Carbon capture and storage update
Matthew E.
Boot-Handford
a,
Juan C.
Abanades
b,
Edward J.
Anthony
c,
Martin J.
Blunt
d,
Stefano
Brandani
e,
Niall
Mac Dowell
a,
José R.
Fernández
b,
Maria-Chiara
Ferrari
e,
Robert
Gross
f,
Jason P.
Hallett
g,
R. Stuart
Haszeldine
h,
Philip
Heptonstall
f,
Anders
Lyngfelt
i,
Zen
Makuch
f,
Enzo
Mangano
e,
Richard T. J.
Porter
j,
Mohamed
Pourkashanian
k,
Gary T.
Rochelle
l,
Nilay
Shah
a,
Joseph G.
Yao
a and
Paul S.
Fennell
*a
aDepartment of Chemical Engineering, Imperial College London, South Kensington, London, SW7 2AZ, UK. E-mail: p.fennell@imperial.ac.uk; Tel: +44 (0)20 7594 6637
bInstituto Nacional del Carbón, (CSIC), Francisco Pintado Fe 26, 33011 Oviedo, Spain
cEnergy and Resource Technology Centre, Cranfield University, Cranfield, Bedford, MK43 0AL, UK
dDepartment of Earth Science and Engineering, Imperial College London, South Kensington, London, SW7 2AZ, UK
eSCCS Centre, School of Engineering, The University of Edinburgh, The King's Buildings, Edinburgh EH9 3JL, UK
fCentre for Environmental Policy, Imperial College London, South Kensington, London, SW7 2AZ, UK
gDepartment of Chemistry, Imperial College London, South Kensington, London, SW7 2AZ, UK
hSCCS, School of Geosciences, The University of Edinburgh, The King's Buildings, Edinburgh EH9 3JW, UK
iChalmers University of Technology, 412 96 Göteborg, Sweden
jEnergy Technology and Innovation Initiative, University of Leeds, Leeds, LS2 9JT, UK
kSchool of Process, Environmental and Materials Engineering, University of Leeds, Leeds LS2 9JT, UK
lMcKetta Department of Chemical Engineering, The University of Texas at Austin, Austin, TX 78712, USA
First published on 13th September 2013
Abstract
In recent years, Carbon Capture and Storage (Sequestration) (CCS) has been proposed as a potential method to allow the continued use of fossil-fuelled power stations whilst preventing emissions of CO2 from reaching the atmosphere. Gas, coal (and biomass)-fired power stations can respond to changes in demand more readily than many other sources of electricity production, hence the importance of retaining them as an option in the energy mix. Here, we review the leading CO2 capture technologies, available in the short and long term, and their technological maturity, before discussing CO2 transport and storage. Current pilot plants and demonstrations are highlighted, as is the importance of optimising the CCS system as a whole. Other topics briefly discussed include the viability of both the capture of CO2 from the air and CO2 reutilisation as climate change mitigation strategies. Finally, we discuss the economic and legal aspects of CCS.
1. Introduction
This paper discusses Carbon Capture and Storage (CCS), as one method to mitigate climate change. This paper will not assess the science behind anthropogenic climate change, the overwhelming evidence is presented by publications such as.1 The rationale for deployment of CCS on fossil-fuelled power stations (and possibly in the future with biomass-fired stations) is that, when deployed in conjunction with other technologies (such as renewables and nuclear), the overall cost of electricity supply is minimised. This is because fossil-fuelled power stations are able to vary their output in response to changes in demand (or indeed to the supply from intermittent sources such as wind) and thus CCS reduces the need for large-scale energy storage to be developed.Carbon capture and storage refers to a number of technologies which capture CO2 at some stage from processes such as combustion (most generally for power generation) or gasification. Many industrial processes, most notably cement manufacture, iron and steel making and natural gas treatment also intrinsically produce CO2 and can be fitted with CO2 capture technologies (and for these industries, CCS offers one of the very few remaining methods to reduce CO2 emissions where the best available technology in terms of e.g. energy efficiency is already used). The captured CO2 is then pressurised to ∼100 bar (or more), prior to being transported to a storage site, where it is injected into one of a number of types of stable geological features, trapping it for multiple hundreds or thousands of years and preventing its subsequent emission into the atmosphere. All of the individual components of the CCS chain, from capture all the way through to (and including) storage, have been demonstrated at or close to industrial scale. However, their integration into a single process is a significant (but ultimately solvable) engineering challenge. There are a large number of different technologies for CCS, some closer to deployment than others. The purpose of this paper is to review the most recent developments in the field, and not to introduce the topics. The interested reader is referred to a previous review, and a special edition of this journal for introductory material.2,3
Here, we discuss solvent scrubbing, oxyfuel combustion (for both pulverised fuel and in a fluidised bed), chemical looping and calcium looping, together with low-temperature sorbents, as exemplars of CCS technologies which might be commercialised within 10–20 years, (solvent scrubbing and oxyfuel potentially being commercialised towards the beginning of the period, with the other technologies towards the end, though we have included ionic liquids as a natural adjunct to solvent scrubbing even though these solvents are unlikely to be commercialised within 20 years). Of course, there are other technologies (such as membranes) which could also be considered, but are not covered here. We then move on to discuss a number of technologies that are either more niche or are further away from commercialisation (CO2 utilisation through mineralisation or in direct production of useful products). Transport of CO2 is then discussed, prior to storage. We then discuss the critical overarching themes: systems integration and policy design and implications for investment.
Throughout this paper, where efficiency penalties are quoted, it should be noted that they are relative to a power station which will have an underlying thermal efficiency of between ∼40 and 60%. This means that an efficiency penalty of (say) 5% requires an increase in fuel-burn of ∼10% in order to produce the same amount of electricity.
1.1 Current power generation
Despite recent global economic turmoil leading to appreciable reductions in global demand for oil and gas, demand for coal has if anything significantly increased in the period since 2005. In 2010, world coal demand was approximately 5000 million tonnes of coal equivalent (Mtce). Under the IEA's “Current Policies Scenario”, this is projected to grow to 7500 Mtce by 2035. It is worth noting that the entirety of this growth (in all scenarios) occurs in non-OECD countries. The share of global coal market arising from the non-OECD countries is expected to rise from 66% to 82%.4Power generation is heavily dependent on coal-fired plants throughout the world; in 2008, 41% of total global electricity was obtained by coal combustion (corresponding to 8273 TWh). While this share is expected to drop to 32% by 2035 (corresponding to 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200 TWh), coal remains the dominant source of energy globally, with non-OECD demand doubling in the period to 2035. OECD demand for coal is expected to drop by as much as 33%—a result of a renewed “dash-for-gas” arising from the exploitation of reserves of shale gas (and other unconventional sources) and policies encouraging the reduction of the carbon intensity of power generation.4 Conventional or so called “sub-critical” coal-fired power generating plants operate with low thermal efficiency (30–45%), which in turn incurs significant fuel costs. This large fuel requirement will in turn increase exposure to fuel price volatility, thus increasing the investment risk associated with this technology. For these reasons, sub-critical power plants are expected to displaced by super-critical and ultra-supercritical power plants, reducing their market share from 73% in 2008 to 31% in 2035.4 Super-critical power plants are considered to be a promising option for future coal-based power generation as they operate with higher base-load efficiency – in the range of 48–52%.5 Super-critical power plants operate with steam parameters in range of 240 bar/600 °C and ultra-super critical plants which operate in the range of 350 bar/700 °C/720 °C or higher are under development.
200 TWh), coal remains the dominant source of energy globally, with non-OECD demand doubling in the period to 2035. OECD demand for coal is expected to drop by as much as 33%—a result of a renewed “dash-for-gas” arising from the exploitation of reserves of shale gas (and other unconventional sources) and policies encouraging the reduction of the carbon intensity of power generation.4 Conventional or so called “sub-critical” coal-fired power generating plants operate with low thermal efficiency (30–45%), which in turn incurs significant fuel costs. This large fuel requirement will in turn increase exposure to fuel price volatility, thus increasing the investment risk associated with this technology. For these reasons, sub-critical power plants are expected to displaced by super-critical and ultra-supercritical power plants, reducing their market share from 73% in 2008 to 31% in 2035.4 Super-critical power plants are considered to be a promising option for future coal-based power generation as they operate with higher base-load efficiency – in the range of 48–52%.5 Super-critical power plants operate with steam parameters in range of 240 bar/600 °C and ultra-super critical plants which operate in the range of 350 bar/700 °C/720 °C or higher are under development.
However, owing to the relatively high-priced materials required for their construction, the capital cost associated with supercritical power plants is relatively high6,7 and this is an active area of on-going research.
For example, Yamamoto et al.8 reported the application of heat resistant material of high creep rupture strength and high oxidation resistance up to 650 °C, which have already been developed for boilers and turbines of ultra-supercritical power plants. Viswanathan5 discussed the materials for ultra-supercritical (USC) plants to withstand operating steam conditions up to 760 °C temperature and 35 MPa pressure, which are under development.
2. Developments in amine scrubbing
2.1 Thermodynamic context
CO2 capture by post-combustion chemisorption relies on the separation of CO2 from flue gas using a chemical solvent. Thus, the thermophysical properties are of paramount importance in determining the potential of absorption, as it specifies interfacial phase equilibrium in addition to speciation in liquid phase and the enthalpy of absorption. Consequently, appropriate selection of a physical property model is of prime importance for the correct modelling of CO2 capture processes.In the context of CO2 capture, aqueous alkanolamine solutions are an extremely complex solution of molecular species, electrolyte species and reaction products and, on certain time scales, reaction intermediates. The physical property model must be applicable to all phases and chemical equilibria for a wide range of thermodynamic states. Several thermodynamic models have been used in the literature to represent the absorption of acid gases in alkanolamine solution, and they can be classified as one of three types: empirical models, equation of state approaches and excess Gibbs energy approaches.
Empirical models are based on empirical mathematical relations, rather than theoretical considerations. Vapour-liquid equilibria (VLE) and chemical equilibria are represented in these models by fitting numerical parameters on experimental data. The resulting correlations, such as that of Gabrielsen et al.9 for the partial pressure of CO2 as a function of the liquid phase CO2 loading, are often easy to implement. However, as with all correlations, owing to their lack of theoretical underpinning, they are typically unsuitable for predictive calculation or extrapolation.
Equations of state can be used to represent both liquid phase and gas phases (including electrolytes). Heterogeneous approaches, using the excess Gibbs energy to obtain activity coefficients in the liquid phase. These models typically need to be coupled with a separate model to describe the gas phase; this is often a cubic equation of state. Homogeneous approaches are based on the Helmholtz energy; such as the formulation of Furst and Renon.10,11 Recently, the Statistical Associating Fluid Theory12,13 (SAFT) for potentials of variable range14 (SAFT-VR) has been applied to aqueous mixtures of amines15 and alkanolamines16,17 and CO2. This new approach provides an implicit treatment of the chemical reactions and ionic speciation in these complex mixtures. Importantly, although the reaction products are also treated in an implicit fashion, it is possible to obtain an accurate description of the equilibrium carbamate/bicarbonate products.17 As a consequence, when these thermodynamic models were incorporated in process models3,18 it was not necessary to describe the reaction products in the process model, nor was an enhancement factor required to describe the accelerating effect of the reactions on the mass transfer. This had the effect of significantly reducing the size of the process models and consequently it was possible to use these detailed dynamic, non-equilibrium models to perform optimisation19 and control20 studies. It is noteworthy that the SAFT approach has been coupled with classical density functional theory approaches and has been used to predict vapour–liquid interfacial properties21 and the soft-SAFT variant22 has also been used to describe the thermophysical properties and phase behaviour of ionic liquids in the context of CO2 capture.23
The third class of models uses the excess Gibbs energy to compute activity coefficients; they are often based on already-existing models for nonelectrolyte systems and extended with the Debye–Huckel theory to address electrolyte species. The model by Deshmukh and Mather24 is one of the simpler models, and parameters have been regressed for some amines25 it assumes ideality for water and calculates the activity coefficient for diluted species with a virial term for interaction between species. The model by Pitzer26 is quite similar and has been used to represent the solubility of CO2 in aqueous methyldiethanolamine (MDEA) and piperazine (PZ).27 Among the more elaborated models using the local composition of the mixture, the electrolyte-NRTL (e-NRTL) and extended UNIQUAC (e-UNIQUAC) models prevail. The e-NRTL model28,29 has been extensively used for CO2 absorption characterisation.30,31 The extended UNIQUAC32 provides the same theoretical basis as e-NRTL, with a simpler formulation, and it has already proved its ability to represent the alkanolamine system for CO2 absorption.33
The development of amine scrubbing has been focused on its application to coal-fired power plants. Unless otherwise noted, the data and discussion on amine scrubbing that follows are based on the application to coal-fired power plants. However, amine scrubbing should be useful for other applications.
2.2 Process flowsheet
The process technology using 30 wt% monoethanolamine (MEA) that has been evaluated by NETL34 to give a baseline for the solvent scrubbing process can no longer be used as a representative baseline for post-combustion capture. A number of vendors, including Fluor35 and MHI36 have developed processes and completed evaluations that give energy performances substantially better than that reported in the NETL analyses. In addition, a recent paper by Ahn et al. has illustrated all the different types of flowsheet configurations for the amine scrubbing process.37Fig. 1 gives an example of a second generation, optimised process for CO2 capture by amine scrubbing using 8 molal (m) piperazine (PZ).38,39 Compared to 30 wt% MEA it has twice the rate of CO2 absorption, 1.8 times the intrinsic working capacity, 5 to 10% lower heat of absorption (a disadvantage), and a maximum stripper T/P of 150 °C/8 bar.40
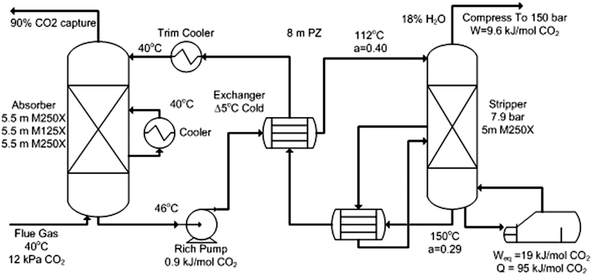 | ||
| Fig. 1 Intercooled Absorber/Interheated stripper with 8 m PZ. Stripper bottom at 150°C/7.9 bar. Weq = 30.5 kJ mol−1 CO2 = 193 kW h per tonne CO2. | ||
In addition to the absorber, the process would probably include SO2 polishing with sodium alkali scrubbing and direct contact cooling of the flue gas before the PZ absorber. It would also usually include a water wash and aerosol removal after the absorber. Much of this additional flue gas contacting could be incorporated into the same vessel as the CO2 absorption.
2.3 Overall energy performance
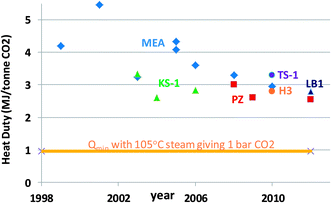 | ||
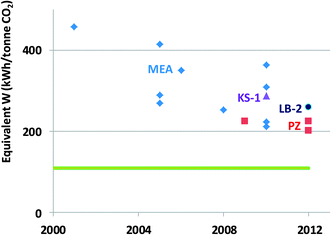
| Fig. 2 Reboiler heat duty for amine scrubbing on coal-fired power plants, taken in part from Rochelle et al.40 MEA = monoethanolamine, KS-1 = proprietary MHI solvent, PZ = 40 wt% piperazine, H3 = proprietary Hitachi solvent,568 LB1 = Proprietary BASF/Linde solvent/process,569 TS-1 = proprietary Toshiba solvent.570 | ||
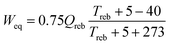 | (1) |
The compression work was estimated by a regression of results from Aspen modelling of an multistage compressor with intercooling to 40 °C:41
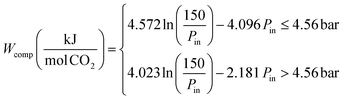 | (2) |
The improvements include thermally stable solvents such as piperazine, that can be stripped at 150 °C to produce CO2 at 8 bar. Rochelle et al.40 present estimates of thermodynamic efficiencies for other common separation processes: desalination by reverse osmosis – 21%, distillation – 14 to 35%, and air separation – 25%. Since the minimum work for this separation is about 110 kWh t−1, it is improbable that further improvement from the current thermodynamic efficiency of about 50% will come easily. A typical coal-fired power plant produces about 1000 kWh tCO2−1 emitted, so CO2 capture by amine scrubbing will reduce the power output by 20 to 30%.
2.4 Features of second-generation processes
Steam pressure should be reversibly reduced before it is used in the reboiler. In this example the steam pressure is 6 bar and could be consistent with steam extracted between the intermediate and low pressure turbine stages of a typical coal-fired power plant. The reboiler approach temperature should be minimised consistent with the tradeoff of reboiler capital cost and equivalent work loss, typically 5 to 10 K. The equivalent work of the stripper and compressor system should be estimated from the work value of the steam heat and the compressor work to a final pressure (typically 150 bar) by equations such as those offered by Van Wagener (above).41
Effective cross exchange between the cold rich and hot lean solvent eliminates much of the energy cost of operating with a large solvent rate. Plate-and-frame exchangers appear to permit an economic approach T of 5 K. A cold rich bypass41 can be used to address imbalance between the heat capacities of the rich and lean streams. With a typical working capacity of 0.8 mol CO2 kg(H2O + amine)−1 and a heat capacity of 3.5 kJ K−1 kg(H2O + amine)−1, the 5 K approach requires only 22 kJheat mol CO2−1 or 3.5 to 4.4 kJequivalent work mol CO2−1 (with stripper at 120 to 150 °C).
2.5 Solvent selection for energy performance
Three aggregated properties of solvents are related to energy performance. These are summarised in Table 1 for a number of potential solvents.| Amine | m | k ′g avg × 107 | Capacity mol kg−1 | −H abs kJ mol−1 | T max °C | P max bar | P H2O/PCO2 |
|---|---|---|---|---|---|---|---|
| Piperazine (PZ) | 8 | 8.5 | 0.79 | 64 | 163 | 14.3 | 0.33 |
| PZ/bis-aminoethylether | 6/2 | 7.3 | 0.67 | 69 | 162 | 16.3 | 0.28 |
| 2-Methyl PZ/PZ | 4/4 | 7.1 | 0.84 | 70 | 155 | 10.3 | 0.41 |
| 2-Methyl PZ | 8 | 5.9 | 0.93 | 72 | 151 | 9.9 | 0.37 |
| 2-Amino-2-methyl propanol (AMP) | 5 | 2.4 | 0.96 | 73 | 140 | 6.1 | 0.49 |
| PZ/aminoethyl PZ | 5/2 | 8.1 | 0.67 | 71 | 138 | 5.0 | 0.55 |
| PZ/AMP | 5/2.3 | 7.5 | 0.7 | 71 | 134 | 4.5 | 0.54 |
| Diglycolamine (registered trademark) | 10 | 3.6 | 0.38 | 81 | 132 | 9.1 | 0.25 |
| Hydroxyethyl PZ | 8 | 5.3 | 0.68 | 69 | 130 | 2.3 | 0.98 |
| PZ/AMP | 2/4 | 8.6 | 0.78 | 72 | 128 | 3.4 | 0.63 |
| 2-Piperidine ethanol | 8 | 3.5 | 1.23 | 73 | 127 | 3.3 | 0.61 |
| Monoethanolamine (MEA) | 11 | 3.6 | 0.66 | 70 | 125 | 2.7 | 0.67 |
| MEA | 7 | 4.3 | 0.47 | 70 | 121 | 2.2 | 0.81 |
| Methydiethanolamine (MDEA)/PZ) | 5/5 | 8.3 | 0.99 | 70 | 120 | 1.8 | 0.92 |
| MDEA/PZ | 7/2 | 6.9 | 0.8 | 68 | 120 | 1.4 | 1.15 |
| Kglycinate | 6 | 3.2 | 0.35 | 69 | 120 | 1.08 | 1.46 |
| Ksarconinate | 6 | 5 | 0.35 | 54 | 120 | 0.73 | 2.17 |
| MEA/PZ | 7/2 | 7.2 | 0.62 | 80 | 104 | 0.7 | 1.38 |
| Pmax = PH2O + PCO2 | (3) |
| PCO2 = 0.5 kPa × exp((ΔHabs/R)(1/Tmax − 1/313)) | (4) |
As reviewed by Freeman45 and Rochelle,46 the piperazine or piperazine derivatives have been identified as solvents with the greatest value of Tmax, resulting in greater values of Pmax. Many amines have lower values of Tmax because they degrade by formation of cyclic urea or by dimerisation through an oxazolidinone.46
Solvents with a low heat of absorption (<60 kJ mol-1) will not be competitive.42 These include systems relying on sodium or potassium carbonate and tertiary or hindered amines with lower pKa values.
Although vacuum stripping works with solvents that have a low heat of absorption, it is not energetically competitive because of the additional compression work for the CO2.41,42
A number of investigators are developing systems that increase the effective heat of absorption by precipitating solids out of the rich solution.47,48 One such system uses aqueous potassium carbonate with precipitation of potassium bicarbonate. These processes will ultimately have to deal with the reliability issues posed by precipitating slurries.
2.6 Normalised capacity – capacity/(μ/10)0.25
The capacity and viscosity of the solvent are reflected in the sensible heat requirement of the stripper, given by: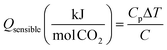 | (5) |
One quantitative measure of the intrinsic solvent capacity is the difference between the equilibrium CO2 concentration at 40 °C at 5 kPa CO2 and the equilibrium concentration at 40 °C at 0.5 kPa. These values allow for a reasonable driving force to provide 90% CO2 removal at conditions of coal-fired power plants. These convenient units of capacity reflect the generalisation that the effective partial molar heat capacity of CO2 loading is typically near zero.
Greater solvent viscosity reduces the heat transfer coefficient in the cross-exchanger. The optimum exchanger design will result in a greater approach ΔT with a greater viscosity. Therefore, it is appropriate to weight the intrinsic capacity by the viscosity to the −0.25 power, as reflected in the normalised capacity given in Table 1, capacity/(μ/10)0.25.49
A number of amine systems provide greater normalised capacity than 7 M MEA. Hindered and tertiary amines usually provide greater capacity because their intrinsic stoichiometry requires only 1 mol amine mol CO2−1, as opposed to two for the MEA system. As shown in Table 1, methyldiethanolamine, (a tertiary amine) with piperazine and aminomethylpiperazine (a hindered amine) with piperazine are quite competitive. Greater capacity is also provided by diamines such as piperazine because more equivalents of amine can be loaded into the solvent before the viscosity becomes unacceptable.
A number of researchers are investigating systems that precipitate solids or separate a lean amine organic phase from the rich solvent.50–52 These phase change systems will usually provide greater capacity, but they must deal with the reliability issues posed by precipitating slurries or two-phase systems.
2.7 Rate of CO2 absorption, k′g
Because the optimisation of the absorber design will require lower rich and lean loading to achieve 90% CO2 removal with a reasonable amount of packing, the rate of CO2 absorption is an important energy parameter of the solvent. A fast rate of CO2 absorption facilitates reversible absorber performance at high rich and lean loading that will minimise energy use in an optimised system. CO2 typically absorbs by the process of diffusion with fast reaction in the boundary layer. The normalised absorption flux of CO2 (k′g, mol m−2 Pa−1) is given approximately by: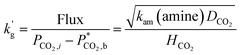 | (6) |
Piperazine or piperazine derivatives provide the greatest values of k′g. Secondary or primary amines are usually necessary to provide an acceptable rate of CO2 absorption. Tertiary amines and hindered amines are usually too slow to be used by themselves.
Several investigators are developing carbonic anhydrase enzymes to catalyse the CO2 kinetics in otherwise slower solvents.53,54 Unfortunately they have not yet developed enzymes that are effective at elevated T (>100 °C). Furthermore, the enzymes are most effective in tertiary amines and carbonate solutions with low heats of CO2 absorption. These systems will probably not be energetically competitive with other second generation amine solvents that can be regenerated at 120 to 150 °C.
2.8 Solvent management
A number of amines are resistant to oxidation at absorber conditions, including piperazine, tertiary amines, and hindered amines. Tertiary amines appear to be oxidation inhibitors when used in blends with other amines. MDEA is effective in inhibiting the oxidation of MEA at absorber conditions.56,57
However, Closmann56 and Voice57 have shown in bench-scale experiments that even resistant amines are subject to reaction with dissolved and entrained oxygen that is carried into the high temperature of the cross-exchanger. This oxidation rate depends on the solubility of oxygen in the solvent and can be substantially less than that in MEA solvents. It can be minimised by stripping the dissolved oxygen from the rich solution with nitrogen or by a low-temperature flash of CO2/H2O.
Vapour losses. Because practical amines usually include at least two or more hydrophilic groups such as amine, alcohol, or ether, residual amine volatility at the top of the stripper can be managed to less than 1 ppm by a water wash. Nguyen66 measured amine volatility in water and showed that two or more hydrophilic groups usually produce an amine volatility less than 100 ppm at absorber lean conditions. In solutions loaded with CO2, diamines such as piperazine are substantially less volatile because of speciation to ions including protonated amine and carbamate.67 Hindered amines and tertiary amines with methyl groups tend to have greater volatility. Aliphatic monoamines without other polar groups have unacceptable volatility.
Several investigators68 have been developing systems with amino acids (partially neutralised by K+) which should be nonvolatile ions. Other vendors may be using amines such as hydroethylpiperazine with three or more hydrophilic groups that have practically no volatility and may not require a water wash.
Amine aerosols. Vapour amine may condense in the absorber on submicron hydrophilic aerosol or particulate to produce small aerosol drops that are not removed by typical contacting internals in the absorber or water wash.69 Several pilot plants have reported amine emissions as high as 200 ppm from pilot plants with 1 to 3 ppm SO3 in the inlet flue gas.70–73 The resulting aerosol can be effectively removed by a fibre filter mist eliminator with a pressure drop of 150 to 250 mmH2O.74 Aker Clean Carbon and MHI claim solutions to this problem.74 This problem could also be addressed by using an amine or amino acid with low or no volatility.
2.9 Development status
Since 1930, hundreds of plants have used amine scrubbing to remove CO2 from hydrogen, natural gas, and other gases that contain little oxygen. The plants use monoethanolamine, diethanolamine, MDEA/PZ, and a number of second and third generation solvents.Amine scrubbing for CO2 capture from natural gas is commercially available. Since 1980, dozens of plants have captured CO2 from combustion of methane or other clean fuels. Most are based on technology provided by Fluor (MEA, Economine) or MHI (KS-1). The Fluor applications include a 70 MWe gas-fired boiler and a gas-fired turbine with a flue gas rate equivalent to 80 MW of a coal-fired boiler.
Two public databases demonstrate that amine scrubbing is near commercial on coal-fired power plants.75,76 More than 25 pilot plants have tested amine absorption/stripping on coal-fired flue gas at 0.1 to 5 MWe. Seven prototype systems have been operated at 10 to 33 MW with coal-fired flue gas and compression of the CO2. There are no larger plants operating on coal-fired gas, but one is under construction at 120 MW and another eight plants at 140 to 765 MW in various states of planning, permitting, and FEED.
2.10 Conclusions
Advanced amine systems will capture CO2 with heat duty less than 2.7 MJ tCO2−1 and equivalent work less than 250 kWh tCO2−1 (including compression to 150 bar).The innovations contributing to reduced energy use include:
(1) Thermally stable amines such as 8 m piperazine that can be regenerated at elevated pressure.
(2) Effective plate-and-frame cross exchangers and high capacity solvents such as PZ/MDEA and PZ/AMP.
(3) Configurations such as the interheated stripper that effectively recover heat from the stripper overhead.
(4) Fast amines such as piperazine and absorber intercooling that provide more reversible absorber operation with greater rich and lean loading.
(5) Amines with high heat of CO2 absorption that maximise the energy performance of thermal swing regeneration.
Remaining issues of secondary environmental impact with advanced amines have acceptable solutions:
(1) Amine oxidation can be minimised by using amines such as piperazine and MDEA that are resistant to oxidation and by stripping dissolved oxygen at <100 °C.
(2) Nitrosamines can be managed by avoiding secondary amines or by thermal or UV decomposition.
(3) Vapour losses of amine can be avoided by water wash with volatile amines or by using non-volatile amines.
(4) Amine aerosol losses can be eliminated by a fibre filter.
3. Ionic liquids as alternative solvents for CCS
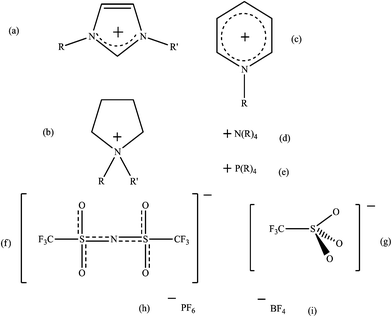
It has been suggested that the use of ionic liquids (ILs) as alternative solvents would have many advantages over conventional amine-based CO2 extraction. The general area of IL use for CCS has been reviewed recently.77–84 In addition to a potentially lower demand for energy in the solvent regeneration step, ILs have lower volatility, lower vapour pressure, are non-flammable, are more thermally stable, and are easier to recycle.The comparison of ILs with molecular organic solvents has been discussed in a recent review3 and also discussed the general implications of changing the cation and anion (see Fig. 4) or employing mixtures. The recent developments in this field will be reviewed here, and the implications of IL physical properties and functionalisation on CO2 solubility will also be explored.
3.1 Relationship between IL physical properties and CO2 solubility
Henry's constant is a quick and useful measure of CO2 solubility in ILs. Henry's law constants for CO2 in a range of different ILs are shown in Table 2. The highest solubility of CO2 recently reported was in [C5C1im][bFAP], which contains a highly fluorinated alkylphosphate anion that is exceedingly non-coordinating, resulting in an open fluid structure that dissolves CO2.85 As can be seen from Table 2, increasing the length of the alkyl side chain on the imidazolium cation improves CO2 solubility.86 However, the high molar solubility of CO2 with increasing n-alkyl chain length is largely a function of the increase in molecular weight of solvent. The volumetric solubility of CO2 does still decrease with increasing cation alkyl chain length, but this effect is less dramatic than the molar solubility change and must be carefully considered when selecting IL cations, as most physical properties suffer when the alkyl chain length exceeds octyl. Density increases roughly linearly with increasing alkyl chain length87 while viscosity increases dramatically.88 This increased viscosity causes poor gas diffusion and slow mass and heat transfer, resulting in larger unit operations, including absorption columns and heat exchangers.89,90 While ILs typically have higher viscosities than common organic solvents and water at the same temperature91 (resulting in slower CO2 absorption kinetics),80 other IL physical properties are potentially better than conventional organic solvents, such as heat capacity, density and surface tension.92 These favourable properties can result in a low energy requirement for solvent regeneration.93 Care is necessary to ensure that overall energy requirements are minimised by the use of any new solvent. As discussed above in the section on solvent scrubbing, this is not a case of finding a solvent with a low heat of regeneration.| Cation | Anion | H (bar) | |
|---|---|---|---|
| 25 °C | 60 °C | ||
| [C4C1im] | [PF6] | 53.4 ± 0.3 | 81.3 ± 0.8 |
| [C4C1im] | [NTf2] | 33.0 ± 0.3 | 48.6 ± 0.9 |
| [C6C1im] | [NTf2] | 31.6 ± 0.2 | 45.6 ± 0.3 |
| [C6(3C1)py] | [NTf2] | 32.8 ± 0.2 | 46.2 ± 0.3 |
| [(C6H4F9)C1im] | [NTf2] | 28.4 ± 0.1 | 48.5 ± 0.4 |
| [(C8H4F13)C1im] | [NTf2] | 27.3 ± 0.2 | 44.7 ± 0.5 |
| [C6C1im] | [eFAP] | 25.2 ± 0.1 | 42.0 ± 0.1 |
| [C6C1im] | [pFAP] | 21.6 ± 0.1 | 36.0 ± 0.3 |
| [C5C1im] | [bFAP] | 20.2 ± 0.1 | 32.9 ± 0.2 |
| [C6C1im] | [ace] | 113.1 ± 16.9 | |
| [C6C1im] | [sac] | 132.2 ± 19.7 | |
| [Et3NBH2C1im] | [NTf2] | 33.1 ± 1.2 | |
3.2 Ion selection
Anion effects on most IL-based solvation processes are dominant.94 This not only includes the solubility of CO2 and the strength of the IL–CO2 interactions in solution,95 but also the solubility and affinity of the IL for water.94 Most IL research has focused on salts with dialkylimidazolium cations ([CnCmim][X]), enabling an easy comparison of various anion effects. For bulk liquid CCS applications, prominence is obviously placed on hydrophobic (water-immiscible) ILs, as many ILs with highly basic anions absorb very large quantities of water.94 This naturally leads to selectivity problems when encountering wet flue gases. The origin of the anion effect on CO2 solubility in ILs has been investigated through molecular dynamics86 where the anion–CO2 interactions were shown to be the strongest solvation forces present. That study also pointed to mixtures on ILs with molecular solvents providing an optimised hybrid solution for CCS.The bistrifluorosulfonylimide [NTf2] anion generally gives the best CO2:N2 selectivity and high overall CO2 solubilities with most IL cations.96 This anion also possesses poor interactions with water (leading to highly hydrophobic ILs) and generally favourable physical properties: relatively low viscosities (20–50 mPa s), very high thermal stabilities (a measure of the thermal stability, Tonset of 400–500 °C) and low melting points (−50–0 °C). As a general rule, this anion can be employed to test designer cations (as it is the most likely to yield favourable physical properties) and yield salts with generally favourable CCS potential. This opens up the cation for specific tailoring to include CO2-philic moieties because it is the easiest portion of an IL to synthetically modify.
3.3 Conventional ILs
Unfortunately, there is currently no comprehensive model for gas solubility in ILs.97 However, some general trends can be observed. Increasing the cation alkyl side chain length increases CO2 solubility, likely by increasing the available volume for CO2 due to a decrease in cation–anion interactions.98,99 It is clear that there are mainly physical phenomena (such as dispersion forces) dominating CO2–IL interactions when unfunctionalised ILs are employed, with only weak chemical complexes forming.100 The enthalpy of CO2 physical absorption by these ILs is generally about 20 kJ mol−1. This results in a lower energy requirement than for amine solutions in the regeneration step, but not overall: as discussed above, a lower heat of absorption can lead to a higher overall energy requirement. The structural flexibility of ILs allow tuning of the enthalpy of absorption by employing basic ionic liquids made by neutralising tetraalkylphosphonium hydroxide with weak proton donors with different pKa values.101 These basic ILs have more rapid absorption rates with little increase in viscosity, though this is likely to be very sensitive to water as these are hydrophilic anions.3.4 Task-specific ILs
Conventional ILs mostly use physical absorption to capture CO2 through the space between ions, while functionalised (task-specific) ILs are usually designed to chemically bond to CO2 in an absorption process, increasing the overall absorption capacity.80 The synthetic flexibility of ILs means that a near-infinite range of functionalisations are possible, though cost and stability become important considerations. However, only some functionalisation strategies have increased CO2 capacity. This field of task-specific ILs (TSILs) for CCS applications has recently undergone rapid growth.82![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CO2 of 2
CO2 of 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 as with amine-based solvents, though the nature of the carbamate complex is still under dispute.103 Unfortunately, these ILs generally have poorer thermal stabilities and higher melting points and viscosities than conventional ILs.105 A variety of cations (imidazolium, pyridinium, ammonium and phosphonium) have been be functionalised with amines for CO2 capture, with (3-aminopropyl)tributylphosphonium ILs (coupled with amino acid anions) exhibiting the best physicochemical properties, such as a low glass transition temperatures (in the range from −69.7 to −29.6 °C) and thermal stability to above 200 °C.105 The salt (2-hydroxyethyl)-trimethyl-ammonium(S)-2-pyrrolidine-carboxylic acid salt or [Choline][Pro] has been demonstrated to be able to capture and release CO2, where CO2 is released by bubbling N2 in the solution106 (of course, further measurements are necessary under a CO2 atmosphere). There is, however, some concern over melting point changes when amino acid anions absorb CO2.82
1 as with amine-based solvents, though the nature of the carbamate complex is still under dispute.103 Unfortunately, these ILs generally have poorer thermal stabilities and higher melting points and viscosities than conventional ILs.105 A variety of cations (imidazolium, pyridinium, ammonium and phosphonium) have been be functionalised with amines for CO2 capture, with (3-aminopropyl)tributylphosphonium ILs (coupled with amino acid anions) exhibiting the best physicochemical properties, such as a low glass transition temperatures (in the range from −69.7 to −29.6 °C) and thermal stability to above 200 °C.105 The salt (2-hydroxyethyl)-trimethyl-ammonium(S)-2-pyrrolidine-carboxylic acid salt or [Choline][Pro] has been demonstrated to be able to capture and release CO2, where CO2 is released by bubbling N2 in the solution106 (of course, further measurements are necessary under a CO2 atmosphere). There is, however, some concern over melting point changes when amino acid anions absorb CO2.82
A variety of imidazolium or tetraalkylphosphonium cations have been combined with amino acid anions to make AAILs.107–109 Potential advantages of using amino acids include their low cost, biodegradability and low toxicity. Also, AAILs can increase CO2 capture because they possess both carboxyl and amine functional groups and the IL can complex CO2 in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 stoichiometric ratio.110 Immobilisation of AAILs into nanoporous PMMA microspheres has recently been shown to increase CO2 uptake rates and ease regeneration.111
1 stoichiometric ratio.110 Immobilisation of AAILs into nanoporous PMMA microspheres has recently been shown to increase CO2 uptake rates and ease regeneration.111
As mentioned above, amine-functionalised ILs tend to be highly viscous, which leads to problems of measuring CO2 capacity and developing handling strategies, and also results in the hindrance of CO2 diffusion rates.102 These ILs also require extra synthetic and purification steps to produce, which will likely increase the expenses.77 One way to overcome the viscosity problem is to use a solid support. However, this requires solid/gas exchange, which is quite challenging in practice. The conventional MEA process solves the viscosity problem by diluting the MEA with water. However, this is not ideal as a large amount of water needs to be evaporated to regenerate the IL.102
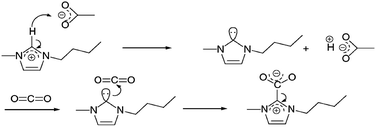 | ||
| Fig. 5 Reaction of CO2 at the C2 position with in situ-generated carbine.113 | ||
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mixture of a proton donor (i.e. alcohol) and organic base, and shows excellent CO2 capacity and good CO2
1 mixture of a proton donor (i.e. alcohol) and organic base, and shows excellent CO2 capacity and good CO2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) N2 selectivities (Scheme 1).101,120–122 These new ILs are interesting CO2 capture options, though the higher volatility of the alcohol component may hinder deployment. To avoid this, the alcohol group can be incorporated into an IL cation side chain.123 CO2 absorbances of 1.04
N2 selectivities (Scheme 1).101,120–122 These new ILs are interesting CO2 capture options, though the higher volatility of the alcohol component may hinder deployment. To avoid this, the alcohol group can be incorporated into an IL cation side chain.123 CO2 absorbances of 1.04![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (relative to base) have been reported for this strategy, which is 20 times higher than the solubility in the neat IL, and can be achieved in under 10 min.123 CO2 release can be achieved by mild heating (90–120 °C) and the IL re-used. It should be noted that for very strong bases (e.g. MTBD, tetramethylguanidine), CO2 may actually react with the IL cation,124 which would imply sensitivity of CO2 absorbance to H2O presence. Functionalisation of the guanidines or amidines, including tethering of the alcohol group to the base,125 may avert these difficulties.
1 (relative to base) have been reported for this strategy, which is 20 times higher than the solubility in the neat IL, and can be achieved in under 10 min.123 CO2 release can be achieved by mild heating (90–120 °C) and the IL re-used. It should be noted that for very strong bases (e.g. MTBD, tetramethylguanidine), CO2 may actually react with the IL cation,124 which would imply sensitivity of CO2 absorbance to H2O presence. Functionalisation of the guanidines or amidines, including tethering of the alcohol group to the base,125 may avert these difficulties.
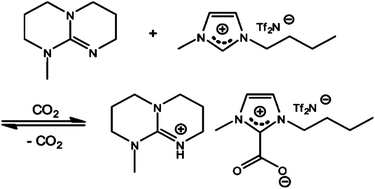 | ||
| Scheme 1 CO2 capture mechanism for reversible ILs.122 | ||
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 IL
1 IL![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CO2 capture capacity through carbamate formation. However, in the presence of small amounts of water (1% by mass), the capture capacity can reach an equimolar ratio as shown in Scheme 2.129 A combination of SILMs and TSILs may be a better choice for CO2 capture at elevated temperatures and pressures.130 However, there remain drawbacks, including leaching of the IL through membrane pores when the pressure drop is higher than the liquid stabilising forces within the matrix. In order to overcome this limitation, polymerisable ILs as membranes could be a possible option for CO2 separation.100
CO2 capture capacity through carbamate formation. However, in the presence of small amounts of water (1% by mass), the capture capacity can reach an equimolar ratio as shown in Scheme 2.129 A combination of SILMs and TSILs may be a better choice for CO2 capture at elevated temperatures and pressures.130 However, there remain drawbacks, including leaching of the IL through membrane pores when the pressure drop is higher than the liquid stabilising forces within the matrix. In order to overcome this limitation, polymerisable ILs as membranes could be a possible option for CO2 separation.100
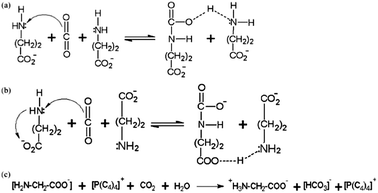 | ||
| Scheme 2 Proposed mechanisms of CO2 capture by AAILs: (a) and (b) without water; (c) with water.129 | ||
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 CO2.135
1 CO2.135
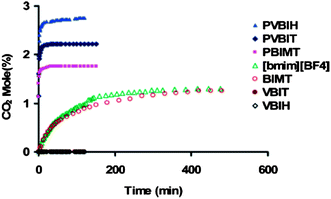 | ||
| Fig. 6 CO2 absorption of three poly(ILs) (PVBIH, PVBIT, PBIMT) and their corresponding monomers (VBIH, VBIT, BIMT) and the IL [bmim][BF4] as a function of time (592.3 mmHg CO2, 22 °C).131 | ||
Biopolymers (chitin and chitosan) also have been used in the process of CO2 capture. These biopolymers are environmentally friendly, renewable, biodegradable and almost non-toxic. There are two hydroxyl groups in chitin while there is an additional amine group in chitosan. The IL [C4C1im]Cl has been used as a solvent to break the strong inter- and intramolecular hydrogen bonds, but it cannot disrupt the crystalline domains of chitosan.136 The result is that chitin–IL and chitosan–IL mixtures have increased CO2 sorption capacity (8.1% higher than the IL) under mild conditions (30 °C, 1 atm CO2 pressure in CO2 fixation and release processes). There are many potential environmental and performance benefits from using such recyclable, non-corrosive and non-volatile CO2 absorption media.93,136
3.5 Molecular simulations of CO2 with ILs
There have been a number of molecular simulation studies focused on the dissolution of CO2 in ILs. A recent review83 highlights the most relevant advances. In conventional ILs, CO2 dissolves in free volume spaces within the IL matrix without greatly affecting the structure, accounting for the rather unusual solubility profiles.137 This is also likely responsible for the lower regeneration energy, as the CO2–IL interactions are relatively weak. By contrast, amine–TSILs form strong chemical complexes with CO2, which has been studied by simulation.1384. Oxyfuel combustion technology
Oxyfuel combustion is one of the most developed technologies for carbon capture and storage. Oxyfuel combustion refers to fuel being burned in a mixture of oxygen and recycled flue gas (RFG). Unlike conventional fossil fuel-fired power stations that use air as the oxidant, an oxy-fired plant employs an Air Separation Unit (ASU) to produce an oxygen stream. The oxygen stream is combined with RFG to produce an oxygen enriched gas for the oxidant. The recycle is necessary to moderate the otherwise excessively high flame temperature that would result from burning in pure oxygen. After the removal of water and other impurities from the flue gas exhaust stream, high-purity CO2 is produced. The combustion of fuel in an oxygen and RFG mixture was proposed in the early 1980s for the purpose of producing a high-purity CO2 stream for use in Enhanced Oil Recovery (EOR)139 and for simultaneously reducing greenhouse gas emissions from fossil fuel energy generation.140 Pilot scale studies were subsequently carried out141–143 in the following decades. During the last decade, the global research activity has increased to the point where several demonstration phase projects have begun and the commercial concept is expected before 2020. Oxyfuel combustion can be applied to several fuels, including coal (oxy-coal combustion), natural gas or blends of biomass and coal. Most interest has focused on oxy-coal combustion due to the abundance, reliability and high carbon content of the fuel. The following sections refer to oxy-coal combustion unless otherwise stated.4.1 Process considerations
In comparison to air-fired plants, the implementation of oxyfuel operation will lead to a number of plant configuration changes and additional unit operations, i.e. recycle loop, ASU, CO2 purification and compression. The optimum recycle ratio is generally 0.7; this yields oxygen levels in the oxidant environment that typically range from 25 to 30% because at these conditions, the flame and heat transfer characteristics reasonably approximate those of air-fired pulverised fuel (PF) boilers. Oxygen excess levels are 15–20% for air-firing conditions but are kept lower for oxyfuel conditions to no more than 10% in order to minimise ASU operational costs. Flue gas oxygen content is typically 3%. The flue gas stream should be cooled, scrubbed and dried before being diverted for the primary recycle. Particulates are removed in order to avoid accumulation of solids in the boiler and prevent the flue gas recirculation fan and gas passages from unnecessary wear due to erosion. Several options for configuration of a secondary recycle stream exist.1444.2 Energy performance
Oxyfuel combustion induces an energy penalty to the process caused by the requirements of producing O2 and compressing CO2. Using current technology, the overall plant efficiency is reduced by 8–12%.145 However, oxyfuel combustion does allow for process flexibility and improved combustion efficiency. One strategy to reduce the energy penalty is the use of pressurised oxyfuel combustion cycles. An advantage of pressurised systems is that the combustion power cycle utilises the higher heating value of the fuel and produces more gross power compared to conventional atmospheric oxyfuel combustion power systems. Elevated dew point and higher available latent enthalpy in the flue gases lead to higher thermal energy recovery from the water in the flue gases. Pilot scale experimental trials by ENEL have shown that pressurised systems146,147 have increased heat transfer rates in the Heat Recovery Steam Generator (HRSG), allowing the possibility of burning cheaper coals and reducing the size of components, which will lead to a reduction in capital costs. The pressurised oxyfuel system is achieved by pre-compressing oxygen in the ASU which leads to high pressure combustion flue gases and a reduction in the work duty of the CO2 compression unit. Overall, the amount of compression work between the ASU and compression unit is reduced in comparison to conventional atmospheric oxyfuel operation. Sensitivity studies by Massachusetts Institute of Technology have determined that maximum efficiency can be achieved in the vicinity of 10 bar combustor operating pressure.148Further studies have been conducted to reduce the energy consumption of the ASU by investigating the use of high-temperature oxygen transport membrane (OTM) technology for oxygen production as an alternative to conventional cryogenic distillation methods.149,150 To conduct oxygen, the temperature of the OTM must be maintained above 800 °C and an oxygen partial pressure gradient must be applied across the membrane. Membranes for oxygen production can be operated with a three-end or four-end design. In the four-end concept, a sweep stream of RFG is applied on the low pressure side of the membrane, increasing the driving force by removing the permeating oxygen and maintaining the necessary operating temperature. In the three-end concept, the driving potential is sustained by applying vacuum to the permeate side or by an increased feed pressure, where the membrane temperature is maintained by preheating the air. Different membrane module types are being investigated: tubular,151 monolithic,152 hollow fibre153 and flat.154 Vente et al.155 compared the different module designs and concluded that tubular systems are the optimal choice for all considered conditions. Current investigations into OTM technologies are at the conceptual or laboratory scale. The different process conditions encountered in the three- and four-end concepts will have implications for the types of materials that can be considered for membranes. The membrane used in the three-end concept is only exposed to air, which allows many different materials to be employed. The membrane in the four-end concept will have direct contact with flue gas, which can make integration in coal powered plants complicated because coal derived flue gas contains corruptive components such as particles and corrosive acid species. Typical membrane materials like Ba0.5Sr0.5Co0.8Fe0.2O3−x (BSCF) and Li2NiO4 that have high permeation rates at the conditions of interest have been found to be unsuitable for four-end operation due to chemical instability after contact with flue gas components.156 Studies of possible implementation of the OTMs have found that their use limits the drop in overall net plant efficiency by 5.2% for four-end concept and 5.8% for three-end concept. Although the four-end concept is preferable due to the higher plant efficiency, the three-end concept will be more technically viable in the near term because no membrane material has yet been identified that can withstand contact with flue gas.149
4.3 Pollutant emission and removal
An operational benefit of oxyfuel combustion is the reduction of NOx and SOx emissions. Oxyfuel combustion offers highly reduced NOx emissions, because NOx in the recycled gas can be reburned by contact with flame-generated hydrocarbons, which act as a reducing agent to produce N-volatiles, consisting of ammonia and cyanide species that may subsequently produce NOx or N2 depending on the conditions. Moreover, as nitrogen from the air is largely eliminated from the process by substitution with RFG, thermal and prompt-NOx formation rates are highly reduced. The amount of NOx emitted per unit of energy generated can be reduced to around a third that of air-firing.157–159 Recent experimental investigations of NOx formation during oxyfuel combustion of pulverised coal160,161 have concluded that fuel-N conversion to NO in O2/CO2 is lower than in O2/CO2. However, very high concentrations of oxygen are often present locally in oxy-coal flames, which can result in enhanced production of NOx from fuel-N if the burner is not suitably designed and operated.162 The reduction in volume throughput of oxyfuel combustion also leads to higher concentration of NOx in the system. Conventional primary and secondary measures can be used for NOx control under oxyfuel operation. Primary measures that reduce NOx formation in the furnace by modifying the combustion environment (i.e. employing low NOx burners, air-staging, fuel staging and flue gas recirculation) are believed to be sufficient for oxyfuel combustion but this will depend on future legislation for CO2 emission and storage.163 Additionally, the development of de-NOx in CO2 compression and purification processes via conversion of NO to NO2 and removal by absorption in condensate is in progress.164The oxyfuel process using RFG results in a higher concentration of SO2 (ppm) in the combustion flue gas due to reduced volumetric flow and the introduction of the recycle loop,165 which can in turn lead to higher concentrations of SO3. However, the overall emission rate of SO2 (mg MJ−1) is lower158,166,167 than air firing due to the increased conversion of SO2 to other species throughout the process. Elevated concentrations of SOx present serious implications for CCS technologies, including boiler and pipeline corrosion, ash deposition and increased acid dew point.168 Mitigation and control strategies for SOx include the use of low sulphur and/or high calcium coals, wall soot blowing, limestone injection, sulphur scrubbing prior to recycle or compression and removal during compression.165 Development of optimal strategies for SOx mitigation and control requires techno-economic evaluation. The emission of mercury in oxy-coal combustion is a corrosion concern because it forms an amalgam with a number of metals, including aluminium used in CO2 compression units.169 Elemental mercury can speciate to the oxidised form (Hg2+) or particulate bound forms HgP during post-combustion quenching. Only a few studies have reported the extent of mercury oxidation or its retention in fly ash. While the change of environment from N2/O2 to CO2/O2 may have little effect on the ratio of Hg0 to Hg2+;170,171 tests in a 30 MW pilot scale facility of Babcock and Wilcox showed mercury concentration to increase in oxy-coal combustion.172 While the increase in Hg concentration is due to the removal of diluent nitrogen, the increase in Hg oxidation may be explained by increased chlorine concentration in oxyfuel combustion. Strategies to control Hg emissions include injection of activated carbon sorbents or forcing oxidation to water soluble Hg2+ forms to then be removed by conventional FGD scrubbing.
Air Products have developed the possibility of co-removal of SOx, NOx and mercury during compression using their “Sour Gas Compression” technology.173 The process relies on the oxidation of NO to NO2 to convert SO2 to H2SO4174 in the presence of water. Mercury will dissolve and react in the nitric acid formed as a condensate. The technology is based on the “lead chamber process”. Further investigations are required to determine the kinetics at higher pressure. Further pilot-scale work in this area has been performed by CANMET175 and at the laboratory scale (e.g. Chalmers University, Sweden176 and Imperial College London177).
Little information on the behaviour under oxyfuel conditions of other pollutants such as particulates, other trace metals (Pb, As, Cd, Se, etc.), volatile organic compounds (VOCs), polyaromatic hydrocarbons, dioxins and other chlorinated compounds are currently available. More investigations of the release and distribution of these substances under oxyfuel conditions are required.
4.4 Computational fluid dynamics modelling
Oxyfuel combustion presents numerous opportunities and challenges for numerical modelling.178 Extensive use of Computational Fluid Dynamics (CFD) modelling tools for the scale-up and advanced design of oxy-coal combustion facilities is expected.179 Utility boilers can be modelled in 3D and the impact of changing various design parameters on fluid flow, heat transfer and chemical reactions in combustion can be investigated. CFD modelling for oxyfuel combustion relies on sub-models that were initially developed for air-firing conditions. While significant progress has been made in adapting the CFD sub-models for application to oxyfuel conditions, some of the models require further modifications and validation in order to be reliably applied in the CO2 rich environment.Char oxidation and burnout is influenced by the high concentrations of CO2 and H2O in oxyfuel combustion. Physical effects (heat capacity and mass transfer) and Arrhenius parameters for homogeneous and heterogeneous reactions must be adapted for accurate prediction of char burnout and the transition between combustion regimes.
Reduced gas-phase chemical kinetic mechanisms which can be adopted within CFD codes at acceptable levels of computational cost require development for CO2 rich environments.
The dominant mode of heat transfer in both air and oxyfuel combustion is radiation. Radiative heat transfer in oxyfuel combustion is very different than air combustion due to altered gas emission and absorption. To calculate radiation within a utility boiler, the radiative transfer equation must be solved and coupled with a radiative properties model that specifies the gaseous and particle properties. Efforts have recently been made to improve the models for gaseous radiative properties by making them applicable to oxyfuel combustion modelling.180
Accurate turbulence models are required since turbulence has important effects on mixing, kinetics and heat transfer with greater significance under oxyfuel conditions. At present, Reynolds Averaged Navier Stokes (RANS) models are considered an acceptable compromise between accuracy and computational cost. Large Eddy Simulations (LES) are more computationally intensive and have recently been applied to oxyfuel combustion.178 LES was found to be capable of capturing the intermittency effects of the coal flame and the importance of gas radiative properties was also demonstrated in the calculations. As computational resources increase, more sophisticated methods such as LES should replace classical turbulence models for CFD.
4.5 Recent trials and developments
Pilot-scale and industrial demonstration projects for oxyfuel combustion are crucial for verifying observations and theories from laboratory and bench-scale, in addition to proving the commercial viability of the technology. Until now, all operated pilot-scale and demonstration projects have been ≤100 MWth in size, and are spread out between several countries. The majority of projects have focused on the CO2 capture process only, without linking to CO2 transport and storage.181 Nevertheless, Vattenfall's Schwartze Pumpe 30 MWth plant in Eastern Germany, which began in 2008, became the world's first full chain oxyfuel pilot demonstration,182 designed for 10 tpd of CO2, transported by refrigerated truck183 to several storage and industrial sites. The 30 MWth Lacq project by Total in France uses natural gas as fuel and commenced in 2009. This was the first time an oxyfuel project has been coupled to pipeline transport for geosequestration. The Lacq project does not include any inerts-removal step,184 so CO2 is transported at 92% purity and 27 bar along an existing pipeline through a populated area. The CO2 is injected down to a depth of 4500 m into a depleted gas field. The largest currently operating integrated CCS chain involving oxyfuel combustion is the Callide 30 MWe oxyfuel project which began in 2011 in Australia.185 The Callide Oxyfuel project is the first demonstration of retrofit to an existing coal-fired boiler with electricity generation supplied to the open market and includes on-line coal milling.Future commercial demonstration-scale oxyfuel plants have recently been announced. Vattenfall's 250 MWth Jäenschwalde plant in Germany will also generate electricity for the open market and has an operational aim of 2015. The FutureGen 2 Merediosa project has been announced. This project aims to convert an oil-fired process into an oxycoal-fired utility at the 200 MWe scale.181
Next generation technologies will include co-firing with biomass, sharing of CO2 transport pipelines and boiler designs optimised for higher O2 concentration. CanmetENERGY are working on oxyfuel systems that aim to minimise or eliminate the RFG. This could lead to drastic plant size reductions, efficiency increases and cost reductions. To achieve this objective, very significant improvements in materials and system design are needed. Technologies which combust coal in a mixture of oxygen and steam/or water, known as hydroxy-fuel combustion are also being investigated. In this technology, RGF is not used, so water or steam will act as a temperature moderator. Similarly, the technology will lead to reduction in equipment size and will utilise novel turbomachinery that can generate power from the expansion of steam–gas mixtures.186
As oxyfuel combustion approaches the commercial demonstration stage of development, some technical uncertainties remain, such as those related to flue gas cleaning; however, no fundamental technical barriers have been encountered with the operation of pilot and demonstration scale test facilities. The successes of demonstration projects will provide practical information and experience needed to push forward oxyfuel technology to commercial realisation.
5. Oxyfuel CFBC
Until recently, the obvious route for oxyfuel combustion was via conventional pulverised coal-fired (PC) boiler technology as discussed above, and there is already one large European oxyfuel PC demonstration plant, with more planned in the future (see Section 4.5). However, recently oxy-fired fluidised bed combustion (FBC) has also become increasingly important as a potential technology offering both fuel flexibility and the possibility of firing or co-firing biomass with CO2 capture. For utility applications, a high velocity version of FBC, in which gas velocities are of the order of 4 to 8 m s−1, called circulating fluidised bed combustion (CFBC) is employed, and this technology is available in the supercritical mode at sizes of up to 460 MWe,187 with larger (550 MWe units) currently being built.188CFBC is now a widely used technology for the power industry for difficult fuels (e.g. low volatiles content or high sulphur, ash or moisture content or for almost any waste material). In this technology, the fuels are burned in a turbulent bed of an inert material, thus ensuring high heat transfer rates, and good solid mixing. Furthermore if sulphur capture is required, limestone can be added to the bed, ensuring SO2 is removed in solid form (CaSO4, anhydrite), which can be landfilled. While this technology was explored 35 years ago189 in its bubbling bed mode (fluidising velocities 1 to 2.5 m s−1), it was not until the last decade that the oxyfuel FBC technology received serious attention, when two large boiler companies, Alstom190 and Foster Wheeler, began to carry out pilot-scale test work and other studies to see if it could be developed as commercial CFBC boiler technology with CO2 capture.
As discussed above, in oxyfiring, flue gases must be recycled in order to keep combustion temperatures to manageable levels (in the case of CFBC, less than 1000 °C). However, unlike oxy-fired PC units, the hot solids which are an integral part of CFBC technology can also be used for extra heat transfer and steam production, either in the primary reaction loop and/or in external fluid bed heat exchangers. This means that unlike PC systems, where perhaps 70–80% of the flue gas must be recycled, lower levels of flue gas recirculation are possible, which would allow oxyfuel CFBCs of any given thermal output to be built; these can potentially be 30 or 40% smaller than the equivalent air-fired units, and thus improve plant cost savings. This lower volume of flue gas means that emissions are best expressed in terms of mg MJ−1 or some similar unit, to avoid misleading comparisons with gas emissions from air-fired units where pollutants are diluted with N2 from the air.
5.1 Pilot plant studies
Although functioning pilot plant units are still limited in number (as indicated in Table 3), studies are being undertaken in numerous countries, and oxyfuel CFBC is being considered in many other industrially important countries such as Russia191 and Australia.192 Also, there is now an international workshop on oxyfuel FBC, which is held annually.193 To date, most test work has been done at small scale (in the <100 kW range), and/or using bottled gases to supply a suitable combustion gas, instead of recycling flue gas to achieve the necessary gas velocity and solid circulation rate in terms of heat transfer requirement. CanmetENERGY in Canada currently has one of the most detailed and well-reported programs, based on results from two pilot plants which are capable of being operated in oxyfuel mode, with full flue gas recycle: a nominal 75 kWth unit and a larger 0.8 MWth unit. Successful runs on the 75 kWth unit were achieved and reported in 2007194 and much of the emissions data195 discussed below come from this unit.| Location | Size | Purpose | Additional information |
|---|---|---|---|
| Alstom, Windsor, CT, USA | 3 MWth | Feasibly studies on O2 fuel FBC technology | Unit did not employ flue gas recycle, but R&D on the unit, which began in 2001, represents the beginning of the company's development of oxy-fuel FBC technology. Alstom also operates a number of smaller facilities including a 4′′ bench-scale FBC (see [Marion et al.572] for an overview of the company's program in oxyfuel FBC) |
| Foster Wheeler, VTT and Lappeenranta University of Technology, Finland | 0.1 MWth | Provided design and operational data for oxyfuel CFB, with flue gas recycle | Foster Wheeler used VTT, Finland along with CanmetENERGY facilities to test numerous fuels and limestones573 as a prelude to their demonstration plant at CIUDEN199,207 |
| CanmetENERGY, Canada | 0.1 and 0.8 MWth | Support of national Canadian program on oxy-fuel CFB | |
| CIRCE, University of Zaragoza, Spain | 100 kWth | Bubbling FBC used to generate fundamental data | |
| University of Utah, Utah, USA | 0.33 MWth | Generation of fundamental knowledge | |
| Metso Power, Finland | 4 MWth | Developing commercial technology | A co-operation between Metso and Fortum, with fundamental studies performed by Chalmers University, Sweden574 |
| Czestochowa University of Technology, Poland | 0.1 MWth | Generation of fundamental data | Unit has also been used to support Foster Wheeler's program |
| ICB-CSIC Spain | 3 kWth | Bubbling bed facility used for generation of fundamental data | |
| Southeast University, China | 100 kWth | Generation of fundamental data | |
| Zhejiang University, China | 30 kWth | Bubbling bed used to generate fundamental knowledge | |
| North China Electric Power University | NA | Batch pressurised bubbling bed facility (using 10 g of fuel) capable of operating up to 4.5 MPa |
5.2 Gas and other emissions from oxyfuel CFBC
Air-fired CFBC technology normally produces low emissions of SO2 on addition of limestone, low NOx due to its low operating temperatures, low emissions of organic species in the form of unburned hydrocarbons, and also low emissions of heavy metals.196,197 At the moment it is far from clear how pure flue gases should be to allow the least-cost production of CO2 for piping and sequestration. However, it is reasonable to assume that if a technology such as oxyfuel CFBC has inherently low emissions then this must represent an advantage.A series of trials194,195 indicated that fuel nitrogen conversions were often about half that seen from air-fired trials, with fuel nitrogen conversions down to 1.5 to 3.5%. More recently, Duan et al.198 investigated the effect of operating parameters on NO formation using a 50 kWth oxy-fired CFB, without flue gas recycle, and also found that NO production with 21% O2/79% CO2 was lower than for the air-fired case.
CFBC can be regarded as a low SO2 emissions technology, due to its ability to use limestone to trap SO2in situ. A key difference between air- and oxy-firing is that unless the CFBC is operated above about 870 °C, the CaO/CaCO3 equilibrium indicates that capture will be with CaCO3 directly (so-called direct sulphation), rather than with CaO produced from the rapid calcination of limestone at temperatures above 790 °C, due to the much higher partial pressures of CO2 in an oxyfuel CFBC. The global reactions which describe sulphur capture in a CFBC are given below:
| CaCO3 + SO2 + 1/2O2 = CaSO4 + CO2 | (7) |
| CaCO3 = CaO + CO2 | (8) |
| CaO + SO2 + 1/2O2 = CaSO4 | (9) |
Typically, limestone conversion in a CFBC is relatively low with the 30–45% utilisation being regarded as acceptable. The work of Jia et al.194,195 has suggested that limestone utilisations are comparable or lower for oxyfuel CFBC combustion. However, to date, insufficient studies have been carried out to determine this issue unequivocally, although recent tests from the 30 MWth CIUDEN demonstration unit have also suggested somewhat lower limestone utilisation.199 CanmetENERGY work200 has suggested that high-temperature steam, at the levels present when burning any hydrocarbon fuel, enhances the sulphation in both the air firing and oxyfuel case, but more so for air firing, so that sulphation is better or comparable in air firing to oxyfuel combustion (Fig. 7).
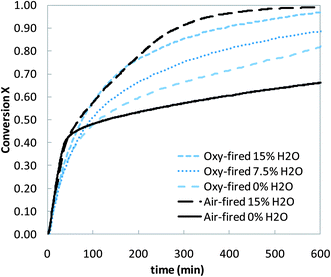 | ||
| Fig. 7 Sulphation conversion profiles under oxy-fired conditions for varied concentrations of H2O (with air-fired profiles overlaid). | ||
The issue of SO3 emissions, given its potential to cause corrosion, is something which is also of interest for oxyfuel systems, for two reasons: the use of recycled flue gases will likely increase SO3 levels; and potentially high oxygen concentrations, particularly at the base of the bed, might also enhance its formation. There is currently a dearth of information on this subject for oxy-FBC systems; however, from preliminary research done by CanmetENERGY on its 0.8 MWth CFBC, levels do not seem excessive at 2 ppmv or less,201 albeit that the bituminous coal used contains only 0.56% sulphur. Ahn et al.202 have also recently examined SO3 concentrations for a 1.5 MW pilot-scale PC combustor and a 330 kW pilot-scale CFB test facility (using a PRB coal with 0.2% S, a Utah coal with 0.5% S and an Illinois coal with 4% S). Unfortunately, they appear only to have examined SO3 levels for the Utah coal, for which they conclude that SO3 levels are similar for both air and oxy-firing under their conditions. They also point out that the presence of particles may provide more opportunities for SO3 formation via catalytic processes, but note again that SO3 concentrations do not appear to be noticeably affected by the amount of limestone addition.
Recently, CanmetENERGY has also examined co-firing of up to 80% wood with a bituminous coal and found that trace elements in the flue gas are negligible.201 On the question of Hg emissions, there are still rather limited data. Font et al.203 have investigated the fate of Hg and other trace elements employing a 90 kWth oxy-fired bubbling FBC. Here the pH of the leachate for the bed ash was in the range of 10.7–11.1, and for the cyclone fly ash even lower (pH = 8). As expected, most trace elements tended to report to the overhead streams (i.e. cyclone and fly ash). Interestingly, in this work the bulk of the Hg was found in the elemental form. By contrast, in some recent work done at CanmetENERGY on a bituminous coal, Hg emissions were 0.8 μg m−3 or less with about 80% of the Hg present in an oxidised form.204
5.3 Larger-scale tests and industrial plans
Foster Wheeler commissioned 8 months of trials at CanmetENERGY and these trials demonstrated excellent control on CO2 levels and combustion conditions.205 Overall performance was excellent, which is a very positive sign for the development of the technology. Foster Wheeler was also the first to commercialise supercritical CFBC technology (Lagisza power plant, Poland) and with this technology as the basis, Foster Wheeler is now working with the power company ENDESA on the development of a 300 MWe supercritical Flexi-Burn® CFBC. The predicted CO2 capture for this technology is 90% of emissions and it is anticipated that it could be available by 2020.206 Foster Wheeler also believes that it could offer such technology at the 600–800 MWe size with 600 °C steam temperature.Demonstration tests are currently underway at the 30 MWth CIUDEN pilot CFB demonstration unit, which will provide a full experimental CCS platform for the demonstration and validation of flexible air/oxyfuel CFB combustion.199,207 An interesting feature of this unit is that it uses NH3 injection into the cyclone to help maintain NOx at ∼120 mg Nm−3.
Finally, it must be noted that Alstom has also announced its intention of carrying out a 100 MWe oxyfuel CFB demonstration; although at the time of writing, no further information appears to be available in the open literature.208 It appears that oxy-fired CFBC technology is making major strides to enter the commercial arena, and it is highly probable that it will also be available as a competitive CCS technology along with oxyfuel PC technology before the end of the decade.
6. Chemical-looping combustion
6.1 Introduction
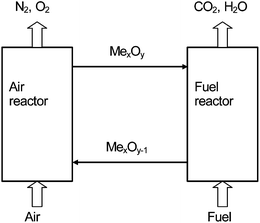
Chemical-looping combustion has emerged as an attractive option for carbon dioxide capture because CO2 is inherently separated from the other flue gas components, i.e. N2 and unused O2, and thus no energy is expended for the gas separation and no gas separation equipment is needed. However, the efficiency of the baseline technology must also be taken into account. If CLC were to be used for power production with gaseous fuels, and not pressurised, CLC would limit the efficiency of the underlying thermodynamic cycle to that of a steam cycle, rather than a more efficient combined cycle. However, where the aim is to produce steam/heat or hydrogen, or if solid fuels are used, this is normally not an issue. Potentially promising technologies, such as chemical looping reforming, which avoid this issue, will also be discussed below. The CLC system is composed of two reactors, an air and a fuel reactor, see Fig. 8.The fuel is introduced in the fuel reactor, which contains a metal oxide, MexOy. The fuel and the metal oxide react according to:
| (2n + m)MexOy + CnH2m → (2n + m)MexOy−1 + mH2O + nCO2 | (10) |
The exit gas stream from the fuel reactor contains CO2 and H2O, and a stream of CO2 is obtained when H2O is condensed. The reduced metal oxide, MexOy−1, is transferred to the air reactor where it is oxidised, reaction (11):
| MexOy−1 + 1/2O2 → MexOy | (11) |
The air, which oxidises the metal oxide, produces a flue gas containing only N2 and some unused O2. Depending on the metal oxide and fuel used, reaction (10) is often endothermic, while reaction (11) is exothermic. The total amount of heat evolved from reaction (10) and (11) is the same as for normal combustion, where the oxygen is in direct contact with the fuel.
The metal oxides used for the oxygen transfer are called oxygen carriers. The reactor system is normally made up by two interconnected fluidised beds, with the oxygen carrier in the form of particles being circulated between the two beds, Fig. 9.
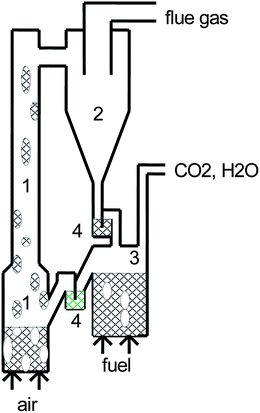 | ||
| Fig. 9 CLC process, example with two interconnected fluidised reactors. (1) Air reactor and riser, (2) cyclone, (3) fuel reactor, (4) loop seals. | ||
CLC was first introduced by Lewis et al. as way to produce pure CO2 from fossil fuels, using two interconnected fluidised beds.209,210 Much later, Ishida et al. proposed the use of chemical-looping combustion for climate mitigation and also started laboratory research on oxygen-carrier materials.211 Ishida also introduced the name of the process, chemical-looping combustion.212 In 2001, a design based on the circulating fluidised-bed principle was presented, see Fig. 9, investigating the critical design parameters of a system such as the solids inventory and recirculation rate of oxygen carriers between the reactors and identifying the relationship between these and the oxygen carrier properties.213
6.2 Applications
Most of the work so far has been focused on gaseous fuels. Gaseous fuels can be used directly as the fluidising medium of the fuel reactor. Important gaseous fuels, e.g. natural gas and refinery gas, contain large amounts of methane. Thus, oxygen carrier development has had significant focus on oxygen carrier materials with high reactivity towards methane.Liquid fuels would also be a possible fuel, but except for the operation involving kerosene in a 300 W unit,214,215 little operational experience is presently available with liquid fuels. Different liquid fuels including heavy fuel oil have been studied in fluidised-bed batch reactor tests.216
The pioneering work of Lewis et al.209,210 utilised copper and iron oxides. Fifty years later, new studies emerged,217–219 revisiting the same oxides. Soon after, Leion et al. investigated different fuels and oxygen carriers in a laboratory fluidised bed,220–222 and today there are a number of publications of laboratory work with solid fuels, as well as from actual operation in smaller pilots.223,224
When using solid fuels, the reaction between the oxygen-carrier and the char remaining after volatiles release is not direct, but involves an intermediate gasification step. This means that CLC with solid fuels will require a different design of the fuel reactor, as well as oxygen carriers with other properties. The following key issues have been identified in relation to fuel reactor performance: solid fuel conversion, gas conversion and CO2 capture.
6.3 Using CLC for hydrogen production with CO2 capture
The chemical-looping technology can also be adapted for the production of hydrogen with inherent CO2 capture. Chemical-looping processes for hydrogen production from gas include, (i) autothermal chemical-looping reforming, (ii) chemical-looping steam reforming, and (iii) chemical-looping with water-splitting.Autothermal chemical-looping reforming, CLR-a, involves utilising chemical-looping for partial oxidation to form a syngas. That, after water–gas shifting, can be separated into CO2 and H2.225–228
Chemical-looping steam reforming, CLR-s, or chemical-looping combustion with steam reforming, is a marriage between conventional steam reforming and CLC.229 Just as in commercial steam reforming, the reactions take place inside tubes using suitable catalysts and at elevated pressures. The steam reforming tubes are placed in a separate fluidised-bed heat exchanger. Hence, the reformer tubes are not heated by direct firing but by oxygen carrier particles, which means extracting the heat generated from the CLC process. The feed gas to the fuel reactor is the offgas from the steam reforming process, which is a gas mixture of CH4, CO2, CO and H2.
The CLR-s process has a number of important advantages: (i) only one gaseous component, i.e. H2, needs to be separated, unlike the CLR-a process, where two essentially pure streams of CO2 and H2 are needed, (ii) the chemical-looping can take place at atmospheric pressure, while the reforming can occur at high pressure, (iii) compared to the gas boilers used in conventional steam reforming, the temperature around the tubes is considerably lower and more uniform. The lower temperature means that a greater fraction of the combustion heat is used for steam reforming, with the consequence that the reforming efficiency is increased. This may well be the only CO2 capture technology which results in increased efficiency (if the efficiency loss of CO2 compression is not included).
Chemical-looping with water-splitting, also known as One-Step Decarbonisation, uses three reactors.230 The process requires an oxygen carrier which is reduced in steps through different oxidation states, e.g. Fe2O3 > Fe3O4 > FeO. In the fuel reactor, the fuel and oxygen carrier needs to move counter-currently. In the top, Fe2O3 is reduced to Fe3O4, while accomplishing complete combustion of the fuel, and in the bottom, Fe3O4 is further reduced to FeO. Then, in the water splitting reactor, the FeO is oxidised to Fe3O4 by steam, yielding hydrogen. Finally the material is led to the air reactor where it is oxidised back to Fe2O3. Note that two changes in oxidation state are needed. Fe2O3 to Fe3O4 is needed to fully oxidise the fuel, while FeO to Fe3O4 is needed for water splitting. The process elegantly avoids any gas separation in the hydrogen production but at the price of an added complexity of the reactor system.
There is also work with chemical-looping of solid fuels directed towards hydrogen production, rather than complete combustion, which is similar to the chemical-looping reforming and water-splitting processes proposed for gaseous fuels. A chemical-looping process for the production of syngas using solid fuels and two interconnected fluidised beds was patented more than 60 years ago.225 Some more recent processes involve using lime to enhance fuel conversion to H2 by in situ CO2 removal, e.g. the Alstom Hybrid Combustion–Gasification Process and the GE Fuel-Flexible Process.231 With respect to water-splitting, it should be mentioned that going back 80–90 years, the main process for hydrogen production was the so-called steam-iron process. In this process, iron oxide was reduced by coal to iron, and the iron was then reacted with steam to form hydrogen.231 Related processes that are concerned with the direct production of hydrogen through water-splitting using Fe/FeO being studied today are the Syngas Chemical-Looping process (SCL) and the coal direct chemical-looping process.231
6.4 Oxygen carrier materials
More than 900 different oxygen carrier materials have been studied in the laboratory,232 and there are several reviews covering oxygen carrier materials,223,232,233 and discussing important criteria and the required thermodynamic properties.234 The first phase of oxygen carrier development focussed mainly on four metal oxides: Ni, Fe, Mn and Cu. However, the development over the last few years has been more diversified; there has been more work on combined metal oxides, on low-cost materials for use with solid fuels, and on materials releasing oxygen, i.e. CLOU materials (see the following section).Combined metal oxides, i.e. where two or more oxides are combined not only physically, but also chemically, produce new oxides, for example, Cu0.95Fe1.05AlO4, Co0.5Ni0.5FeAlO4, CoFeAlO4, CuFeGaO4, NiFeAlO4.235 Some of these materials have the perovskite structure, e.g. La1−xSrxFe1−yCoyO3−δ, and Sr(Mn1−xNix)O3.236,237 Other types of oxide that should be mentioned are combined Mn oxides with partial CLOU properties, i.e. with the ability to release some oxygen. This includes Mn combined with Ca, Mg, Ni and Fe.238–240 Many of these combined materials are promising, but fewer have been successfully tested during actual operation. An exception is CaMn0.875Ti0.125O3.241 Another is ilmenite, FeTiO3, a naturally occurring low-cost combined oxide commonly used with solid fuels.
Low-cost materials have been investigated mainly for use with solid fuels, these studies include iron ore,242–244 manganese ore,245 ilmenite, CaSO4/CaS,246–253 industrial waste materials,254,255 as well as comparisons of materials of different sources.256,257 Most of the studies have used ilmenite,258–262 because it is a cheap ore, has a reasonably high reactivity towards syngas and has shown good fluidisation behaviour.
Most materials studied have only been investigated in laboratory, but a significant number of different materials have actually been used in continuous operation in CLC pilots. These include oxides of nickel, copper, iron, manganese and cobalt, as well as natural minerals like ilmenite, iron ore and manganese ore.
6.5 Chemical-looping with oxygen uncoupling (CLOU)
Chemical-Looping with Oxygen Uncoupling (CLOU) is closely related to chemical-looping combustion but differs from CLC through the spontaneous release of oxygen in the fuel reactor. For instance, the CuO/Cu2O system has an equilibrium oxygen partial pressure of 0.02 bar at a temperature of 913 °C. This means that, at this temperature, the O2 concentration could be reduced down to a minimum of 2% in the air reactor, while oxygen could be released up to maximum 2% in the fuel reactor. As the presence of fuel in the fuel reactor will consume oxygen released, a very rapid release of oxygen is possible. CLOU using CuO has been shown to work not only in laboratory batch fluidised-bed tests with CuO and solid fuel,263,264 but also in continuous operation with solid fuel.265 Also, combined manganese oxides have the ability to release oxygen240 and successful operation with calcium manganates has been reported.2416.6 Fluidised bed reactor system for CLC
In order to investigate fluidised systems for CLC, a number of studies have utilised cold-flow modelling to identify stable and suitable operating conditions for various designs.266–272Actual operation in 12 CLC units of sizes 0.3 to 140 kW involving 29 oxygen-carrier materials was reported by Lyngfelt.273 The units are presented in Table 4, including eight additional units. Thus, more than 4800 h of operation in 20 units of sizes 0.5 to 140 kW, using a number of different oxygen-carriers and fuels have been accomplished. This includes more than 600 h in seven units using solid fuels. The successful operation in a number of small units with different designs, different fuels, and different oxygen carriers, clearly demonstrates that the process works and is viable, and that there are suitable oxygen-carrier materials for this new combustion technology.
| Location | Unit | Oxides tested | Time | Fuel\references | Year |
|---|---|---|---|---|---|
| a SF – solid fuel, GSF – gaseous & solid fuel, Pr – pressurised, LF – liquid fuel. | |||||
| Chalmers | 10 kW | NiO, Fe2O3 | 1410 | Nat. gas\575–578 | 2004 |
| KIER | 50 kW | NiO, CoO | 28 | Nat. gas\579,580 | 2004 |
| CSIC | 10 kW | CuO, NiO | 120 | Nat. gas\581,582 | 2006 |
| Chalmers | 0.3 kW | NiO, Mn3O4, Fe2O3, ilmenite, CaMnO3 | 810 | Nat. gas, syngas\227,241,583–591 | 2006 |
| Chalmers | 10 kW-SF | Ilmenite, manganese ore | 149 | Coal, petcoke\245,259,592–595 | 2008 |
| CSIC | 0.5 kW | CuO, NiO, Fe2O3 | 820 | Nat. gas\228,254,596–606 | 2009 |
| KAIST | 1 kW | NiO + Fe2O3 | ? | CH4\607 | 2009 |
| Vienna UT | 140 kW | Ilmenite, NiO | 390 | Nat. gas, CO, H2\262,608–617 | 2009 |
| Alstom | 15 kW | NiO | 100 | Nat. gas\4 | 2009 |
| Nanjing | 10 kW-SF | NiO, Fe2O3 | 230 | Coal, biom.\618–621 | 2009 |
| KIER | 50 kW | NiO, CoO | 300 | Nat. gas, syngas\622 | 2010 |
| Nanjing | 1 kW-SF | Fe2O3 (ore) | >10 | Coal, biomass\244,623 | 2010 |
| IFP-Lyon | 10 kW-GSF | NiO | >90 | CH4, coal, syngas\624,625 | 2010 |
| Stuttgart | 10 kW | Ilmenite | ? | Syngas\261 | 2010 |
| Xi'an Jiaotong | 10 kW-Pr | CuO/Fe2O3 | 15 | Coke oven gas\626 | 2010 |
| CSIC | 0.5 kW-SF | Ilmenite, CuO, Fe2O3 | 164 | Coal\260,265,627,628 | 2011 |
| Chalmers | 0.3 kW-LF | NiO, Mn3O4, CuO | 116 | Kerosene\214,215 | 2011 |
| Chalmers | 100 kW-SF | Ilmenite | 24 | Coal\629–632 | 2012 |
| Hamburg | 25 kW-SF | Ilmenite | 21 | Coal\633 | 2012 |
| Ohio | 25 kW-SF | Fe2O3 | ∼72 | Coal\634 | 2012 |
6.7 Modelling
For gaseous fuels, the main performance criterion is fuel conversion in the fuel reactor, and the work primarily involves estimations of the required solids inventory to gain a given conversion to CO2, and its comparison to actual achievements.223,274–277For solid fuels, the performance is more complex, and normally three performance criteria are used, (i) solid fuel conversion, (ii) gas conversion and (iii) CO2 capture efficiency. These can essentially be modelled separately (as seen in publications available).278–282
6.8 Conclusions for CLC
Although more development work is needed, it should be pointed out that the CLC technology provides unique advantages for avoiding the large costs and energy penalties inherent in gas separation. In the case of gaseous fuels, the following conclusions can be made:• The technology has been successfully demonstrated in a number of smaller pilots and the technology should be ready to scale up to 1 or 10 MW size.
• The presently studied technology, i.e. systems that operate under atmospheric conditions and temperatures of 800–950 °C, would have significantly lower efficiency in power production as compared to natural gas combined cycle (NGCC) plants. CLC for higher pressures and temperatures need significant development efforts. However, there are a number of applications where gaseous fuel CLC could be used for steam/heat production.
The following conclusion can be made for CLC with solid fuels:
• The technology is similar to established combustion of coal in circulating fluidised beds.
• There is a unique potential for dramatic reduction in cost and energy penalty for CO2 capture.
• CLC operation with low-cost mineral ilmenite works well, but to reach high performance, additional development is needed, either with regards to reactor system or the oxygen carrier material used.
• Oxygen carrier materials other than ilmenite could provide significant improvement of performance, but it is not clear if are they available at reasonable costs.
• The following options to have a complete conversion of the gas to CO2/H2O in the fuel reactor are available: (i) oxygen polishing, (ii) separation/recycling of unconverted gas (iii) using two fuel reactors in series and (iv) CLOU oxygen carriers.
• For scale-up, a more detailed understanding of the processes in the fuel reactor is needed to design and optimise the fuel reactor system, in order to assess how the performance will be affected by the properties of the oxygen carrier and the reactor design.
• The optimisation of the fuel reactor system will primarily need to consider three costs, i.e. costs for oxygen carrier, costs for the fuel reactor system, and costs downstream of the fuel reactor to accommodate for incomplete conversion, e.g. oxygen polishing. Consequently, a good understanding of these costs is needed to find the optimal solution and realise the great potential of this technology.
7. Calcium looping, CaL
Calcium looping is a family of CO2 capture technologies that use CaO as a regenerable sorbent of CO2:| CaO(s) + CO2(g) ⇆ CaCO3(s), ΔH298K = −178.8 kJ mol−1 | (12) |
Both the carbonation and calcination reactions are carried out at high temperatures (650–700 °C and 900 °C, respectively), allowing for efficient heat recovery in the process or steam cycle of a power generation system. The technology has attracted, in the last 10 years, a great deal of attention, and several comprehensive reviews have been recently published.283–287 Only the main aspects and newest developments are discussed in this section.
The use of this chemical loop was first attempted in the 19th century as it was noted that gasification gases would have a higher heating power when coal was gasified in the presence of CaO. This idea was exploited in the acceptor gasification process, which tested the principle in a continuous pilot rig using an interconnected fluidised bed coal gasifier and a combustor operated at high pressure.288 Other hydrogen generation processes have been investigated from the 90s, focusing on the sorption enhanced reforming principle.289 The first application of Ca-looping as a post-combustion CO2 capture process was patented by Hirama et al.290 They also proposed a practical solution for the calcination problem: the oxy-combustion of an additional flow of fuel in a fluidised-bed calciner to provide the “Heat” arrow of Fig. 10, that can be effectively recovered in a steam cycle to generate more power.291 This section briefly reviews the status of these two main groups of CO2 capture processes. A third subsection reviews recent developments on sorbent performance issues that are common for both process routes.
 | ||
| Fig. 10 General scheme of calcium looping cycle for CO2 capture in postcombustion or precombustion (between brackets) applications. | ||
7.1 Post-combustion CO2 capture by CaL
For large scale novel energy processes such as the CaL system, it is essential to carry out detailed process simulation and thermal integration exercises to assess the viability of the full system under expected operating conditions, evaluate power generation efficiencies and conduct a transparent benchmarking exercise against more mature CO2 capture technology options. A variety of research groups have recently confirmed the inherent thermodynamic advantages of the post-combustion Ca-looping concept using oxyfuel combustion, with efficiency penalties between 6 and 8% points with respect to reference plants without CO2 capture.292–295 The calcination of the fresh make up flow of limestone can take up to 3–10% of the total energy input to the Ca looping system. But this may be considered not to be an energy penalty if the solid purge is used for cement applications, desulphurisation, or other large scale uses of CaO. The synergy between CaL and cement industry has long been recognised, but only recently detailed specific process proposals and experimental investigations have been reported.284,296 Energy penalties can be further reduced in advanced CaL concepts that avoid the need of an air separation unit, by transferring heat from a high-temperature source to the calciner, which may be operated with lower partial pressures of CO2 by introducing steam. Although the basic idea is not new,297,298 only recently there have been works investigating in detail these processes.299,300 Recent work has also included experimental studies.301Several projects have been running in order to prove experimentally the concept of post-combustion CaL using interconnected carbonator and calciner reactors. INCAR-CSIC designed and operated a 30 kWth test facility made up of two interconnected circulating fluidised-bed reactors (0.1 m ID) that reported capture efficiencies between 70 and 97% under realistic flue gas conditions in the carbonator reactor. This reactor functioned as an effective absorber of CO2 as long as there was a sufficient bed inventory (400 kg m−2) and solids circulation rate (0.5–2.2 kg m−2 s−1), even with highly deactivated calcium oxide.302,303 This test facility was also used to test the principle of low-temperature combustion of biomass (700 °C) for in situ CO2 capture.304,305 Capture rates were limited in this rig to 4 mol CO2 m−2 s−1 because of the need to limit gas velocities to ensure sufficient holdup of solids in the 6 m riser. This limitation was removed at IFK, University of Stuttgart that designed and operated a 10 kWth pilot plant, consisting of a CFB carbonator (12.4 m height) and a bubbling fluidised-bed calciner. Experimental results showed capture rates close to those expected in large scale commercial systems (up to 10 mol m−2 s−1).306 A 200 kWth pilot plant was also recently completed at IFK, with three interconnected circulating fluidised-bed reactors, which were designed to accommodate a wide range of solid looping and make-up flow rates307 and test a variety of process routes. The CANMET Energy and Technology Centre designed and constructed a 75 kWth dual fluidised-bed combustion system able to test CaL and oxy-combustion conditions. The reactors (5 m of total height) have an ID of 0.1 m and can be operated at up to 1000 °C at atmospheric pressure. Their most recent studies have been focused on evaluating the effect of steam and SO2 during calcium looping cycles.308 Ohio State University developed a 120 kWth plant to perform the Carbonation–Calcination Reaction (CCR) process, which consists of a CaL system with an intermediate hydration stage to prevent the decay in sorbent reactivity over multiple carbonation–calcination cycles. The pilot test rig involves an entrained bed carbonator, a rotary kiln calciner and a bubbling fluidised-bed hydrator. The CCR process has been demonstrated to be highly effective and efficient in removing both carbon dioxide (over 90%) and sulphur dioxide (near 100%) under realistic conditions.309,310 In Taiwan, ITRI has plans to build (in the near term) a 1 MW pilot plant specially adapted for cement application (rotary kiln calciner).311
In Spain, the largest pilot globally for post-combustion CaL testing (1.7 MWth) has been completed and successfully operated. The plant includes two CFB reactors interconnected (15 m height) and is able to treat up to 2400 kg h−1 of flue gas from an existing 50 MWe CFBC power plant. The CFB calciner has been operated in air-combustion and in oxy-fired mode. Effective CO2 capture (80–90% capture efficiency) with a conservative value of calcium conversion to CaCO3 in the carbonator (10%) has been achieved.312Fig. 11 shows the evolution of the CO2 carrying capacity (Xave), sulphation conversion (XCaSO4) and total sorbent utilisation (sum of both) measured on solid samples from a long duration experiment in La Pereda pilot plant. The trend is consistent but slightly better than expected from lab scale testing.313 Capture efficiencies over 80% were obtained even with low activity and highly sulphated material, as shown on the right hand side of the plot. Also, successful commissioning and positive initial results have been reported in a 1 MWth test facility (11.35 m height) located at Darmstadt University. Experimental campaigns using propane and pulverised coal as fuels to supply the heat for sorbent calcination provided CO2 capture efficiencies above 90%.314,315 Finally, a new 300 kWth facility for biomass combustion with in situ CO2 capture with CaO is being commissioned in Spain.316
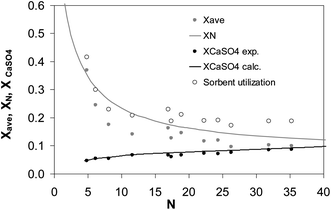 | ||
| Fig. 11 Evolution of sorbent utilisation with the average number of carbonation–calcination cycles of particles in the 1.7 MWth pilot plant of la Pereda (see Arias et al.312 for details). | ||
This stream of new experimental data from increasing scales should provide a strong basis for improved models at the particle, reactor and process level. Simple CFB reactor models have been used to interpret results from a test facility at CSIC and IFK.302,303,317 More elaborated carbonator models have been recently reported that take into account basic hydrodynamics in the riser and including the effect of SO2 co-capture and ash presence in the reactor.318–320 Hyppänen and co-workers319,321 have recently adapted their comprehensive model for CFB combustors to the carbonation reactor. These will be essential tools for future scaling up of the technology.
7.2 Pre-combustion CO2 capture by CaL
As noted above, calcium looping can be combined with reforming and/or gasification processes to produce a hydrogen-rich gas, high fuel conversions and minimal CO formation. These processes offer significant potential for efficiency and economic improvements, but they usually involve higher technical complexity. For natural gas, the benefits of the sorption enhanced reforming process, SER, and the main experimental results investigating the concept at laboratory scale are reviewed by Harrison.289 In principle, the overall process is reduced to a single reaction step, close to thermal neutrality, that can be operated at intermediate temperatures (around 650 °C) and can yield over 90% of H2 purity on a dry basis. Several studies have been recently reported on mathematical modelling of natural gas SER processes322–324 and the SER concept has been also proposed for alternative fuels, such as propane, methanol or ethanol.325–327 Romano et al.328 investigated the potentiality of a SER process coupled to a combined cycle, calculating a net efficiency of 50.2% with a carbon capture ratio of 88%, which are comparable with those values obtained for a competitive technology based on autothermal reforming, but with higher plant simplicity and lower plant cost. Meyer et al.329 evaluated the feasibility of a novel ZEG power concept, featuring the production of electrical power via a close integration of the SER process with a high-temperature Solid Oxide Fuel Cell (SOFC), obtaining efficiencies close to 77% with 100% CO2 capture and no NOx emissions.As mentioned above, CO2 capture with CaO during solid fuel gasification can be considered the first application of CaL. In the gasifier, the presence of CaO drives the WGS equilibrium towards H2 formation and the heat released from the carbonation reaction provides heat for the endothermic gasification reactions. Furthermore, CaO and CaCO3 catalytically enhance the beneficial destruction of tars.330 Different configurations for the gasification of coal have been proposed in order to improve thermal and CO2 capture efficiencies, such as the HyPr-RING process,331 the LEGS process332 or the “Calcium Looping Process” (CLP).333 The gasification of biomass in the presence of CaO has also been investigated as sustainable path for the production of hydrogen.334 In the AER process, a biomass gasifier of 8 MWth interconnected with a circulating fluidised-bed combustor has been successfully operated,335 yielding a product gas with a high H2 content of 35–40% (dry basis) and low content of condensable higher hydrocarbons, tar, below 5 g m−3. However, it must be noted that this is not designed as a CO2 capture system since the CO2 captured by CaO is released in the air-combustor. In general, the calcination step of CaCO3 in a rich atmosphere of CO2 remains a serious challenge for CaL practical applications, especially for those that require high-pressure operation to access higher efficiencies. On the basis of the unmixed reforming concept,336 a novel process has recently been proposed that employs the exothermic reduction of CuO with a fuel to supply the heat required for CO2 sorbent calcination, obtaining a flue gas rich in CO2 and readily separable H2O. By coupling an endothermic and an exothermic reaction in the same solid matrix, a higher efficiency and lower equipment cost can be achieved, since the heat is directly transferred from the metal to the carbonate.337,338 Recent works have demonstrated the theoretical viability of the novel Ca/Cu looping process,339,340 and suitable materials are also being developed.341,342
7.3 CaO performance as a CO2 sorbent
In CaL processes, particles of CaO will experience repeated cycles of carbonation and calcination. Although particles or pellets can continuously be replaced by fresh (low cost) material, it is obvious that the design and operation of any CaL system is highly sensitive to the quality (in terms of CO2 carrying capacity, reaction rate, mechanical resistance, etc.) of the functional material in the system. The carbonation reaction is characterised by a fast chemically-controlled rate followed by a slower reaction stage controlled by the diffusion through the CaCO3 layer. It has also been observed that the transition between the fast and slow regimes takes place quite suddenly at a given level of conversion and that this level of conversion decreases when the number of carbonation–calcination cycles is increased. It is generally accepted that a fast decay in CO2 carrying capacity in the first 20 cycles is almost unavoidable for natural sorbents, following a similar tendency for a wide range of particle sizes and reaction conditions. Sorbent capacity tends to stabilise at very high cycle number at a residual conversion, which is around 8–10% for natural limestones.313 The observed loss in sorbent reactivity has been attributed to the drop in internal surface area and associated increase in pore size by sintering, competing sulphation/sulphidation reactions, attrition of the sorbent and the subsequent elutriation of fines, and by ash fouling. It is noteworthy that even when fully degraded in reactivity, CaO from natural limestone still takes up ∼0.16 g CO2 per gram, a high value compared to many other potential sorbents. The thickness of the carbonate layer formed on the free internal surfaces of CaO is a critical parameter to explain the end of the fast reaction period. However, some important phenomena are not well explained by this simple theory, such as the temperature dependency of the CO2 carrying capacity and steam effects,343 which can be very significant.344 Li et al.345 have recently provided an elegant carbonation reaction rate model that seems to be able to explain most observations using a mechanism well established for other gas–solid reactions: they model the CaCO3 growth as islands on the CaO surface. The fast carbonation stage is completed when these islands merge. With the increase of reaction temperature, the sizes and heights of the CaCO3 product island increases while the island density decreases. Only a few parameters are needed to fit the observed carbonation rate curves in a wide range of temperatures. The calcination reaction of highly cycled particles in a CaL is assumed to be very fast, but this has only recently been confirmed experimentally.346 Sulphation rates of CaO in CaL reactors (carbonator or calciner) are also a recent subject of investigation. The large make-up flow of fresh limestone characteristic of most CaL systems allows for lower CaSO4 content in the system than in equivalent desulphurisation units using CaO. Open pore structures of the sorbent in a CaL can also alter the rate and the extent of sulphation. Arias et al.347 reported sulphation kinetics of CaO particles at low levels of conversion to CaSO4, whilst Anthony and co-workers348 focused their investigations on the performance of synthetic Ca-based sorbents. Attrition is another important issue in CaL because it affects capture efficiency, sorbent cost and operational cost. Particles have been shown to break up mainly during the first calcination. In addition, attrition has been found to be highly sensitive to limestone choice.349,350Several approaches have been investigated to improve initial sorbent properties and/or reactivate spent sorbents, but many still require detailed studies at the process level to ensure their viability for large-scale commercial applications. Lisbona et al.351 studied the integration of the sorbent cost and its carrying capacity and mechanical performance for different options applied to an existing coal-fired power plant. They demonstrated that the optimum CO2 carrying capacity that involves minimal heat requirements in the calciner is relatively modest (at around 20% of Ca conversion). However, for pre-combustion applications higher activities may be desirable. In general, R&D activity continues on the main techniques explored for sorbent improvement: hydration,352–354 doping,283,284,287,355 thermal pre-activation,283,286 and synthetic sorbents.286,287 Low cost methods based on co-precipitation can yield synthetic sorbents with high melting points and a carbonation conversion above 75% after 50 cycles.356,357 The use of supports like alumina358 or cements containing CaO and Al2O3359, 360 have recently been shown to improve the durability of CaO sorbent (some of them above 0.50 g g−1 sorbent after 30 carbonation–calcination cycles under severe calcination conditions).361 A different method for reactivation has also been proposed recently:362 a small regeneration reactor (recarbonator) is added between the carbonator and calciner vessels to re-carbonate the particles leaving the carbonator (calcination–carbonation–recarbonation cycles) using a small flow of pure CO2 from the calciner's off-gas. The slight increase in the carbonate conversion in each cycle sustains the residual activity at around 0.15 to 0.2, which is close to the optimum design target for post-combustion systems.
8. Low temperature adsorbents
A large number of adsorbents have been recently proposed and investigated as possible candidates for carbon capture at low temperature. The selection of the best samples cannot be based only on their adsorption properties (i.e. capacity, heat of adsorption, kinetics) because other factors may play a crucial role in the overall process. For this reason, economic criteria have to always be taken into account, not only with regards to the costs of synthesis but also to the size of the equipment (i.e. the volume of the adsorbent needed); the regeneration energy demand (i.e. the heat of adsorption); the cycle time (i.e. equilibrium and kinetic properties and process selected, pressure or vacuum swing adsorption (PSA or VSA) vs. temperature swing adsorption (TSA)); the hydrothermal stability; the loss of performances due to the presence of impurities in the feed stream.Based on these observations the best adsorbent should have high CO2 capacity at low pressure, high selectivity for CO2, fast adsorption/desorption kinetics, good mechanical properties, high hydrothermal and chemical stability, as well as low costs of synthesis.
Zeolites, as well as carbon-based materials, are the most widely investigated classes of adsorbents. In recent years a considerable research effort has been put in the development of a new class of adsorbent, MOFs (metal–organic frameworks), as promising candidates for CO2 separation. In addition, a large variety of functionalised (mostly amine-based) adsorbents has been recently produced. The very encouraging results obtained are opening a new field for the investigation of new possible adsorbents for carbon capture applications.
8.1 Zeolites
Zeolites are crystalline aluminosilicates characterised by a highly ordered open structure. They can differ greatly for the framework type, the size and shape of the channels and cages as well as the Si/Al ratio. With regard to carbon capture applications, type X and A zeolites have been widely investigated.363 They are generally characterised by a relatively high CO2 capacity at low pressure, which makes them very promising candidates for CO2 separation from flue gases.364–371 Generally 13X is indicated as the best candidate for post combustion PSA applications with values of the CO2 uptake between 2 and 3 mol kg−1 at 0.1 bar, at temperatures between 15 and 35 °C. Despite the relative high heat of adsorption (36–37 kJ mol−1),364,368 the high working capacity and selectivity make zeolite 13X one of the best choice for CO2 capture from flue gas streams.364,365,368,369 For this reason 13X is generally used as a benchmark material for low-temperature adsorbents for carbon capture applications.Despite the good adsorption properties for CO2, zeolites are generally highly hydrophilic; the presence of water induces an alteration of the electric field reducing the strength of interaction between the quadrupole of CO2 and the cations, resulting in a lower uptake.372,373 Detailed studies on the effect of the presence of small amounts of water on the CO2 uptake of zeolites were presented by Brandani and Ruthven372,373 and, more recently by Li et al.374 and Lee et al.,375 concluding that the presence of even very small amount of water greatly reduces the adsorption performance of zeolites.
The nature and the distribution of the cations inside the zeolite framework play a crucial role in the final CO2 adsorption properties. Their presence not only induces modifications of the electrical field inside the pores, but it can also change the morphological structure of the zeolites, influencing the adsorption kinetics. Ideally, the higher charge density of the smaller cations should increase the electrostatic interaction between the CO2 and the cations, resulting in a higher uptake. This trend of the CO2 uptake with the increasing charge density has been observed by several authors.375–379 Deviations from the expected trend have also been reported for some types of zeolites due to the high basicity of the framework, which has a predominant role relative to the strength of the quadrupole interaction.380,381 On the other hand, the size, the position and the grade of occupancy of the extra-framework cations may be responsible for hindering diffusion of CO2 due to the blockage of the windows of the structure by the cations.376 In this regard, an interesting case is represented by the Rho zeolites, for which the presence of extra-framework cations has been proved to induce considerable distortions in the structure.382–387 A recent study of Lozinska et al.379 reported that the combination of the framework distortion and the hindering effect of the cations resulted in an extremely slow diffusion of CO2 (measured using the ZLC technique). In addition, a gating effect was detected for the Na-Rho type due to the presence of CO2, similarly to what reported by Palomino et al.378
8.2 MOFs
The structure of MOFs consists of organic–inorganic hybrid networks formed by metal ligand bonds.388 One of their main attractive features is the possibility to modify their structures and functional properties by changing the building blocks used in their construction: this gives the incredible advantage of finely controlling pore dimension, shape of the channels, and chemical potential of the surface, which ultimately gives the possibility to build adsorbents with the desired adsorption properties.389 MOFs generally show higher CO2 capacity at high pressures compared to zeolites, but, despite their relatively low capacity at low partial pressures, their high thermal stability and the fully reversible CO2 adsorption make them very promising materials for pressure-swing processes.390,391 With regard to the low pressure applications, MOF-74 and its politypes have shown attractive features for carbon capture. The trend of the CO2 uptake follows the sequence, Mg > Ni ∼ Co > Zn, with values of the CO2 capacity for Mg-MOF-74 being almost double than that for 13X.389,392–394 Mg-MOF-74 is characterised by a high selectivity for CO2395 and the heat of adsorption is generally higher than the one of zeolites with values of about 47 and 41 kJ mol−1 for the Mg and Ni form respectively.389,393,396,397 The higher ionic character of the Mg–O bond improves the affinity with CO2, but on the other hand, it makes the Mg form more hydrophilic than the analogous Ni form. Studies to compare the effect of water on CO2 adsorption for Ni-MOF-74 and commercial zeolites were performed by LeVan et al.;391 although the CO2 capacity was found to reduce in presence of water for all the materials, the effect was less pronounced for Ni-MOF-74. Liu et al.398 reported that the H2O molecules interact specifically with the strong adsorption sites of Ni-MOF-74, causing a non-recoverable loss of CO2 capacity. An extensive study on different MOF-74 samples (Zn-, Co-, Ni- and Mg-MOF-74) was carried out by Hu394 at the conditions of interest for post-combustion carbon capture at 38 °C and 0.1 bar using the ZLC method. Tests in the presence of impurities (water, SOx and NOx) showed a significant deactivation of the samples with the Ni-MOF-74 demonstrating a greater resistant to degradation.8.3 Carbon-based adsorbents
Carbon-based adsorbents are synthesised by the thermal decomposition of carbonaceous materials and have been investigated and used for a wide range of gas separations. Siriwardane et al.,399 compared the adsorption properties of commercial activated carbon with 13X and 4A. From the study, it emerged that, relative to the zeolites, activated carbon showed a lower uptake and selectivity at lower pressures, but they maintained higher hydrothermal stability. Values of the heat of adsorption are generally lower for the activated carbons than for other adsorbents with values in the range from 15 to 30 kJ mol−1.399–402Shen et al.403,404 investigated the use of activated carbon in a VPSA process to capture CO2 from flue gas, obtaining relatively high values for the recovery and purity of CO2. The possibility of application of carbon molecular sieves in a PSA process for CO2 from flue gas was recently investigated by Carruthers et al.402 The study concluded that despite the lower capacity relative to other adsorbents the low heats of adsorption and the stability of carbon-based adsorbents make them competitive for CO2 capture from flue gas.
The adsorption properties of activated carbon can be significantly improved by the incorporation of amine functional groups into their porous structure. The CO2 chemically reacts with the amine groups forming bicarbonate and/or carbamate,405,406 which is promoted at higher temperatures. As a result, an increase of the CO2 capacity is observed at higher temperatures while at lower temperatures, physisorption is predominant and the loss of porosity due to the amine functionalisation has a crucial role in the final CO2 uptake.406 Moreover, relatively slow kinetics are generally observed.405
As part of the carbon based materials new developments are in progress with regard to the carbon nanotubes (CNT) for low-pressure carbon capture. Multi-walled CNT functionalised with APTES have shown a significant improvement of the CO2 uptake compared to the pristine sample.407–409
8.4 Mesoporous silicas
Mesoporous silicas are generally characterised by low CO2 uptake due to the weak surface interaction with CO2 molecules. What makes them attractive for carbon capture is the possibility to introduce functional groups (usually amine-based groups) to increase the affinity with CO2. The advantage of having large and uniform pores is that it is possible to introduce surface modification, reducing possible steric hindrance of the adsorption sites. As a result of the introduction of functional groups, a significant increase of CO2 uptake at low pressure has been shown compared to the pure silica; very promising results have been obtained recently, but the increased complexity of the adsorption process may, in some cases, lead to an overestimation of the adsorption capacity.410 The adsorption properties are mostly influenced by the density of amine active sites and by the accessibility to the sites (pore size).411Belmabkhout et al.412–414 induced a series of modifications on MCM-41: they synthesised a pore-expanded form (PE-MCM-41) and successively introduced amine groups in the expanded form (TRI-PE-MCM-41). The PE-MCM-41 exhibited a higher CO2 uptake at high pressure than the non-modified MCM-41; however, there was not a significant improvement in the low concentration region. On the other hand, the TRI-PE-MCM-41 sample, which combined the advantages of a large pore structure due to the presence of amine groups, showed a dramatic improvement of the adsorption capacity, especially in the low pressure region. The value of the CO2 uptake at 0.1 bar and 25 °C was comparable with the one of a typical zeolite, 13X (2.2 mol kg−1). Even though the capacity is comparable with 13X, the amine-modified sample exhibited a significant increase of the CO2 uptake in presence of water, which is a very important advantage for the possible application of the sample for CO2 capture applications. Xu et al.415,416 studied the adsorption performances of PEI-impregnated MCM-41 under different conditions, reporting an increase of the CO2 capacity with the PEI loading and temperature (with a maximum at 75 °C for the sample with 75 wt% of PEI), while the adsorption process was found to be strongly kinetically controlled.
8.5 Pilot-plants development and testing
At present, a few pilot scale demonstrations are investigating the effectiveness of low temperature adsorbents for CO2 capture. One of the first pilot plant projects was the CO2CRC H3 project417 lignite-fired power plant based at International Power's Hazelwood Power Plant, and was commissioned in 2009 Latrobe Valley, Victoria, Australia. The research project was completed in 2011 and the performance of commercial and novel adsorbents was investigated at high humidity levels in the presence of SOx and NOx with a 3-bed multi-layered vacuum swing adsorption process. Multi-layered adsorbents were used to remove, first, the water and subsequently SOx/NOx from the flue gas. A layer of CO2-selective materials was then added. A purity of about 71% and a recovery of about 60% were achieved after continuous running of the process using a simple 6-step cycle (without purge) for a week.The Science and Engineering Research Council (SERC) of Singapore in 2009 launched a research programme on Carbon Capture and Utilisation (CCU), which includes a collaborative project418 between the adsorption and process systems research groups at National University of Singapore (NUS), Nanyang Technological University (NTU) and Institute of Chemical and Engineering Sciences (ICES). A pilot plant that was designed based on the results from a detailed simulation study, has been constructed. 1 m long columns with 0.3 m internal diameter were used and the plant is expected to capture around 3 tCO2 madsorbent−3 per day using a simple 4-step Vacuum Swing Adsorption (VSA) with Zeochem 13X and synthetic dry flue gas. Special attention is focused on the power consumption by the vacuum pumps so that a reliable estimate of the energy penalty may be obtained.
Based on a lab scale 1 kW plant with supported amine sorbents in a circulating fluidised bed developed by ADA Environmental Solutions, the US DOE419,420 has funded a 1 MW pilot plant. The pilot plant will be located in the Southern Company – Alabama power Co. plant and should be completed by the end of 2013.
Inventys claims that their VeloxoTherm™421 process can capture CO2 for 15 US$ t−1. The technology involves an intensified temperature swing adsorption process with structured adsorbent and steam regeneration in a rotating adsorbent wheel. ETI422 just announced the award of £20 million funding for a 5 MW project that can be used for a new-build CCGT or retrofitted onto one. The consortium will be led by Inventys with Howden, MAST Carbon International and Doosan Power Systems as partners, as well as Rolls Royce for specialist engineering support. The initial stage of the project is lab scale studies, but the final aim is to have a commercial technology by 2020.
The ATMI/SRI BrightBlack423 microporous carbon was recently tested at a coal-fired steam production facility operated by the University of Toledo in Ohio, USA. The test results were presented in Pittsburgh, Pennsylvania, USA at the 2012 NETL CO2 Capture Technology Meeting. The material exceeded the DOE targets of >90% CO2 capture with >90% CO2 purity during tests with 200 standard l min−1 of flue gas. Additionally, the column operated for approximately 7000 adsorption–regeneration cycles with no loss in process or adsorbent performance and no signs of adsorbent degradation. The project partners are now looking at scaling up to pilot scale testing.
Not much information is available at the moment on the pilots due to the early stages of development of most of them; the availability of data on these projects in the future will represent a crucial step towards the deployment of adsorption processes at commercial scale.
Having reviewed a number of technologies for carbon capture from industrial and power station sources, this article will now focus on more long-term options. This will include carbon capture from the ambient atmosphere, CO2 utilisation and mineralisation.
9. Direct air capture technology
9.1 Introduction
Direct air capture is the process of removing CO2 from the air and generating a concentrated stream of CO2 for sequestration or re-use. It belongs to a group of technologies referred to as negative emissions, or carbon dioxide removal (CDR) technologies. Other negative emissions technologies include bioenergy enhanced carbon capture and storage (BECCS); augmented ocean disposal (or ocean liming); biochar production and utilisation; the dispersion of naturally occurring bases such as serpentine and olivine across the land; and the enhancement of biological CO2 sinks such as reforestation, afforestation and aquatic biomass via ocean fertilisation. These technologies are beyond the scope of this paper, though the interested reader may refer to a recent techno-economic analysis of negative emissions technologies by McGlashan et al. for more information.424 In fact, we will only very briefly review recent developments in air capture technologies here.Compared with traditional CO2 capture from concentrated point sources; direct air capture offers a number of purported advantages. Firstly, air capture provides a means of adjusting the atmospheric CO2 concentration in the increasingly more likely event that mitigation efforts fall short of targets and the atmospheric greenhouse gas inventory reaches dangerous levels or takes a trajectory towards stabilisation at dangerous levels. Air capture could also offer an option for addressing CO2 emissions from mobile and distributed sources, such as vehicles, fuel use in buildings and geographically isolated industry, where direct capture and integration into a centralised CCS network would be either impractical and/or uneconomical. Furthermore, direct air capture technology could be installed by storage site operators to manage fugitive emissions from the CCS network and leakage from geological formations. In addition, it has been suggested that direct air capture technology could potentially be situated anywhere, such as deserts, wasteland and the ocean, provided there is access to an available energy source and sequestration sites. However, there are also significant disadvantages to the technology. Removing and concentrating CO2 from air at ∼390 ppm to a pure stream (>90%) implies a greater energy input, and treatment of a vastly greater volume of gas than CO2 capture from concentrated point sources. For example, the thermodynamic minimum energy required to extract CO2 from ambient air is ∼20 kJ mol−1 compared with 8.4 kJ mol−1 and 5.3 kJ mol−1 to capture and concentrate CO2 from the flue gases of natural gas-, and coal-fired power stations containing 5% and 15% CO2 respectively at 65 °C. Furthermore, the actual energy consumed by air capture technology will be significantly larger than the thermodynamic minimum, as is the case for CCS systems. Zeman et al. estimated that the energy demand of a large-scale MEA-based process for CO2 capture from concentrated sources would be 181 kJ mol−1, which is far greater than the thermodynamic minimum energy requirement.425
Direct air capture has been practiced on a small scale for decades for the purpose of maintaining safe levels of CO2 in submarines426 and spaceships427 though it is important to note that the concentration of CO2 is in these locations is significantly higher than that within the atmosphere. CO2 must also be removed from air prior to air liquefaction to avoid operational issues associated with dry ice formation.428 However, the volume of air that must be handled to capture comparable amounts of CO2 to traditional CCS technologies is far greater. This has significant implications on energy consumption and the required plant size. As a consequence, capture technologies that require pre-processing of air, such as drying, heating, cooling or pressurising will not be economical.429 This rules out technologies typically used for small-scale air capture such as membrane separation (large pressure gradients and multiple passes required to achieve a high-purity CO2 stream);430 cryogenic separation (cooling and compression required); and zeolite, activated carbon and alumina-based molecular sieves (adversely affected by moisture and low adsorption capacities at ambient conditions). Two main approaches have been proposed for direct air capture (i) wet air capture systems and (ii) dry air capture systems. Whilst both capture processes require energy to regenerate the sorbent, the energy demand scales proportionally to the mass of CO2 captured as opposed to the volume of air processed. For those seeking more information than this brief overview, the reader is encouraged to read a recent review by Goeppert et al.431
9.2 Wet air capture systems-the soda/lime process
The most developed approach for wet air capture is the soda/lime process.431 This uses aqueous sodium hydroxide (NaOH)-based solutions to extract CO2 from ambient air in a packed-column,432 convection tower433 or spray-tower contactor system.434 After the contactor, the NaOH solution is regenerated via caustic recovery (or causticisation), where slaked lime (an aqueous solution of Ca(OH)2) is reacted with the dissolved sodium carbonate (Na2CO3) product to form a calcium carbonate (CaCO3) precipitate mud. The CaCO3 mud is filtered, dried and transferred to a rotary kiln where it is calcined at temperatures in excess of 900 °C to produce a concentrated stream of CO2 and a calcium oxide (CaO) powder. The CaO powder is then dissolved in water to regenerate the slaked lime solution.The requirement of substantial thermal energy for lime regeneration represents a significant drawback of this process. Baciocchi et al. estimated that process energy demands are likely to range between 7.6 and 11.6 GJ tCO2−1 (334–510 kJ mol CO2−1) with drying, pre-heating and calcining of the CaCO3 accounting for the majority of the total energy demand.435 Other energy intensive processes considered in the Baciocchi et al. estimates were CO2 compression and air separation to produce O2 for an oxyfuel kiln. As a consequence, CO2 abatement costs for this process are high, typically quoted as 240–500 US$ tC−1 (65–136 US$ tCO2−1);436 much higher than some estimated cost of CCS at 30–50 US$ tCO2−1437 (some authors of this paper might suggest a more conservative range of costs, from $50–$120 tCO2−1 depending upon source and capture technology). Recent papers by the American Physical Society438 and House et al.439 have stated costs may be even higher, in the region of US$600 and US$1000 per ton of CO2 respectively, though it should be noted that these costings are disputed by researchers within the air capture community.429,440
9.3 Alternative wet air capture systems
In attempts to eliminate the energy intensive lime regeneration step, the paper and pulping industry have been developing and piloting an alternative approach that involves direct causticisation with titanates.441 The energy required to regenerate titananates is much lower than for CaO; 90 kJ mol−1 compared to 179 kJ mol−1.9.4 Dry air capture systems
Dry air capture systems typically employ solid organoamine-based adsorbents where amine functional groups are either physically or chemically bound to the surface of a porous-silica; carbon, metal oxide; or polymer support.442 Much of the work to date has focused on developing sorbents with high CO2 capacities and has neglected to use realistic desorption conditions, opting instead for desorption at elevated temperatures in an inert gas stream generating a dilute CO2 stream. Lackner et al. have developed an alternative material for extracting CO2 from ambient air, comprising of an anionic ion-exchange resin with quaternary amine functionality dispersed onto a polypropylene membrane.443 The positive charge associated with the quaternary amines is balanced by mobile hydroxyl or carbonate counter-ions, which adsorb CO2 when dry, and release CO2 when wet. Desorption can be achieved via either contacting the material with a humidified gas stream or directly with water. Air capture costs for a system employing this adsorbent have been estimated by the purveyors as 15 US$ tCO2−1 with initial costs including infrastructure and maintenance costs of 200 US$ tCO2−1. It is considered by a number of the authors of this article that these costs are unrealistic, on the basis of highly contentious assumptions concerning mass production and autonomous operation. A simple analysis by Brandani indicates that the cost of air capture relative to CO2 capture from a power station should be around a factor of ten higher.4449.5 Conclusions and future scope
Laboratory scale research has demonstrated that direct air capture is technically feasible. Wet air capture is the most developed approach. However, the high energy requirements for sorbent regeneration, particularly in the case of the soda/lime process, has led to very high estimated mitigation costs. Some progress has been made towards reducing the energy demand associated with sorbent regeneration through the use of alternative causticising agents and other improvements; however, further work is required to address and manage issues associated with high energy requirements, heat integration, large evaporative losses during liquid–air contacting and the high corrosivity of the strong-base absorbents solutions.At present, the future of large-scale direct air capture as a climate change mitigation technology remains uncertain. Air capture R&D is still in its infancy, far behind the more conventional climate change mitigation technologies. Cost estimates vary substantially ranging from as low as 20 US$ tCO2−1 to as high as 1000 US$ tCO2−1 and it is highly likely that air capture will offer one of the most expensive options for mitigating climate change. For this reason, other, cheaper options for addressing climate change such as reducing the carbon intensity of electricity generation through efficiency savings in existing power plants, increased deployment of renewable energy technologies, nuclear power and CCS should be aggressively pursued before air capture is considered. Ultimately, commercial deployment of air capture technology will depend on whether the technology can be proven on a large scale and at a cost that makes it profitable to do so. For this to be realised, the future carbon price must also be high, i.e. more than the cost of extracting and storing atmospheric CO2.
10. Retrofitting CCS to power stations – the case for flexible operation
10.1 Introduction
The aim of this section is to review the recent literature pertaining to the retrofitting of post-combustion CO2 capture technology to fossil fuel-fired power stations and then discuss the impact of this retrofit on the merit order of such a decarbonised power station. The temporal, economic and policy context of this discussion is in that of the UK in the 2030s where the current electricity market reform445 (EMR) discussion has been completed and there are significant amounts of intermittent renewable power446 in the UK energy system. One important target is that of having 15% of the UK's energy supplied by renewable resources by 2020.446At the time of writing (early 2013), the UK is undergoing an EMR exercise which is intended to create a policy environment conducive to sufficiently de-risk the capital investment associated with the installation of new power generation capacity to support investment by the international capital markets in UK power generation, and avoid the foreseen energy gap if this investment is not made.
From the perspective of a potential investor, risk is associated with the probability and magnitude of an unfavourable outcome (e.g. profit below expectations). Taking profit as simply the difference between annualised revenue and cost, in the context of a power station, and it is a function of:
• Electricity price (feeding into a revenue stream).
• Load factor and dispatch frequency (how much and how often a power plant can sell energy to the grid).
• Annualised capital cost (a function of the initial installed cost, payback time and discount rate).
• Fuel cost.
• Carbon cost.
However, given that one will typically select a payback time and discount rate such that 80% of the original investment is paid back within 10 years,447,448 carbon prices are likely to be mandated by the EMR (at least in the UK, although there may be some market element to this as well) and electricity prices are essentially pegged to fuel prices (gas in the UK), the main sources of risk associated with investing in a power plant, with or without CCS, are the load factor, dispatch frequency, fuel and carbon costs. It is, therefore, evident that the least risky option will be to invest in a power station that is fuel flexible, has low greenhouse gas (GHG) emission per unit of output and can operate in a flexible (i.e. load following) manner. The authors would emphasise that this rationale (fuel and operational flexibility) should hold for investment into any fungible energy network, i.e. the arguments presented herein are held to be equally applicable to any energy system comprising diverse generation sources.
The remainder of this section is laid out as follows; we first provide a high level description of post-combustion CO2 capture processes, sub- and super-critical coal-fired power stations and of a gas-fired power station. We then go on to describe the optimal means for the integration of the capture and power plants and discuss some operational strategies that are held to minimise the operational risk associated with these systems.
10.2 Description of sub-systems
In this section, we provide high-level descriptions of the various unit operations and sub-processes which come together to compose a decarbonised power plant.Amine-based CO2 capture processes comprise two distinct unit operations—absorption and desorption (or solvent regeneration), for further details, see the section above on solvent absorbtion.
| Pressure, P, (bar) | Temperature, T, (°C) | Enthalpy, H, (kJ kg−1) | |
|---|---|---|---|
| HP in | 130.00 | 535.0 | 3430 |
| HP out | 38.00 | 351.2 | 3100 |
| IP in | 35.00 | 535.0 | 3530 |
| IP out | 2.50 | — | 2861 |
| LP in | 2.50 | — | 2861 |
| LP out | 0.08 | 41.5 | 2366 |
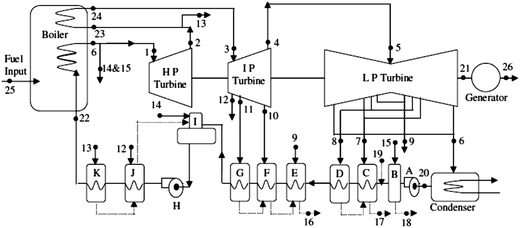 | ||
| Fig. 12 Schematic of sub-critical power station. From Asthana and Panigrahi.449 | ||
It is evident that the specific enthalpy in the steam in the HP, IP and LP are not equal (ΔHIP > ΔHLP > ΔHHP). This is important when calculating the opportunity cost associated with steam extraction for solvent regeneration.19
10.3 Integration strategy
In integrating the power and capture plants, at the simplest level of integration, one simply needs to connect the exhaust gas stream from the FGD process with the inlet to the absorption process. This will require passing the exhaust gas through a fan, in order to add a small amount of pressure (>0.02 MPa), to ensure that the exhaust gas has sufficient mechanical energy to overcome the pressure drop associated with the absorption column.As discussed previously, the CO2 rich solvent needs to be heated in order to recover the CO2 and reuse the solvent. This requires the application of appreciable quantities of energy, QRegen. This energy may be, in turn, partitioned into contributions required to heat the solvent, QSens, and that required to break the chemical bonds between the CO2 and the amine solvent, QChem. In part, this energy penalty is offset via heat exchange between the hot, lean solvent exiting the reboiler and the cold, rich solvent exiting the absorber. This occurs in the so-called “rich-lean heat exchanger”, or RLHX. The temperature of the rich solvent stream exiting the RLHX is clearly a function of the available heat transfer area, thermal driving force and the efficiency with which the RLHX operates. However, an average exit temperature for the rich solvent stream would be approximately 87 °C.451 This stream will still require 3.8–4.2 GJ tCO2−1 recovered as energy input.
Some of the early integration studies proposed the addition of a separate natural gas ancillary boiler to provide steam for solvent regeneration on a post-combustion capture retrofit on a coal power plant,452 as is common practice for natural gas treating plants. However, this option was found to have a relatively low efficiency and was subsequently abandoned as a viable option in decarbonising power plants. Subsequently, direct extraction of steam from the steam cycle of the main power plant has become the preferred option. Owing to the desired conditions within the reboiler (T = 120 °C, P = 0.2 MPa), this energy is best obtained via condensation of saturated steam at P ≈ 0.25 MPa. In the case of a sub-critical coal-fired power station, it has been shown that the optimal location for the extraction of this steam from the steam cycle is between the IP and LP turbines.453 As before, one can partition the contributions to QRegen into that obtained by the condensation of steam, QCond, and that obtained by sensible heat transfer by sub-cooling the condensate, QSub-cool. Once this steam has been condensed, the resulting condensate is then returned to the steam cycle. It has been shown19 that the fraction of QRegen obtained from QSub-cool is negligible. Thus, the condensate should, therefore, be returned with the minimum degree of sub-cooling in order to avoid an additional penalty on the power plant associated with returning large quantities of sub-cooled liquid to steam cycle. Specifically, the condensate should be returned to the condensate heating train, as opposed to the power cycle condenser.453 More sophisticated approaches to heat integration between power and capture plants and the CO2 compression train have also been investigated, for example, a recent contribution by Duan et al.454 shows how this can appreciably reduce the total energy penalty associated with the decarbonisation of the power plant. In particular, the recovery of heat from the inter-coolers of the compression train was found to be especially important. The vast majority of integration studies concentrate on the modification of the solvent phase, either by designing new solvents or by carrying out heat integration and recovery studies. However, a recent paper illustrates how the humidity of the inlet exhaust gas stream at the base of the absorber will play an important role in the process operation. It would appear that a dry exhaust gas will require a shorter column than a wet gas, leading to an appreciable reduction in capital cost.18
10.4 Flexible operation of decarbonised power plants – towards risk mitigation
As discussed in the introduction, in any power generation network, there are clear benefits associated with being able to generate power in a flexible way. Furthermore, we suggest that the position of fossil fuel-based power plants in the power generation merit order is changing. With the increasing intermittency associated with the diverse energy network envisioned for the UK in the 2030's and beyond, this capacity for flexible operation will command a special premium.The concept of storing CO2-rich solvent on-site was originally proposed by Chalmers and Gibbins.455 This concept has recently been quantitatively proven456 to provide an important reduction in operating cost456 and as a buffer between the dynamic behaviour of the power plant and the required steady state operation of a CO2 transport network. In particular, Arce et al. have shown that adjusting the degree of solvent regeneration in sympathy with prevailing market prices for energy, fuel and carbon (i.e. increased solvent regeneration or a lower lean solvent loading at times of low energy prices, and reduced levels of solvent regeneration at times of high energy prices) can lead to an appreciable reduction in operating cost. Furthermore, allowing CO2 to accumulate in an on-site solvent inventory can enhance this effect. As alluded to above, this idea can be used in conjunction with super-critical power plants to enhance their flexibility, and, therefore, their profitability in a diverse energy generation system.
11. CO2 transport
11.1 Introduction
CO2 can be transported by pipeline, ship, rail or road. The choice of transport will depend on the quantity of CO2 that needs to be transported, the distance and terrain to be travelled, and the specifications of the CO2 stream produced at the capture facility.457 In most cases, transporting CO2via pipeline will be the most cost effective mode of transport. The instances where transport by ship may prove more economical would be if CO2 needs to be moved over very large distances (>1000 km) or over large bodies of water. Transport via rail or road is only expected to be feasible for moving CO2 on a small scale for specialist applications.11.2 Basic operation
Prior to pipeline transport, CO2 is compressed to a supercritical fluid (sc-CO2) or liquid state (i.e. a dense-phase fluid). CO2 exists as a supercritical fluid above its critical point, 31.1 °C and 74 bar. This is the most efficient phase for transporting CO2 by pipeline as it has both the high density of a liquid and the favourable flow characteristics of a gas. However, it is not possible to maintain pipeline temperatures above the critical temperature in all situations. It is, therefore, important to ensure pressures drops are managed and pipeline pressures are kept above vapour–liquid equilibrium conditions to maintain a single-dense-phase flow and avoid liquid slugs and other operational problems that may eventuate if conditions fall within the region where a two-phase (gas–liquid) flow may occur. Operating pressures of existing CO2 pipelines are in the range of 85 to 210 bar where CO2 is a dense-phase fluid over a wide range of temperatures. To maintain sufficiently high pressures over long distances, intermediate pumping (or booster) stations are required at certain intervals along the pipeline. For shorter distances, booster stations may be avoided by increasing the pipeline inlet pressure; however, more energy would be consumed for compression and thicker walled pipeline would be required.In terms of CO2 transport by ship, it is most efficient to transport CO2 as a cryogenic liquid. Aspelund et al. has calculated optimum conditions for CO2 transport by ship of 6.5 bar and −51.2 °C.458 Large scale liquefaction would primarily involve cooling via compression and expansion of the feed gas. Some loss of CO2 is expected as a consequence of boil off and the ship's emissions would be in the region of 3 to 4% per 1000 km.457 Losses can be minimised by utilising a refrigerated container ship or by re-capturing and liquefying the boil-off gas.
Ship-based transport systems require intermediate storage facilities, and the equipment and infrastructure for loading and unloading CO2 at the loading docks, and storage sites to link continuous production of CO2 at capture facilities with discrete transport of CO2 by ship.
Ships are more flexible than pipelines as they are able to transport CO2 in volumes far below the design capacity. Ships therefore offer the potential to collect CO2 from multiple sites on the way to the storage site and can better adapt to fluctuations in the CO2 production rate of the emitter.
11.3 Existing experience
Globally, there is approximately 6000 km of pipeline infrastructure in operation for CO2 transportation purposes, most of which is based in the US and Canada for transporting CO2 to sites for enhanced oil recovery (EOR).459 At present, most of the CO2 used for EOR is sourced from natural deposits, though there are a few projects utilising CO2 from anthropogenic sources. Table 6 provides a summary of the major long-distance CO2 pipelines currently operating. There is also limited operational experience of onshore and offshore pipelines transporting CO2, derived from natural gas production, for sequestration in saline aquifers at the Snøhvit LNG facility, Sleipner, and In Salah.| Pipeline | Operator | Location | Capacity [Mt per year] | Length [km] | Pressure [bar] | Source | Purpose | Start year |
|---|---|---|---|---|---|---|---|---|
| Canyon Reef (SACROC) | Kinder Morgan | USA | 4.4 | 352 | 140 | Gasification Plant | EOR | 1972 |
| Bati Raman | Turkish Petroleum | Turkey | 1.1 | 90 | 170 | Dodan Field | EOR | 1983 |
| Sheep Mountain North | BP AMOCO | USA | 9.2 | 772 | 132 | Sheep Mountain | EOR | 1983 |
| Cortez | Kinder Morgan | USA | 19.3 | 803 | 186 | McElmo Dome | EOR | 1984 |
| Bravo | Kinder Morgan | USA | 7.3 | 350 | 165 | Bravo Dome | EOR | 1984 |
| Central Basin | Kinder Morgan | USA | 20 | 278 | 170 | Denver City Hub | EOR | 1985 |
| Bairoil | — | USA | 8.3 | 180 | — | Gas manufacturing plant | EOR | 1986 |
| Snøhvit | Statoil | Norway | 1 | 160 | — | Separation from natural gas | Storage | 1996 |
| Weyburn | North Dakota Gasification Co. | USA & Canada | 5 | 328 | 186 & 204 | Gasification Plant | EOR | 2000 |
| In Salah | BP | Algeria | 1.0 | 17 | 185 | Separation from natural gas | Storage | 2004 |
| Sleipner | Statoil | Norway | 0.7 | 153 | 100 | Separation from natural gas | Storage | 2006 |
Ship-based CO2 transport experience is far more limited. At present, there are only four small ships, transporting food-grade CO2 in northern Europe.457 Anthony Veder Group operates the first purpose-built CO2 tanker, which can ship up to 1825 tonnes of CO2 at −40 °C and 18 barg. Yara International charters the other three ships which have capacities between 900 and 1200 tCO2−1.191,460 Work is on-going to develop ships with the capacities required for large-scale CCS deployment. Maersk are currently working on pressurised, semi-refrigerated CO2 tankers with capacities up to 45![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 tonnes.461
000 tonnes.461
11.4 Pipeline design and operation considerations
CO2 pipelines must be designed and constructed at an optimal cost in such a way that they are reliable and safe to operate, posing minimal risk to local populations and the environment.462 Pipeline design is primarily influenced by the required throughput and hydrodynamic properties of the CO2, such as density, phase behaviour, viscosity and compressibility. Therefore, factors affecting the hydrodynamic properties of CO2, including temperature, pressure, flow rate and composition, need to be modelled and characterised as part of the design process. The presence of impurities, particularly water, may also lead to operational problems concerning corrosion, gas hydrate and ice formation. As a consequence, pipeline entry specifications are set in order to minimise or even avoid these problems.The level of impurities that can be tolerated will depend on the storage method (or end use) and the transportation method. EOR specifications tend to be strict (Table 7) as certain impurities will have detrimental effects on the process. In the case of EOR, there is an economic incentive to remove certain impurities down to very low levels and design CO2 specifications for optimum oil recovery efficiency. However, transporting CO2 for storage purposes does not share this relationship and removing impurities in anthropogenic CO2 down to very low levels imposes a significant energy and cost penalty on the process. Therefore, CO2 specifications for storage purposes will most likely be determined on the basis of cost-benefit analyses, regulatory and legislative requirements, and health and safety considerations.
| Constituent | Specification | Reason |
|---|---|---|
| CO2 | >95% | MMP |
| N2 | 4% | MMP |
| Hydrocarbons | 5% | MMP |
| H2O | 480 mg m−3 | Corrosion |
| O2 | 10 ppm | Corrosion |
| H2S | 10–200 ppm | Safety |
| Glycol | 0.4 ml m−3 | Operations |
| Temperature | 65 °C | Material integrity |
A few studies have attempted to define CO2 specifications for transport and storage purposes. The Dynamis project463 updated specifications that were initially proposed for pre-combustion CO2 capture technologies as part of the ENCAP project464 to take into account safety and toxicity limits. As a consequence, allowable levels of H2S, CO, SOx and NOx gases have been lowered in accordance with their short term exposure limits (STELs). To meet these specifications, additional gas treatment measures such as regenerative absorption columns may be required in addition to the standard SOx and NOx removal (FGD, low NOx burners and SCR), increasing gas treatment energy and infrastructure costs.
Ecofys have also produced CO2 transport/storage specifications based on impurities that are likely to be present in CO2 from a coal-fired power plant.465 The Ecofys specifications are similar to those defined by the Dynamis project, although they did not define tolerance limits for SOx and NOx based on their assumption that these impurities will not cause operational problems in the absence of a separate water phase. Both the Dynamis and Ecofys specifications recommend a maximum water level of 500 ppm to avoid precipitation of a separate water phase, which has been identified as the main factor influencing corrosion, gas hydrate and ice formation.
The presence of impurities also reduces the density of dense-phase CO2, particularly at conditions close to the vapour–liquid equilibrium boundary.465,466 As a consequence, pipelines transporting CO2 containing large amounts of impurities must be operated at higher inlet pressures to achieve the desired throughput. A thicker walled pipeline or tougher pipeline material may also be required at a higher infrastructure cost.
Pressure and temperature drops within pipelines are also affected by the CO2 composition. The presence of impurities with lower critical temperatures and pressures than CO2 enhance pressure and temperature drops whilst impurities with higher critical temperatures and pressures, such as H2S, SO2 and NO2, reduce pressure and temperature drops along a set length of pipeline.467 To account for increased pressure drops, more booster stations would be required at shorter intervals to keep the pressure sufficiently high to maintain a dense-phase flow. However, adding more booster station substantially increases pipeline infrastructure costs, and in any case, this option is not feasible for subsea pipelines. Alternatively, the pipeline would have to be operated at a higher inlet pressure.
Yan et al. carried out a cost-benefit analysis to determine the effect of removing typical non-condensable impurities found in anthropogenic CO2 (N2, H2, O2 and CH4) to different levels (1%, 4% and 10%).468 They found that limiting the amount of non-condensable compounds to <4% was optimal in terms of balancing gas treatment and compression costs, although a higher limit of <10% may be acceptable for short distances.
A reasonable amount of work has been published on the corrosive properties of CO2–H2O systems at conditions relevant to natural gas pipelines; however, there is far less published research at conditions relevant to CO2 pipeline transport. Corrosion rates are dependent on the operating conditions, including pressure, temperature, water vapour content, flow rate, steel composition and exposure time. The presence of other impurities will also influence corrosion rates. Corrosion rates as high as 20 mm per year have been reported for carbon steel exposed to water-saturated CO2 at relatively high temperatures and pressures, although the literature is not in general agreement, with different authors reporting significantly different corrosion rates at quite similar conditions.469,470 The primary cause of these discrepancies is most likely due to the differences in the flow rates, exposure time, material-to-water surface areas, and steel composition used by different research institutions.
Investigations into the corrosion mechanisms have found that under stagnant conditions, corrosion rates are initially fast but decrease with increasing exposure time.471 This is due to formation of a protective iron carbonate (FeCO3) product layer/scale that precipitates out of the aqueous phase once it becomes supersaturated with iron. Product layer formation is inhibited under free flowing conditions as mobile aqueous phases are less likely to become supersaturated with iron. Furthermore, in the case where a protective layer has formed, the frictional forces of the flowing gas are likely to cause destabilisation or removal of the protective film, which could lead to severe localised corrosion.
Corrosion can still occur in the absence of a distinct aqueous phase; however, rates are much lower due to the rapid precipitation of a protective FeCO3 scale. In this case, corrosion and scale formation is most likely due to the formation of residual or transient aqueous phases, or stabilised aqueous surface films.
The presence of other impurities complicates matters somewhat as certain impurities will interact in the presence of an aqueous phase to enhance, or in a few cases hinder, internal corrosion within a CO2 transport system.472 Of particular concern are SOx, NOx and O2, which react with water to form sulphuric or nitric acid. O2 will also react with FeCO3 scales to form oxides and hydroxides which are not protective. These acids along with other acidic impurities that may be present in anthropogenic CO2 such as HCl (a common impurity in flue gases from coal power stations), will act to further reduce the pH of any aqueous phases that have formed. The solubility of FeCO3 is increased at the lower pH values, rendering the formation of an iron carbonate protective layer unlikely, thus enhancing the induced corrosion rate. Furthermore, sulphuric and nitric acids can form in the vapour phase, even at very low water vapour contents of <200 ppm, causing notable corrosion in the absence of a distinct aqueous phase.471 Ruhl et al. found that HNO3 and HCl were the most mobile in supercritical CO2, causing the highest corrosion rates. H2SO4 on the other hand, appeared to be much less mobile and did not cause significant corrosion in the absence of an aqueous phase.473 On the other hand, the presence of basic impurities such as glycols, NH3 and amines such as MEA will have the opposite effect on the pH of an aqueous phase and thus hinder corrosion. In fact, glycols are commonly used corrosion inhibitors in natural gas pipelines.
Experience from the petrochemical industry has found that H2S will also cause corrosion. At partial pressures <0.0035 bar, H2S interacts with carbon steel in the presence of an aqueous phase to form iron sulphide.465 As is the case with FeCO3, FeS can precipitate onto the surface of the carbon steel, forming a protective layer that inhibits further corrosion. However, H2S at partial pressures >0.0035 bar can cause “sour corrosion” (or sour cracking), primarily via sulphide stress induced cracking (SSIC) and hydrogen induced cracking (HIC). It has also been reported that the presence of CO in CO2–H2O mixtures can cause transgranular stress cracking corrosion. Cracking corrosion is particularly problematic as it can lead to material failure within a timescale of days.
Impurities may also affect the solubility of water in CO2. A few accounts suggest that the presence of CH4 and other hydrocarbons reduce the water solubility of CO2,474 whilst H2S has the opposite effect.463 At present, published research concerning the effect of impurities on the water solubility of dense-phase CO2 is very limited. More work is required to investigate the effects of anthropogenic CO2 impurities on corrosion and the water solubility of dense-phase CO2 at a greater range of conditions relevant to CO2 transport.
Rogers and Mayhew defined the threshold water vapour limit, below which corrosion and other water related issues are deemed negligible, as <60% relative humidity.477 Others have defined threshold water limits between 300 and 600 ppm.463,475 Kinder Morgan, the largest CO2 pipeline operator, specifies a maximum water level of 640 ppm.465 Considering water can be removed down to 400–500 ppm in vapour–liquid separator drums, a 400–600 ppm water level limit seems appropriate. Furthermore, this is well below the 60% relative humidity threshold at typical pipeline operating conditions (0–50 °C and 85–200 bar), where the minimum water solubility of dense-phase CO2 is ∼1500 ppm.478 However, it is likely that more conservative water level requirements may have to be specified when transporting CO2 containing high concentrations of NOx, H2S and CH4. Furthermore, water level specifications for ship transport will be less flexible owing to the lower operating temperature. Water level specifications will most likely remain at <50 ppm to avoid gas hydrate and ice formation during compression and liquefaction.476
Supercritical CO2 is known to be detrimental to polymers and lubricants used in pipeline components, such as valves, o-rings, gaskets and coatings. It diffuses into the polymer, which expands when the pressure is reduced, blistering the material. As the blistering worsens, components may fail, which in the case of seals could lead to rapid release of CO2 into the surrounding atmosphere. The polymeric components used in smart pigs are also affected, making in situ monitoring and cleaning of CO2 pipelines very difficult. In the period up until 2008, there were only two instances in North America where smart pigs survived in situ monitoring and/or cleaning operations in CO2 pipelines.459 Alternatively, more durable polymers such as Teflon and Viton can be used to reduce the problem; however, the costs are greater and degradation is not eliminated completely. Further research is required to develop polymeric materials and lubricants that are resistant to the super-solvent effects of supercritical CO2.
12. Geological storage of CO2 by injection into deep porous rock
While CO2 capture is likely to represent the major cost—in both money and energy—of the whole CCS process, CO2 storage poses a great deal of uncertainty. This is uncertainty in the quantification of storage potential, in the confirmation of outline assessments to a standard suitable for investment, the tracking verification and monitoring of injected CO2, and finally the fail-safe retention of CO2, so that a storage site can be transferred to government as a low-risk proposition for long-term care and maintenance. There are significant engineering challenges to ensure that the injected CO2 remains in the subsurface for hundreds or thousands of years. The CO2 is injected at high pressures deep underground; the principal storage sites are saline aquifers, depleted oil and gas fields, and deep coal seams. Most assessments of storage capacity consider that saline aquifers have the largest storage potential, while oil and gas fields offer the economic incentive of additional hydrocarbon recovery when the CO2 is injected. CO2 injection is routine in the oil industry for improved recovery with many projects around the world, while CO2 storage itself has been successfully implemented at several sites. The best publicised example is at Sleipner, offshore in the Norwegian North Sea, where around 1 Mt per year of CO2 separated from produced condensate hydrocarbon has been injected each year since 1996, to avoid the payment of a carbon tax.There are four principal mechanisms by which the CO2 remains underground: physical trapping below impermeable or low-permeability rock, such as shale; dissolution trapping, where the CO2 dissolves in brine—this CO2-laden brine is dense and tends, slowly, to sink through the storage aquifer; mineral trapping, where CO2 reacts with the host rock precipitating carbonate; and capillary trapping where—at the trailing edge of the CO2 plume—CO2 can be trapped as pore-space bubbles in the pore space. Fig. 13 illustrates this process schematically:480 at the regional scale, several tens of Mt of CO2 will be injected each year, leading to a plume in the subsurface that will extend many km. The injection has to be carefully monitored and controlled to prevent excessive rises in fluid pressure that could fracture the rock and produce leakage pathways to the surface. At the small scale, capillary and dissolution trapping lead—over time—to increased storage security.
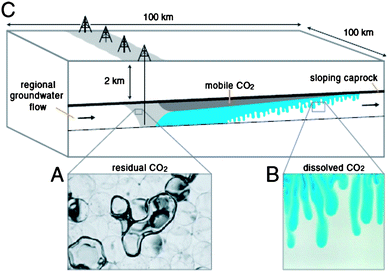 | ||
| Fig. 13 A schematic of CO2 storage and trapping mechanisms.480 Here extensive storage of tens of Mt of CO2 per year from several power stations and other industrial plants is considered. The CO2 is injected through an array of wells that penetrate deep in the subsurface. The CO2 and the associated pressure increase has a footprint underground that may extend 100 km or more. The effectiveness of long-term storage is controlled by limiting the pressure increase to avoid fracturing of the rock that could lead to leakage, and the interplay of trapping mechanisms at the small scale. Capillary trapping (A: residual CO2 in the pore space at the scale of around 100 μm) and dissolution (B) are illustrated here. Capillary trapping occurs at the trailing edge of the CO2 plume, where brine displaces CO2. This limits the spread of CO2 in its own phase. Dissolution occurs throughout the plume: the CO2-saturated brine is dense and sinks. Both processes prevent the escape of CO2 to the surface. | ||
The main research in the storage area has been principally devoted to three types of study: investigating in detail the different trapping mechanisms outlined above, understanding through analytical or simplified models the likely migration of CO2 injected into the subsurface, and detailed assessments of safe storage capacity in different geological and industrial settings. Rather than attempt to list this vast literature in a very brief overview, we will highlight some important work on these topics to illustrate recent activity and highlight the progress that is being made towards the understanding and design of effective CO2 storage.
12.1 Capillary trapping and multiphase flow
Fig. 14 shows the results of micro-flow experiments at typical aquifer storage conditions (9 MPa fluid pressure and a temperature of 70 °C) where the CO2 is a supercritical fluid. Trapped CO2 is imaged in the pore space with a resolution of approximately 10 μm. The experiments confirm that indeed a significant fraction of the pore space (25% in this case) can contain disconnected ganglia of CO2 surrounded by water that cannot move further. These results have been confirmed by traditional core flood experiments on larger rock samples.481–483 Over time the CO2 may dissolve, but it cannot escape. At the field scale, this mechanism severely limits the spread of the CO2 plume.
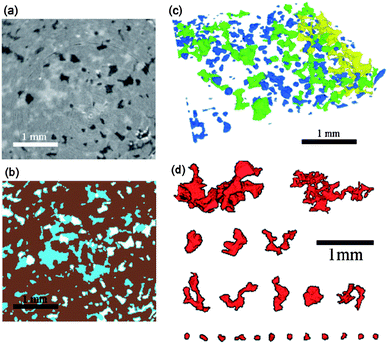 | ||
| Fig. 14 Pore-scale images of trapped CO2 in sandstone.571 (a) This is a two-dimensional cross-section of a three-dimensional image showing in grey scale the rock (grey), CO2 (light) and brine (dark). The segmented image, used to identify the fluid phases, is shown in (b). The trapped CO2 ganglia in three dimensions are shown in (c). The colours indicate the size of the clusters. A collection of trapped clusters, illustrating the wide range of size and shape is shown in (d). | ||
It is not just the amount of trapping, but also the multiphase flow of CO2 in the presence of brine that is important when attempting to predict and design CO2 injection. This is quantified through the relative permeability: it measures the flow conductance of a phase as a function of the fraction of the pore space it occupies (the saturation). Fig. 15 shows a compilation of relative permeability measurements of a supercritical CO2–brine system.482 It shows the relative permeabilities for CO2 injection (CO2 displaces brine) to some maximum CO2 saturation, followed by CO2 displacement by brine, leaving a trapped saturation of CO2—that is, the relative permeability of CO2 is zero even though the CO2 saturation is finite.
 | ||
| Fig. 15 Relative permeability curves – the fractional conductance for flow – as a function of brine saturation.482 A compilation of experiments are shown for CO2 injection into brine to a given saturation, followed by displacement of CO2 by brine. The curves have a zero value even for finite CO2 and brine saturations, indicating trapping. These curves are used to predict the movement of CO2 at the large scale in the subsurface. | ||
These curves can then be used for a quantitative, predictive assessment of the extent and speed of CO2 movement in the subsurface.
12.2 Regional assessments of storage capacity
To make a significant contribution to reducing atmospheric emissions, it will be necessary to store several Gt of CO2 each year worldwide, and many Mt in large regional aquifers in areas with significant industry and fossil-fuel power generation. Assessment of the storage capacity takes into account the factors mentioned above: the likely increase in pressure, CO2 movement and trapping processes. These are normally incorporated into analytical or numerical models to estimate how much CO2 can be safely stored and the likely extent of CO2 migration in the subsurface.As an example, Fig. 16 shows the estimated storage capacity in large regional aquifers in the continental US.480 A total capacity of over 100 Gt is calculated, sufficient to make a major contribution to mitigating CO2 emissions in North America. The methodology combines an assessment of both the storage capacity and how fast the CO2 can be injected. However, the actual capacity in CO2 storage reservoirs at present remains essentially unvalidated, as we discuss next.
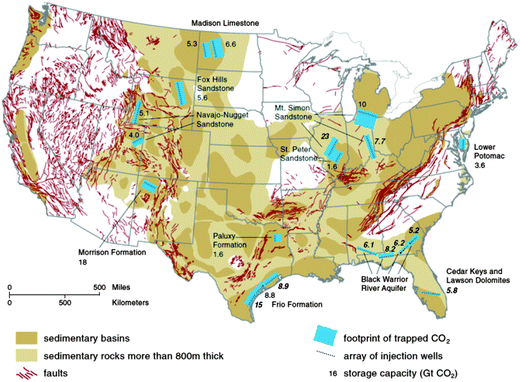 | ||
| Fig. 16 A US-wide assessment of CO2 storage capacity.480 Analytical models have been combined with regional geological models to estimate a total storage capacity of over 100 Gt in the continental US. | ||
Additional complexities can arise when licensing and regulation activities collide with natural subsurface geometries of reservoir and seal.491 Regulators and lawyers are concerned to avoid damage or trespass into adjacent subsurface property, whilst also attempting to ensure maximum certainty in identifying injected CO2. In many states of the USA, this may be resolved by the acquisition of rights to pore space utilisation across surrounding properties, although it remains unclear if this will be to protect against a simple case of physical CO2 movement, or will the much greater geographical area of pressure increase be regarded as adversely affected? Different European states have different historical approaches. For example, in Germany, different regions are bound by ancient mining rights, such that even the federal state cannot interfere. By contrast, the UK has taken a centralised approach where the rights to all pore space are managed for the Crown Estate. However, federal European law has not helped, in that the European Emissions Allowance Directive defines a very local storage site, consisting of the defined reservoir and caprock. By contrast, the European CCS Directive allows definition of a much greater subsurface volume, which can contain multiple layers of reservoirs and multiple seals. Unplanned CO2 migration from a European Emissions Allowance reservoir need not be a leakage from a CCS Directive complex. These legal approaches will need to be harmonised during the progress of early CCS injection demonstrations.
Zoback and Gorelick496 inferred an increased risk that seismicity will be induced, especially onshore, by injection of large volumes of CO2. These earthquakes, it is claimed would cause multiple storage sites to rupture and leak CO2. However, this has been widely critiqued on the basis that (i) licensing of a storage site before injection and monitoring after injection will eliminate known tectonic sites and (ii) will detect anomalous small tremors before buildup to larger events, (iii) that a pressure anomaly can be managed by water production, (iv) that physical leakage of CO2 as a consequence of seismicity would only occur if the tremor site coincided in space and depth with the physical CO2—which is very unlikely, (v) the pressure pulse anomaly will decay within 50 years from the time of peak CO2 injection rate.
From a practical point of view, it still remains very unclear how large-scale CCS storage reservoirs may be evaluated and tested. Initial experimental projects, even on industrial scale, inevitably choose high-quality reservoirs; assessing the regional impact of injection, and assessing the interaction of multiple injections into the same reservoir remains a problem with no clear solution. This causes some analysts to propose that large-scale, high-quality investigations of saline aquifer regional geology will be required before any licensing can occur.497 Clearly this can take 5 or even 10 years, and may cost many tens, probably hundreds, of millions of dollars. If undertaken in a failsafe and stage-gated process, such investigations could act as a terminal slowing of the rollout for commercial CCS. This remains a real problem, as there are numerous unpublished examples of commercial investigations worldwide for CO2 storage which have failed to meet the required performance targets and have resulted in the cancellation of project developments. Determination of adequate storage, suitable for the CO2 tonnages envisaged during the entire power-plant lifetime, is likely to be the rate determining step for CCS worldwide. Innovation is badly needed in the technical evaluation of storage, linked to suitable business models and regulatory permissions.
Even with these practical difficulties of emissions offsetting, it may still be worth undertaking CO2-EOR as a stepping stone to rapid building of large numbers of capture plants connected to pipeline networks, connected to multiple storage sites which will reach their full potential after the additional oil production is exhausted.
12.3 Conclusions (CO2 storage)
The overwhelming consensus is that large-scale storage of CO2 is feasible, with storage security increasing over time. The main concern is to ensure that the fluid pressure does not increase sufficiently to induce fracturing, and to ensure that the mobile CO2 does not find a permeable path to the surface. Over time—with capillary, dissolution and mineral trapping—storage becomes more secure and the CO2 less likely to escape. It is wrong to think of the CO2 as having some typical storage time or leakage rate: the (low) risk of leakage occurs mainly during the injection period and declines with time as pressure dissipates and the CO2 becomes less mobile. Even if natural seals are breached, then leakage rates in natural examples are slow and impact at the surface is small. The development of CO2-EOR projects may accelerate the development of efficient capture engineering but will do little for net CO2 reduction over the life cycle of a project. Benefits from those projects may be a legacy of pipeline to access abundant proven storage sites.CO2 storage can be engineered to deal with potential problems. If injectivity is poor, new wells can be drilled; water can be abstracted from the subsurface to relieve pressure, or re-injected to promote capillary trapping. Diverse objections raised against storage, suggesting uncontrolled pressure increase, or induced seismic tremors leading to extensive leakage, are potential difficulties which can be managed with known techniques. CO2 storage is not a passive process but one that with responsive monitoring and engineering can be achieved at scale, efficiently and securely. More work is needed on how to provide sufficient confidence in storage site evaluation, to positively inform the process of starting a CCS power-plant development. Additionally, there is a need to produce greater certainty in the fail-safe retention of CO2, which is shown both by trapping processes measured at laboratory scale and by calculations from natural analogues.
13. CO2 sequestration via ex situ mineral carbonation
13.1 Background
Ex situ mineral carbonation is a suggested CO2 sequestration option for geological storage. The process involves carbonating materials containing alkali or alkaline earth metal oxides or hydroxides, fixing captured CO2 as thermodynamically stable and environmentally benign carbonate minerals (eqn (13) and (14)). Storage is considered permanent so no post-storage monitoring would be required.457,499 In addition, the carbonation process is exothermic, so the theory is that the process could be utilised to produce useful energy if heat is released at high enough temperatures. There is enough alkaline earth metal oxide-containing material on earth to sequester all of the CO2 that could ever be emitted from fossil fuel use.457 Mineral carbonation also provides an option for storing CO2 at locations without access to geological storage sites.| CaO(s) + CO2(g) → CaCO3(s), ΔH = −179 kJ mol−1 | (13) |
| MgO(s) + CO2(g) → MgCO3(s), ΔH = −118 kJ mol−1 | (14) |
A feasibility study carried out by O'Connor et al.504 estimated that an industrial-scale mineral carbonation operation based on the NETL process would impose a 30–50% energy penalty on the power generation process; 75% of this penalty was attributed to grinding the feedstock to <37 μm. On the basis of these calculations, sequestration costs alone were estimated at 50–100 US$ tCO2−1, an order of magnitude higher than the current cost of geological storage at 5–10 US$ tCO2−1.437 O'Connor's costings are still used as the official best-case scenario estimated costs for mineral carbonation by many of the important organisations and agencies advising on climate change and mitigation strategies, such as the IEA and IPCC.437,457
Pilot-scale testing of this technology is likely to follow in the near future.508 However, like fly ash and indeed most other potential waste-derived feedstocks, the CO2 storage potential of iron and steel slags is low—estimated at up to 170 MtCO2 per year509 compared with total global CO2 emissions from the iron and steel sector of 2.3 Gt per year.510
14. Carbon dioxide re-use
14.1 Background
Carbon dioxide re-use (CDR) is a purported alternative to storage that involves the production of saleable products from captured CO2. CDR includes the use of CO2 as a technological fluid and as a reagent for the production of chemicals (CO2-to-chemicals), plastics (CO2-to-plastics), or fuels (CO2-to-fuels). The combined system of CO2 capture with CO2 re-use is typically referred to as carbon capture and utilisation (CCU). In a few cases, CDR can result in some permanent storage and removal of CO2 from the carbon cycle (e.g. enhanced oil recovery or mineral carbonisation); however, in most cases, CDR will result in re-emission further down the line (e.g. the use of CO2 as a technological fluid or as a precursor for fuel production). In the latter case, the lifetime in which CO2 is removed from the carbon cycle will vary: some uses, such as the use of CO2 as a fuel precursor, are very short term (days to months); whilst others, such as its use as a precursor for plastics, have a longer term. In fact, the use of CO2 as a precursor for some plastics may result in the CO2 being fixed away from the atmosphere for decades and can, therefore, be considered a form of storage.The primary advantage of CDR compared with CCS is that its end product is of value. It has been argued that increased deployment of CDR processes will therefore drive up the market price of CO2, incentivising development and deployment of CO2 capture technologies. CCS on the other hand requires market intervention by governments through the application of strict penalties or economic support and subsidies to achieve the same result. However, the argument that CDR will meaningfully increase the price of CO2 fails to take into account the vast supply of CO2 and the comparatively small demand for CO2.
14.2 Current status of CDR technology
Despite the fact that CO2 is a renewable, widely available, low-cost and low-toxicity C1 feedstock, current industrial demand is relatively low, amounting to around 232 Mt per year, with only a few commercial processes currently using CO2 as a raw material (Table 8). Most of the current demand is met by naturally derived CO2 with only 40 Mt obtained from anthropogenic sources of which 70% is used for EOR purposes and another significant fraction used for urea production.511 At present, the market for CO2 is several orders of magnitude smaller than the amount of CO2 released into the atmosphere each year from anthropogenic sources and approximately 60 times smaller than the amount of CO2 emitted from large point sources (∼14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 Mt per year).512 The following sections provide a brief overview of the current status of CDR technology.
000 Mt per year).512 The following sections provide a brief overview of the current status of CDR technology.
| Process | Industrial volume Mt per year | Global CO2 usage Mt per year | Lifetime of storage |
|---|---|---|---|
| a Whilst EOR offers the potential of permanent storage, most of the CO2 used for EOR is currently not stored. | |||
| Urea | 159.4 | ∼119.6 | Months |
| Methanol | 55 | 14 | Months |
| Inorganic carbonates | 80 | 30 | Decades – permanent |
| Organic carbonates | 4 | 0.2 | Decades |
| Technological | 10 | 10 | Days to years |
| Food | 8 | 8 | Days to years |
| EOR | 50 | 50 | Permanenta |
| Total | — | 232 Mt | |
EOR offers one of the largest potential markets for CO2. In a recent report, Advanced Resources International estimated that at least 8 billion tons of CO2 could be sequestered using EOR in the US alone.511 This, in turn, would produce between 4 and 47 billion barrels of additional domestic resources. Given that the US has only 1.6% of the World's proven oil reserves, there is potential for significant growth in CO2 use and subsequent sequestration via EOR, particularly as oil production declines in existing wells in the Gulf States. Despite this, the future of EOR is uncertain. High CAPEX and OPEX costs and uncertainty over long-term oil prices, unclear and ill-defined regulations governing EOR activities, and wavering public support have all impeded deployment of EOR.514 Furthermore, offshore EOR has yet to be proven. Over recent years, a number of fields in the North Sea have been assessed as potential sites for EOR by Shell, BP and Norsk-Hydro and have failed commercial hurdles due to high offshore platform retrofit costs, cash flow issues, and alternative, cheaper options for maintaining oil production in depleting reservoirs, such as by drilling additional targeted and deviated bore-holes. EOR also competes with other methods of enhanced resource production, such as unconventional gas and tight oil, coal-to-liquids (CTL) and gas-to-liquid (GTL) technologies.
CO2 to chemicals. There are a vast amount of different chemicals that in theory could be produced using CO2 as a C1 feedstock; however, many would either prove impractical to produce from CO2 on an industrial scale or have limited market potential. Scheme 3 outlines a number of promising CO2-derived synthetic targets and their current global market. Of particular interest are alkylene carbonates and polycarbonates, which have current global markets of several hundred kt per year515–517 and 4 Mt per year3 respectively, inorganic carbonates (∼60 Mt per year),518,519 urea (∼160 Mt per year);520 polyurethane (∼18 Mt per year),521 and acrylic acid and acrylates (10 Mt per year).522
 | ||
| Scheme 3 Chemical transformations of CO2 into synthetic targets with large current or potential markets (adapted and updated from Mac Dowell et al.3 | ||
According to the International Fertiliser Association, current global production of urea is 159.4 Mt per year.520 Given that between 0.735 and 0.75 tonnes of CO2 are consumed per tonne of urea, CO2 consumption as a consequence of urea production is around 119.6 Mt per year. The overwhelming market for urea is that of the fertiliser industry, which consumes over 80% of total global urea production.3
The climate change mitigation potential of ramping up urea production is poor. As discussed by Fennell,503 the storage of CO2 within urea is short, since the chemical breaks down upon application as fertiliser, releasing the CO2 into the atmosphere.523 In addition, N2O emissions, of which fertiliser use is the main source, correspond to around a third of anthropogenic N2O emissions, further reducing the case for increasing urea production as a means for stimulating the development and deployment of CCS/CCU technology.
14.3 CO2-to-fuels
The production of fuels from CO2 is ostensibly an attractive goal, given that the global fuels market is roughly two orders of magnitude greater than that of chemicals. In order for the production and use of CO2-derived fuels to contribute to climate change mitigation efforts, the energy requirements must be supplied from non-fossil sources, i.e. RETs or nuclear. The idea is that the application of such technology may provide a way of storing excess electrical or intermittent electricity production. The fuels produced could be used to fire a power plant, generator or fuel cell during periods when RETs relying on intermittent energy sources (such as the sun or wind) are not able to meet demand; alternatively, the fuels could be used for mobile or distributed applications. Furthermore, the production of easy to transport fuels from CO2, utilising renewable energy at a remote location (such as geothermal or solar) might prove a more convenient and cost-effective way of delivering otherwise stranded resources to market than constructing transmission lines or finding other potential uses for such resources.CO2 is the lowest energy state of any binary neutral carbon species and the ultimate product of energy-releasing hydrocarbon combustion and metabolic pathways. Therefore, a significant energy input is required to overcome the substantial thermodynamic and kinetic barriers of converting CO2 into a useable fuel. When considering the energetics of CO2 activation, only a very few synthetic fuel targets can possibly be considered viable, these targets include syngas, methane, methanol and formic acid.3
Methanol can be produced via the catalytic hydrogenation of CO2 utilising similar conditions catalysts to those used for the production of methanol in the conventional commercial approach.524 In this case, the use of hydrocarbons can be avoided by using H2 from renewable sources, i.e. water splitting via electrolysis. However, the drawbacks with this approach are that equilibrium yields are much lower compared with methanol production from syngas. In addition, a third of the hydrogen is converted to water; this process is inefficient, particularly when considering that H2 production via electrolysis of water is a very energy intensive process. Furthermore, both methods of producing methanol consume a great deal of thermal energy, and the lifecycle efficiency, particularly considering that the original source of energy for the production of H2 is electricity, is exceptionally low. It has been argued503 that pumped storage of the electricity, combined with the use of electric vehicles is around five times more efficient in terms of miles driven than using the electricity to produce hydrogen, then methanol, then using this to run an internal combustion engine.
14.4 Future outlook
At present, utilisation of CO2, particularly CO2 from anthropogenic sources, is low. The use of anthropogenic CO2 in place of CO2 derived from natural deposits will offer small emissions reductions, although in the context of climate change mitigation, the impact will be insignificant. In the short term, increased deployment of EOR has the potential to offer the largest economic stimulus for large point sources to capture and subsequently store their CO2. However, a clear framework for regulating and incentivising wide-scale deployment of CCS and EOR that also addresses potential liability issues associated with EOR coupled with permanent storage needs to be established. Further work is also required to assess the feasibility and any potential deployment issues associated with EOR and permanent storage particularly at off-shore locations.CO2-to-fuels technology is far from commercial status and will have to compete with other unconventional methods of producing liquid fuels, such as GTL and CTL in addition to other means of storing and utilising energy such as high efficiency batteries and ultracapacitors. The future of CO2-to-fuels technology, therefore, remains highly uncertain because of the inherent thermodynamic efficiency penalties.
15. Policy design and implications for investment
15.1 Introduction
The preceding sections of this paper have discussed the range of CCS technologies under development. Whilst it is perhaps too early to say which set of technologies will come to dominate the field, it is certainly the case that any CCS technology will require policy support to ensure deployment at the scale and volume required to deliver on climate change goals.525 This section reviews the recent history of policy support for CCS, focusing on the power generation sector in the UK and EU, and discusses the continuing policy challenges which are faced, together with the implications for potential investment in CCS projects. The focus reflects the key role that decarbonisation of the electricity generation sector is expected to have in meeting CO2 emissions reduction goals, the ‘leading role’ that the UK is taking with a package of measures intended to support CCS projects both in, and beyond, the demonstration phase, together with the EU-wide policies to support demonstration projects.526It is not the purpose of this section to review estimated costings for different technologies, since this subject is highly contestable and frequently quite subjective. A recent review of costs in industrial settings deals with this subject527 and an excellent summary of recent pilot and large-scale costing data is available from the Global Carbon Capture and Storage Institute on a yearly basis.528
16. A recent history of UK and EU CCS policy
In 2007, the UK Government launched a competition for demonstrating post-combustion capture on a coal-fired power station, to be operational by 2014, aiming to ‘make the UK a world leader in this globally important technology’.529,530 Two of the applicants for the competition were awarded funding for Front End Engineering and Design (FEED) work. The 2010 Spending Review confirmed that Government would provide up to £1 billion for the successful project, but on the same day as this announcement was made, one of the remaining applicants withdrew from the competition on the grounds that the economic conditions were not right, leaving only one remaining applicant—a post-combustion retrofit to part of ScottishPower's Longannet coal-fired plant. During the same year, the UK Government reaffirmed its commitment to a further three CCS demonstration projects, and completed a market sounding exercise to ‘help the department to explore workable options for the CCS demonstration project selection and funding processes, and learn about projects being considered by industry’.531 A key development was the decision to make gas fired generation eligible for the competition, following recommendations by the Committee on Climate Change.532It was originally intended that funding for the further three projects would be financed by a levy on consumer bills, but this levy was later shelved. Longer term, the CCS funding mechanism is bound up in the Electricity Market Reform (EMR) process.533 At the EU level, it was anticipated that the New Entrant Reserve funding (commonly referred to as the NER 300) would be available from the auctioning of 300 million EU ETS allowances, at the time expected to raise between €4.5 and €9.0 billion in total with a substantial fraction of this to be available for CCS projects across the EU. To be eligible, all prospective NER 300 projects are required to secure 50% co-funding from other sources.534
In December 2010, the UK Government launched a consultation on the EMR which set out a proposed package of policies to ‘ensure that low-carbon technologies become a more attractive choice for investors, and adequately reward back up capacity to ensure the lights stay on’. These reforms were driven by Government's belief that ‘the current market will not deliver on the Government's objectives for decarbonisation, security of supply or affordability for consumers’.535 The four key EMR mechanisms are:
(1) A Feed-in Tariff (FiT) to stabilise and top-up the revenues of low-carbon generators including CCS, transferring electricity price risk from generators to consumers, through a Contract for Difference (CfD).
(2) A carbon price floor to reduce uncertainty for investors and incentivise low-carbon generation by topping up the EU ETS carbon price.
(3) An Emissions Performance Standard (EPS) to put an annual limit on the amount of CO2 that a plant can emit, equivalent to 450 gCO2 kW h−1 for plant operating at baseload, thereby effectively prohibiting new unabated coal-fired plant but new allowing new unabated CCGT plant.
(4) A capacity mechanism to ensure that there is sufficient generating capacity to meet peak demand.
The EMR consultation process was followed by a White Paper during 2011536 and in 2012 by a draft Energy Bill537 which set out the legislative framework for the proposals. In the meantime, however, ScottishPower pulled out of the first demonstration plant competition in October 2011, with the UK Government citing increased costs and the inability to reach a commercial agreement as the reasons. At the same time, it was confirmed that the £1 billion of public funds set aside for the first demonstration would be ‘available for a new process’.538
This new process was launched as a ‘Commercialisation Programme’ and involves a competition through which successful applicants will receive direct funding from the £1 billion budget and also the possibility of further revenue-based support under the CfD FiT mechanism proposed in the EMR. Applicants for funding from the new process must be able to demonstrate at commercial scale and be operational by 2016–2020.539 The separate process by which the EC selected projects for funding under the NER 300 scheme continues in parallel to the UK Government's Commercialisation Programme. However, under the first round of the NER 300, no CCS projects were granted funding, though funding is still potentially available in the second round. The level of funding available through the NER 300 process will be considerably less than was originally hoped because the market price of EU ETS allowances is well below the level envisaged when the process was set up. The EC currently anticipate that the total level of funding from the process will be between €1.3 billion and €1.5 billion, with funding for any individual project capped at 15% of the total, meaning a maximum of €292–337 million for any one project.
At the time of writing (May 2013), the UK Government has announced the two preferred bidders for the Commercialisation Programme competition. These are the White Rose project, an oxyfuel-based project based at Drax, North Yorkshire and proposed by a consortium of companies including Alstom, Drax and BOC, working closely with National Grid and who together form “Capture Power”;540 and the Peterhead project in Aberdeenshire, Scotland, involving Shell and SSE. Both projects are currently performing front-end engineering design studies. Table 9 details projects which were shortlisted for funding by both the EU and UK CCS competitions.
| EC NER 300 CCS projects (ranked order) | UK CCS projects (alphabetical order) | |||
|---|---|---|---|---|
| Project | Type, fuel | Country | Project | Type, fuel |
| Don Valley Power Project | Pre-combustion, coal | UK | Captain Clean Energy Project | Pre-combustion, coal |
| Belchatow CCS Project | Post-combustion, coal | Poland | Peterhead Gas CCS Project | Post-combustion, gas |
| Green Hydrogen | Industrial application, gas | Netherlands | The Teeside CCS Project | Pre-combustion, coal |
| The Teeside CCS Project | Pre-combustion, coal | UK | White Rose Project | Oxyfuel, coal |
| UK Oxy CCS Demo (White Rose Project) | Oxyfuel, coal | UK | ||
| C.GEN North Killingholme Power Station | Pre-combustion, coal, petcoke, biomass | UK | ||
| Zero Emission Porto Tolle | Post-combustion, coal, biomass | Italy | ||
| ULCOS-BF | Industrial application, gas | France | ||
| EC NER 300 Reserve list | ||
|---|---|---|
| Getica CCS Demo Project | Post-combustion, coal | Romania |
| Peterhead Gas CCS Project | Post-combustion, gas | UK |
16.1 Implications for investment in CCS
The generic investment challenges faced by renewable energy and nuclear power plants operating in liberalised energy markets are well understood. The combination of high capital costs and very low operating costs means that such plant are typically ‘price takers’ because once constructed, it typically makes most sense to run them whenever they are physically able to do so, almost regardless of electricity prices. It is conventional gas and coal-fired plant that have the ‘price maker’ role and have the dominant influence over electricity prices as they are generally able to remain profitable over a wider set of operating regimes and pass variations in fuel costs through to consumers.541 In practice this means that potential investors will tend to prefer low capital cost conventional gas-fired power station projects with operating costs linked closely to electrical output, even if the lifetime levelised costs of electricity from higher capital cost projects are similar.541CCS introduces another set of challenges because it also carries relatively high fuel and operation and maintenance costs, a carbon cost for the residual CO2 emissions which cannot be captured, and a potential long-term liability associated with the stored CO2. Whilst the support offered through the FiT mechanism proposed in the EMR suits the characteristics of low operating cost plant such as nuclear and wind power, the fuel costs associated with CCS plants suggest that it may require a premium payment that is linked to those fuel costs.
The relatively high variable costs of CCS (when compared to nuclear or wind power for example) mean that CCS plants can generally be expected to have a lower position in the electricity market merit order, which may lead to lower load factors for some CCS plant. This ‘load following’ role in the UK electricity market has typically been filled by a combination of relatively new, low-capital-cost CCGT plants, and coal plants whose build costs were sunk several decades ago. A CCS plant in the current market may therefore be squeezed between low variable cost ‘price takers’ (nuclear and wind) and low capital cost ‘price makers’ (CCGT). Of course, conventional CCGT, whilst lower carbon than conventional coal, is still not low-carbon, which presents an opportunity for low-carbon, potentially load-following plant such as CCS. Depending on the contribution of nuclear and wind power to the generation mix, a proportion of any CCS fleet may be able to run at, or near, baseload, but the characteristics of CCS described above still present a significant challenge, particularly if, as some suggest, levelised costs for CCS are likely to show only small reductions over the next few decades as potential reductions in capital cost are offset by carbon price increases.542
In the relatively small literature on CCS as an investment proposition, there appears to be something of a consensus emerging that the policy support mechanisms under consideration, both internationally and in the UK, are unlikely to deliver the level of CCS deployment that many suggest will be required.543 In their 2009 paper, Abadie and Chamorro544 concluded that in the face of the risks associated with uncertain returns, investment in CCS on coal plants will be delayed. They also concluded from a real-options based assessment that the CO2 price required to overcome these risks and incentivise CCS investment was more than four times that which is suggested by a typical Net Present Value (NPV) assessment, and still more than three times even if the additional capital cost is covered by full subsidy.545 From their analysis of CCS investments in the US policy context, Hamilton and colleagues546 suggest that given ‘nth of a kind’ cost estimates available and the projected value of avoided carbon emissions under the then proposed US carbon cap and trade bills, Super Critical Pulverised Coal (SCPC) plant with CCS would not present a breakeven proposition until after 2030. Osmundsen and Emhjellen547 argue in their 2010 paper that CCS does not offer a profitable proposition and delivers CO2 abatement at ‘very high cost’. Others contend that the EU ETS on its own won't lead to large scale CCS deployment,548 a view that has some support from within the industry.549 Flannery550 contends that ‘CCS today lacks both an economically viable policy framework and a business model’. With a different analytical approach, Evar assessed stakeholder perceptions of the uncertainties over CCS technology development and whether support levels will be sufficient: he concluded that ‘experts express certitude in the prospects for deploying large-scale CCS technology in the UK, all the while questioning several underlying technical and policy premises that are necessary to ensure this goal’.551
Further issues which concern analysts are pipeline network sizing and the potential long-term liability that CO2 storage represents. It is argued by some stakeholders that with current policy there is a danger of a piece-meal build-up of pipelines, when a more coordinated approach might be more cost effective in the long run,531 a view which has some support.552 The effect of a sub-optimal pipeline network is to make overall costs per unit of output (MWh of electricity or tonne of CO2 stored) higher than they could otherwise be. Concerns over the long term liabilities associated with CO2 storage are often raised in the context of the investment proposition of CCS,550,553 whilst others question the degree to which the long term CO2 storage liability is a commercially insurable risk.554 On the other hand, the EU directive on CO2 storage, which seems likely to mandate a long term liability fund, may go some to way to addressing these concerns.555
16.2 Continuing challenges facing CCS policy support mechanisms
The UK and EU clearly do have substantial policy support offerings for CCS but the key question is whether they will be sufficient to deliver both the early-stage investment in demonstration plants and the large-scale CCS deployment that will be required if the technology is to make a meaningful contribution to meeting climate policy goals. Research undertaken by the UK Energy Research Centre556–558 suggests that significant concerns remain and these can summarised as follows:Technology and construction risk is a particularly important factor deterring investment at present. The multiplicity of CCS technologies, each of which has differing technological characteristics, makes this factor especially difficult to tackle. The high up-front capital costs of CCS projects (and the uncertainty around those costs), together with delays in the UK demonstration programme, and the EMR's emphasis on premium payments for electricity generated rather than up-front capital grants, are exacerbating this risk.
The infrastructural barriers to CCS investment include the first-of-a-kind costs associated with developing a CO2 transportation network, and the lack of a systematic policy approach to coordinating and optimising the network through, for example, pipeline oversizing. There are also more general uncertainties about the legal liabilities of CO2 storage.
CCS has significant and variable fuel-related operating costs, which creates a fuel price risk. Although fossil fuel plants are typically ‘price makers’, with the ability to pass fuel price increases on to consumers, there are concerns that the FiT CfD support mechanism for UK projects may remove this natural hedge unless the mechanism is also linked to fuel prices.
CCS has relatively high operating and fuel costs, which may mean that load factor risk could become important by the late 2020s. In particular, CCS plant might be required to operate flexibly (and therefore at lower load factors) when there is increased penetration of very-low-marginal-cost nuclear and wind power plants. This has the potential to increase the unit costs (£/MWh) of CCS generation, thus undermining the attractiveness of CCS investments unless investors can be sure of receiving high prices when plants do run, or can be compensated in some other way, for example through the proposed capacity payment mechanism. This risk is potentially greater for coal CCS than gas CCS, due to the higher capital intensity of coal plant.
Whilst other low carbon power generation technologies such as nuclear, wind and solar photovoltaics also generally require support, the unique characteristics of CCS present both significant opportunities and policy challenges. The opportunities include the potential for dispatchable, flexible, low carbon generation—which will have particular value in electricity systems with large contributions from technically or economically inflexible generation such as wind and nuclear power. Combining these attributes with the potential for geographically diversified fuels sources explains why CCS technologies feature so strongly in many countries' CO2 emissions reduction strategies. However, policy design does need to recognise the specific techno-economic characteristics of CCS and the need for substantial capital grants for early projects, address the inherent fuel price risk, and ensure that the CO2 transportation networks are built up in the most efficient long-term manner. The Global CCS Institute have called for ‘substantial, timely and stable’ policy support,526 reinforcing the IEA's call for a ‘stable but flexible’ policy framework.525 What is also clear is that time is of the essence if CCS technologies are to be developed and deployed at the scale implied by global policy aspirations.
17. UK and EU legislative responses to CCS
This section explores the main features of the EU and UK legislative frameworks for CCS. This analysis is cast against a not inconsiderable concern over the financial and regulatory risk management dimensions of the technology. What is revealed in particular is the need for greater regulatory certainty and legal and financial assurances for would be CCS investors and operators of CCS storage sites.17.1 The CCS directive
The Directive559 provides the legislative basis for safe geological storage of carbon dioxide. It makes passing references to capture and transport activities. There are comprehensive requirements for storage addressing the life cycle of prospective storage sites. In particular, there is coverage of: storage site selection (Article 4); permits for exploration (Article 5), storage permits (Article 6); and operation, closure and post-closure obligations (Chapter 4). Finally, there are rules prescribed for transfer of site-based responsibilities (Article 18).In summary, the CCS Directive provides a significant number of risk management opportunities for UK regulators while placing significant costs on storage operators. For example, not to approve storage sites with risky geological profiles, to seek strict permit conditions such that human error will be reduced in respect of technical compliance, etc. Additionally, among the regulatory risk management opportunities available to governments are the rights of authorities to require the following:
• That no storage site which may leak or create undue environmental or health risks shall be permitted;
• That no storage site shall be permitted without requisite levels of financial security and technical excellence;
• That a storage site shall not operate without a permit and observance of all permit conditions;
• That a storage site must feature effective monitoring and reporting requirements to the regulatory authority;
• That the regulator must be notified immediately of leakages or irregularities at the site;
• That a storage site will be closed for breach of permit conditions;
• That the storage site operator will comply with strict closure and aftercare requirements;
• That all environmental and related financial liabilities may be placed on the storage site operator;
• That there shall be proportionate penalties for regulatory infractions;
• That emission allowances be purchased to cover leakage events.
The sheer weight and nature of risk management opportunities available to the regulator and the commensurate risk management standards, procedures and financial and related liability requirements placed upon the storage site operators suggests that a “cooperation” or “partnership” approach between industry and regulators to risk management and related long-term and financial liability for leakage is necessary.
In respect of commercial scale storage sites, it is worth recalling that geological storage will extend over long periods of time. As such the CCS Directive spells out framework requirements to ensure the long-term stewardship of storage sites. The Directive thus provides for sites to be transferred to Member State control in the long term, however, that can only occur once the Competent Authority has been assured that no leakage is likely to occur. (The operator retains responsibility for a site whilst it presents a significant risk of leakage.) Under the CCS Directive, a storage site shall be transferred (legal liabilities included) to the state when:
• All available evidence indicates that the CO2 will be completely contained for the indefinite future;
• A minimum period before transfer to be determined by the competent authority has elapsed;
• A financial contribution for the post-transfer period covering at least the costs for monitoring for 30 years has been made and;
• The site has been sealed and the injection facilities have been removed. As this is the second key decision in the lifecycle of a storage site (the first being the decision to permit the site for use), a Commission review is foreseen at this stage too.
There is a perception (CCS Directive, Article 18) that potential storage site operator liabilities and financial obligations end within approximately 20 years (given as a minimum period). However, the nature of Directive Article 18.1-2 language is such that the conditions 1(a) “complete and permanent storage” may not be proven by that time, (b) the 20 year period is a minimum, and 2(c) site evolution “towards a situation of long-term stability” may not be proven by that time. As such, this loose Directive language offers regulators an open door to deny the transfer of responsibility from the storage site operator to the competent authority at the 20 year threshold. In such circumstances, it has previously been demonstrated that regulators do not accept such a transfer of responsibility in analogous environmental law fields (in Canada and the United States) pertaining to waste management facilities and contaminated land sites. Transfers can be infinitely stalled by competent authorities, through requests for more monitoring data for example. This issue ought to be considered by firms operating in particularly risk adverse government jurisdictions.
17.2 The Environmental Liability Directive560
The CCS Directive itself does not address the specific mechanics of liability. Hence, we must look to the Environmental Liability Directive and the Emissions Trading Scheme Directive given that the CCS Directive delegates this matter to them.Further to Article 34 of the CCS Directive, the Environmental Liability Directive brings storage site operations within the liability framework of the European Union. As such, operators of CCS sites have obligations in respect of the prevention and remediation of environmental damage associated with such sites. This applies to all relevant “environmental damage” and corresponding duties of prevention (Article 5) and remediation/mitigation (Article 6) under the Environmental Liability Directive. Financial security measures are also to be undertaken by storage site operators further to Article 14 of the Environmental Liability Directive. A flexible interpretation of Article 14 allows for the use of ceilings on financial instruments. It also allows for the exclusion of liability on behalf of operators, where they are not at fault or are otherwise not negligent.
17.3. The Emissions Trading Directive561
If we move on to the Emissions Trading Directive, by virtue of the inclusion of geological storage sites under Annex I of the Emissions Trading Directive, installations will be required to surrender allowances for any emissions from the site, including leakage, as calculated pursuant to the Monitoring and Reporting Guidelines for CCS. The amount of the Financial Security (FS) for this obligation can be based on the potential total tons of emissions, including those due to leakage(s), multiplied by the market cost of purchasing an equivalent amount of allowances. This calculation will require (i) estimates for the total tons of emissions that may be released, including those due to leakage(s), (ii) the timing of emissions, and (iii) costs of allowances when releases occur.Guidance Document 4 observations aside, there is unavoidable uncertainty about the future price of EU Allowances (EUA) at the time of any potential leakage. There is no cap on the EUA price; the penalty for excess emission (100 € t−1) does not relieve the operator of the need to provide allowances to cover the emissions, and is not, therefore, a cap on EUA prices.
The need to hedge against such risk becomes important when it is likely that liability for allowances would entail greater costs over time as carbon prices rise. Furthermore, the assumption of long-term emissions credits liability would mean that allowances which are bought in the future, as a compensatory measure for loss of CO2 stored, would be with a significantly higher price tag than those bought today, which would further defer investments.
As such, a liability of this kind is not insurable and presents an incalculable risk to potential storage site operators.
In terms of financial risk derived from liability, it is worth noting that the purchase of emissions credits serves as a climate change mitigation and prevention strategy in itself. Arguably, damage in terms of failed climate change mitigation is already covered in respect of the types of damage listed in the EU Environmental Liability Directive (2004/35/EC) (including, but not limited to species loss, marine ecosystem damage, fundamental changes in land use, damage to land, damage to water, etc.). These types of damage occur as a result of anthropogenic climate change as well, which is why CO2 as a pollutant has already been determined to be remediated under climate change mitigation measures. Thereby, if CCS operators are legally required to buy emissions credits and CCS operators also bound to cover liability of the same leakage event, there is a clear double-payment by the private sector. This problem of double-counting liability has to be addressed by counterbalanced regulatory solutions that push forward CCS technology investment.
17.4 EU state aids/competition law
There may be those that point to state aids/competition law restrictions on regulatory solutions for financial instrument and long-term liability regulation further to the CCS Directive. It is noted to date that the UK and German Governments have taken a favourable position in this regard by adopting a flexible approach to state aids and it would appear that the European Commission is similarly disposed. There is also a strong argument to suggest that in its essence, carbon dioxide storage represents a public good or service that fulfils a government function of mitigating climate change. By storing carbon that would otherwise have been inevitably produced in order to satisfy energy demand, storage serves to mitigate climate change and to meet binding emissions reduction targets that are placed upon Governments within an EU and international legal context. Given the additional point that carbon storage may well turn out to be a cost vs. revenue neutral activity some easing of state aids rules/competition law should apply. This argumentation is supported by the EC Treaty obligation of competition law not to obstruct the performance, in law or in fact, of the particular tasks assigned to services of general economic interest (i.e. the provision of carbon storage for climate change prevention and mitigation).Thus far, leading Member State Governments have taken a sensible approach to state aid regulation and CCS. For this reason, it is not suggested that a formal procedure be commenced to review the EU General Block Exemption Regulation or Guidelines for State Aid for Environmental Protection562 with the aim of codifying new principles and rules in respect of CCS. This would constitute a drawn-out and cumbersome process. Given the history of CCS Directive negotiation, there would be further uncertainty about the result and Member States and non-State interests that are without direct and active interests and projects in the field of CCS would still be in a position to influence the outcome in a manner that may not best serve Member States and private sector actors that wish to advance CCS technology. There is also the observation that the revision of EU state aids regulation and guidance for CCS should have taken place at a time that was commensurate with the creation of the CCS Directive. Re-opening the debate would only lead to further market uncertainty at a time when the CCS Directive is just now being enforced.
17.5 The UK regulatory response
Legislative developments at European level have created a regulatory framework for offshore CCS within the European Community, whilst amendments to the London Protocol on the Prevention of Marine Pollution by the Dumping of Waste and Other Matter (1972) and the Convention for the Protection of the marine Environment of the North-East Atlantic (OSPAR Convention) to allow for sub-seabed geological storage, provide an international regulatory dimension. Whilst supporting the amendments to the London Protocol and OSPAR Convention in 2007, UK announced a competition for funding a full-scale demonstration project.The Energy Bill was unveiled in 2008 which detailed a framework for the licensing, enforcement and registration of CCS. The Department for Business, Enterprise & Regulatory Reform (BERR) expected that the Bill would provide a sound system, which would enable private sector investment in CCS projects and along with the Planning and Climate Change Bills, ensure legislation that underpins the long term delivery of our energy and climate change strategy. Creative legislative solutions in addition to the provision of finance will need to operate in tandem if first-mover gains in the emerging CCS industry in the UK are to be won in the CCS field.
17.6 The Energy Act 2008563
In summary, the Energy Review of 2006 concluded that should it be proved that CCS is cost effective, the next stage would need to be commercial demonstration. In Budget 2007, the Government announced a competition to design and build full-scale demonstration of CCS projects and it was launched in November 2007. The Energy Act 2008 established the enabling provisions for regulating offshore CO2 storage in the UK in November 2008. Furthermore, consultation on the proposed offshore CO2 licensing regime, including draft regulations to implement that regime was done in September 2009.The Energy Act introduces a regulatory framework for the licensing of the offshore storage aspect of CCS. Furthermore, the Act states that there is a right of the Crown to have sole jurisdiction from the UK coast line for up to 200 miles out to sea (the so-called Exclusive Economic Zone—EEZ) in relation to the storing of gas. The Government may also designate ‘Gas Importation and Storage Zones’ within the EEZ. For operators seeking to undertake CCS activities within the newly designated EEZ, a lease and presumably a rental payment will be required from the Crown Estate. According to this Act, all natural resources belong to the coastal state (i.e. the UK) including storage space under the sea bed.
The Act also provides a regulatory regime for CO2 storage for certain relevant existing offshore oil and gas legislation. For example, the oil and gas installation decommissioning regime found in the Petroleum Act (1998) will be applied to facilities used for CO2 storage. The licensing regime also regulates storage in depleted and partially depleted hydrocarbon fields under the sea bed and in non-hydrocarbon geological features, such as deep saline formations.
A regime based upon licensing is introduced and requires a licence from the relevant authority for activities relating to the storage of CO2 (with a view to its permanent disposal). According to the Act, the Secretary of State or the Scottish Ministers grant a licence that may also attach a set of particular requirements for a specific applicant. The licence may include provisions relating to financial security in respect of future obligations, as well as obligations between the closure of an installation and the termination of a licence.
The Act also introduces a detailed section about the enforcement of licences and criminal offences and sanctions when activities are undertaken without a licence or where a licence holder fails to abide by its prescribed conditions. The 1998 Act also prescribes detailed plans and approvals that require persons seeking to abandon an installation offshore to provide an ‘abandonment programme’ which sets out the ‘measures proposed to be taken in connection with the abandonment of an offshore installation or submarine pipeline’.
17.7 Energy Act 2010564
This legislation implements elements of: The UK Low Carbon Transition Plan – a national strategy for climate and energy.This Plan will deliver emission cuts of 18% on 2008 levels by 2020 (and over a one third reduction on 1990 levels) on the way to achieving a reduction of at least 80% by 2050. The Plan makes it clear that we need to cut emissions in a way that helps the sustainable development of our economy, society and environment. This means keeping energy supplies safe and secure, maximising economic opportunities and protecting the most vulnerable consumers.
For instance, the Secretary of State has the power to modify supply licences so that it can be made certain that suppliers will let their consumers know about any potential changes to their contract in terms of pricing or any other changes as such within a certain period of time.
17.8 The storage of carbon dioxide (licensing etc.) regulations 2010565
These Regulations introduce a permitting regime for offshore CCS activities under the authority of Energy Act 2008. Furthermore, they set out a range of requirements that operators need to fulfil in order to obtain a storage site permit from the Secretary of State. The Regulations cover the conditions for granting licences and exploration permits, obligations of the storage operator, closure of the storage site, post-closure period, and financial security.17.9 Environmental Permitting (EP)566
The EP Regulations 2010 comprise a common set of definitions, processes and controls for the permitting of specified activities to prevent pollution. In doing so, it has rationalised various permitting regimes into a common framework that is easier to understand and use. For example, it only requires businesses to have one permit instead of several permits for activities falling under the regulations on one site and by doing so, it allows the regulators to focus recourses on higher risk activities.Four amendments were made to the EP Regulations 2010 and took effect in 2011. The first two amendments arise from the need to transpose certain parts and provisions of the CCS directive. The third amendment is in respect to offshore CCS activities, and the final amendment is regarding the gas produced by anaerobic digestion plants.
17.10 Liability implications and developments in the USA
The proposed license is very similar to the licences granted to the petroleum production industry. The licence would—subject to specific consent for drilling of any well—permit the conduction of intrusive exploration. Furthermore, it expresses time limited rights to apply for storage permits which would allow site operators to construct storage facilities—including offshore facilities—in order to store the liquefied CO2. Moreover, the licence provides the necessary framework to demonstrate the legal obligations that site operators have with respect to ensuring the safe/secure containment of CO2 under geological formations, decommissioning the site after use and the monitoring of the stored material's behaviour during and after the completion of storage operations.Interestingly, the US Environmental Protection Agency has adopted a more flexible regulatory approach. It has finalised requirements for CCS through the development of permitting for a new class of storage wells (Class VI) to be used specifically for geological storage of CO2. The EPA has proposed a default 50-year time-frame for CCS liability with the provision that the acting EPA Director may shorten or lengthen that period based on risk data gathered during the permitting process. Additionally, this new permitting system will allow for financial guarantees for CCS to be chosen from a variety of different options which would allow for greater market competition and rapid deployment of lower-cost solutions in the CCS industry.
Complementing this approach, as of 27 March 2012, New Source Performance Standards567 (NSPS) addressing carbon emissions are to be applied to new and, rather confusingly, existing power plants. No new standards have yet been set for existing power plants. Although it is anticipated that the majority of new power plants that will become operational are natural gas combined cycle plants, new coal-fired power plants could meet the NSPS by capturing and permanently sequestering their GHG emissions using CCS technologies. Under the regulations, plants would have the flexibility to phase in CCS using a 30-year emissions average. This would allow for both improvements to CCS technology equipment to be introduced at the plant or to delay implementation until after plant construction when CCS technologies become more ubiquitous and technology investment costs are at the right level.
18. Conclusions
Carbon capture and storage is a key climate change mitigation technology and is currently in the process of being demonstrated worldwide. There exist a large number of different technologies for CO2 capture, ranging from currently available technologies such as amine-scrubbing through to 2nd or 3rd generation technologies with potentially superior thermodynamics, such as chemical or carbonate looping. Safe and secure CO2 storage has been demonstrated, and is still being demonstrated, at a number of sites across the world, with multi-year injections of around 1 Mt per year at a number of sites. Total CO2 storage capacity is also being proven, but will be sufficient for many years of CO2 emissions. In addition, CO2 is regularly transported safely in pipelines across large parts of the USA and Canada.A number of technologies have been proposed which would potentially allow CO2 to be captured directly from the air, or to utilise captured CO2 to produce useful products. Extreme care should be exercised when evaluating the climate benefits and scalability of such processes.
The financial case for CCS requires that it operates in a flexible manner, load-following ability is extremely important to the long-term economics.
Acknowledgements
PSF thanks the RCUK Energy Programme and Engineering and Physical Sciences Research Council for support under EP/K000446 and Matt Boot-Handford for a PhD studentship under EP/I010912/1. Joseph Yao thanks the Grantham Institute at Imperial College for their support.References
- IPCC, Climate Change 2007: Synthesis Report. Contribution of Working Groups I, II and III to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change, Geneva, Switzerland, 2007 Search PubMed.
- F. C. Krebs, Energy Environ. Sci., 2012, 5, 7238–7239 Search PubMed.
- N. MacDowell, N. Florin, A. Buchard, J. Hallett, A. Galindo, G. Jackson, C. S. Adjiman, C. K. Williams, N. Shah and P. Fennell, Energy Environ. Sci., 2010, 3, 1645–1669 CAS.
- International Energy Agency, World Energy Outlook 2010, OECD Publishing, 2010 Search PubMed.
- R. Viswanathan, P. Alto, J. F. Henry, A. Chattanooga, J. Tanzosh, G. Stanko, J. Shingledecker and K. Weighardt, presented in part at the Power-Gen Europe, Brussels, 2001 Search PubMed.
- H. Nalbandian, Energeia, 2009, 20, 1–3 Search PubMed.
- H. Nalbandian, Performance and risks of advanced pulverised coal plant, Report CCC/135, IEA, 2008 Search PubMed.
- K. Yamamoto, I. Kajigaya and H. Umaki, Mater. High Temp., 2003, 20, 15–18 CrossRef.
- J. Gabrielsen, M. L. Michelsen, E. H. Stenby and G. M. Kontogeorgis, Ind. Eng. Chem. Res., 2005, 44, 3348–3354 CrossRef CAS.
- W. Fürst and H. Renon, AIChE J., 1993, 39, 335–343 CrossRef.
- G. Vallée, P. Mougin, S. Jullian and W. Fürst, Ind. Eng. Chem. Res., 1999, 38, 3473–3480 CrossRef.
- W. G. Chapman, K. E. Gubbins, G. Jackson and M. Radosz, Fluid Phase Equilib., 1989, 52, 31–38 CrossRef CAS.
- W. G. Chapman, K. E. Gubbins, G. Jackson and M. Radosz, Ind. Eng. Chem. Res., 1990, 29, 1709–1721 CrossRef CAS.
- A. Gil-Villegas, A. Galindo, P. J. Whitehead, S. J. Mills, G. Jackson and A. N. Burgess, J. Chem. Phys., 1997, 106, 4168–4186 CrossRef CAS.
- N. Mac Dowell, F. E. Pereira, F. Llovell, F. J. Blas, C. S. Adjiman, G. Jackson and A. Galindo, J. Phys. Chem. B, 2011, 115, 8155–8168 CrossRef CAS PubMed.
- N. Mac Dowell, F. Llovell, C. S. Adjiman, G. Jackson and A. Galindo, Ind. Eng. Chem. Res., 2010, 49, 1883–1899 CrossRef CAS.
- J. Rodriguez, N. Mac Dowell, F. Llovell, C. S. Adjiman, G. Jackson and A. Galindo, Mol. Phys., 2012, 110, 1325–1348 CrossRef CAS.
- N. Mac Dowell, N. J. Samsatli and N. Shah, Int. J. Greenhouse Gas Control, 2013, 12, 247–258 CrossRef CAS PubMed.
- N. Mac Dowell and N. Shah, Int. J. Greenhouse Gas Control, 2013, 13, 44–58 CrossRef CAS PubMed.
- A. Arce, N. Mac Dowell, N. Shah and L. F. Vega, Int. J. Greenhouse Gas Control, 2012, 11, 236–250 CrossRef PubMed.
- F. Llovell, N. Mac Dowell, F. J. Blas, A. Galindo and G. Jackson, Fluid Phase Equilib., 2012, 336, 137–150 CrossRef CAS PubMed.
- F. J. Blas and L. F. Vega, Mol. Phys., 1997, 92, 135–150 CrossRef CAS.
- F. Llovell, R. M. Marcos, N. MacDowell and L. F. Vega, J. Phys. Chem. B, 2012, 116, 7709–7718 CrossRef CAS PubMed.
- R. D. Deshmukh and A. E. Mather, Chem. Eng. Sci., 1981, 36, 355–362 CrossRef CAS.
- R. H. Weiland, T. Chakravarty and A. E. Mather, Ind. Eng. Chem. Res., 1993, 32, 1419–1430 CrossRef CAS.
- K. S. Pitzer, J. Phys. Chem., 1973, 77, 268–277 CrossRef CAS.
- W. Böttinger, M. Maiwald and H. Hasse, Ind. Eng. Chem. Res., 2008, 47, 7917–7926 CrossRef.
- C.-C. Chen and L. B. Evans, AIChE J., 1986, 32, 444–454 CrossRef CAS.
- C.-C. Chen, H. I. Britt, J. F. Boston and L. B. Evans, AIChE J., 1982, 28, 588–596 CrossRef CAS.
- D. M. Austgen, G. T. Rochelle, X. Peng and C. C. Chen, Ind. Eng. Chem. Res., 1989, 28, 1060–1073 CrossRef CAS.
- E. T. Hessen, T. Haug-Warberg and H. F. Svendsen, Chem. Eng. Sci., 2010, 65, 3638–3648 CrossRef CAS PubMed.
- K. Thomsen and P. Rasmussen, Chem. Eng. Sci., 1999, 54, 1787–1802 CrossRef CAS.
- L. Faramarzi, G. M. Kontogeorgis, K. Thomsen and E. H. Stenby, Fluid Phase Equilib., 2009, 282, 121–132 CrossRef CAS PubMed.
- DOE and NETL, Cost and Performance Baseline for Fossil Energy Plants Volume 1: Bituminous Coal and Natural Gas to Electricity, Report DOE/NETL 2010/1397, Department of Energy (DOE), National Energy Technology Laboratory (NETL), 2010 Search PubMed.
- S. Reddy, J. Scherffius, S. Freguia and C. Roberts, Fluor's Econamine FG PlusSM Technology An Enhanced Amine-Based CO2 Capture Process, Paper presented at the Second National Conference on Carbon Sequestration NETL/DOE, Alexandria, VA, USA, May 5–8, 2003 Search PubMed.
- Y. Yagi, Development and Improvement of CO2-Capture System, Paper presented at GHGT-8, Trondheim, Norway, 19–22 June 2006 Search PubMed.
- H. Ahn, M. Luberti, Z. Liu and S. Brandani, Int. J. Greenhouse Gas Control, 2013, 16, 29–40 CrossRef CAS PubMed.
- P. T. Frailie, T. Madan, B. Sherman and G. T. Rochelle, Energy Performance of Advanced Stripper Configurations, Kyoto, Japan, 18–22 November 2012 Search PubMed.
- A. J. Sexton, K. S. Fisher, R. A. Jones, T. Madan, D. Sachde and G. T. Rochelle, Techno-Economic Analysis for CO2 Capture by Concentrated Piperazine with Regeneration by High Temperature Two Stage Flash: Budget Period 1, Prepared for US DOE Cooperative Agreement No: DE-FE0005654, June 2012 Search PubMed.
- G. Rochelle, E. Chen, S. Freeman, D. Van Wagener, Q. Xu and A. Voice, Chem. Eng. J., 2011, 171, 725–733 CrossRef CAS PubMed.
- D. H. Van Wagener, Ph.D. Dissertation, The University of Texas at Austin, TX, USA, 2011.
- B. A. Oyenekan and G. T. Rochelle, AIChE J., 2007, 53, 3144–3154 CrossRef CAS.
- M. S. Jassim and G. T. Rochelle, Ind. Eng. Chem. Fundam., 2006, 45, 2465–2472 CAS.
- J. M. Plaza, Ph.D. Dissertation, The University of Texas at Austin, TX, USA, 2012.
- S. A. Freeman, Ph.D. Dissertation, The University of Texas at Austin, TX, USA, 2011.
- G. T. Rochelle, Curr. Opin. Chem. Eng., 2012, 1, 183–190 CrossRef CAS PubMed.
- G. Stevens, K. Mumford, K. Endo, D. Quyn, H. Thee, K. Smith, S. Kentish, University of Melbourne, C. Anderson, B. Hooper, A. Qadar and Co-operative research centre for greenhouse gas technologies, Precipitating carbonate solvent process for carbon dioxide capture, Kyoto, Japan, November, 2012 Search PubMed.
- R. Moene, L. Schoon and F. Geuzenbroek, Shell Global Solutions International B.V, J. v. Streel and Shell (Petroleum Mining) Co. Ltd (NZ), Precipitating carbonate process for low-energy post-combustion CO2 capture technology development and pilot-plant operation, Kyoto, Japan, November, 2012.
- L. Li, Y. Du, H. Li, O. Namjoshi and G. T. Rochelle, Absorption rates and CO2 solubility in new piperazine blends, Kyoto, Japan, November, 2012 Search PubMed.
- L. Hu, Post-Combustion CO2 Capture for Existing PC Boilers by Self-Concentrating Absorbent, Presented at 2012 NETL CO2 Capture Technology Meeting in Pittsburgh, PA, USA, 2012 Search PubMed.
- I. Kim and S. Ma'mum, Selection and characterization of phase-change solvent for CO2 capture: precipitating system, Kyoto, Japan, November, 2012 Search PubMed.
- U. Liebenthal, A. Kather, Hamburg University of Technology, D. Pinto, J. Monteiro, H. Svendsen and Norweigian University of Science and Technology, Overall process analysis and optimization for CO2 capture from coal fired power plants based on phase change solvents forming two liquid phases, Kyoto, Japan, November, 2012 Search PubMed.
- L. Nguyen, Low-Cost Enzyme-based Technology for Carbon Capture, Presented at 2012 NETL CO2 Capture Technology Meeting, Pittsburgh, July 9–12, 2012 Search PubMed.
- N. J. M. C. P.-v. Elk, P. W. J. Derks, S. Fradette and G. F. Versteeg, Int. J. Greenhouse Gas Control, 2012, 9, 385–392 CrossRef PubMed.
- A. J. Sexton, Ph.D. Dissertation, The University of Texas at Austin, TX, USA, 2008.
- F. B. Closmann, Ph.D. Dissertation, The University of Texas at Austin, TX, USA, 2011.
- A. K. Voice, B. C. Closmann and G. T. Rochelle, Energy Procedia, 2013, 37, 2118–2132 CrossRef CAS PubMed.
- Nathan Fine, G. Rochelle, M. Ashouripashaki, A. Voice, S. Fulk, L. Li, O. Namjoshi and University of Texas at Austin, Nitrosamine Management in Aqueous Piperazine for CO2 Capture, Kyoto, Japan, November, 2012 Search PubMed.
- P. Nielsen, L. Li, G. Rochelle and The University of Texas at Austin, Piperazine Degradation in Pilot Plants, Kyoto, Japan, November, 2012 Search PubMed.
- A. K. Voice and G. T. Rochelle, Environ. Sci. Technol., 2012 Search PubMed , submitted.
- B. Fostås, A. Gangstad, B. Nenseter, S. Pedersen, M. Sjøvoll and A. L. Sørensen, Energy Procedia, 2011, 4, 1566–1573 CrossRef PubMed.
- B. Schallert, unpublished work.
- H. Knuutila, H. Svendsen, N. Asif and NTNU/Department of Chemical Engineering, Destruction of nitrosoamines with UV-light, Kyoto, Japan, November, 2012 Search PubMed.
- M. Attalla, P. Jackson and CSIRO, Ultra-violet treatment as a strategy for destruction of degradation products from amine based post combustion CO2 capture, Kyoto, Japan, November, 2012 Search PubMed.
- E. Gjernes, L. I. Helgesen, S. F. Gassnova, S. Nepstad and TCM DA, Health and environmental impact of amine based post combustion CO2 capture, Kyoto, Japan, November, 2012 Search PubMed.
- T. Nguyen, M. Hilliard and G. Rochelle, Energy Procedia, 2011, 4, 1624–1630 CrossRef CAS PubMed.
- T. Nguyen, M. Hilliard and G. T. Rochelle, Int. J. Greenhouse Gas Control, 2010, 4, 707–715 CrossRef CAS PubMed.
- S. C.-C. Wei, G. Puxty, P. Feron and CSIRO Energy Technology, Amino acids salts for CO2 capture at flue gas temperatures, Kyoto, Japan, November, 2012 Search PubMed.
- S. Fulk, G. Rochelle and The University of Texas at Austin, Volatile Contaminant Control in Amine-Based CO2 Capture Systems, Kyoto, Japan, November, 2012 Search PubMed.
- J. Mertensa, J. Knudsen, M.-L. Thielens and J. Anderson, Int. J. Greenhouse Gas Control, 2012, 6, 2–11 CrossRef PubMed.
- E. F. da Silva, K. A. Hoff, T. Mejdell and H. Kolderup, presented in part at the 1st Post Combustion Capture Conference, Abu Dhabi, 2011 Search PubMed.
- S. Holton, Project Update of 500 TPD Demonstration Plant for Coal-fired Power Plant and MHI Amine Emission Reduction technology, Will be presented at the 12th Annual Conference on Carbon Capture Utilization & Sequestration, May 13–16, 2013, Pittsburgh, PA, USA, 2013 Search PubMed.
- T. Carter, National Carbon Capture Center: Post-Combustion, Presented at 2012 NETL CO2 Capture Technology Meeting, Pittsburgh, PA, USA, 2012 Search PubMed.
- J. N. Knudsen, O. M. Bade, M. Anheden, O. Gorset, R. Bjorklund and Aker Clean Carbon AS, Novel concept for emission control in post combustion capture, Kyoto, Japan, November, 2012 Search PubMed.
- MIT, Power Plant CCS Projects, http://sequestration.mit.edu/tools/projects/index_capture.html, accessed 18 September 2012.
- GCCSI, CO2 Capture Technologies: Post Combustion Capture (PCC), http://cdn.globalccsinstitute.com/sites/default/files/publications/29721/co2-capture-technologies-pcc.pdf, accessed 30 March 2013.
- F. Karadas, M. Atilhan and S. Aparicio, Energy Fuels, 2010, 24, 5817–5828 CrossRef CAS.
- Y. Ding and E. Alpay, Chem. Eng. Sci., 2000, 55, 3461–3474 CrossRef CAS.
- J. E. Bara, T. K. Carlisle, C. J. Gabriel, D. Camper, A. Finotello, D. L. Gin and R. D. Noble, Ind. Eng. Chem. Res., 2009, 48, 2739–2751 CrossRef CAS.
- J. H. Huang and T. Ruther, Aust. J. Chem., 2009, 62, 298–308 CrossRef CAS.
- M. Hasib-ur-Rahman, M. Siaj and F. Larachi, Chem. Eng. Process., 2010, 49, 313–322 CrossRef CAS PubMed.
- Z. Z. Yang, Y. N. Zhao and L. N. He, RSC Adv., 2011, 1, 545–567 RSC.
- X. P. Zhang, X. C. Zhang, H. F. Dong, Z. J. Zhao, S. J. Zhang and Y. Huang, Energy Environ. Sci., 2012, 5, 6668–6681 CAS.
- M. Ramdin, T. W. de Loos and T. J. H. Vlugt, Ind. Eng. Chem. Res., 2012, 51, 8149–8177 CrossRef CAS.
- M. J. Muldoon, S. N. V. K. Aki, J. L. Anderson, J. K. Dixon and J. F. Brennecke, J. Phys. Chem. B, 2007, 111, 9001–9009 CrossRef CAS PubMed.
- C. Cadena, J. L. Anthony, J. K. Shah, T. I. Morrow, J. F. Brennecke and E. J. Maginn, J. Am. Chem. Soc., 2004, 126, 5300–5308 CrossRef CAS PubMed.
- J. E. Bara, C. J. Gabriel, E. S. Hatakeyama, T. K. Carlisle, S. Lessmann, R. D. Noble and D. L. Gin, J. Membr. Sci., 2008, 321, 3–7 CrossRef CAS PubMed.
- K. R. Seddon, A. Stark and M. J. Torres, ACS Symp. Ser., 2002, 819, 34–49 CrossRef CAS PubMed.
- A. Sharma, C. Julcour, A. A. Kelkar, R. M. Deshpande and H. Delmas, Ind. Eng. Chem. Res., 2009, 48, 4075–4082 CrossRef CAS.
- W. L. McCabe, J. C. Smith and P. Harriott, Unit Operations of Chemical Engineering, McGraw-Hill, New York, 6th edn, 2001 Search PubMed.
- R. L. Gardas and J. A. P. Coutinho, Fluid Phase Equilib., 2008, 266, 195–201 CrossRef CAS PubMed.
- M. S. Shannon and J. E. Bara, Ind. Eng. Chem. Res., 2011, 50, 8665–8677 CrossRef CAS.
- E. Gmelin, Thermochim. Acta, 1997, 305, 1–26 CrossRef.
- J. P. Hallett and T. Welton, Chem. Rev., 2011, 111, 3508–3576 CrossRef CAS PubMed.
- L. Cammarata, S. G. Kazarian, P. A. Salter and T. Welton, Phys. Chem. Chem. Phys., 2001, 3, 5192–5200 RSC.
- J. L. Anthony, J. L. Anderson, E. J. Maginn and J. F. Brennecke, J. Phys. Chem. B, 2005, 109, 6366–6374 CrossRef CAS PubMed.
- L. A. Blanchard, Z. Y. Gu and J. F. Brennecke, J. Phys. Chem. B, 2001, 105, 2437–2444 CrossRef CAS.
- Y. Hou and R. E. Baltus, Ind. Eng. Chem. Res., 2007, 46, 8166–8175 CrossRef CAS.
- M. B. Shiflett and A. Yokozeki, Ind. Eng. Chem. Res., 2005, 44, 4453–4464 CrossRef CAS.
- J. E. Bara, D. E. Camper, D. L. Gin and R. D. Noble, Acc. Chem. Res., 2010, 43, 152–159 CrossRef CAS PubMed.
- C. M. Wang, X. Y. Luo, H. M. Luo, D. E. Jiang, H. R. Li and S. Dai, Angew. Chem., Int. Ed., 2011, 50, 4918–4922 CrossRef CAS PubMed.
- J. E. Brennecke and B. E. Gurkan, J. Phys. Chem. Lett., 2010, 1, 3459–3464 CrossRef CAS.
- E. D. Bates, R. D. Mayton, I. Ntai and J. H. Davis, J. Am. Chem. Soc., 2002, 124, 926–927 CrossRef CAS PubMed.
- A. E. Visser, R. P. Swatloski, W. M. Reichert, R. Mayton, S. Sheff, A. Wierzbicki, J. H. Davis and R. D. Rogers, Chem. Commun., 2001, 135–136 RSC.
- K. E. Gutowski and E. J. Maginn, J. Am. Chem. Soc., 2008, 130, 14690–14704 CrossRef CAS PubMed.
- X. Y. Li, M. Q. Hou, Z. F. Zhang, B. X. Han, G. Y. Yang, X. L. Wang and L. Z. Zou, Green Chem., 2008, 10, 879–884 RSC.
- K. Fukumoto, M. Yoshizawa and H. Ohno, J. Am. Chem. Soc., 2005, 127, 2398–2399 CrossRef CAS PubMed.
- G. H. Tao, L. He, N. Sun and Y. Kou, Chem. Commun., 2005, 3562–3564 RSC.
- H. Ohno and K. Fukumoto, Acc. Chem. Res., 2007, 40, 1122–1129 CrossRef CAS PubMed.
- Y. Q. Zhang, S. J. Zhang, X. M. Lu, Q. Zhou, W. Fan and X. P. Zhang, Chem.–Eur. J., 2009, 15, 3003–3011 CrossRef CAS PubMed.
- X. Wang, N. Akhmedov, Y. Duan, D. Luebkea and B. Li, J. Mater. Chem. A, 2013, 1, 2978–2982 CAS.
- A. Brandt, J. Gräsvik, J. P. Hallett and T. Welton, Green Chem., 2013, 15, 550–583 Search PubMed.
- G. Gurau, H. Rodriguez, S. P. Kelley, P. Janiczek, R. S. Kalb and R. D. Rogers, Angew. Chem., Int. Ed., 2011, 50, 12024–12026 CrossRef CAS PubMed.
- D. J. Heldebrant, C. R. Yonker, P. G. Jessop and L. Phan, Chem.–Eur. J., 2009, 15, 7619–7627 CrossRef CAS PubMed.
- D. J. Heldebrant, P. G. Jessop, C. A. Thomas, C. A. Eckert and C. L. Liotta, J. Org. Chem., 2005, 70, 5335–5338 CrossRef CAS PubMed.
- P. G. Jessop, D. J. Heldebrant, X. W. Li, C. A. Eckert and C. L. Liotta, Nature, 2005, 436, 1102 CrossRef CAS PubMed.
- L. Phan, D. Chiu, D. J. Heldebrant, H. Huttenhower, E. John, X. W. Li, P. Pollet, R. Y. Wang, C. A. Eckert, C. L. Liotta and P. G. Jessop, Ind. Eng. Chem. Res., 2008, 47, 539–545 CrossRef CAS.
- Y. X. Liu, P. G. Jessop, M. Cunningham, C. A. Eckert and C. L. Liotta, Science, 2006, 313, 958–960 CrossRef CAS PubMed.
- D. J. Heldebrant, C. R. Yonker, P. G. Jessop and L. Phan, Energy Environ. Sci., 2008, 1, 487–493 CAS.
- C. M. Wang, H. M. Luo, D. E. Jiang, H. R. Li and S. Dai, Angew. Chem., Int. Ed., 2010, 49, 5978–5981 CrossRef CAS PubMed.
- C. M. Wang, S. M. Mahurin, H. M. Luo, G. A. Baker, H. R. Li and S. Dai, Green Chem., 2010, 12, 870–874 RSC.
- C. M. Wang, H. M. Luo, X. Y. Luo, H. R. Li and S. Dai, Green Chem., 2010, 12, 2019–2023 RSC.
- J. L. Anderson, J. K. Dixon and J. F. Brennecke, Acc. Chem. Res., 2007, 40, 1208–1216 CrossRef CAS PubMed.
- B. R. Van Ausdall, J. L. Glass, K. M. Wiggins, A. M. Aarif and J. Louie, J. Org. Chem., 2009, 74, 7935–7942 CrossRef CAS PubMed.
- P. K. Koech, J. Zhang, I. V. Kutnyakov, L. Cosimbescu, S. J. Lee, M. E. Bowden, T. D. Smurthwaite and D. J. Heldebrant, RSC Adv., 2013, 3, 566–572 RSC.
- L. J. Lozano, C. Godinez, A. P. de los Rios, F. J. Hernandez-Fernandez, S. Sanchez-Segado and F. J. Alguacil, J. Membr. Sci., 2011, 376, 1–14 CrossRef CAS PubMed.
- J. E. Bara, S. Lessmann, C. J. Gabriel, E. S. Hatakeyama, R. D. Noble and D. L. Gin, Ind. Eng. Chem. Res., 2007, 46, 5397–5404 CrossRef CAS.
- S. Hanioka, T. Maruyama, T. Sotani, M. Teramoto, H. Matsuyama, K. Nakashima, M. Hanaki, F. Kubota and M. Goto, J. Membr. Sci., 2008, 314, 1–4 CrossRef CAS PubMed.
- J. M. Zhang, S. J. Zhang, K. Dong, Y. Q. Zhang, Y. Q. Shen and X. M. Lv, Chem.–Eur. J., 2006, 12, 4021–4026 CrossRef CAS PubMed.
- P. Scovazzo, D. Havard, M. McShea, S. Mixon and D. Morgan, J. Membr. Sci., 2009, 327, 41–48 CrossRef CAS PubMed.
- J. B. Tang, W. L. Sun, H. D. Tang, M. Radosz and Y. Q. Shen, Macromolecules, 2005, 38, 2037–2039 CrossRef CAS.
- J. B. Tang, H. D. Tang, W. L. Sun, H. Plancher, M. Radosz and Y. Q. Shen, Chem. Commun., 2005, 3325–3327 CAS.
- J. B. Tang, H. D. Tang, W. L. Sun, M. Radosz and Y. Q. Shen, Polymer, 2005, 46, 12460–12467 CrossRef CAS PubMed.
- J. B. Tang, Y. Q. Shen, M. Radosz and W. L. Sun, Ind. Eng. Chem. Res., 2009, 48, 9113–9118 CrossRef CAS.
- S. H. Ren, Y. C. Hou, W. Z. Wu, S. D. Tian and W. N. Liu, RSC Adv., 2012, 2, 2504–2507 RSC.
- H. B. Xie, S. B. Zhang and S. H. Li, Green Chem., 2006, 8, 630–633 RSC.
- X. H. Huang, C. J. Margulis, Y. H. Li and B. J. Berne, J. Am. Chem. Soc., 2005, 127, 17842–17851 CrossRef CAS PubMed.
- G. G. Yu and S. J. Zhang, Fluid Phase Equilib., 2007, 255, 86–92 CrossRef CAS PubMed.
- B. M. Abraham, J. G. Asbury, E. P. Lynch and A. P. S. Teotia, Oil Gas J., 1982, 80, 68–75 CAS.
- F. L. Horn and M. Steinberg, Fuel, 1982, 61, 415–422 CrossRef CAS.
- A. E. Weller, B. W. Rising, A. A. Boiarski, R. J. Nordstrom, R. E. Barrett and R. G. Luce, Experimental evaluation of firing pulverized coal in a CO2/O2 atmosphere, Report ANL/CNSV-TM-168, Argonne National Laboratory, 1985 Search PubMed.
- D. M. Woycenko, W. L. van de Kemp and P. A. Roberts, Combustion of pulverised coal in a mixture of oxygen and recycled flue gas, Report JOU2-CT92-0093, 1995 Search PubMed.
- M. A. Douglas, E. Chui, Y. Tan, G. K. Lee, E. Croiset and K. V. Thambimuthu, Oxy-fuel combustion at the CANMET vertical combustor research facility, Washington, DC, USA, 2001 Search PubMed.
- M. B. Toftegaard, J. Brix, P. A. Jensen, P. Glarborg and A. D. Jensen, Prog. Energy Combust. Sci., 2010, 36, 581–625 CrossRef CAS PubMed.
- B. J. P. Buhre, L. K. Elliott, C. D. Sheng, R. P. Gupta and T. F. Wall, Prog. Energy Combust. Sci., 2005, 31, 283–307 CrossRef CAS PubMed.
- G. Benelli, D. Cumbo, M. Gazzino and E. Morgani, Pressurized oxy-combustion of coal with flue gas recirculation: pilot scale demonstration, Milan, Italy, 2008 Search PubMed.
- M. Gazzino, G. Riccio, N. Rossi and G. Benelli, presented in part at the The third international conference on energy sustainability, San Francisco, CA, USA, 2009 Search PubMed.
- J. Hong, R. Field, M. Gazzino and A. F. Ghoniem, Energy, 2010, 35, 5391–5399 CrossRef CAS PubMed.
- H. Stadler, F. Beggel, M. Habermehl, B. Persigehl, R. Kneer, M. Modigell and P. Jeschke, Int. J. Greenhouse Gas Control, 2011, 5, 7–15 CrossRef CAS PubMed.
- S. Engels, F. Beggel, M. Modigell and H. Stadler, J. Membr. Sci., 2010, 359, 93–101 CrossRef CAS PubMed.
- J. Wilson, M. Christie, N. Degenstein, M. Shah, J. Li, V. Venkateswaran, E. Eddings and J. Adams, OTM based oxycombustion for CO2 capture from coal power plants, Clearwater, FL, US, 2009 Search PubMed.
- S. G. Sundkvist, S. Julsrud, B. Viyeland, T. Naas, M. Budd, H. Leistner and D. Winkler, Int. J. Greenhouse Gas Control, 2007, 1, 180–187 CrossRef CAS.
- C. Buysse, A. Kovalevsky, F. Snijkers, A. Buekenhoudt, S. Mullens, J. Luyten, J. Kretzschmar and S. Lenaerts, J. Membr. Sci., 2010, 359, 86–92 CrossRef CAS PubMed.
- Air Products and Chemicals, Inc, Patent EP1676811A2, 2006.
- J. F. Vente, W. G. Haije, R. IJpelaan and F. T. Rusting, J. Membr. Sci., 2006, 278, 66–71 CrossRef CAS PubMed.
- S. Engels and M. Modigell, presented in part at the International Conferences on Inorganic Membranes, Tokyo, Japan, 2008 Search PubMed.
- H. L. Cao, S. Z. Sun, Y. H. Liu and T. F. Wall, Energy Fuels, 2010, 24, 131–135 CrossRef CAS.
- T. Wall, Y. H. Liu, C. Spero, L. Elliott, S. Khare, R. Rathnam, F. Zeenathal, B. Moghtaderi, B. Buhre, C. D. Sheng, R. Gupta, T. Yamada, K. Makino and J. L. Yu, Chem. Eng. Res. Des., 2009, 87, 1003–1016 CrossRef CAS PubMed.
- K. Andersson, F. Normann, F. Johnsson and B. Leckner, Ind. Eng. Chem. Res., 2008, 47, 1835–1845 CrossRef CAS.
- S. Z. Sun, H. L. Cao, H. Chen, X. Y. Wang, J. A. Qian and T. Wall, Proc. Combust. Inst., 2011, 33, 1731–1738 CrossRef CAS PubMed.
- C. R. Shaddix and A. Molina, Proc. Combust. Inst., 2011, 33, 1723–1730 CrossRef CAS PubMed.
- E. H. Chui, A. J. Majeski, M. A. Douglas, Y. Tan and K. V. Thambimuthu, Energy, 2004, 29, 1285–1296 CrossRef CAS PubMed.
- F. Normann, K. Andersson, B. Leckner and F. Johnsson, Prog. Energy Combust. Sci., 2009, 35, 385–397 CrossRef CAS PubMed.
- J. Y. Yan, M. Anheden, R. Faber, F. Starfelt, R. Preusche, H. Ecke, N. Padban, D. Kosel, N. Jentsch and G. Lindgren, 10th International Conference on Greenhouse Gas Control Technologies, 2011, vol. 4, pp. 900–907 Search PubMed.
- R. Stanger and T. Wall, Prog. Energy Combust. Sci., 2011, 37, 69–88 CrossRef CAS PubMed.
- E. Croiset and K. V. Thambimuthu, Fuel, 2001, 80, 2117–2121 CrossRef CAS.
- Y. W. Tan, E. Croiset, M. A. Douglas and K. V. Thambimuthu, Fuel, 2006, 85, 507–512 CrossRef CAS PubMed.
- T. Wall, R. Stanger and J. Maier, presented in part at the IEA GHG 1st Oxfuel Combustion Conference, Cottbus, Germany, 2009 Search PubMed.
- S. Santos, Challenges in Understanding the Fate of Mercury during Oxyfuel Combustion, http://www.ieaghg.org/docs/General_Docs/IEAGHG_Presentations/SSantos_-_Hg_Presentation_MEC7_-_June_18.pdf, accessed 30 March 2013.
- A. Suriyawong, M. Gamble, M. H. Lee, R. Axelbaum and P. Biswas, Energy Fuels, 2006, 20, 2357–2363 CrossRef CAS.
- L. G. Zheng and E. Furimsky, Fuel Process. Technol., 2003, 81, 23–34 CrossRef CAS.
- H. Farzan, D. McDonald, R. Varagani, N. Docquier and N. Perrin, presented in part at the 1st Oxy-Coal Combustion Conference Cottbus, Germany, 2009 Search PubMed.
- V. White, L. Torrente-Murciano, D. Sturgeon and D. Chadwick, Int. J. Greenhouse Gas Control, 2010, 4, 137–142 CrossRef CAS PubMed.
- T. Wall, R. Stanger and Y. H. Liu, Fuel, 2013, 108, 85–90 CrossRef CAS PubMed.
- K. E. Zanganeh, A. Shafeen, C. Salvador, A. Beigzadeh and M. Abbassi, 10th International Conference on Greenhouse Gas Control Technologies, 2011, vol. 4, pp. 1018–1025 Search PubMed.
- D. Kühnemuth, Absorption of NOx and SO2 in the flue gas treatment of an oxyfuel power plant, in Environmental Engineering, Chalmers University of Technology, 2007 Search PubMed.
- L. Torrente-Murciano, V. White and D. Chadwick, presented in part at the 1st international oxy-fuel combustion conference, Cottbus, Germany, 2009 Search PubMed.
- P. Edge, M. Gharebaghi, R. Irons, R. Porter, R. T. J. Porter, M. Pourkashanian, D. Smith, P. Stephenson and A. Williams, Chem. Eng. Res. Des., 2011, 89, 1470–1493 CrossRef CAS PubMed.
- L. Chen, S. Z. Yong and A. F. Ghoniem, Prog. Energy Combust. Sci., 2012, 38, 156–214 CrossRef CAS PubMed.
- R. Johansson, K. Andersson, B. Leckner and H. Thunman, Int. J. Heat Mass Transfer, 2010, 53, 220–230 CrossRef CAS PubMed.
- T. Wall, R. Stanger and S. Santos, Int. J. Greenhouse Gas Control, 2011, 5, S5–S15 CrossRef CAS PubMed.
- R. Faber, J. Y. Yan, F. Stark and S. Priesnitz, Int. J. Greenhouse Gas Control, 2011, 5, S210–S223 CAS.
- L. Stromberg, G. Lindgrena, J. Jacoby, R. Giering, M. Anheden, U. Burchhardt, H. Altmann, F. Kluger and G. N. Stamatelopoulos, Greenhouse Gas Control Technologies 9, 2009, vol. 1, pp. 581–589 Search PubMed.
- N. Aimard, C. Prebende, D. Cieutat, I. Sanchez-Molinero and R. Tsiava, presented in part at the 3rd Workshop IEA GHG International Oxy-fuel Combustion Network, Yokohama, Japan, 2008 Search PubMed.
- C. Spero, T. Yamada, E. Sturm and D. McGregor, presented in part at the 3rd Meeting of the Oxyfuel Combustion Network, Yokohama, Japan, 2008 Search PubMed.
- CanmetENERGY, Next Generation Oxyfuel Systems, http://canmetenergy.nrcan.gc.ca/clean-fossils-fuels/carbon-capture-storage/zero-emission-combustion/1790, accessed 30 March 2013.
- Power Technology, 2011.
- R. T. Dahowski, J. J. Dooley, C. L. Davidson and N. Mahasenan, in Greenhouse Gas Control Technologies 7, ed. E. S. Rubin, D. W. Keith, C. F. Gilboy, M. Wilson, T. Morris, J. Gale and K. Thambimuthu, Elsevier Science Ltd, Oxford, 2005, pp. 2459–2462 Search PubMed.
- L. Yaverbaum, Fluidized bed combustion of coal and waste materials, Noyes Data Corp., 1977 Search PubMed.
- G. N. Liljedahl, D. G. Turek, N. Y. Nsakala, N. C. Mohn and T. E. Fout, Alstom's Oxygen-Fired CFB Technology Development Status for CO2 Mitigation, 31st International Technical Conference on Coal Utilization and Fuel Systems, Clearwater, FL, USA, May 21–25, 2006 Search PubMed.
- V. M. Supranov, V. A. Batorshin, A. V. Shtegman and D. A. Mel'nikov, Therm. Eng., 2012, 59, 580–588 CrossRef.
- T. Wall, Y. Liu and S. Bhattacharya, A Scoping Study on Oxy-CFB Technology as an Alternative Carbon Capture Option for Australian Black and Brown Coals, ANLEC R&D, Monash University, 2012 Search PubMed.
- M. Zieba, 2nd International Workshop on Oxyfuel FBC Technology, http://www.eu-projects.de/Default.aspx?alias=www.eu-projects.de/oxyfbc%26;, accessed 17 September 2012.
- L. Jia, Y. Tan, C. Wang and E. J. Anthony, Energy Fuels, 2007, 21, 3160–3164 CrossRef CAS.
- L. Jia, Y. Tan and E. J. Anthony, Energy Fuels, 2010, 24, 910–915 CrossRef CAS.
- Circulating Fluidized Beds, ed. J. R. Grace, A. A. Avidan and T. M. Knowlton, Blackie Academic & Professional, 1997 Search PubMed.
- E. J. Anthony, Fluidized Bed Combustion of Waste Fuels Contaminated with Chlorine or Sulphur, Proceedings of the 21st International Conference on Fluidized Bed Combustion, Naples, Italy, 2012, pp. 3–11 Search PubMed.
- L. Duan, C. Zhao, W. Zhou, C. Qu and X. Chen, Fuel Process. Technol., 2011, 92, 379–384 CrossRef CAS PubMed.
- H. Hack, M. Lupion, A. Hotta, J. Lantto, R. Kuivalainen and J. Alvarez, presented in part at the The 37th International Technical Conference on Clean Coal and Fuel Systems, Clearwater, FL, USA, June 3–7, 2012 Search PubMed.
- M. C. Stewart, V. Manovic, E. J. Anthony and A. Macchi, Environ. Sci. Technol., 2010, 44, 8781–8786 CrossRef CAS PubMed.
- L. Jia, Y. Tan, Y. Wu and E. J. Anthony, Co-firing of Coal and Biomass in a Pilot-Scale Oxyfuel CFB, 64th IEA FBC Workshop, Naples, Italy, June 3, 2012 Search PubMed.
- J. Ahn, R. Okerlund, A. Fry and E. G. Eddings, Int. J. Greenhouse Gas Control, 2011, 5(suppl. 1), S127–S135 CrossRef CAS PubMed.
- O. Font, P. Córdoba, C. Leiva, L. M. Romeo, I. Bolea, I. Guedea, N. Moreno, X. Querol, C. Fernandez and L. I. Díez, Fuel, 2012, 95, 272–281 CrossRef CAS PubMed.
- L. Jia, Y. Tan, Y. Wu and E. J. Anthony, Oxy-fuel Combustion Tests using a 0.8 MWth Pilot-Scale Circulating Fluidized Bed, Proceedings of the 21st International Conference on Fluidized Bed Combustion, Naples, Italy, June 3–6, 2012, pp. 381–388 Search PubMed.
- R. Kuivalainen, T. Eriksson, A. Hotta, A. S.-B. Sacristán, J. M. Jubitero, J. C. Ballesteros, M. Lupion, V. Cortes, B. Anthony, L. Jia, D. McCalden, Y. Tan, I. He, Y. Wu and R. Symonds, Development and Demonstration of Oxy-Fuel CFBC Technology, The 35th International Technical Conference on Clean Coal & Fuel Systems, Clearwater, FL, USA, June 6–10, 2010 Search PubMed.
- T. Eriksson, O. Sippu, A. Hotta, Z. Fan, J. A. Ruis, A. S.-B. Sacristán, J. M. Jubitero, J. C. Ballesteros, M. Shah, N. Prosser, J. Haley and R. Guidici, Development of Flexi-BurnTM CFB Technology Aiming at Fully Integrated CCS Demonstration, Power Gen Europe, Cologne, Germany, May 26–28, 2009 Search PubMed.
- H. Hack, I. Alvarez, R. Diego, M. Lupion, V. Cortes, A. Hotta, J. Lantto and R. Kuivalainen, Initial Operation of the CIUDEN Oxy-CFB Boiler Demonstration Project, The 11th Annual Carbon Capture, Utilization & Sequestration Conference, Pittsburgh, PA, USA, April 30–May 3, 2012 Search PubMed.
- S. L. Suraniti, N. y. Nsakala and S. L. Darling, Energy Procedia, 2009, 1, 543–548 CrossRef CAS PubMed.
- W. Lewis, E. Gilliland and M. Sweeney, Chem. Eng. Prog., 1951, 47, 251–256 CAS.
- W. K. Lewis and E. R. Gilliland, US Pat., No. 2,665,972, 1954.
- M. Ishida and H. Jin, J. Chem. Eng. Jpn., 1994, 27, 296–301 CrossRef CAS.
- M. Ishida, D. Zheng and T. Akehata, Energy, 1987, 12, 147–154 CrossRef CAS.
- A. Lyngfelt, B. Leckner and T. Mattisson, Chem. Eng. Sci., 2001, 56, 3101–3113 CrossRef CAS.
- P. Moldenhauer, M. Ryden, T. Mattisson and A. Lyngfelt, Int. J. Greenhouse Gas Control, 2012, 9, 1–9 CrossRef CAS PubMed.
- P. Moldenhauer, M. Ryden, T. Mattisson and A. Lyngfelt, Fuel Process. Technol., 2012, 104, 378–389 CrossRef CAS PubMed.
- A. Hoteit, A. Forret, W. Pelletant, J. Roesler and T. Gauthier, Oil Gas Sci. Technol., 2011, 66, 193–199 CrossRef CAS PubMed.
- R. K. Lyon and J. A. Cole, Combust. Flame, 2000, 121, 249–261 CrossRef CAS.
- Y. Cao, B. Casenas and W. P. Pan, Energy Fuels, 2006, 20, 1845–1854 CrossRef CAS.
- J. S. Dennis, S. A. Scott and A. N. Hayhurst, J. Energy Inst., 2006, 79, 187–190 CrossRef CAS PubMed.
- H. Leion, T. Mattisson and A. Lyngfelt, Fuel, 2007, 86, 1947–1958 CrossRef CAS PubMed.
- H. Leion, T. Mattisson and A. Lyngfelt, Int. J. Greenhouse Gas Control, 2008, 2, 180–193 CrossRef CAS.
- H. Leion, E. Jerndal, B. M. Steenari, S. Hermansson, M. Israelsson, E. Jansson, M. Johnsson, R. Thunberg, A. Vadenbo, T. Mattisson and A. Lyngfelt, Fuel, 2009, 88, 1945–1954 CrossRef CAS PubMed.
- J. Adánez, A. Abad, F. Garcia-Labiano, P. Gayan and L. de Diego, Prog. Energy Combust. Sci., 2012, 38, 215–282 CrossRef PubMed.
- A. Lyngfelt, Chemical-looping combustion of solid fuels – status of development, Appl. Energy Search PubMed , accepted.
- C. Arnold, Br. Pat., 636, 206, 1950.
- M. Rydén and A. Lyngfelt, Hydrogen and power production with integrated carbon dioxide capture by chemical-looping reforming, Vancouver, Canada, 2004 Search PubMed.
- M. Rydén, A. Lyngfelt and T. Mattisson, Fuel, 2006, 85, 1631–1641 CrossRef PubMed.
- L. F. de Diego, M. Ortiz, F. García-Labiano, J. Adánez, A. Abad and P. Gayán, J. Power Sources, 2009, 192, 27–34 CrossRef CAS PubMed.
- M. Rydén and A. Lyngfelt, Int. J. Hydrogen Energy, 2006, 31, 1271–1283 CrossRef PubMed.
- F. Mizia, S. Rossini, M. Cozzolino, U. Cornaro, S. Tlatlik, I. Kaus, E. Bakken and Y. Larring, in Carbon Dioxide Capture for Storage in Deep Geologic Formations – Results from the CO2 Capture Project, ed. L. I. Eide, CPL Press, Newbury, 2009, vol. 3 Search PubMed.
- L.-S. Fan, Chemical Looping Systems for Fossil Energy Conversions, John Wiley & Sons, 2010 Search PubMed.
- A. Lyngfelt and T. Mattisson, in Efficient Carbon Capture for Coal Power Plants, ed. D. Stolten and V. Scherer, WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim, 2011, ch. 17, pp. 475–504 Search PubMed.
- M. Hossain and H. de Lasa, Chem. Eng. Sci., 2008, 63, 4433–4451 CrossRef CAS PubMed.
- E. Jerndal, T. Mattisson and A. Lyngfelt, Chem. Eng. Res. Des., 2006, 84, 795–806 CrossRef CAS.
- A. Lambert, P. Briault and E. Comte, 10th International Conference on Greenhouse Gas Control Technologies, 2011, vol. 4, pp. 318–323 Search PubMed.
- M. Ryden, A. Lyngfelt, T. Mattisson, D. Chen, A. Holmen and E. Björgum, Int. J. Greenhouse Gas Control, 2008, 2, 21–36 CrossRef CAS.
- E. Ksepko, E. Talik and J. Figa, presented in part at the 25th Annual International Pittsburgh Coal Conference, Pittsburgh, PA, September 29–October 2, 2008 Search PubMed.
- A. Shulman, E. Cleverstam, T. Mattisson and A. Lyngfelt, Energy Fuels, 2009, 23, 5269–5275 CrossRef CAS.
- A. Shulman, E. Cleverstam, T. Mattisson and A. Lyngfelt, Fuel, 2011, 90, 941–950 CrossRef CAS PubMed.
- G. Azimi, M. Rydén, H. Leion, T. Mattisson and A. Lyngfelt, AIChE J., 2013, 59, 582–588 CrossRef CAS.
- M. Rydén, A. Lyngfelt and T. Mattisson, Int. J. Greenhouse Gas Control, 2011, 5, 356–366 CrossRef PubMed.
- T. Mattisson, A. Lyngfelt and P. Cho, Fuel, 2001, 80, 1953–1962 CrossRef CAS.
- R. Xiao, Q. Song, S. Zhang, W. Zheng and Y. Yang, Energy Fuels, 2010, 24, 1449–1463 CrossRef CAS.
- H. Gu, L. Shen, J. Xiao, S. Zhang and T. Song, Energy Fuels, 2011, 25, 446–455 CrossRef CAS.
- C. Linderholm, A. Lyngfelt, A. Cuadrat and E. Jerndal, Fuel, 2012, 102, 808–822 CrossRef CAS PubMed.
- L. Shen, M. Zheng, J. Xiao and R. Xiao, Combust. Flame, 2008, 154, 489–506 CrossRef CAS PubMed.
- Q. Song, R. Xiao, Z. Deng, W. Zheng, L. Shen and J. Xiao, Energy Fuels, 2008, 22, 3661–3672 CrossRef CAS.
- Q. Song, R. Xiao, Z. Deng, H. Zhang, L. Shen, J. Xiao and M. Zhang, Energy Convers. Manage., 2008, 49, 3178–3187 CrossRef CAS PubMed.
- Q. Song, R. Xiao, Z. Deng, L. Shen, J. Xiao and M. Zhang, Ind. Eng. Chem. Res., 2008, 47, 8148–8159 CrossRef CAS.
- H. Tian, Q. Guo and J. Chang, Energy Fuels, 2008, 22, 3915–3921 CrossRef CAS.
- Z. Deng, R. Xiao, B. Jin and Q. Song, Int. J. Greenhouse Gas Control, 2009, 3, 368–375 CrossRef CAS PubMed.
- H. Tian and Q. Guo, Ind. Eng. Chem. Res., 2009, 48, 5624–5632 CrossRef CAS.
- R. Xiao, Q. L. Song, W. G. Zheng, Z. Y. Deng, L. H. Shen and M. Y. Zhang, presented in part at the Proceedings of the 20th International Conference on Fluidized Bed Combustion, 2009 Search PubMed.
- M. Ortiz, P. Gayán, L. F. De Diego, F. García-Labiano, A. Abad, M. A. Pans and J. Adánez, J. Power Sources, 2011, 196, 4370–4381 CrossRef CAS PubMed.
- E. Jerndal, H. Leion, L. Axelsson, T. Ekvall, M. Hedberg, K. Johansson, M. Källén, R. Svensson, T. Mattisson and A. Lyngfelt, Oil & Gas Science and Technology – Revue IFP Energies nouvelles, 2011, 66, pp. 235–248 Search PubMed.
- H. Leion, T. Mattisson and A. Lyngfelt, Energy Fuels, 2009, 23, 2307–2315 CrossRef CAS.
- A. Fossdal, E. Bakken, B. A. Øye, C. Schøning, I. Kaus, T. Mokkelbost and Y. Larring, Int. J. Greenhouse Gas Control, 2011, 5, 483–488 CrossRef CAS PubMed.
- H. Leion, A. Lyngfelt, M. Johansson, E. Jerndal and T. Mattisson, Chem. Eng. Res. Des., 2008, 86, 1017–1026 CrossRef CAS PubMed.
- N. Berguerand and A. Lyngfelt, Int. J. Greenhouse Gas Control, 2008, 2, 169–179 CrossRef CAS PubMed.
- A. Cuadrat, A. Abad, F. García-Labiano, P. Gayán, L. F. de Diego and J. Adánez, Int. J. Greenhouse Gas Control, 2012, 6, 153–163 CrossRef CAS PubMed.
- A. R. Bidwe, F. Mayer, C. Hawthorne, A. Charitos, A. Schuster and G. Scheffknecht, Energy Procedia, 2011, 4, 433–440 CrossRef CAS PubMed.
- T. Pröll, K. Mayer, J. Bolhàr-Nordenkampf, P. Kolbitsch, T. Mattisson, A. Lyngfelt and H. Hofbauer, Energy Procedia, 2009, 1, 27–34 CrossRef PubMed.
- T. Mattisson, H. Leion and A. Lyngfelt, Fuel, 2009, 88, 683–690 CrossRef CAS PubMed.
- H. Leion, T. Mattisson and A. Lyngfelt, presented in part at the The Clearwater Coal Conference – The 33rd International Technical Conference on Coal Utilization & Fuel Systems, Clearwater, Florida, 2008 Search PubMed.
- A. Abad, I. Adánez-Rubio, P. Gayán, F. García-Labiano, L. F. de Diego and J. Adánez, Int. J. Greenhouse Gas Control, 2012, 6, 189–200 CrossRef CAS PubMed.
- E. Johansson, A. Lyngfelt, T. Mattisson and F. Johnsson, A circulating fluidized bed combustor system with inherent CO2 separation – application of chemical looping combustion, Niagara Falls, Ontario, 2002 Search PubMed.
- E. Johansson, A. Lyngfelt, T. Mattisson and F. Johnsson, Powder Technol., 2003, 134, 210–217 CrossRef CAS.
- B. Kronberger, E. Johansson, G. Löffer, T. Mattisson, A. Lyngfelt and H. Hofbauer, Chem. Eng. Technol., 2004, 27, 1318–1326 CrossRef CAS.
- B. Kronberger, A. Lyngfelt, G. Löffler and H. Hofbauer, Ind. Eng. Chem. Res., 2005, 44, 546–556 CrossRef CAS.
- M. Xu, N. Ellis, C. J. Lim and H. J. Ryu, Chem. Eng. Technol., 2009, 32, 404–409 CrossRef CAS.
- A. Bischi, O. Langorgen, I. Saanum, J. Bakken, M. Seljeskog, M. Bysveen, J. X. Morin and O. Bolland, Int. J. Greenhouse Gas Control, 2011, 5, 467–474 CrossRef CAS PubMed.
- P. Markström and A. Lyngfelt, Powder Technol., 2012, 222, 182–192 CrossRef PubMed.
- A. Lyngfelt, Oil & Gas Science and Technology – Revue d'IFP Energies Nouvelles, 2011, vol. 66, pp. 161–172 Search PubMed.
- A. Abad, J. Adánez, F. García-Labiano, L. F. de Diego and P. Gayán, Combust. Flame, 2010, 157, 602–615 CrossRef CAS PubMed.
- A. Abad, J. Adánez, F. García-Labiano, L. F. de Diego, P. Gayán and J. Celaya, Chem. Eng. Sci., 2007, 62, 533–549 CrossRef CAS PubMed.
- F. García-Labiano, L. F. de Diego, J. Adánez, A. Abad and P. Gayán, Ind. Eng. Chem. Res., 2004, 43, 8168–8177 CrossRef.
- T. Mattisson, E. Jerndal, C. Linderholm and A. Lyngfelt, Chem. Eng. Sci., 2011, 66, 4636–4644 CrossRef CAS PubMed.
- A. Cuadrat, A. Abad, P. Gayan, L. F. de Diego, F. Garcia-Labiano and J. Adanez, Fuel, 2012, 97, 536–551 CrossRef CAS PubMed.
- N. Berguerand, A. Lyngfelt, T. Mattisson and P. Markström, Oil & Gas Science and Technology – Revue IFP Energies Nouvelles, 2011, vol. 66, pp. 181–191 Search PubMed.
- K. Mahalatkar, J. Kuhlman, E. D. Huckaby and T. O'Brien, Chem. Eng. Sci., 2011, 66, 469–479 CrossRef CAS PubMed.
- P. Markström, N. Berguerand and A. Lyngfelt, Chem. Eng. Sci., 2010, 65, 5055–5066 CrossRef PubMed.
- J. Ströhle, M. Orth and B. Epple, Simulation of the fuel reactor of a 1 MWth chemical-looping plant for coal, Lyon, 2010 Search PubMed.
- J. Blamey, E. J. Anthony, J. Wang and P. S. Fennell, Prog. Energy Combust. Sci., 2010, 36, 260–279 CrossRef CAS PubMed.
- C. C. Dean, J. Blamey, N. H. Florin, M. J. Al-Jeboori and P. S. Fennell, Chem. Eng. Res. Des., 2011, 89, 836–855 CrossRef CAS PubMed.
- L.-S. Fan, L. Zeng, W. Wang and S. Luo, Energy Environ. Sci., 2012, 5, 7254–7280 CAS.
- W. Liu, H. An, C. Qin, J. Yin, G. Wang, B. Feng and M. Xu, Energy Fuels, 2012, 26, 2751–2767 CrossRef CAS.
- F. C. Yu, N. Phalak, Z. Sun and L.-S. Fan, Ind. Eng. Chem. Res., 2012, 51, 2133–2142 CrossRef CAS.
- G. P. Curran, C. E. Fink and E. Gorin, Adv. Chem. Ser., 1967, 69, 141–165 CrossRef CAS PubMed.
- D. P. Harrison, Ind. Eng. Chem. Res., 2008, 47, 6486–6501 CrossRef CAS.
- GB 2291051 (A), 1996.
- T. Shimizu, T. Hirama, H. Hosoda, K. Kitano, M. Inagaki and K. Tejima, Chem. Eng. Res. Des., 1999, 77, 62–68 CrossRef CAS.
- L. M. Romeo, S. Uson, A. Valero and J. M. Escosa, Int. J. Greenhouse Gas Control, 2010, 4, 647–654 CrossRef CAS PubMed.
- S. Lin, T. Kiga, Y. Wang and K. Nakayama, Energy Procedia, 2011, 4, 356–361 CrossRef CAS PubMed.
- D. Daval, O. Sissmann, N. Menguy, G. D. Saldi, F. Guyot, I. Martinez, J. Corvisier, B. Garcia, I. Machouk, K. G. Knauss and R. Hellmann, Chem. Geol., 2011, 284, 193–209 CrossRef CAS PubMed.
- A. Martinez, Y. Lara, P. Lisbona and L. M. Romeo, Int. J. Greenhouse Gas Control, 2012, 7, 74–81 CrossRef CAS PubMed.
- N. Rodriguez, R. Murillo and J. C. Abanades, Environ. Sci. Technol., 2012, 46, 2460–2466 CrossRef CAS PubMed.
- J. C. Abanades and J. E. Oakey, Int. Pat., CA 2479886, 2003.
- J. C. Abanades, J. E. Oakey, D. Alvarez and J. Hamalainen, Novel combustion cycles incorporating capture of CO2 with CaO, Kyoto, Japan, 2003 Search PubMed.
- I. Martinez, R. Murillo, G. Grasa, N. Rodriguez and J. C. Abanades, Int. J. Greenhouse Gas Control, 2011, 5, 498–504 CrossRef CAS PubMed.
- J. Ströle, A. Lasheras, A. Galloy and B. Epple, Chem. Eng. Technol., 2009, 32, 435–442 CrossRef.
- C. C. Dean, D. Dugwell and P. S. Fennell, Energy Environ. Sci., 2011, 2050–2053 CAS.
- N. Rodriguez, M. Alonso and J. C. Abanades, AIChE J., 2011, 57, 1356–1366 CrossRef CAS.
- M. Alonso, N. Rodriguez, B. Gonzalez, G. Grasa, R. Murillo and J. C. Abanades, Int. J. Greenhouse Gas Control, 2010, 4, 167–173 CrossRef CAS PubMed.
- J. C. Abanades, M. Alonso and N. Rodriguez, Ind. Eng. Chem. Res., 2011, 50, 6972–6981 CrossRef CAS.
- M. Alonso, N. Rodriguez, B. Gonzalez, B. Arias and J. C. Abanades, Ind. Eng. Chem. Res., 2011, 50, 6982–6989 CrossRef CAS.
- A. Charitos, C. Hawthorne, A. R. Bidwe, S. Sivalingam, A. Schuster, H. Spliethoff and G. Scheffknecht, Int. J. Greenhouse Gas Control, 2010, 4, 776–784 CrossRef CAS PubMed.
- C. Hawthorne, H. Dieter, A. Bidwe, A. Schuster, G. Scheffknecht, S. Unterberger and M. Käβ, Energy Procedia, 2011, 4, 441–448 CrossRef CAS PubMed.
- R. T. Symonds, D. Y. Lu, V. Manovic and E. J. Anthony, Ind. Eng. Chem. Res., 2012, 51, 7177–7184 CrossRef CAS.
- W. Wang, S. Ramkumar, D. W. S. Li, M. Iyer, B. Sakadjian, R. M. Statnick and L. S. Fan, Ind. Eng. Chem. Res., 2010, 49, 5094–5101 CrossRef CAS.
- W. Wang, S. Ramkumar, D. Wong and L. S. Fan, Fuel, 2012, 92, 94–106 CrossRef CAS PubMed.
- C. M. Huang, H. W. Hsu, W. H. Liu, J. Y. Cheng, W. C. Chen, T. W. Wen and W. Chen, Energy Procedia, 2011, 4, 1268–1275 CrossRef CAS PubMed.
- B. Arias, M. E. Diego, J. C. Abanades, M. Lorenzo, L. Diaz, D. Martínez, J. Alvarez and A. Sánchez-Biezma, Int. J. Greenhouse Gas Control, 2013, 18, 237–245 CrossRef CAS PubMed.
- G. Grasa and J. C. Abanades, Ind. Eng. Chem. Res., 2006, 45, 8846–8851 CrossRef CAS.
- A. Galloy, A. J. Ströhle and B. Epple, in VGB PowerTech, 2011, vol. 9, pp. 64–68 Search PubMed.
- J. Kremer, A. Galloy, J. Ströhle and B. Epple, presented in part at the 2nd Int. Conference on Chemical Looping, Darmstadt, Germany, September, 2012 Search PubMed.
- J. R. Chamberlain and C. P. Ros, presented in part at the ICCS&T, Oviedo, Spain, October, 2011 Search PubMed.
- A. Charitos, N. Rodriguez, C. Hawthorne, M. Alonso, M. Zieba, B. Arias, G. Kopanakis, G. Scheffknecht and J. C. Abanades, Ind. Eng. Chem. Res., 2011, 50, 9685–9695 CrossRef CAS.
- M. C. Romano, Chem. Eng. Sci., 2012, 69, 257–269 CrossRef CAS PubMed.
- J. Yälato, J. Ritvanen, B. Arias, T. Tynjälä and T. Hyppänen, Int. J. Greenhouse Gas Control, 2012, 9, 130–135 CrossRef PubMed.
- A. Lasheras, J. Strohle, A. Galloy and B. Epple, Int. J. Greenhouse Gas Control, 2011, 5, 686–693 CrossRef CAS PubMed.
- S. Takkinen, J. Saastamoinen and T. Hyppänen, AIChE J., 2012, 58, 2563–2572 CrossRef CAS.
- J. Solsvik and H. A. Jakobsen, Chem. Eng. J., 2011, 178, 407–422 CrossRef CAS PubMed.
- K. R. Rout and H. A. Jakobsen, Fuel Process. Technol., 2012, 99, 13–34 CrossRef CAS PubMed.
- Y. F. Wang, Z. X. Chao, D. Chen and H. A. Jakobsen, Int. J. Greenhouse Gas Control, 2011, 5, 489–497 CrossRef CAS PubMed.
- L. He, H. Berntsen and D. Chen, J. Phys. Chem. A, 2010, 114, 3834–3844 CrossRef CAS PubMed.
- A. L. da Silva and I. L. Muller, Int. J. Hydrogen Energy, 2011, 36, 2057–2075 CrossRef PubMed.
- X. D. Wang, N. Wang and L. A. Wang, Int. J. Hydrogen Energy, 2011, 36, 466–472 CrossRef CAS PubMed.
- M. C. Romano, E. N. Cassotti, P. Chiesa, J. Meyer and J. Mastin, Energy Procedia, 2011, 4, 1125–1132 CrossRef CAS PubMed.
- J. Meyer, J. Mastin, T. K. Bjornebole, T. Ryberg and N. Eldrup, Energy Procedia, 2011, 4, 1949–1956 CrossRef CAS PubMed.
- N. H. Florin and A. T. Harris, AIChE J., 2008, 54, 1096–1109 CrossRef CAS.
- S. Lin, T. Kiga, K. Nakayama and Y. Suzuki, Energy Procedia, 2011, 4, 378–384 CrossRef CAS PubMed.
- T. Weimer, R. Berger, C. Hawthorne and J. C. Abanades, Fuel, 2008, 87, 1678–1686 CrossRef CAS PubMed.
- S. Ramkumar, M. V. Iyer and L. S. Fan, Ind. Eng. Chem. Res., 2011, 50, 1716–1729 CrossRef CAS.
- N. H. Florin and A. T. Harris, Chem. Eng. Sci., 2008, 63, 287–316 CrossRef CAS PubMed.
- T. Proll, R. Rauch, C. Aichernig and H. Hofbauer, Int. J. Chem. React. Eng., 2007, 5, DOI:10.2202/1542-6580.1398.
- V. Dupont, A. B. Ross, E. Knight, I. Hanley and M. V. Twigg, Chem. Eng. Sci., 2008, 63, 2966–2979 CrossRef CAS PubMed.
- J. R. Fernandez, J. C. Abanades, R. Murillo and G. Grasa, Int. J. Greenhouse Gas Control, 2012, 6, 126–141 CrossRef CAS PubMed.
- I. L. Martínez, R. Murillo, G. Grasa, J. R. Fernández and J. C. Abanades, Integrated combined cycle from natural gas with CO2 capture using a Ca–Cu chemical loop, AIChE J., 2013, 59, 2750–2794 CrossRef.
- J. R. Fernandez, J. C. Abanades and R. Murillo, Chem. Eng. Sci., 2012, 84, 1–11 CrossRef CAS PubMed.
- J. R. Fernandez, J. C. Abanades and G. Grasa, Chem. Eng. Sci., 2012, 84, 12–20 CrossRef CAS PubMed.
- V. Manovic and E. J. Anthony, Energy Fuels, 2011, 25, 4846–4853 CrossRef CAS.
- A. M. Kierzkowska and C. R. Muller, Energy Environ. Sci., 2012, 5, 6061–6065 CAS.
- V. Manovic and E. J. Anthony, Ind. Eng. Chem. Res., 2010, 49, 9105–9110 CrossRef CAS.
- F. Donat, N. H. Florin, E. J. Anthony and P. S. Fennell, Environ. Sci. Technol., 2011, 46, 1262–1269 CrossRef PubMed.
- Z. S. Li, F. Fang, X. Y. Tang and N. S. Cai, Energy Fuels, 2012, 26, 2473–2482 CrossRef CAS.
- I. Martinez, G. Grasa, R. Murillo, B. Arias and J. C. Abanades, Energy Fuels, 2012, 26, 1432–1440 CrossRef CAS.
- B. Arias, J. M. Cordero, M. Alonso and J. C. Abanades, AIChE J., 2012, 58, 2262–2269 CrossRef CAS.
- V. Manovic and E. J. Anthony, Energy Fuels, 2010, 24, 1414–1420 CrossRef CAS.
- F. Scala and P. Salatino, Fuel, 2010, 89, 827–832 CrossRef CAS PubMed.
- B. Gonzalez, M. Alonso and J. C. Abanades, Fuel, 2010, 89, 2918–2924 CrossRef CAS PubMed.
- P. Lisbona, A. Martinez, Y. Lara and L. M. Romeo, Energy Fuels, 2010, 24, 728–736 CrossRef CAS.
- V. Materic and S. I. Smedley, Ind. Eng. Chem. Res., 2011, 50, 5927–5932 CrossRef CAS.
- I. Martinez, G. Grasa, R. Murillo, B. Arias and J. C. Abanades, Energy Fuels, 2011, 25, 1294–1301 CrossRef CAS.
- P. S. Fennell, J. F. Davidson, J. S. Dennis and A. N. Hayhurst, J. Energy Inst., 2007, 80, 116–119 CrossRef CAS PubMed.
- M. J. Al-Jeboori, M. Nguyen, C. Dean and P. S. Fennell, Ind. Eng. Chem. Res., 2013, 52, 1426–1433 CrossRef CAS.
- H. C. Chen and C. S. Zhao, Chem. Eng. J., 2011, 171, 197–205 CrossRef CAS PubMed.
- R. Filitz, A. M. Kierzkowska, M. Broda and C. R. Muller, Environ. Sci. Technol., 2012, 46, 559–565 CrossRef CAS PubMed.
- P. Gruene, A. G. Belova, T. M. Yegulalp, R. J. Farrauto and M. J. Castaldi, Ind. Eng. Chem. Res., 2011, 50, 4042–4049 CrossRef CAS.
- V. Manovic and E. J. Anthony, Ind. Eng. Chem. Res., 2010, 49, 6916–6922 CrossRef CAS.
- N. H. Florin, J. Blamey and P. S. Fennell, Energy Fuels, 2010, 24, 4598–4604 CrossRef CAS.
- M. Broda and C. R. Muller, Adv. Mater., 2012, 24, 3059–3064 CrossRef CAS PubMed.
- B. Arias, G. S. Grasa, M. Alonso and J. C. Abanades, Energy Environ. Sci., 2012, 5, 7353–7359 CAS.
- N. Hedin, L. Chen and A. Laaksonen, Nanoscale, 2010, 2, 1819–1841 RSC.
- K. T. Chue, J. N. Kim, Y. J. Yoo and S. H. Cho, Ind. Eng. Chem. Res., 1995, 34, 591–598 CrossRef CAS.
- P. J. E. Harlick and F. H. Tezel, Microporous Mesoporous Mater., 2004, 76, 71–79 CrossRef CAS PubMed.
- R. V. Siriwardane, M. S. Shen and E. P. Fisher, Energy Fuels, 2003, 17, 571–576 CrossRef CAS.
- R. V. Siriwardane, M. S. Shen and E. P. Fisher, Energy Fuels, 2005, 19, 1153–1159 CrossRef CAS.
- S. Cavenati, C. A. Grande and A. E. Rodrigues, J. Chem. Eng. Data, 2004, 49, 1095–1101 CrossRef CAS.
- S. Dasgupta, N. Biswas, Aarti, N. G. Gode, S. Divekar, A. Nanoti and A. N. Goswami, Int. J. Greenhouse Gas Control, 2012, 7, 225–229 CrossRef CAS PubMed.
- H. Yi, H. Deng, X. Tang, Q. Yu, X. Zhou and H. Liu, J. Hazard. Mater., 2012, 203–204, 111–117 CrossRef CAS PubMed.
- H. Deng, H. Yi, X. Tang, Q. Yu, P. Ning and L. Yang, Chem. Eng. J., 2012, 188, 77–85 CrossRef CAS PubMed.
- F. Brandani, D. M. Ruthven and C. G. Coe, Ind. Eng. Chem. Res., 2003, 42, 1451–1461 CrossRef CAS.
- D. M. Ruthven and F. Brandani, Ind. Eng. Chem. Res., 2004, 43, 8339–8344 CrossRef.
- G. Li, P. Xiao, P. Webley, J. Zhang, R. Singh and M. Marshall, Adsorption, 2008, 14, 415–422 CrossRef CAS.
- K. M. Lee, Y. H. Lim and Y. M. Jo, Environ. Technol., 2012, 33, 77–84 CrossRef CAS.
- F. N. Ridha and P. A. Webley, J. Colloid Interface Sci., 2009, 337, 332–337 CrossRef CAS PubMed.
- S. T. Yang, J. Kim and W. S. Ahn, Microporous Mesoporous Mater., 2010, 135, 90–94 CrossRef CAS PubMed.
- M. Palomino, A. Corma, J. L. Jordá, F. Rey and S. Valencia, Chem. Commun., 2012, 48, 215–217 RSC.
- Magdalena M. Lozinska, Enzo Mangano, John P. S. Mowat, Ashley M. Shepherd, Russell F. Howe, Stephen P. Thompson, Julia E. Parker, Stefano Brandani and Paul A. Wright, Understanding Carbon Dioxide Adsorption on Univalent Cation Forms of the Flexible Zeolite Rho at Conditions Relevant to Carbon Capture from Flue Gases, J. Am. Chem. Soc., 2012, 134(42), 17628–17642 CrossRef CAS PubMed.
- G. D. Pirngruber, P. Raybaud, Y. Belmabkhout, J. Cejka and A. Zukal, Phys. Chem. Chem. Phys., 2010, 12, 13534–13546 RSC.
- K. S. Walton, M. B. Abney and M. D. LeVan, Microporous Mesoporous Mater., 2006, 91, 78–84 CrossRef CAS PubMed.
- G. M. Johnson, B. A. Reisner, A. Tripathi, D. R. Corbin, B. H. Toby and J. B. Parise, Chem. Mater., 1999, 11, 2780–2787 CrossRef CAS.
- J. B. Parise, L. Abrams, T. E. Gier and D. R. Corbin, J. Phys. Chem., 1984, 88, 2303–2307 CrossRef CAS.
- D. R. Corbin, L. Abrams, G. A. Jones, M. M. Eddy, G. D. Stucky and D. E. Cox, Flexibility of the zeolite rho framework. Neutron powder structural characterization of Ca-exchanged zeolite rho, J. Chem. Soc., Chem. Commun., 1989, 42–43 Search PubMed.
- M. L. Smith, D. R. Corbin, L. Abrams and C. Dybowski, J. Phys. Chem., 1993, 97, 7793–7795 CrossRef CAS.
- J. B. Parise, T. E. Gier, D. R. Corbin and D. E. Cox, J. Phys. Chem., 1984, 88, 1635–1640 CrossRef CAS.
- L. B. McCusker, Acta Crystallogr., Sect. A: Found. Crystallogr., 1991, 47, 297–313 CrossRef.
- C. W. Jones, S. Choi and J. H. Drese, ChemSusChem, 2009, 2, 796–854 CrossRef PubMed.
- P. D. C. Dietzel, V. Besikiotis and R. Blom, J. Mater. Chem., 2009, 19, 7362–7370 RSC.
- A. R. Millward and O. M. Yaghi, J. Am. Chem. Soc., 2005, 127, 17998–17999 CrossRef CAS PubMed.
- M. D. LeVan, J. Liu, Y. Wang, A. I. Benin, P. Jakubczak and R. R. Willis, Langmuir, 2010, 26(17), 14301–14307 CrossRef PubMed.
- D. A. Yang, H. Y. Cho, J. Kim, S. T. Yang and W. S. Ahn, Energy Environ. Sci., 2012, 5, 6465–6473 CAS.
- S. R. Caskey, A. G. Wong-Foy and A. J. Matzger, J. Am. Chem. Soc., 2008, 130, 10870–10871 CrossRef CAS PubMed.
- X. Hu, PhD, University of Edinburgh, 2012.
- Z. R. Herm, R. Krishna and J. R. Long, Microporous Mesoporous Mater., 2012, 151, 481–487 CrossRef CAS PubMed.
- L. Valenzano, B. Civalleri, S. Chavan, G. T. Palomino, C. O. Areán and S. Bordiga, J. Phys. Chem., 2010, 114, 11185–11191 CAS.
- L. Valenzano, J. G. Vitillo, S. Chavana, B. Civalleri, F. Bonino, S. Bordiga and C. Lamberti, Catal. Today, 2012, 182, 67–79 CrossRef CAS PubMed.
- J. Liu, J. Tian, P. K. Thallapally and B. P. McGrail, J. Phys. Chem., 2012, 116, 9575–9581 CAS.
- J. A. Poston, R. V. Siriwardane, M. Shen and E. P. Fisher, Energy Fuels, 2001, 15, 279–284 CrossRef.
- S. Himeno, T. Komatsu and S. Fujita, J. Chem. Eng. Data, 2005, 50, 369–376 CrossRef CAS.
- C. Shen, C. A. Grande, P. Li, J. Yua and A. E. Rodrigues, Chem. Eng. J., 2010, 160, 398–407 CrossRef CAS PubMed.
- J. D. Carruthers, M. A. Petruska, E. A. Sturm and S. M. Wilson, Microporous Mesoporous Mater., 2012, 154, 62–67 CrossRef PubMed.
- C. Shen, Z. Liu, P. Li and J. Yu, Ind. Eng. Chem. Res., 2012, 51, 5011–5021 CrossRef CAS.
- C. Shen, J. Yu, P. Li, C. A. Grande and A. E. Rodrigues, Adsorption, 2011, 17, 179–188 CrossRef CAS.
- A. Samanta, A. Zhao, G. K. H. Shimizu, P. Sarkar and R. Gupta, Ind. Eng. Chem. Res., 2012, 51, 1438–1463 CrossRef CAS.
- M. M. Maroto-Valer, Z. Lu, Y. Zhang and Z. Tang, Waste Manag., 2008, 28, 2320–2328 CrossRef PubMed.
- M. M. Gui, Y. X. Yap, S. P. Chai and A. R. Mohamed, Int. J. Greenhouse Gas Control, 2013, 14, 65–73 CrossRef CAS PubMed.
- F. Su, C. Lu and H.-S. Chen, Langmuir, 2011, 27, 8090–8098 CrossRef CAS PubMed.
- C. Lu, H. Bai, B. Wu, F. Su and J. F. Hwang, Energy Fuels, 2008, 22, 3050–3056 CrossRef CAS.
- G. Qi, Y. Wang, L. Estevez, X. Duan, N. Anako, A. H. A. Park, W. Li, C. W. Jones and E. P. Giannelis, Energy Environ. Sci., 2011, 4, 444–452 CAS.
- V. Zeleňák, M. Badaničová, D. Halamová, J. Čejka, A. Zukal, N. Murafa and G. Goerigk, Chem. Eng. J., 2008, 144, 336–342 CrossRef PubMed.
- R. Serna-Guerrero, Y. Belmabkhout and A. Sayari, Chem. Eng. J., 2010, 158, 513–519 CrossRef CAS PubMed.
- Y. Belmabkhout, R. Serna-Guerrero and A. Sayari, Ind. Eng. Chem. Res., 2010, 49, 359–365 CrossRef CAS.
- Y. Belmabkhout and A. Sayari, Adsorption, 2009, 15, 318–328 CrossRef CAS PubMed.
- X. C. Xu, C. S. Song, J. M. Andresen, B. G. Miller and A. W. Scaroni, Microporous Mesoporous Mater., 2003, 62, 29–45 CrossRef CAS.
- X. C. Xu, C. S. Song, J. M. Andresen, B. G. Miller and A. W. Scaroni, Int. J. Environ. Technol. Manage., 2004, 4, 32–52 CAS.
- A. Qader, B. Hooper, T. Innocenzi, G. Stevens, S. Kentish, C. Scholes, K. Mumford, K. Smith, P. A. Webley and J. Zhang, 10th International Conference on Greenhouse Gas Control Technologies, 2011, vol. 4, pp. 1668–1675 Search PubMed.
- V. Rama Rao, S. Krishnamurthy, S. K. Guntuka, A. Rajendran, M. A. Ullah, P. Sharratt, I. A. Karimi and S. Farooq, A Pilot Plant Study of a VSA Process for CO2 Capture From Power Plant Flue Gas, Pittsburgh, PA, 2012 Search PubMed.
- S. Sjostroma, H. Krutkaa, T. Starnsa and T. Campbella, Energy Procedia, 2011, 4, 1584–1592 CrossRef PubMed.
- ADA-ES, Evaluation of Solid Sorbents as a Retrofit Technology for CO2 Capture, http://www.netl.doe.gov/publications/proceedings/12/co2capture/presentations/2-Tuesday/2-Travis%20Starns-ADA.pdf, accessed 24 April 2013.
- Inventys, http://www.inventysinc.com/, accessed 24 April 2013.
- Energy Technologies Institute (ETI), Ed Davey announces new £20 million ETI project set to advance carbon capture technologies, http://www.internationalsustainableenergy.com/6666/news/ed-davey-announces-new-20-million-eti-project-set-to-advance-carbon-capture-technologies/, accessed 24 April 2013.
- G. Krishnan, Development Novel Carbon Sorbents for Carbon Dioxide Capture, http://www.atmi-brightblack.com/uploads/document/doe–-netl-co2-capture-presentation_sri_atmi.pdf, accessed 24 April 2013.
- N. McGlashan, N. Shah, B. Caldecott and M. Workman, Process Saf. Environ. Prot., 2012, 90, 501–510 CrossRef CAS PubMed.
- F. Zeman, Environ. Sci. Technol., 2007, 41, 7558–7563 CrossRef CAS.
- R. Carey, A. Gomezplata and A. Sarich, Ocean Eng., 1983, 10, 227–233 CrossRef.
- L. A. DallBauman and J. E. Finn, Adsorption and Its Applications in Industry and Environmental Protection, Vol Ii: Applications in Environmental Protection, 1999, vol. 120, pp. 455–471 Search PubMed.
- W. F. Castle, in Cryogenic Engineering, ed. K. Timmerhaus and R. Reed, Springer, New York, 2007, ch. 8, pp. 146–160 Search PubMed.
- K. S. Lackner, S. Brennan, J. M. Matter, A. H. A. Park, A. Wright and B. van der Zwaan, Proc. Natl. Acad. Sci. U. S. A., 2012, 109, 13156–13162 CrossRef CAS PubMed.
- A. Brunetti, F. Scura, G. Barbieri and E. Drioli, J. Membr. Sci., 2010, 359, 115–125 CrossRef CAS PubMed.
- A. Goeppert, M. Czaun, G. K. S. Prakash and G. A. Olah, Energy Environ. Sci., 2012, 5, 7833–7853 CAS.
- G. Holmes and D. W. Keith, Philos. Trans. R. Soc., A, 2012, 370, 4380–4403 CrossRef CAS PubMed.
- R. B. Polak and M. Steinberg, Pat., US20120003722A1, 2012.
- J. K. Stolaroff, D. W. Keith and G. V. Lowry, Environ. Sci. Technol., 2008, 42, 2728–2735 CrossRef CAS.
- R. Baciocchi, G. Storti and M. Mazzotti, Chem. Eng. Process., 2006, 45, 1047–1058 CrossRef CAS PubMed.
- D. Keith, M. Ha-Duong and J. Stolaroff, Clim. Change, 2006, 74, 17–45 CrossRef CAS.
- G. Simbolotti, IEA ETSAP – Technology Brief E14 – CO2 Capture & Storage, Internation Energy Association Energy Technology Network, 2010 Search PubMed.
- R. Socolow, M. Desmond, R. Aines, J. Blackstock, O. Bolland, T. Kaarsberg, N. Lewis, M. Mazzotti, A. Pfeffer and K. Sawyer, Report, American Physical Society (APS), 2011 Search PubMed.
- K. Z. House, A. C. Baclig, M. Ranjan, E. A. van Nierop, J. Wilcox and H. J. Herzog, Proc. Natl. Acad. Sci. U. S. A., 2011, 108, 20428–20433 CrossRef CAS PubMed.
- D. Keith and G. Holmes, Comments on the APS report onDirect Air Capture, http://www.carbonengineering.com/wp-content/uploads/2011/05/CE_APS_DAC_Comments.pdf, accessed 03 January 2013.
- E. Dahlquist and A. Jones, Tappi J., 2005, 4, 15 CAS.
- S. Choi, J. H. Drese, P. M. Eisenberger and C. W. Jones, Environ. Sci. Technol., 2011, 45, 2420–2427 CrossRef CAS PubMed.
- K. S. Lackner, Eur. Phys. J.: Spec. Top., 2009, 176, 93–106 CrossRef.
- S. Brandani, Energy Environ., 2012, 23, 319–328 Search PubMed.
- DECC, Electricity Market Reform, http://www.decc.gov.uk/en/content/cms/meeting_energy/renewable_ener/re_roadmap/re_roadmap.aspx, accessed 07 January 2013.
- DECC, UK Renewable Energy Roadmap, http://www.decc.gov.uk/en/content/cms/meeting_energy/markets/electricity/electricity.aspx, accessed 07 January 2013.
- D. S. Remer and A. P. Nieto, Int. J. Prod. Econ., 1995, 42, 79–96 CrossRef.
- D. S. Remer and A. P. Nieto, Int. J. Prod. Econ., 1995, 42, 101–129 CrossRef.
- V. Asthana and P. K. Panigrahi, presented in part at the 5th European Thermal Sciences Conference, The Netherlands, 2008 Search PubMed.
- T. Sanpasertparnich, R. Idem, I. Bolea, D. deMontigny and P. Tontiwachwuthikul, Int. J. Greenhouse Gas Control, 2010, 4, 499–510 CrossRef CAS PubMed.
- M. B. Shiflett, D. W. Drew, R. A. Cantini and A. Yokozeki, Energy Fuels, 2010, 24, 5781–5789 CrossRef CAS.
- D. Singh, E. Croiset, P. L. Douglas and M. A. Douglas, Energy Convers. Manage., 2003, 44, 3073–3091 CrossRef CAS.
- M. Lucquiaud and J. Gibbins, Chem. Eng. Res. Des., 2011, 89, 1553–1571 CrossRef CAS PubMed.
- L. Q. Duan, M. D. Zhao and Y. P. Yang, Energy, 2012, 45, 107–116 CrossRef CAS PubMed.
- H. Chalmers and J. Gibbins, Fuel, 2007, 86, 2109–2123 CrossRef CAS PubMed.
- A. Arce, N. Mac Dowell and N. Shah, Int. J. Greenhouse Gas Control, 2012, 11, 236–250 CrossRef PubMed.
- B. Metz and I. P. o. C. C. W. G. III., Carbon Dioxide Capture And Storage: IPCC Special Report, Cambridge University Press, 2005 Search PubMed.
- A. Aspelund, M. J. Mølnvik and G. De Koeijer, Chem. Eng. Res. Des., 2006, 84, 847–855 CrossRef CAS.
- J. Watt, Journal of Pipeline Engineering, 2010, 9, 213–222 Search PubMed.
- R. Steeneveldt, B. Berger and T. A. Torp, Chem. Eng. Res. Des., 2006, 84, 739–763 CrossRef CAS.
- A. Bradt, Maersk Tankers – a pioneer in CO2 shipping, Carbon Capture Journal, 2010, http://www.carboncapturejournal.com/news/maersk-tankers-a-pioneer-in-co2-shipping/2714.aspx?Category=all Search PubMed.
- M. Mohitpour, A. Jenkins and G. Nahas, Journal of Pipeline Engineering, 2008, 7, 237–251 Search PubMed.
- E. de Visser, C. Hendriks, M. Barrio, M. J. Mølnvik, G. de Koeijer, S. Liljemark and Y. Le Gallo, Int. J. Greenhouse Gas Control, 2008, 2, 478–484 CrossRef CAS PubMed.
- M. Anheden, A. Andersson, C. Bernstone, S. Eriksson, S. S. Liljemark and C. Wall, CO2 quality requirement for a system with CO2 capture, transport and storage, Vancouver, 2005 Search PubMed.
- J. M. Race, B. Wetenhall, P. N. Seevam and M. J. Downie, Journal of Pipeline Engineering, 2012, 11, 173–190 Search PubMed.
- P. N. Seevam, J. M. Race and M. J. Downie, Journal of Pipeline Engineering, 2007, 6, 140–141 Search PubMed.
- J. Serpa, J. Morbee and E. Tzimas, Technical and Economic Characteristics of a CO2 Transmission Pipeline Infrastructure, European Commission Joint Research Centre Institute for Energy, Petten, Netherlands, 2011 Search PubMed.
- J. Yan, M. Anheden, C. Liljemark, S. Bemstone, H. Pettersson, D. Simonsoon and H. Li, presented in part at the IEA CO2 Specification Working Group Meeting, Stockholm, October 2008 Search PubMed.
- Y.-S. Choi and S. Nešić, Int. J. Greenhouse Gas Control, 2011, 5, 788–797 CrossRef CAS PubMed.
- S. Papavinasam, J. Li, A. Doiron, J. Krausher, C. Shi, J.-P. Gravel, K. Zanganesh, D. Emadi, C. Salvador, A. Shafeen and A. Pratt, presented in part at the NACE International 2012 Corrosion Conference and Expo, 2012 Search PubMed.
- D. Sandana, M. Hadden, J. Race and E. A. Charles, Journal of Pipeline Engineering, 2012, 11, 229–238 Search PubMed.
- I. S. Cole, P. Corrigan, S. Sim and N. Birbilis, Int. J. Greenhouse Gas Control, 2011, 5, 749–756 CrossRef CAS PubMed.
- A. S. Ruhl and A. Kranzmann, J. Supercrit. Fluids, 2012, 68, 81–86 CrossRef CAS PubMed.
- A. B. Chapoy, R. W. Burgass, B. Tohidi Kalorazi, J. M. Austell and C. Eickhoff, SPE J., 2011, 16, 921–930 CrossRef CAS.
- M. Mohitpour, H. Golshan and M. A. Murray, Pipeline design & construction: a practical approach, ASME Press, 2003 Search PubMed.
- A. Aspelund and K. Jordal, Int. J. Greenhouse Gas Control, 2007, 1, 343–354 CrossRef CAS.
- G. F. C. Rogers and Y. R. Mayhew, Engineering thermodynamics: work and heat transfer: SI units, Longman, 1980 Search PubMed.
- L. Buit, M. Ahmad, W. Mallon and F. Hage, Energy Procedia, 2011, 4, 3056–3062 CrossRef CAS PubMed.
- S. Paul, R. Shepherd, A. Bahrami and P. Woollin, presented in part at the The First International Forum on the transportation of CO2 by Pipeline, Gateshead, UK, 2010 Search PubMed.
- M. L. Szulczewski, C. W. MacMinn, H. J. Herzog and R. Juanes, Proc. Natl. Acad. Sci. U. S. A., 2012, 109, 5185–5189 CrossRef CAS PubMed.
- C. H. Pentland, R. El-Maghraby, S. Iglauer and M. J. Blunt, Geophys. Res. Lett., 2011, 38, DOI:10.1029/2011GL046683.
- M. Akbarabadi and M. Piri, Adv. Water Resour., 2013, 52, 190–206 CrossRef CAS PubMed.
- S. C. M. Krevor, R. Pini, B. X. Li and S. M. Benson, Geophys. Res. Lett., 2011, 38, DOI:10.1029/2011GL048239.
- R. S. Haszeldine, O. Quinn, G. England, M. Wilkinson, Z. K. Shipton, J. P. Evans, J. Heath, L. Crossey, C. J. Ballentine and C. Graham, Oil Gas Sci. Technol., 2005, 60, 33–49 CAS.
- M. Wigley, N. Kampman, B. Dubacq and M. Bickle, Geology, 2012, 40, 555–558 CrossRef CAS PubMed.
- N. Heinemann, M. Wilkinson, R. S. Haszeldine, A. E. Fallick and G. E. Pickup, Geology, 2013, 41, 411–414 CrossRef CAS PubMed.
- J. M. Lu, M. Wilkinson, R. S. Haszeldine and A. E. Fallick, Geology, 2009, 37, 35–38 CrossRef CAS PubMed.
- S. M. V. Gilfillan, B. S. Lollar, G. Holland, D. Blagburn, S. Stevens, M. Schoell, M. Cassidy, Z. J. Ding, Z. Zhou, G. Lacrampe-Couloume and C. J. Ballentine, Nature, 2009, 458, 614–618 CrossRef CAS PubMed.
- N. M. Burnside, Z. K. Shipton, B. Dockrill and R. M. Ellam, Geology, 2013, 41, 471–474 CrossRef CAS PubMed.
- J. J. Roberts, R. A. Wood and R. S. Haszeldine, Proc. Natl. Acad. Sci. U. S. A., 2011, 108, 16545–16548 CrossRef CAS PubMed.
- I. Havercroft, R. Macrory and R. B. Stewart, Carbon Capture and Storage – Emerging Legal and Regulatory Issues, Hart Press, Oxford, 2011 Search PubMed.
- C. Ehlig-Economides and M. J. Economides, J. Pet. Sci. Eng., 2010, 70, 118–125 CrossRef CAS PubMed.
- A. J. Cavanagh, R. S. Haszeldine and M. J. Blunt, J. Pet. Sci. Eng., 2010, 74, 107–110 CrossRef CAS PubMed.
- A. Hosa, M. Esentia, J. Stewart and S. Haszeldine, Chem. Eng. Res. Des., 2011, 89, 1855–1864 CrossRef CAS PubMed.
- A. J. Cavanagh, IEAGHG report 210/15, Cheltenham, UK, 2011.
- M. D. Zoback and S. M. Gorelick, Proc. Natl. Acad. Sci. U. S. A., 2012, 109, 10164–10168 CrossRef CAS PubMed.
- B. Senior, J. Bradshaw, A. Chikkatur and M. Wright, 10th International Conference on Greenhouse Gas Control Technologies, 2011, vol. 4, pp. 4575–4582 Search PubMed.
- P. Jaramillo, W. M. Griffin and S. T. McCoy, Environ. Sci. Technol., 2009, 43, 8027–8032 CrossRef CAS PubMed.
- K. S. Lackner, Science, 2003, 300, 1677–1678 CrossRef CAS PubMed.
- W. J. J. Huijgen and R. N. J. Comans, Carbon Dioxide Sequestration by Mineral Carbonation: Literature Review, Energy research Centre of the Netherlands ECN, 2003 Search PubMed.
- H. Herzog, Carbon sequestration via mineral carbonation: overview and assessment, MIT Laboratory for Energy and the Environment, 2002 Search PubMed.
- R. Zevenhoven and J. Fagerlund, in Developments and innovations in carbon dioxide (CO2) capture and storage, ed. M. Maroto-Valer, Woodhead Publishing, 2010, vol. 2, ch. 16 Search PubMed.
- P. S. Fennell, N. Florin, M. J. Al-Jeboori and I. Mckenzie, Institution of Mechanical Engineers meeting. “What's the future for CCS”. London. 19th–20th October, 2011 Search PubMed.
- W. K. O'Connor, D. C. Dahlin, S. J. Rush, S. J. Gerdermann and L. R. Penner, Aqueous mineral carbonation: Mineral availability, pretreatment, reaction parametrics and process studies, U.S. Department of Energy, Albany Research center, Albany, NY, 2005 Search PubMed.
- A. Sanna, M. R. Hall and M. Maroto-Valer, Energy Environ. Sci., 2012, 5, 7781–7796 CAS.
- J. Sipilä, S. Teir and R. Zevenhoven, Carbon dioxide sequestration by mineral carbonation – literature review update 2005–2007, Report VT 2008-1, Åbo Akademi Univ., Heat Engineering Lab., Turku, Finland, 2007 Search PubMed.
- R. C. Sahu, R. K. Patel and B. C. Ray, J. Hazard. Mater., 2010, 179, 28–34 CrossRef CAS PubMed.
- S. Eloneva, A. Said, C.-J. Fogelholm and R. Zevenhoven, Appl. Energy, 2012, 90, 329–334 CrossRef CAS PubMed.
- S. Eloneva, S. Teir, H. Revitzer, J. Salminen, A. Said, C.-J. Fogelholm and R. Zevenhoven, Steel Res. Int., 2009, 80, 415–421 CAS.
- T. Brown, A. Gambhir, N. Florin and P. Fennell, Briefing paper No. 7 Reducing CO2 emissions from heavy industry: a review of technologies and considerations for policy makers, Grantham Institute for Climate Change, Imperial College London, London, 2012 Search PubMed.
- S. Liang, H. Liu, T. Jiang, J. Song, G. Yang and B. Han, Chem. Commun., 2011, 47, 2131–2133 RSC.
- Intergovernmental Panel on Climate Change: Special Report on Carbon Dioxide Capture and Storage, IPCC, Cambridge University Press, Cambridge, 2005.
- M. Aresta and A. Dibenedetto, in Developments and Innovation in Carbon Dioxide (CO2) Capture and Storage Technology, ed. M. Maroto-Valer, Woodhead Publishing Limited, 2010, vol. 2, ch. 14 Search PubMed.
- R. S. Haszeldine, Science, 2009, 325, 1647–1652 CrossRef CAS PubMed.
- T. Sakakura and K. Kohno, Chem. Commun., 2009, 1312–1330 RSC.
- R. Pacciani, C. R. Muller, J. F. Davidson, J. S. Dennis and A. N. Hayhurst, Can. J. Chem. Eng., 2008, 86, 356–366 CrossRef CAS.
- T. Supap, R. Idem, A. Veawab, A. Aroonwilas, P. Tontiwachwuthikul, A. Chakma and B. Kybett, Ind. Eng. Chem. Res., 2001, 40, 3445–3450 CrossRef CAS.
- Roskill, Ground & Precipitated Calcium Carbonate: Global Industry Markets & Outlook, Roskill, 1st edn, 2012 Search PubMed.
- D. S. Kostick, 2013 Minerals Yearbook, United States Geological Survey, 2013 Search PubMed.
- Global CCS Institute and P. Brinckerhoff, Accelerating the Uptake of CCS: Industrial Use of Captured Carbon Dioxide, Global CCS Institute, Canberra, Australia, 2011 Search PubMed.
- Research In China, Global and China Polyurethane Industry Chain Report, 2011–2012, Research In China, Beijing, China, 2012 Search PubMed.
- Research In China, Global and China Acrylic Acid and Esters Industry Report, 2012–2015, Research in China, Beijing, China, 2012 Search PubMed.
- C. S. Snyder, T. W. Bruulsema, T. L. Jensen and P. E. Fixen, Agric., Ecosyst. Environ., 2009, 133, 247–266 CrossRef CAS PubMed.
- A. O. George, G. Alain and G. K. S. Prakash, J. Org. Chem., 2009, 74, 487–498 CrossRef PubMed.
- IEA, A Policy Strategy for Carbon Capture and Storage, International Energy Agency, Paris, 2012 Search PubMed.
- GCCSI, The Global Status of CCS, Global CCS Institute, Canberra, 2012 Search PubMed.
- P. S. Fennell, N. Florin, T. Napp and T. Hills, Sustainable Technologies, Systems & Policies, 2012, 17 Search PubMed.
- GCCI, 2012.
- DECC, A framework for the development of clean coal: consultation document, Department of Energy & Climate Change, London, 2009 Search PubMed.
- DTI, Meeting the Energy Challenge: A White Paper on energy, Department of Trade and Industry, London, 2007 Search PubMed.
- DECC, Responses to the market sounding for CCS demonstration projects 2–4, Department of Energy & Climate Change, London, 2010 Search PubMed.
- CCC, Letter from CCC to Chris Huhne re: CCC advice on the approach to investment in fossil fuel power generation, Committee on Climate Change, London, 2011 Search PubMed.
- HMT, Carbon price floor consultation: the Government response, HM Treasury, London, 2011 Search PubMed.
- EC, NER300-Moving towards a low carbon economy and boosting innovation, growth and employment across the EU, European Commission, Brussels, 2012 Search PubMed.
- DECC, Electricity Market Reform Consultation Document, Department of Energy & Climate Change, London, 2010 Search PubMed.
- DECC, Planning our electric future: a White Paper for secure, affordable and low carbon electricity, Department of Energy & Climate Change, London, 2011 Search PubMed.
- HM Government, Draft Energy Bill, The Stationery Office, London, 2012 Search PubMed.
- DECC, Press Notice: 11/084 ‘Government reaffirms commitment to CCS’, Department of Energy & Climate Change, London, 2011 Search PubMed.
- DECC, CCS Commercialisation Programme, http://www.decc.gov.uk/en/content/cms/emissions/ccs/ukccscomm_prog/commercialise/commercialise.aspx, accessed 30 March 2013.
- White Rose, White Rose Selected for Carbon Capture and Storage Funding 20 March 2013, http://www.whiteroseccs.co.uk, accessed 30 May 2013.
- R. Gross, W. Blyth and P. Heptonstall, Energ. Econ., 2010, 32, 796–804 CrossRef PubMed.
- Mott MacDonald, Costs of low carbon generation technologies, Committee on Climate Change, London, 2011 Search PubMed.
- C. von Stechow, J. Watson and B. Praetorius, Global Environ. Change, 2011, 21, 346–357 CrossRef PubMed.
- L. M. Abadie and J. M. Chamorro, Energy Policy, 2009, 37, 5304–5316 CrossRef PubMed.
- L. M. Abadie and J. M. Chamorro, Energ. Econ., 2008, 30, 2992–3015 CrossRef PubMed.
- M. R. Hamilton, H. J. Herzog and J. E. Parsons, Energy Procedia, 2009, 1, 4487–4494 CrossRef CAS PubMed.
- P. Osmundsen and M. Emhjellen, Energy Policy, 2010, 38, 7818–7826 CrossRef PubMed.
- H. Groenenberg and H. de Coninck, Int. J. Greenhouse Gas Control, 2008, 2, 653–664 CrossRef CAS PubMed.
- R. Gaisford, A view on the operation of the carbon market and its effect on CO2 abatement & electricity pricing, RCM Consulting Ltd, London, 2009 Search PubMed.
- B. P. Flannery, Energ. Econ., 2011, 33, 605–607 CrossRef PubMed.
- B. Evar, Energy Policy, 2011, 39, 3414–3424 CrossRef PubMed.
- I. Chrysostomidis, P. Zakkour, M. Bohm, E. Beynon, R. de Filippo and A. Lee, Energy Procedia, 2009, 1, 1625–1632 CrossRef PubMed.
- ERM, Update on selected regulatory issues for CO2 capture and geological storage, CO2 Capture Project, 2010 Search PubMed.
- McKinsey, Carbon Capture & Storage: Assessing the Economics, McKinsey & Company, 2008 Search PubMed.
- EU, Directive 2009/31/EC of the European Parliament and of the Council, European Parliament and the Council of the European Union, Brussels, 2009 Search PubMed.
- J. Watson, F. Kern, M. Gross, G. Gross, P. Heptonstall, F. Jones, S. Haszeldine, F. Ascui, H. Chalmers, N. Ghaleigh, J. Gibbins, N. Markusson, W. Marsden, D. Rossati, S. Russell, M. Winskel, P. Pearson and S. Arapostathis, Carbon Capture and Storage, Realising the Potential?, UK Energy Research Centre, London, 2012 Search PubMed.
- F. Jones, White Paper, green light? The impact of UK Electricity Market Reform on carbon capture and storage investment, MSc Thesis, Imperial College, London, 2011.
- P. Heptonstall, R. Gross and F. Jones, Carbon Capture and Storage: Realising the Potential? Work Package 3, Task 5 Working Paper, UK Energy Research Centre, London, 2011 Search PubMed.
- EU, Directive 2009/31/EC of the European Parliament and of the Council of 23 April 2009 on the geological storage of carbon dioxide and amending Council Directive 85/337/EEC, European Parliament and Council Directives 2000/60/EC, 2001/80/EC, 2004/35/EC, 2006/12/EC, 2008/1/EC and Regulation (EC) No 1013/2006, http://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=CELEX:32009L0031:EN:%20NOT, accessed 30 May 2013.
- EU, Directive 2004/35/CE of the European Parliament and of the Council of 21 April 2004 on environmental liability with regard to the prevention and remedying of environmental damage, http://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=CELEX:32004L0035:EN:%20NOT, accessed 30 May 2013.
- EU, Directive 2009/29/EC of the European Parliament and of the Council of 23 April 2009 amending Directive 2003/87/EC so as to improve and extend the greenhouse gas emission allowance trading scheme of the Community, http://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=CELEX:32009L0029:EN:%20NOT, accessed 30 May 2013.
- EU, Commission Regulation (EC) No 800/2008 of 6 August 2008 declaring certain categories of aid compatible with the common market in application of Articles 87 and 88 of the Treaty (General block exemption Regulation), http://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=CELEX:32008R0800:EN:%20NOT, accessed 30 May 2013.
- UK Government, Energy Act 2008, http://www.legislation.gov.uk/ukpga/2008/32/contents, accessed 30 May 2013.
- UK Government, Energy Act 2010, http://www.legislation.gov.uk/ukpga/2010/27/contents.
- UK Government, S.I. 2010 No. 2221 ENVIRONMENTAL PROTECTION The Storage of Carbon Dioxide (Licensing etc.) Regulations 2010, http://www.legislation.gov.uk/uksi/2010/2221/introduction/made, accessed 30 May 2013.
- UK Government, The Environmental Permitting (England and Wales) Regulations 2010, http://www.legislation.gov.uk/uksi/2010/675/contents/made, accessed 30 May 2013.
- US Government, Code of Federal Regulations Title 40-Protection of Environment Part 60—STANDARDS OF PERFORMANCE FOR NEW STATIONARY SOURCES, http://www.gpo.gov/fdsys/pkg/CFR-2011-title40-vol6/xml/CFR-2011-title40-vol6-part60.xml, accessed 30 May 2013.
- S. Eswaran, S. Wu and R. Nicolo, Advanced Amine-based CO2 Capture for Coal-fired Power Plants, COAL-GEN 2010, Pittsburgh, PA, USA, August 10–12, 2010 Search PubMed.
- S. Jovanovic, T. Stoffregen, I. Clausen and G. Sieder, Slipstream pilot-scale demonstration of a novel amine-based post-combustion technology for carbon dioxide capture from coal-fired power plant flue gas, Topical report: Techno-economic analysis of a 550 MWe Subcritical PC power plant with CO2 capture, DE-FOA-0000403, May 4, 2012 Search PubMed.
- K. Miyaike, Toshiba's Activity in Post Combustion CO2 Capture Technology, Power Systems Company, Toshiba Corporation, October 28, 2010 Search PubMed.
- S. Iglauer, A. Paluszny, C. H. Pentland and M. J. Blunt, Geophys. Res. Lett., 2011, 38, DOI:10.1029/2011GL049680.
- J. Marion, A. Brautsch, F. Kluger, M. Larsson and T. Pourchot, Overview of a Manufacturer's Efforts to Commercialize Oxy-Combustion for Steam Power Plants, 2nd Oxyfuel Combustion Conference, IEAGHG International Combustion Research Oxyfuel Network, Queensland, Australia, 12–16 September, 2011 Search PubMed.
- T. Eriksson, O. Sippu, A. Hotta, K. Myohanen, T. Hyppänen and T. Pikkarainen, Oxyfuel CFB Boiler as a Route to Near Zero CO2 Emission Coal Firing, Power Generation Europe, Madrid, Spain, June 3–6, 2007 Search PubMed.
- M. Varonen, I. Hyytiäinen, M. Palonen and V. Ylä-Outinen, Results of Oxyfuel Combustion Tests in a 4 MWth CFB Pilot Boiler, Proceedings of the 21st International Conference on Fluidized Beds, Naples, Italy, June 3–6, 2012, pp. 325–331 Search PubMed.
- A. Lyngfelt, B. Kronberger, J. Adanez, J.-X. Morin and P. Hurst, The GRACE project. Development of oxygen carrier particles for chemical-looping combustion. Design and operation of a 10 kW chemical-looping combustor, Vancouver, 2004 Search PubMed.
- A. Lyngfelt and H. Thunman, Carbon Dioxide Capture for Storage in Deep Geologic Formations–Results from the CO2 Capture Project, 2005, vol. 1, pp. 625–645 Search PubMed.
- C. Linderholm, T. Mattisson and A. Lyngfelt, Fuel, 2009, 88, 2083–2096 CrossRef CAS PubMed.
- C. Linderholm, A. Abad, T. Mattisson and A. Lyngfelt, Int. J. Greenhouse Gas Control, 2008, 2, 520–530 CrossRef CAS PubMed.
- H.-J. Ryu, G.-T. Jin and C.-K. Yi, Demonstration of inherent CO2 separation and no NOx emission in a 50 kW chemical-looping combustor: continuous reduction and oxidation experiment, Vancouver, 2004 Search PubMed.
- H.-J. Ryu, G.-T. Jin, D.-H. Bae and C.-K. Yi, presented in part at the 5th China-Korea Joint Workshop on Clean Energy Technology, Qingdao University, China, October 25–28, 2004 Search PubMed.
- J. Adanez, P. Gayan, J. Celaya, L. F. de Diego, F. Garcia-Labiano and A. Abad, Ind. Eng. Chem. Res., 2006, 45, 6075–6080 CrossRef CAS.
- L. F. de Diego, F. García-Labiano, P. Gayán, J. Celaya, J. M. Palacios and J. Adánez, Fuel, 2007, 86, 1036–1045 CrossRef CAS PubMed.
- E. Johansson, T. Mattisson, A. Lyngfelt and H. Thunman, Fuel, 2006, 85, 1428–1438 CrossRef CAS PubMed.
- E. Johansson, T. Mattisson, A. Lyngfelt and H. Thunman, Chem. Eng. Res. Des., 2006, 84, 819–827 CrossRef CAS.
- A. Abad, T. Mattisson, A. Lyngfelt and M. Rydén, Fuel, 2006, 85, 1174–1185 CrossRef CAS PubMed.
- A. Abad, T. Mattisson, A. Lyngfelt and M. Johansson, Fuel, 2007, 86, 1021–1035 CrossRef CAS PubMed.
- M. Rydén, A. Lyngfelt and T. Mattisson, Energy Fuels, 2008, 22, 2585–2597 CrossRef.
- M. Rydén, M. Johansson, A. Lyngfelt and T. Mattisson, Energy Environ. Sci., 2009, 2, 970–981 Search PubMed.
- C. Linderholm, E. Jerndal, T. Mattisson and A. Lyngfelt, Chem. Eng. Res. Des., 2010, 88, 661–672 CrossRef CAS PubMed.
- P. Moldenhauer, M. Rydén and A. Lyngfelt, Fuel, 2012, 93, 351–363 CrossRef CAS PubMed.
- M. Rydén, A. Lyngfelt and T. Mattisson, Energy Procedia, 2011, 4, 341–348 CrossRef PubMed.
- N. Berguerand and A. Lyngfelt, Fuel, 2008, 87, 2713–2726 CrossRef CAS PubMed.
- N. Berguerand and A. Lyngfelt, Energy Fuels, 2009, 23, 5257–5268 CrossRef CAS.
- N. Berguerand and A. Lyngfelt, Energy Procedia, 2009, 1, 407–414 CrossRef CAS PubMed.
- A. Cuadrat, C. Linderholm, A. Abad, A. Lyngfelt and J. Adánez, Energy Fuels, 2011, 25, 4818–4828 CrossRef CAS.
- J. Adánez, C. Dueso, L. F. D. Diego, F. García-Labiano, P. Gayán and A. Abad, Energy Fuels, 2009, 23, 130–142 CrossRef.
- J. Adánez, F. Garcá-Labiano, P. Gayán, L. F. de Diego, A. Abad, C. Dueso and C. R. Forero, Effect of gas impurities on the behavior of Ni-based oxygen carriers on chemical-looping combustion, 2009 Search PubMed.
- J. Adánez, C. Dueso, L. F. De Diego, F. García-Labiano, P. Gayán and A. Abad, Ind. Eng. Chem. Res., 2009, 48, 2509–2518 CrossRef.
- L. F. de Diego, M. Ortiz, F. García-Labiano, J. Adánez, A. Abad and P. Gayán, Energy Procedia, 2009, 1, 3–10 CrossRef CAS PubMed.
- C. Dueso, F. García-Labiano, J. Adánez, L. F. de Diego, P. Gayán and A. Abad, Fuel, 2009, 88, 2357–2364 CrossRef CAS PubMed.
- C. R. Forero, P. Gayán, L. F. de Diego, A. Abad, F. García-Labiano and J. Adánez, Fuel Process. Technol., 2009, 90, 1471–1479 CrossRef CAS PubMed.
- F. García-Labiano, L. F. de Diego, P. Gayán, J. Adánez, A. Abad and C. Dueso, Ind. Eng. Chem. Res., 2009, 48, 2499–2508 CrossRef.
- P. Gayán, C. R. Forero, L. F. de Diego, A. Abad, F. García-Labiano and J. Adánez, Int. J. Greenhouse Gas Control, 2010, 4, 13–22 CrossRef PubMed.
- C. R. Forero, P. Gayán, F. García-Labiano, L. F. de Diego, A. Abad and J. Adánez, Int. J. Greenhouse Gas Control, 2011, 5, 659–667 CrossRef CAS PubMed.
- P. Gayán, M. A. Pans, M. Ortiz, A. Abad, L. F. de Diego, F. García-Labiano and J. Adánez, Fuel Process. Technol., 2012, 96, 37–47 CrossRef PubMed.
- P. Gayán, C. R. Forero, A. Abad, L. F. de Diego, F. García-Labiano and J. Adánez, Energy Fuels, 2011, 25, 1316–1326 CrossRef.
- S. R. Son and S. D. Kim, Ind. Eng. Chem. Res., 2006, 45, 2689–2696 CrossRef CAS.
- J. Bolhàr-Nordenkampf, T. Pröll, P. Kolbitsch and H. Hofbauer, Energy Procedia, 2009, 1, 19–25 CrossRef PubMed.
- J. Bolhàr-Nordenkampf, T. Pröll, P. Kolbitsch and H. Hofbauer, Chemical looping autothermal reforming at a 120 kW pilot rig, 2009 Search PubMed.
- P. Kolbitsch, T. Pröll, J. Bolhar-Nordenkampf and H. Hofbauer, Chem. Eng. Technol., 2009, 32, 398–403 CrossRef CAS.
- P. Kolbitsch, T. Pröll, J. Bolhar-Nordenkampf and H. Hofbauer, Energy Procedia, 2009, 1, 1465–1472 CrossRef CAS PubMed.
- P. Kolbitsch, T. Proll, J. Bolhar-Nordenkampf and H. Hofbauer, Energy Fuels, 2009, 23, 1450–1455 CrossRef CAS.
- P. Kolbitsch, J. Bolhàr-Nordenkampf, T. Pröll and H. Hofbauer, Ind. Eng. Chem. Res., 2009, 48, 5542–5547 CrossRef CAS.
- T. Pröll, P. Kolbitsch, J. Bolhàr-Nordenkampf and H. Hofbauer, AIChE J., 2009, 55, 3255–3266 CrossRef.
- T. Pröll, J. Bolhàr-Nordenkampf, P. Kolbitsch and H. Hofbauer, Fuel, 2010, 89, 1249–1256 CrossRef PubMed.
- P. Kolbitsch, J. Bolhàr-Nordenkampf, T. Pröll and H. Hofbauer, Int. J. Greenhouse Gas Control, 2010, 4, 180–185 CrossRef CAS PubMed.
- T. Pröll, P. Kolbitsch, J. Bolhàr-Nordenkampf and H. Hofbauer, Oil & Gas Science and Technology – Revue d'IFP Energies Nouvelles, 2011, vol. 66, pp. 173–180 Search PubMed.
- L. Shen, J. Wu and J. Xiao, Combust. Flame, 2009, 156, 721–728 CrossRef CAS PubMed.
- L. Shen, J. Wu, J. Xiao, Q. Song and R. Xiao, Energy Fuels, 2009, 23, 2498–2505 CrossRef CAS.
- L. Shen, J. Wu, Z. Gao and J. Xiao, Combust.ion and Flame, 2009, 156, 1377–1385 CrossRef CAS PubMed.
- J. Wu, L. Shen, J. Xiao, L. Wang and J. Hao, CIESC J., 2009, 60, 2080–2088 CAS.
- H.-J. Ryu, S.-H. Jo, Y. C. Park, D.-H. Bae and S. Kim, presented in part at the 1st International Conference on Chemical Looping, Lyon, 2010 Search PubMed.
- J. Wu, L. Shen, J. Hao and H. Gu, presented in part at the 1st International Conference on Chemical Looping, Lyon, 2010 Search PubMed.
- S. Rifflart, A. Hoteit, M. M. Yazdanpanah, W. Pelletant and K. Surla, 10th International Conference on Greenhouse Gas Control Technologies, 2011, vol. 4, pp. 333–340 Search PubMed.
- T. Sozinho, W. Pelletant, T. Gauthier and H. Stainton, presented in part at the 2nd Int. Conf. on Chemical Looping, Darmstadt, 26–28 September, 2012 Search PubMed.
- S. Wang, G. Wang, F. Jiang, M. Luo and H. Li, Energy Environ. Sci., 2010, 3, 1353–1360 CAS.
- A. Cuadrat, A. Abad, F. García-Labiano, P. Gayán, L. F. de Diego and J. Adánez, Int. J. Greenhouse Gas Control, 2011, 5, 1630–1642 CrossRef CAS PubMed.
- A. Cuadrat, A. Abad, F. García-Labiano, P. Gayán, L. F. de Diego and J. Adánez, Energy Procedia, 2011, 4, 362–369 CrossRef CAS PubMed.
- P. Markström, A. Lyngfelt and C. Linderholm, Paper presented at 21st International Conference on Fluidized Bed Combustion, Naples, June 3–6, 2012, 2012 Search PubMed.
- P. Markström, C. Linderholm and A. Lyngfelt, Operation of a 100 kW chemical-looping combustor with Mexican petroleum coke and Cerrejón coal, Appl. Energy, DOI:10.1016/j.apenergy.2013.04.066.
- P. Markstrom, C. Linderholm and A. Lyngfelt, Int. J. Greenhouse Gas Control, 2013, 15, 150–162 CrossRef PubMed.
- P. Markström, C. Linderholm and A. Lyngfelt, Chem. Eng. Sci., 2013, 96, 131–141 Search PubMed.
- A. Thon, M. Kramp, E.-U. Hartge, S. Heinrich and J. Werther, presented in part at the 2nd Int. Conf. on Chemical Looping, Darmstadt, 26–28 September, 2012 Search PubMed.
- A. Tong, S. Bayham, M. Kathe, L. Zeng, S. Luo and L.-S. Fan, presented in part at the 2nd International Conference on Chemical Looping, Darmstadt, 2012 Search PubMed.
- M. L. Godec, Global technology roadmap for CCS in industry sectoral assessment CO2 enhanced oil recovery, Prepared by Advanced Resources International, Inc. for United Nations Industrial Development Organization, Arlington, VA, USA, 2011 Search PubMed.
- B. Metz and Intergovernmental Panel on Climate Change. Working Group III, Carbon Dioxide Capture And Storage: IPCC Special Report, Cambridge University Press, 2005 Search PubMed.
- D. Johnson, Presentation on: Global Methanol Market Review, http://www.ptq.pemex.com/productosyservicios/eventosdescargas/Documents/Foro%20PEMEX%20Petroqu%C3%ADmica/2012/PEMEX_DJohnson.pdf, accessed 07 March 2013.
- M. Mikkelsen, M. Jorgensen and F. C. Krebs, Energy Environ. Sci ., 2010, 3, 43–81 CAS.
| This journal is © The Royal Society of Chemistry 2014 |