0.15 | 0.15 | 0.21 | 0.15 |
| PM2.5 | 0.11 | 0.16 | 0.13 | 0.14 | 0.20 | 0.12 | 0.14 | 0.15 | 0.13 | 0.20 | 0.11 | 0.21 | 0.13 | 0.16 | 0.14 | 0.18 | 0.22 | 0.13 | 0.16 | 0.16 | 0.12 |
We have previously reported that the time activity diaries for the DEARS participants show that approximately 80% of their time is spent indoors at the residence (Rodes et al. 2010 (ref. 12)). Personal exposures to particles are frequently dominated by exposure to non-ambient particles and originate from indoor sources. Therefore, understanding how well residential indoor mass concentrations of these PM components relate to ambient measures is critical in reducing exposure uncertainty. Indoor PM2.5 component concentrations revealed a high degree of variability when compared to those from the ambient monitoring site (Table 3). A p value ≤ 0.05 is once again indicative of some degree of mass concentration heterogeneity associated with the mean mixed model value for the PM component across all EMAs when adjusted for the day to day variability of ambient-based mass concentrations. Seasonal residential indoor PM2.5 mass concentrations were observed to range from 8.8 to 31.5 μg m−3. Indoor PM2.5 total mass concentration associated with participants from EMA 7 represented the lowest means observed regardless of season. Some degree of Fe, Mn, Pb, Zn, NO3, OC, and S (SO4) indoor mass spatiality occurred over both the summer and winter seasons. While there are numerous indoor sources of OC (cooking aerosols being one example) and thus a ready explanation for the observed spatial effect, the observed spatiality for indoor S needs to be explained. The indoor OC and S have high spatial and temporal variability using the periodic central site monitoring for these evaluations. While it is a regional pollutant, we have identified environmental tobacco smoke in the participant's homes as being an influencing factor on overall indoor S concentrations in the DEARS (Williams et al., 2012 (ref. 36)), and thus the effect observed here. K was the only component not observed to exhibit some degree of indoor residential statistical significant or low spatial and temporal (p = 0.6) variability, although near significance was observed for the winter season. Ca was significantly different during the summer.
| Component | Season | Enumeration Monitoring Areas (EMA) | p-Value | |||||
|---|---|---|---|---|---|---|---|---|
| 1 | 3 | 4 | 5 | 6 | 7 | |||
| a Conversions used in the manuscript to quantify SO4: SO4 = S × 4.125. b Conversions used in the table OC = OC × 1.4. | ||||||||
| Calcium | Summer | 145 | 100 | 67 | 196 | 80 | 59 | <0.01 |
| Winter | 262 | 74 | 59 | 146 | 60 | 54 | 0.17 | |
| Iron | Summer | 254 | 143 | 71 | 284 | 91 | 46 | <0.01 |
| Winter | 177 | 110 | 63 | 257 | 69 | 47 | <0.01 | |
| Potassium | Summer | 86 | 68 | 75 | 99 | 66 | 43 | 0.13 |
| Winter | 97 | 50 | 136 | 80 | 45 | 37 | 0.06 | |
| Manganese | Summer | 7 | 5 | 3 | 9 | 3 | 3 | <0.01 |
| Winter | 6 | 5 | 3 | 8 | 2 | 2 | <0.01 | |
| Lead | Summer | 10 | 8 | 4 | 12 | 4 | 2 | <0.01 |
| Winter | 6 | 5 | 3 | 14 | 3 | 2 | <0.01 | |
| Zinc | Summer | 68 | 41 | 44 | 117 | 21 | 17 | <0.01 |
| Winter | 43 | 31 | 23 | 61 | 18 | 14 | <0.01 | |
| Silicon | Summer | 262 | 190 | 194 | 263 | 322 | 101 | 0.59 |
| Winter | 288 | 112 | 99 | 218 | 86 | 68 | <0.01 | |
| Nitrates | Summer | 905 | 565 | 755 | 735 | 575 | 380 | <0.01 |
| Winter | 1214 | 888 | 1110 | 2186 | 755 | 346 | 0.02 | |
| Sulfur | Summer | 1680 | 1458 | 1308 | 1584 | 1440 | 1008 | <0.01 |
| Winter | 802 | 755 | 664 | 762 | 572 | 493 | <0.01 | |
| EC | Summer | 861 | 904 | 576 | 807 | 674 | 411 | <0.01 |
| Winter | 723 | 453 | 602 | 368 | 351 | 366 | 0.33 | |
| OC | Summer | 18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 525 525 |
15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 239 239 |
20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 280 280 |
20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 130 130 |
17![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 117 117 |
<0.01 |
| Winter | 24![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 244 244 |
18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 749 749 |
34![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 921 921 |
27![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 147 147 |
24![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 457 457 |
20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 245 245 |
0.01 | |
| PM2.5 | Summer | 19![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 636 636 |
15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 067 067 |
25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 234 234 |
20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 245 245 |
18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 474 474 |
12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 054 054 |
0.02 |
| Winter | 17![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 786 786 |
9756 | 31![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 573 573 |
26![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 411 411 |
15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 538 538 |
8797 | 0.02 | |
We compared matched residential indoor versus residential outdoor PM2.5 composition ratios (ESI Table 10†). Such ratios are often considered as enrichment factors when ratios exceed unity (>1.0). Total PM2.5 indoor/outdoor ratios 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 indicate the significant contribution to the infiltration of outdoor air had on total mass concentrations as a whole. Even so, it must be realized that indoor sources of PM2.5 also contributed to the totals. We have previously reported that the mean residential PM2.5 infiltration factor in the DEARS was ∼0.7 (Williams et al., 2009 (ref. 6)). Therefore, it is suggested that on average, residential indoor sources contributed ∼30% of the total PM2.5 mass observed and the resulting 1
1 indicate the significant contribution to the infiltration of outdoor air had on total mass concentrations as a whole. Even so, it must be realized that indoor sources of PM2.5 also contributed to the totals. We have previously reported that the mean residential PM2.5 infiltration factor in the DEARS was ∼0.7 (Williams et al., 2009 (ref. 6)). Therefore, it is suggested that on average, residential indoor sources contributed ∼30% of the total PM2.5 mass observed and the resulting 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratios. While a clear majority of the comparisons had lower indoor concentrations of any respective component, some enrichment was observed. This was most notable for Ca (winter), K (summer and winter), Zn (summer), and Si (summer and winter). One might speculate on a variety of either indoor or indoor infiltrated/deposited sources responsible for each of those named immediately above. Descriptive statistics detail the residential indoor PM2.5 components variability across the summer and winter seasons. The large degree of variability (often exceeding 100%) as measured by the RSD across the various components and by EMA suggests the difficulty that might exist in trying to associate ambient-based measures of these pollutants as surrogates for indoor concentrations in most instances. Future work will attempt to associate residential indoor and outdoor concentrations of these elements along with survey information obtained in the DEARS to investigate potential influencing human and environmental exposure factors.
1 ratios. While a clear majority of the comparisons had lower indoor concentrations of any respective component, some enrichment was observed. This was most notable for Ca (winter), K (summer and winter), Zn (summer), and Si (summer and winter). One might speculate on a variety of either indoor or indoor infiltrated/deposited sources responsible for each of those named immediately above. Descriptive statistics detail the residential indoor PM2.5 components variability across the summer and winter seasons. The large degree of variability (often exceeding 100%) as measured by the RSD across the various components and by EMA suggests the difficulty that might exist in trying to associate ambient-based measures of these pollutants as surrogates for indoor concentrations in most instances. Future work will attempt to associate residential indoor and outdoor concentrations of these elements along with survey information obtained in the DEARS to investigate potential influencing human and environmental exposure factors.
Data reported in Table 4 examines the effect of spatial variability on personal measures after adjustment for day to day variability of ambient-based measures. As can be seen in ESI Table 11,† matched personal and residential indoor component mass concentration ratios were often within 20% of unity. This is not surprising considering the time activity pattern of the DEARS participants indicated a significant (∼75%) amount of time spent home indoors each day (Rodes et al. 2010,12). Therefore, the residential indoor environment would have the largest time opportunity to influence the total daily personal exposure profile. Mn and Zn exhibited the greatest divergence from unity, and are suggestive of non-residential indoor source impacts on some of the participants. The p-value statistics (p ≤ 0.05) reported in ESI Table 12† indicates that both spatial and temporal effects are evident relative to ambient-adjusted personal exposures. In other words, the day to day variability observed in personal exposure PM2.5 mass component heterogeneity across the EMAs cannot be accounted for by changes in the ambient conditions alone. The least degree of heterogeneity or spatial variability was observed for Ca (winter), K (winter), Zn (summer), and Si (summer). The observed heterogeneity for S observed here would appear to be due to the much lower mass concentrations observed in EMA 7 with respect to the other EMAs. One possible explanation for this observance would be that EMA 7 is upwind of the majority of industrial emissions in the DEARS study area and therefore less impacted by secondary organic aerosol products (e.g., SO4) impacting total personal exposures.
| Component | Season | Enumeration Monitoring Areas (EMA) | p-Value | |||||
|---|---|---|---|---|---|---|---|---|
| 1 | 3 | 4 | 5 | 6 | 7 | |||
| a Conversions used in the manuscript to quantify SO4: SO4 = S × 4.125. | ||||||||
| Calcium | Summer | 159 | 141 | 111 | 200 | 97 | 103 | <0.01 |
| Winter | 199 | 103 | 66 | 128 | 74 | 119 | 0.178 | |
| Iron | Summer | 246 | 167 | 99 | 262 | 134 | 73 | <0.01 |
| Winter | 192 | 120 | 68 | 233 | 77 | 61 | <0.01 | |
| Potassium | Summer | 104 | 87 | 75 | 120 | 71 | 56 | 0.040 |
| Winter | 88 | 60 | 153 | 76 | 48 | 50 | 0.123 | |
| Manganese | Summer | 8 | 7 | 4 | 8 | 5 | 3 | <0.01 |
| Winter | 7 | 5 | 3 | 8 | 3 | 3 | <0.01 | |
| Lead | Summer | 11 | 8 | 4 | 11 | 5 | 4 | <0.01 |
| Winter | 6 | 6 | 3 | 13 | 4 | 3 | <0.01 | |
| Zinc | Summer | 66 | 47 | 55 | 94 | 29 | 33 | 0.12 |
| Winter | 51 | 31 | 26 | 51 | 25 | 24 | 0.02 | |
| Silicon | Summer | 289 | 289 | 209 | 304 | 222 | 361 | 0.08 |
| Winter | 240 | 151 | 132 | 205 | 111 | 208 | 0.03 | |
| Sulfur | Summer | 1596 | 1435 | 1197 | 1586 | 1396 | 899 | <0.01 |
| Winter | 742 | 707 | 638 | 743 | 535 | 523 | 0.02 | |
| PM2.5 | Summer | 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 400 400 |
17![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 800 800 |
24![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 900 900 |
24![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 100 |
19![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 600 600 |
0.031 |
| Winter | 16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200 200 |
12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 400 400 |
34![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 300 300 |
26![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 900 900 |
16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
9700 | 0.014 | |
Information reported in the ESI Tables 8 and 9† provide spatial and temporal descriptive statistics of personal PM2.5 mass component observed in the DEARS. These tables give more insight into the variability of the personal measures. The large values associated with the RSD are an indication of the high spatial and temporal variability of personal exposures to the various PM components across the various EMAs. Taken into context with data presented it is evident that local and certainly some indoor-related sources often play a large role in total personal exposures to these PM mass components. It has been reported that indoor particulates are generated or re-suspended from everyday activities such as cooking, dusting, vacuuming, etc. (Wallace et al. 1996 (ref. 37)). “The source strengths were found to be a function of the number of persons performing the activity, the vigor of the activity, the type of activity, and the type of flooring (Ferro et al. 2004 (ref. 38)).” The impact of indoor activities on total personal exposures mentioned above vary between households and individuals which explains the variability in exposure. In addition to indoor residential activities, microenvironments affect personal exposures. These microenvironments include workplaces, outdoor surroundings, personal cloud, etc. (Wallace et al. 1996 (ref. 37)). Landis et al.39 have reported on possible non-ambient related personal activities that appear to influence total personal PM2.5 component exposures.
4 Conclusion
The DEARS represented an extensive matched personal, residential indoor, residential outdoor, and ambient-based spatial and temporal study design and provided hundreds of PM2.5 mass component comparison opportunities. It is evident in the data provided that attempting to use ambient measures as effective surrogates of exposures to specific PM2.5 mass components might be problematic and could lead to substantial exposure measurement uncertainty or potentially even health outcomes misclassification in health-based risk assessments. Even adjusting for day to day changes in the ambient environment often failed to negate the observed differences at the residential outdoor, residential indoor, and especially at the personal level. Local (unknown) sources are impacting many of the EMAs investigated in the DEARS. The report by Bereznicki et al. 2012 (ref. 9) provides some insight as to these local source impacts. Examination of the extensive time activity and residential survey information obtained in the DEARS will now be used in future efforts to elucidate the specific activities that impacted the study population's exposure to non-ambient PM2.5 mass components. Additional information concerning the DEARS can be found at http://www.epa.gov/DEARS/. EPA is working toward a web-based public release of the DEARS data in the future that holds the potential for collaboration on additional data analyses.Acknowledgements
The U.S. Environmental Protection Agency through the Office of Research and Development funded and conducted the research described in this article under contract 68-D-00-012 (RTI International), EP-D-00-068 (Battelle Columbus Laboratory), 68-D-00-206 and EP-05-D-065 (Alion Science and Technology). It has been subjected to Agency administrative review and approved for publication. Mention of trade names or commercial products does not constitute endorsement or recommendation for use. We especially thank Carry Croghan, Terri Conner and Robert Willis of the U.S. EPA; Charles Rodes, Jonathan Thornburg and Randy Newsome of RTI, International for their assistance in field or laboratory components of this effort.References
- F. Dominici, R. Peng, M. Bell, L. Pham, A. McDermott, S. L. Zeger and J. M. Samet, JAMA, J. Am. Med. Assoc., 2006, 295, 1127–1134 CrossRef CAS PubMed.
- R. Brook, R. Bard, R. Burnett, H. Shin, A. Vette, C. Croghan, C. Stevens, M. Phillips and R. Williams, Occup. Environ. Med., 2011, 68, 224–230 CrossRef PubMed.
- S. Zeger, D. Thomas, F. Dominici, J. Samet, J. Schwartz, D. Dockery and A. Cohen, Environ. Health Perspect., 2000, 108, 419 CrossRef CAS.
- W. Navidi, D. Thomas, D. Stram and J. Peters, Environ. Health Perspect., 1994, 102(suppl 8), 25–32 CrossRef.
- F. Lipfert and R. Wyzga, J. Air Waste Manage. Assoc., 1997, 47(4), 517–523 CAS.
- R. Williams, A. Rea, A. Vette, C. Croghan, D. Whitaker, C. Stevens, S. McDow, R. Fortmann, L. Sheldon, H. Wilson, J. Thornburg, M. Phillips, P. Lawless, C. Rodes and H. Daughtrey, J. Exposure Sci. Environ. Epidemiol., 2009, 19, 643 CrossRef CAS PubMed.
- EPA 2012, http://www.epa.gov/dears/.
- R. Duvall, D. Olson, G. Norris, J. Burke, R. Vedantham and R. Williams, Atmos. Environ., 2012, 47, 491 CrossRef CAS PubMed.
- S. Bereznicki, J. Sobus, A. Vette, M. Stiegel and R. Williams, Atmos. Environ., 2012, 61, 159 CrossRef CAS PubMed.
- B. George, D. Whitaker, R. Gilliam, J. Swall and R. Williams, J. Air Waste Manage. Assoc., 2010, 60, 1094 Search PubMed.
- J. Thornburg, C. Rodes, P. Lawless and R. Williams, Atmos. Environ., 2009, 43, 4251 CrossRef CAS PubMed.
- C. Rodes, P. Lawless, J. Thornburg, R. Williams and C. Croghan, Atmos. Environ., 2010, 44(11), 1386 CrossRef CAS PubMed.
- D. Martuzeviciusa, S. Grinshpuna, T. Reponena, R. Gornya, R. Shuklaa, J. Lockeya, S. Hub, R. McDonald, P. Biswas, L. Kliucininkas and G. LeMasters, Atmos. Environ., 2004, 38, 1091 CrossRef PubMed.
- B. Ostro, W. Feng, R. Broadwin, B. Malig, R. Green and M. Lipsett, Occup. Environ. Med., 2008, 65, 750 CrossRef CAS PubMed.
- W. Gauderman, E. Avol, F. Gilliland, H. Vora, D. Thomas, K. Berhane, R. McConnell, N. Kuenzli, F. Lurmann, E. Rappaport, H. Margolis, D. Bates and J. Peters, N. Engl. J. Med., 2004, 351(11), 1057 CrossRef CAS PubMed.
- A. Peters, E. Liu, R. Verrier and J. Schwartz, Am. J. Epidemiol., 2000, 11, 11 CrossRef CAS PubMed.
- R. B. Schlesinger and F. Cassee, Inhalation Toxicol., 2003, 15, 197 CrossRef CAS PubMed.
- J. C. Seagrave, J. D. McDonald, E. Bedrick, E. S. Edgerton, A. P. Gigliotti and J. J. Jansen, et al. , Environ. Health Perspect., 2006, 114, 1387 CrossRef CAS.
- T. J. Grahame and R. B. Schlesinger, Inhalation Toxicol., 2007, 19, 457 CrossRef CAS PubMed.
- J. Cavallari, E. Eisen, S. Fang, J. Schwartz, R. Hauser, R. Herrick and D. Christiani, Environ. Health, 2008, 9, 7 Search PubMed.
- M. Valko, C. Rhodes, J. Moncol, M. Izakovic and M. Mazur, Chem.-Biol. Interact., 2006, 160, 1 CrossRef CAS PubMed.
- L. Oglesby, N. Künzli, M. Röösli and C. Braun-Fahrländer, J. Air Waste Manage. Assoc., 2000, 50, 1251 CAS.
- J. Lau, W. Hung and C. Cheung, Atmos. Environ., 2009, 43, 769 CrossRef CAS PubMed.
- A. Kousa, L. Oglesby, K. Koistinen, N. Kunzli and M. Jantunen, Atmos. Environ., 2002, 36, 3031 CrossRef CAS.
- F. Violante, A. Barbieri, S. Curti, G. Sanguinetti, F. Graziosi and S. Mattioli, Chemosphere, 2006, 64, 1722 CrossRef CAS PubMed.
- K. Olofson, P. Anderson, M. Hallquist, E. Ljungstraem, L. Tang, D. Chen and J. Peterson, Atmos. Environ., 1994, 43, 340 CrossRef PubMed.
- N. Motallebi, C. Taylor, K. Turkiewicz and B. Croes, J. Air Waste Manage. Assoc., 2003, 53, 1517 CAS.
- F. Ledoux, L. Courcot, D. Courcot, A. Aboukais and E. Puskaric, Atmos. Res., 2006, 82, 633 CrossRef CAS PubMed.
- T. Barzyk, B. George, A. Vette, R. Williams, C. Croghan and C. Stevens, Atmos. Environ., 2009, 43, 787 CrossRef CAS PubMed.
- MDOT Traffic Monitoring Information System (TMIS), 2010, http://mdotnetpublic.state.mi.us/tmispublic/.
- M. Phillips, C. Rodes, J. Thornburg, F. Shamo, R. Whitmore, D. Chowdhury, J. Allpress, A. Vette and R. Williams, Research Triangle Institute Press, 2010, DOI:10.3768/rtipress.2010.2010.mr0021.1011.
- R. Littell, P. Henry and C. Ammerman, J. Anim. Sci., 1998, 76, 1216 CAS.
- J. Pinto, A. Lefohn and D. Shadwick, J. Air Waste Manage. Assoc., 2004, 54, 440 CAS.
- J. Chow, J. Watson, D. Lowenthal, P. Solomon, K. Magliano, S. Ziman and L. Richards, Aerosol Sci. Technol., 1993, 18, 105 CrossRef CAS.
- J. Chow, J. Watson, E. Fujita, Z. Lu, D. Lawson and L. Ashbaugh, Atmos. Environ., 1994, 28, 2061 CrossRef CAS.
- R. W. Williams, P. A. Jones, C. W. Croghan, J. Thornburg and C. E. Rodes, J. Exposure Sci. Environ. Epidemiol., 2012, 22(2), 109 CrossRef CAS PubMed.
- L. Wallace, J. Air Waste Manage. Assoc., 1996, 46(2), 98 CAS.
- A. Ferro, R. Kopperud and L. Hildermann, Environ. Sci. Technol., 2004, 38, 1759 CrossRef CAS.
- M. Landis, G. Norris, R. Williams and J. Weinstein, Atmos. Environ., 2001, 35, 6551 CrossRef.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c3em00364g |
| This journal is © The Royal Society of Chemistry 2014 |