Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Ferromagnetic interaction and slow magnetic relaxation in a Co3 cluster-based three-dimensional framework†
Bing-Yan
Wu
a,
Chen-I
Yang
*a,
Motohiro
Nakano
b and
Gene-Hsiang
Lee
c
aDepartment of Chemistry, Tunghai University, Taichung 407, Taiwan. E-mail: ciyang@thu.edu.tw; Fax: +886-4-23590426
bDivision of Applied Chemistry, Graduate School of Engineering, Osaka University, 2-1 Yamada-oka, Suita, 565-0871, Japan
cInstrumentation Center, College of Science, National Taiwan University, Taipei, 106, Taiwan
First published on 14th October 2013
Abstract
A Co3 cluster-based three-dimensional (3D) framework, [Co3(4-ptz)4(N3)2(H2O)2]·4DMF (1; 4-Hptz = 4-(1H-tetrazol-5-yl)pyridine), exhibits ferromagnetic interactions and slow-magnetic relaxation behavior.
The development of assembling molecules in a predetermined multidimensional coordination environment with the use of intermolecular interactions is a major topic of increasing scientific interest.1 In the past decade, this methodology has given birth to several novel areas of research, including the preparation of rotaxanes,2 molecular building blocks,3 host–guest chemistry,4 and metal–organic frameworks (MOFs).5 A potential application of this research resides in high-capacity data storage for electronic devices,6 which can be achieved by organizing molecular nanomagnets, which are referred to as single-molecule magnets (SMMs), in a multidimensional environment.7 Theoretically, such an arrangement of molecular magnets in a crystalline material would enable magnetization probing without interference by magnetic ordering, allowing researchers to study the exact nature of the retention of magnetization for SMMs.8 They display slow magnetic relaxation phenomena such as magnetization hysteresis loops, and frequency-dependent signal measurements of out-of-phase alternating current (AC) magnetic susceptibility arise from a large anisotropy energy barrier of KV = |D|Sz2 which results from the combination of a large-spin ground state (S) and an Ising-type magnetic anisotropy (negative zero-field splitting parameter D).9 Compounds that contain Co2+ ions have been reported as SMMs recently, including some multi-nuclear Co2+ clusters and a complex containing a single-ion of Co2+,10 which are considered as good candidates for use in preparing multi-dimensional magnetic materials; however, only one example of a Co-SMM-based 3D compound has been reported.11
We herein report on the preparation of a 3D Co3-based framework, [Co3(4-ptz)4(N3)2(H2O)2]·4DMF (1), obtained by the bridging of 4-Hptz and azide. 4-Hptz and azide, the selected ligands, not only connect the Co2+ into a multidimensional structure but also dominate a significant magnetic exchange which results in the formation of a ferromagnetic Co3 building unit that exhibits slow magnetic relaxation behavior.
The reaction of Co(OAc)2·4H2O (1 equiv.), 4-Hptz (1 equiv.), with NaN3 (1 equiv.) in DMF at 80 °C in a Teflon-lined stainless autoclave for 3 days resulted in the formation of very fine purple crystals of [Co3(4-ptz)3(N3)2(H2O)2]·4DMF (1) in 68% yield.
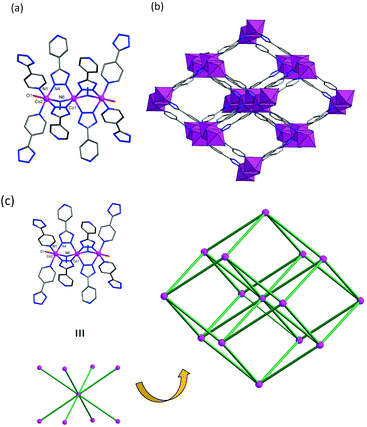
An X-ray crystal‡ structure analysis (see ESI†) showed that 1 crystallizes in the orthorhombic space group Pnnm. The structure of [Co3(4-ptz)4(N3)2(H2O)2]·4DMF (1) is shown in Fig. 1. The asymmetric unit consists of two Co2+ centers, one bridging the azido ligand, one the 4-ptz ligand, one a coordinated water molecule, and two disordered DMF guest molecules (Fig. 1a). The two nonequivalent Co atoms adopt a distorted octahedral CoN6 and CoN5O coordination geometry for Co1 and Co2, respectively. Co1 is bound to four tetrazole nitrogen atoms and two nitrogen atoms from two end-on (EO) N3 ligands, and the Co2 is bound to two tetrazole nitrogen atoms, two pyridyl nitrogen atoms, one nitrogen atom from EO-N3, and one coordinated water molecule. The cobalt–cobalt separation, bridged by the azido and tetrazole moiety of the 4-ptz ligands, is 3.394(1) Å. The tetrazole ligands adopt a bridging mode with a coordinating pyridyl group and an η2,μ2-tetrazolato bridge with its 1,2-nitrogen atoms. This connects each Co3 unit to a 3D framework (Fig. 1b). Except for the coordinated water molecules, all of the donor atoms form reasonably strong bonds with the cobalt atoms. All of the Co–N and Co–O distances are in the range between 2.062(8) and 2.153(6) Å.
In view of the diverse network structure of compound 1, it is best elucidated on the basis of the types of connectivities of the Co3 cluster moieties through the 4-ptz linker. As shown in Fig. 1c, the Co3 moieties of compound 1 are arranged such that each Co3 unit is connected to eight neighboring Co3 clusters, and can be considered to be a planar 8-connected node. As a result, the net assembly based on the 8-connected node leads to the formation of a CsCl-like 3D network with a bcu-type topology.
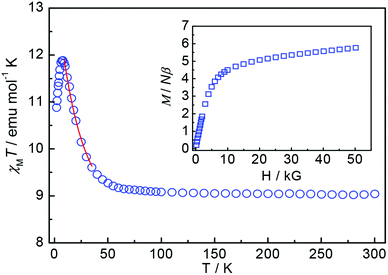
Solid-state, variable-temperature magnetic susceptibility measurements were performed on a microcrystalline sample of complex 1 in the 2.0–300 K range in a 1 kG magnetic field, which was suspended in eicosane to prevent torquing. The temperature dependence of the χMT value of 1 is shown in Fig. 2. The χMT value at 300 K is 9.04 emu mol−1 K, much larger than the 5.63 emu mol−1 K value for three non-interacting CoII ions. This could be a result of the depopulation of the higher Kramers doublets, caused by the splitting of the 4T1g ground triplet under the combined action of the spin–orbit coupling and non-cubic crystal-field terms.12 Upon cooling, the χMT value remains constant until the temperature reaches approximately 50 K, where it then begins to increase to a maximum value of 11.88 emu K mol−1 at 8 K before dropping sharply below this temperature. The sharp increase in the χMT value at temperatures above 8 K is a characteristic of ferromagnetic behavior, leading to a non-zero ground state, and the decrease below 8 K can be attributed to the combined effect of zero-field splitting, the Zeeman effect and weak interunit interactions through the 4-ptz ligand, which has been reported as a weak antiferromagnetic interaction in the literature.13 The inverse of the dc susceptibility decreases linearly from 300 to 50 K following Curie–Weiss behavior with C = 9.01 emu mol−1 K and θ = 1.1 K (Fig. 4S†). The positive Weiss constant (θ) indicates the existence of a ferromagnetic interaction within complex 1. Because of the complications introduced by the orbital contributions of CoII ions, a quantitative treatment of the magnetic susceptibility data of 1 in the whole 2.0–300 K temperature range was not attempted. However, the low temperature region (10–35 K) of magnetic susceptibility can be roughly fitted using a linear CoII3 Heisenberg–van Vleck model of H = −2J(S1S2 + S2S3) based on an Seff = 1/2 for each CoII ion (solid line in Fig. 2), which gives the best fit parameters of g′ = 5.2, J = 11.2 cm−1. The positive J value confirms the intraunit ferromagnetic interaction and the maximum χMT value of 11.88 emu mol−1 K at 8 K is consistent with the theoretical value of 12.7 calculated for g′ = 5.2 and ST = 3/2. The isotherm magnetization of 1 at 2 K was collected and no hysteresis loop was observed (Fig. 5S†). The magnetization shows the onset of saturation above 10 kG (Fig. 2, inset) and the saturation is not complete up to 50 kG; its value of 5.9Nβ is lower than the theoretical value of 7.8Nβ (calculated by g′ = 5.2 and ST = 3/2), which is indicative of very close levels in 1. Moreover, the field-dependent magnetization (0.25–5 T at 1.8–4 K) and χMT vs. T under different applied fields confirm the participation of low-lying excited states and the very close levels and crossing of the levels, respectively (Fig. 6S and 7S†).
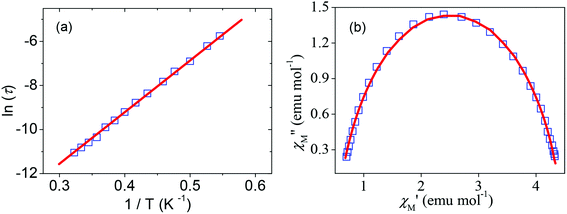
Alternating current (AC) susceptibility measurements were carried out for complex 1 under a zero DC field. As shown in the ESI, Fig. 8S,† complex 1 shows frequency-dependent in-phase (χM′) and out-of-phase (χM′′) signals, indicating a significant barrier to magnetization relaxation and precludes 3D ordering. However, no maxima were observed for χM′′ in the temperature and frequency range studied. This behavior can be attributed to the zero-field fast tunneling of the magnetization, and the application of an external field is usually sufficient to partially suppress this fast tunneling.10,14 The χM′′ signals under an applied field of 500 G were collected and these data are shown in Fig. 3. As the frequency of the AC field changed from 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 to 10 Hz, the χM′′ peak shifted from 3.4 to 2.1 K. The Arrhenius equation was applied to fit the data with the rate for the reversal of magnetization and the effective energy barrier of this complex was obtained. The plot of the relaxation time as a function of the inverse of temperature for complex 1 is shown in Fig. 4a. The best fitting of the experimental data gives parameters for the effective energy barriers for the reversal of magnetization of 24 K and τ0 = 5.3 × 10−9 s. This energy barrier may originate from the combination of the high-spin ground state and magnetic anisotropy of CoII ions of complex 1, which is consistent with those reported for CoII SMMs.10 Furthermore, at a fixed temperature of 2.2 K, we obtained a semicircle Cole–Cole diagram (χM′′ vs. χM′), which could be fitted to a generalized Debye model,15 with α parameters of 0.18, which indicates a moderate distribution in relaxation time (Fig. 4b).
000 to 10 Hz, the χM′′ peak shifted from 3.4 to 2.1 K. The Arrhenius equation was applied to fit the data with the rate for the reversal of magnetization and the effective energy barrier of this complex was obtained. The plot of the relaxation time as a function of the inverse of temperature for complex 1 is shown in Fig. 4a. The best fitting of the experimental data gives parameters for the effective energy barriers for the reversal of magnetization of 24 K and τ0 = 5.3 × 10−9 s. This energy barrier may originate from the combination of the high-spin ground state and magnetic anisotropy of CoII ions of complex 1, which is consistent with those reported for CoII SMMs.10 Furthermore, at a fixed temperature of 2.2 K, we obtained a semicircle Cole–Cole diagram (χM′′ vs. χM′), which could be fitted to a generalized Debye model,15 with α parameters of 0.18, which indicates a moderate distribution in relaxation time (Fig. 4b).
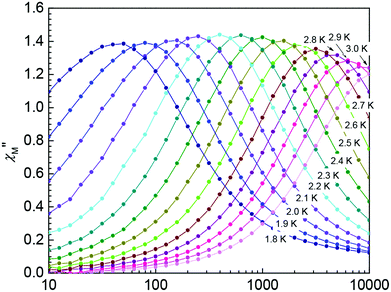 | ||
| Fig. 3 Plots of out-of-phase (χM′′) of AC susceptibility vs. frequency in a 3.5 G AC field oscillating and 500 G DC field at the indicated temperatures for complex 1. | ||
For a reported Co4-SMM-based 3D compound, a relatively stronger interunit interaction is evident from a shorter syn–anti carboxlate bridging and a crossover of the χM′′ curves has been seen in the AC data. In contrast, the longer 4-ptz ligand participated in a weak interunit interaction in 1 and no crossover of χM′′ curves was observed.
In the heat capacity measurements of 1 that were performed on powdered crystals, no λ peak was observed in Cp on cooling to 2 K (Fig. 11S†), suggesting the absence of bulk magnetic ordering.
Conclusions
A Co3 cluster-based 3D framework was built up with CoII ions using N3− and the nitrogen donor ligand 4-pyridinely-tetrazo5-(4-pyridyl)-tetrazole resulting in a bcu-type topology. A magnetic analysis reveals that the compound shows ferromagnetic interactions within the Co3 cluster and slow-magnetic relaxation behavior.Notes and references
- (a) J.-M. Lehn, Supramolecular Chemistry: Concepts and Perspectives, VCH, Weinheim, Germany, 1995 Search PubMed; (b) F. Diederich, Angew. Chem., Int. Ed., 2007, 46, 68 CrossRef CAS PubMed; (c) M. A. Pitt and D. W. Johnson, Chem. Soc. Rev., 2007, 36, 1441 RSC; (d) L. Fabbrizzi and A. Poggi, Chem. Soc. Rev., 1995, 24, 197 RSC; (e) T. Kudernac, S. Lei, J. A. A. W. Elemans and S. D. Feyter, Chem. Soc. Rev., 2009, 38, 402 RSC; (f) J. W. Steed and J. L. Atwood, Supramolecular Chemistry, John Wiley and Sons, New York, 2009 Search PubMed; (g) J.-M. Lehn, Science, 1993, 260, 1762 CAS; (h) G. V. Oshovsky, D. N. Reinhoudt and W. Verboom, Angew. Chem., Int. Ed., 2007, 46, 2366 CrossRef CAS PubMed.
- (a) V. Balzani, A. Credi and M. Venturi, Chem. Soc. Rev., 2009, 38, 1542 RSC; (b) J. A. Faiz, V. Heitz and J.-P. Sauvage, Chem. Soc. Rev., 2009, 38, 422 RSC; (c) S. J. Loeb, Chem. Soc. Rev., 2007, 36, 226 RSC; (d) B. Champin, P. Mobian and J.-P. Sauvage, Chem. Soc. Rev., 2007, 36, 358 RSC; (e) H. Tian and Q.-C. Wang, Chem. Soc. Rev., 2006, 35, 361 RSC.
- (a) F. Wurthner, A. Sautter and C. Thalacker, Angew. Chem., Int. Ed., 2000, 39, 1243 CrossRef CAS; (b) M. B. Nielsen, C. Lomholt and J. Becher, Chem. Soc. Rev., 2000, 39, 153 RSC; (c) R. Gómez, C. Seoane and J. L. Segura, Chem. Soc. Rev., 2007, 36, 1305 RSC; (d) J. J. Perry, J. A. Perman and M. J. Zaworotko, Chem. Soc. Rev., 2009, 38, 1400 RSC.
- (a) V. G. Organo and D. M. Rudkevich, Chem. Commun., 2007, 3891 RSC; (b) A. Petitjean, R. G. Khoury, N. Kyritsakas and J.-M. Lehn, J. Am. Chem. Soc., 2004, 126, 6637 CrossRef CAS PubMed; (c) A. Sygula, F. R. Fronczek, R. Sygula, P. W. Rabideau and M. M. Olmstead, J. Am. Chem. Soc., 2007, 129, 3842 CrossRef CAS PubMed; (d) F. Diederich and M. Gómez-López, Chem. Soc. Rev., 1999, 28, 263 RSC.
- (a) J. R. Long and O. M. Yaghi, Chem. Soc. Rev., 2009, 38, 1213 RSC; (b) S. T. James, Chem. Soc. Rev., 2003, 32, 276 RSC; (c) S. Kitagawa, R. Kitaura and S.-I. Noro, Angew. Chem., Int. Ed., 2004, 43, 2334 CrossRef CAS PubMed; (d) G. Férey, Chem. Soc. Rev., 2008, 37, 191 RSC.
- (a) A. U. Czaja, N. Trukhan and U. Muller, Chem. Soc. Rev., 2009, 38, 1284 RSC; (b) T. Duren, Y.-S. Bae and R. Q. Snurr, Chem. Soc. Rev., 2009, 38, 1237 RSC; (c) J.-R. Li, R. J. Kuppler and H.-C. Zhou, Chem. Soc. Rev., 2009, 38, 1477 RSC; (d) L. J. Murray, M. Dincă and J. R. Long, Chem. Soc. Rev., 2009, 38, 1294 RSC; (e) S. S. Han, J. L. Mendoza-Cortés and W. A. Goddard, Chem. Soc. Rev., 2009, 38, 1460 RSC.
- (a) A. D. Burrows, C. G. Frost, M. F. Mahon, M. Winsper, C. Richardson, J. P. Attfield and J. A. Rodgers, Dalton Trans., 2008, 6788 RSC; (b) J. H. Cavka, S. Jakobsen, U. Olsbye, N. Guillou, C. Lamberti, S. Bordiga and K. P. Lillerud, J. Am. Chem. Soc., 2008, 130, 13850 CrossRef PubMed; (c) M. H. Alkordi, Y. Liu, R. W. Larsen, J. F. Eubank and M. Eddaoudi, J. Am. Chem. Soc., 2008, 130, 12639 CrossRef CAS PubMed.
- (a) D. Gatteschi and R. Sessoli, Angew. Chem., Int. Ed., 2003, 42, 268 CrossRef CAS PubMed and references therein; ; (b) C. J. Milios, I. A. Gass, A. Vinslava, L. Budd, S. Parsons, W. Wernsdorfer, S. P. Perlepes, G. Christou and E. K. Brechin, Inorg. Chem., 2007, 46, 6215 CrossRef CAS PubMed; (c) C. J. Milios, A. Prescimone, A. Mishra, S. Parsons, W. Wernsdorfer, G. Christou, S. P. Perlepes and E. K. Brechin, Chem. Commun., 2007, 153 RSC; (d) C. J. Milios, A. Vinslava, P. A. Wood, S. Parsons, W. Wernsdorfer, G. Christou, S. P. Perlepes and E. K. Brechin, J. Am. Chem. Soc., 2007, 129, 8 CrossRef CAS PubMed; (e) C. J. Milios, R. Inglis, R. Bagai, W. Wernsdorfer, A. Collins, S. Moggach, S. Parsons, S. P. Perlepes, G. Christou and E. K. Brechin, Chem. Commun., 2007, 3476 RSC; (f) A. J. Tasiopoulos, A. Vinslava, W. Wernsdorfer, K. A. Abboud and G. Christou, Angew. Chem., Int. Ed., 2004, 43, 2117 CrossRef CAS PubMed.
- (a) R. Sessoli, H.-L. Tsai, A. R. Schake, S. Wang, J. B. Vincent, K. Folting, D. Gatteschi, G. Christou and D. N. Hendrickson, J. Am. Chem. Soc., 1993, 115, 1804 CrossRef CAS; (b) R. Sessoli, D. Gatteschi, A. Caneschi and M. A. Novak, Nature, 1993, 365, 141 CrossRef CAS; (c) D. Gatteschi, A. Caneschi, L. Pardi and R. Sessoli, Science, 1994, 265, 1054 CAS.
- (a) D. Gatteschi, R. Sessoli and J. Villain, Molecular Nanomagnets, Oxford Press, New York, 2006 Search PubMed; (b) M. Murrie, Chem. Soc. Rev., 2010, 39, 1986 RSC; (c) Q. Chen, M.-H. Zeng, L.-Q. Wei and M. Kurmoo, Chem. Mater., 2010, 22, 4328 CrossRef CAS; (d) T. Jurca, A. Farghal, P. H. Lin, I. Korobkov, M. Murugesu and D. S. Richeson, J. Am. Chem. Soc., 2011, 133, 15814 CrossRef CAS PubMed; (e) J. M. Zadrozny, J. Liu, N. A. Piro, C. J. Chang, S. Hill and J. R. Long, Chem. Commun., 2012, 48, 3927 RSC.
- K. W. Galloway, M. Schmidtmann, J. Sanchez-Benitez, K. V. Kamenev, W. Wernsdorfer and M. Murrie, Dalton Trans., 2010, 39, 4727 RSC.
- (a) M.-H. Zeng, M.-C. Wu, H. Zhou, Y.-L. Liang, X.-M. Chen and S. W. Ng, Inorg. Chem., 2007, 46, 7241 CrossRef CAS PubMed; (b) M.-H. Zeng, B. Wang, X. Y. Wang, W.-X. Zhang, X.-M. Chen and S. Gao, Inorg. Chem., 2006, 45, 7069 CrossRef CAS PubMed; (c) Y.-L. Zhou, F.-Y. Meng, J. Zhang, M.-H. Zeng and H. Liang, Cryst. Growth Des., 2009, 9, 1402 CrossRef CAS.
- (a) W. Ouellette, H. Liu, C. J. O'Connor and J. Zubieta, Inorg. Chem., 2009, 48, 4655 CrossRef CAS PubMed; (b) K. Darling, W. Ouellette, S. Pellizzeri, J. Vargas, C. J. O'Connor, T. Smith, S. Tomaszfski and J. Zubieta, Inorg. Chim. Acta, 2012, 392, 417 CrossRef CAS PubMed.
- (a) S. L. Castro, Z. Sun, C. M. Grant, J. C. Bollinger, D. N. Hendrickson and G. Christou, J. Am. Chem. Soc., 1998, 120, 2365 CrossRef CAS; (b) A. M. Ako, M. Mereacre, I. J. Hewitt, R. Clérac, L. Lecren, C. E. Anson and A. K. Powell, J. Mater. Chem., 2006, 16, 2579 RSC; (c) T. Kajiwara, M. Nakano, S. Takaishi and M. Yamashita, Inorg. Chem., 2008, 47, 8604 CrossRef CAS PubMed.
- (a) K. S. Cole and R. H. Cole, J. Chem. Phys., 1941, 9, 341 CrossRef CAS; (b) S. M. J. Aubin, Z. Sun, L. Pardi, J. Krzystek, K. Folting, L.-C. Brunel, A. L. Rheingold, G. Christou and D. N. Hendrickson, Inorg. Chem., 1999, 38, 5329 CrossRef CAS.
Footnotes |
| † Electronic supplementary information (ESI) available: Detailed experimental procedures, additional crystallographic diagrams and magnetic diagram. CCDC 941507. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c3dt51997j |
| ‡ The complex analyzed as (C, H, N) 1, calcd (found): C, 36.82 (36.58); H, 4.13 (4.12); N, 35.85 (35.60)%. Crystal-structure data for 1, C36H48Co3N30O6, M = 1173.83, orthorhombic, Pnnm, a = 12.2143(13) Å, b = 13.8051(15) Å, c = 16.5293(17) Å, V = 2987.2(5) Å3, T = 150(2) K, Z = 2. (Rint = 0.0847), 2557 parameters, R(Rw) = 0.1039(0.2530) with [I > 2σ(I)]. |
| This journal is © The Royal Society of Chemistry 2014 |