Shape effects of goethite particles on their photocatalytic activity in the decomposition of acetaldehyde
Seiji
Kakuta
*,
Taiki
Numata
and
Toru
Okayama
New Energy Technology Section, Aomori Prefectural Industrial Technology Research Center, 221-10, Yamaguchi, Nogi, Aomori, 030-0142, Japan. E-mail: seiji_kakuta@aomori-itc.or.jp
First published on 29th October 2013
Abstract
The relationship between the photocatalytic activity of goethite (α-FeOOH) and the shape of its particles was investigated. Goethite particles with various morphologies (rod-like, array-like, and pancake-like) were synthesized through the hydrolysis of a ferric nitrate solution or the oxidation of Fe(OH)2. All the particles synthesized consisted of {110} and {021} facets. The photocatalytic activities of the different samples were examined for the decomposition of acetaldehyde under irradiation. It was found that the photocatalytic activity of goethite is affected by both the shape and the specific surface area of its particles. Further investigation of the effects of the particle shape indicated that the photocatalytic activity of the {021} facets is higher than that of the {110} facets of goethite. The goethite particles (short rod-like and pancake-like particles), therefore, exhibited higher photocatalytic activities than other particle types (long rod-like and array-like particles) owing to the greater {021} facets.
Introduction
Iron oxides and iron oxyhydroxides have been studied extensively as photocatalysts for various types of reactions (e.g., the splitting of water and the decomposition of organic pollutants)1–10 because of their visible-light responsiveness, low cost, nontoxic nature, and natural abundance. Of the various such compounds investigated, goethite (α-FeOOH) is known to be a visible-light-responsive photocatalyst (band gap: 2.0–2.5 eV).11 In contrast to other iron oxides and oxyhydroxides, goethite can be synthesized easily under ambient conditions and, thus, has been studied for use in various environmental applications, such as the photocatalytic decomposition of dyes, organic acids, and aldehydes.5–7,12–15 Therefore, the preparation of highly active goethite as photocatalyst is an important goal in modern materials chemistry and green chemistry, and much effort has been devoted to the synthesis of goethite nanoparticles with high specific surface areas such that they exhibit high photocatalyst activity.6,7In the last decade, it has been recognized that the activity of a photocatalyst depends not only on the specific surface area of the photocatalyst particles but also on their shape. This is because the anisotropy of the photocatalyst crystals results in the photocatalytic activity being different on different faces of the crystals. For instance, octahedral Cu2O particles, which consist of {111} facets, show higher photocatalytic activity than cubic ones, in which {100} facets are dominant.16 The higher photocatalytic activity of the {111} facets is attributed to the presence of dangling bonds from the Cu atoms. In the case of anatase TiO2, it has been reported that the {001} and {100} facets exhibit high activity with respect to photocatalytic reactions.17–19 Having determined that the {100} facets are dominant in TiO2 has led to the development of highly active TiO2 as a photocatalyst.20 Goethite particles with various shapes have been synthesized;6,13 however, the effects of the shape of the goethite particles on their photocatalytic activity from a crystallographic point of view still remain unknown. Knowing the relationship between the particle shape of goethite and its photocatalytic activity is important in order to be able to synthesize a more active and efficient photocatalyst.
In the present study, goethite particles with diverse morphologies (i.e., rod-like, pancake-like, and array-like particles) were synthesized. The photocatalytic activities of these particles were evaluated in the oxidation of acetaldehyde under irradiation, with the focus being on the morphological factors affecting the photocatalytic activities of the goethite particles.
Experimental
Preparation of goethite particles
The goethite particles investigated in this study were prepared through two methods: the oxidation of Fe(OH)2 (method A)21,22 and the hydrolysis of a ferric nitrate solution (method B).7,23Characterization of goethite particles
The shapes of the different goethite samples were observed using scanning electron microscopy (SEM) (Hitachi, SU6600). The specific surface area of the samples was determined by the single point Brunauer–Emmett–Teller method using a Micromeritics FlowSorb II 2300, AutoMate 23 instrument. Approximately 0.1–0.2 g of the samples was taken for measurements. The measurements were performed using a mixture of nitrogen and helium gases at a molar volume ratio of 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7. Prior to analysis, the samples were degassed at 130 °C for 4 h. Powder X-ray diffraction (XRD) patterns of the samples were obtained using a Shimadzu XD-610 diffractometer with CuKα radiation (0.154 nm). The diffuse reflectance spectra of the samples were measured using an ultraviolet–visible spectrophotometer (Jasco, V-570) equipped with an integrating sphere (Jasco, ISN-470). The diffuse reflectance data were converted into the corresponding Kubelka–Munk functions. The size distributions of the different types of particles in solution were measured using a dynamic light-scattering particle-size analyzer (HORIBA, LB-550).
7. Prior to analysis, the samples were degassed at 130 °C for 4 h. Powder X-ray diffraction (XRD) patterns of the samples were obtained using a Shimadzu XD-610 diffractometer with CuKα radiation (0.154 nm). The diffuse reflectance spectra of the samples were measured using an ultraviolet–visible spectrophotometer (Jasco, V-570) equipped with an integrating sphere (Jasco, ISN-470). The diffuse reflectance data were converted into the corresponding Kubelka–Munk functions. The size distributions of the different types of particles in solution were measured using a dynamic light-scattering particle-size analyzer (HORIBA, LB-550).
Photocatalytic experiments
The photocatalytic activity of each sample was investigated in terms of the oxidation of acetaldehyde. The photocatalytic experiments were performed in air in a 20 cm3 airtight glass vessel containing 10 cm3 of 50 mmol dm−3 acetaldehyde (pH of 4.8) and 10 mg of the photocatalyst (A-1, A-2, B-1, or B-2). A 150 W xenon lamp (Ushio, UXL-151D-0) with a light intensity of ~100 mW cm−2 was used as the light source. Additionally, a cut-off filter (Suruga Seiki, S76-L42) was mounted in the irradiation window so as to irradiate the samples with light of wavelength λ > 420 nm. The light intensity was measured using a power meter (Gentec, PSV-3103V2). The gaseous products were analyzed using a gas chromatograph with a thermal conductivity detector (Shimadzu, GC-14B) and a packed column (Restek, ShinCarbon ST). Ar was used as the carrier gas.Results and discussion
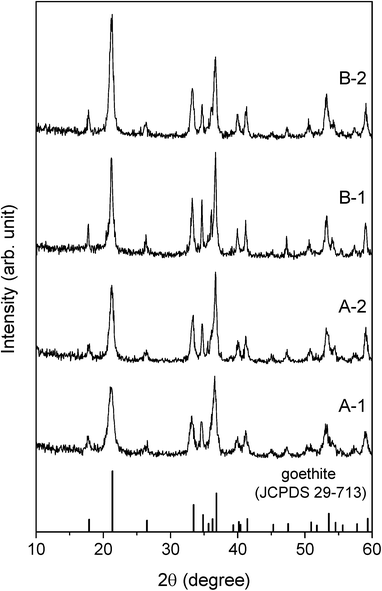
The structural and optical characteristics of each sample were identified from the corresponding XRD patterns, SEM images, specific surface areas, and diffuse reflectance spectra.The XRD patterns of all the samples (Fig. 1) could be indexed to pure goethite (JCPDS 29-713) since no peaks characteristic of any other Fe species were noticed. The intensities of the peaks in the spectrum for A-1 were similar to those of the corresponding peaks in the spectrum for A-2. Therefore, A-1 and A-2 can be said to possess essentially the same crystallinity. The same was true for B-1 and B-2 as well.
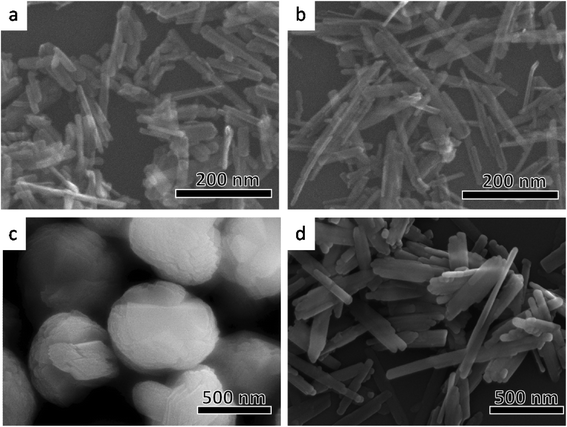
Fig. 2 shows the shapes of the different types of goethite particles observed using scanning electron microscopy. The particles of samples A-1 (Fig. 2a) and A-2 (Fig. 2b) were rod-like in shape and had a length of 100–200 nm. Commonly, goethite crystals are often acicular and elongated along the crystallographic c-axis direction,7,24 terminating in {110} facets on the elongated faces, and are capped by {021} surfaces (Fig. 3a). In case of both samples A-1 and A-2 (Fig. 2a and b), the shape of the edge of the rod was in good agreement with the acicular elongated shape of common goethite crystals. This indicates that the rod-like particles in A-1 and A-2 consist of {110} and {021} facets. The length of the A-2 particles was greater than that of the particles of A-1. The width of the A-1 particles was, however, almost the same as that of the A-2 particles. The differences in the shapes of the two types of particles are discussed in detail later. Particles of B-1 had a pancake-like (oblate) shape (Fig. 2c). Goethite with a particle structure similar to that of B-1 has been reported previously.25,26 As shown in Fig. 3b, this structure consists of six rod-shaped goethite particles (similar to both A-1 and A-2 particles) that intersect at 60° in the horizontal plane, coupling over one another to form an asterisk-like sheet. This sheet is stacked on top of another such sheet parallel to the crystallographic c-axis direction, resulting in the pancake-like particles. Therefore, the pancake-like particles essentially consisted of {110} and {021} facets; that is, the sides of the particles consisted of {021} facets, while the top and bottom were {110} facets. On the other hand, the B-2 particles had an array-like structure (Fig. 2d). Rod-shaped goethite particles (similar to both A-1 and A-2 particles) that are longer than the A-2 particles combine along the crystallographic b-axis (minor axis) to form array-like structures (Fig. 3c).6 Hence, the array-like particles also consisted of {110} and {021} facets. These results indicate that goethite particles of a different shape were successfully prepared by the above-mentioned methods and that these particles were made up of {110} and {021} facets.
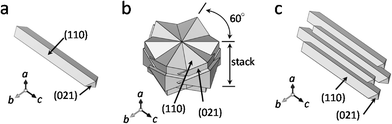 | ||
| Fig. 3 Schematic illustrations of the different shapes of the photocatalyst particles made using the software VESTA.38 a: Rod-like particles (A-1 and A-2), b: pancake-like particles (B-1), and c: array-like particles (B-2). | ||
The specific surface areas of the different types of particles were as follows: ~73 m2 g−1 for A-1, ~70 m2 g−1 for A-2, ~59 m2 g−1 for B-1, and ~48 m2 g−1 for B-2. The diffuse reflectance spectra of A-1, A-2, B-1, and B-2 are shown in Fig. 4. The absorption edges of all the samples had a wavelength of ~550 nm. The band gap of goethite has been reported to be 2.0–2.5 eV;11 the results obtained in this study were in good agreement with this value. In addition, it has been shown that a decrease in the size of photocatalyst particles can lead to a blue shift of the absorption edge of the photocatalyst owing to a quantum confinement effect.27 Hence, the band gap energies as determined from Fig. 4 may also suggest that the intrinsic oxidative/reductive powers of all samples were similar.
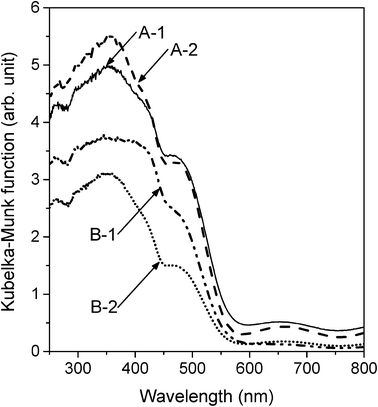 | ||
| Fig. 4 Diffuse reflectance spectra of A-1, A-2, B-1, and B-2 converted into the corresponding Kubelka–Munk functions. | ||
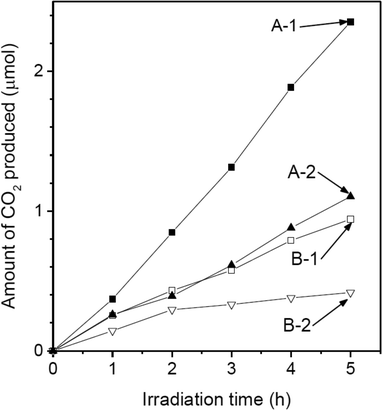
The photocatalytic activities of the different samples were investigated with respect to the decomposition of acetaldehyde (CH3CHO + 5/2O2 → 2CO2 + 2H2O).7Fig. 5 shows the time courses of the amounts of CO2 produced using the different samples under irradiation. During photocatalysis, all samples oxidatively induced the formation of CO2. However, CO2 was not produced in the dark or under irradiation without a photocatalyst present. Hence, it was verified that acetaldehyde degrades through a photocatalytic process. The amount of CO2 produced exhibited a linear dependence on the irradiation time; this was true for all the samples, indicating that all the samples acted as stable photocatalysts. The rates of CO2 formation for the different samples are listed in Table 1. The sample with the highest activity was A-1. The rate of CO2 production in the case of A-2 was almost the same as that in the case of B-1. The sample with the lowest activity was B-2. However, the order in which different samples ranked in terms of their photocatalytic activities was different from the order of their specific surface areas. Table 1 also shows the rates of CO2 formation per unit surface area for the different samples. A-1 had a specific surface area similar to that of A-2; however, the photocatalytic activity per unit surface area of A-1 was higher than that of A-2. Similarly, the photocatalytic activity per unit surface area of B-1 was higher than that of B-2. These results indicated that the photocatalytic activity of the samples was affected by their particle shape as well as by their specific surface area.
| Sample | k 1 (μmol h−1) | k s (μmol h−1 m−2) |
|---|---|---|
| a k s = k1/(the amount of photocatalyst used (i.e., 10 mg) × SSA). | ||
| A-1 | 0.46 | 0.63 |
| A-2 | 0.20 | 0.28 |
| B-1 | 0.22 | 0.37 |
| B-2 | 0.10 | 0.20 |
To elucidate the effects of the particle shape of the samples on their photocatalytic activity, the particle shapes of the different samples were investigated in detail. First, the shapes of A-1 and A-2, both of which were rod-like, were compared. Fig. 6 shows the results of a statistical analysis of the distribution of the dimensions of the rod-like particles of A-1 and A-2 based on SEM observations. The length and width were 101 ± 34 and 30 ± 8 nm, respectively, in the case of sample A-1 and 187 ± 63 and 29 ± 7 nm, respectively, for sample A-2. The width of the A-1 particles was almost the same as that of the A-2 particles; however, the length of the A-2 particles was almost twice that of the A-1 particles. Considering that the crystals of both sample A-1 and sample A-2 consisted of {110} and {021} facets (Fig. 2 and 3a), the shortening of the particle length in the case of sample A-1 increased the relative surface area of the {021} facets. That is, the relative surface area of the {021} facets in A-1 was greater than that in A-2. Next, to investigate the dispersion states of the different samples in the reaction solution, the distributions of the sizes of the particles in the reaction solution were determined using a dynamic light-scattering particle size analyzer. The aggregation of the particles of a photocatalyst in solution often causes a decrease in its photocatalytic activity, since the surface area decreases owing to the aggregation.28,29Fig. 7 shows the particle sizes of samples A-1 and A-2 dispersed in a 50 mmol dm−3 acetaldehyde solution after 5 h of irradiation. The median diameters of the A-1 and A-2 particles were ~115 nm and ~179 nm, respectively; these values were in good agreement with the particle sizes estimated from the SEM images (Fig. 5). This result indicated that the particles of A-1 and A-2 are monodispersed in the reactive solution and that the effects of particle aggregation could be ignored for both samples. It also suggested that the photocatalytic activities shown in Fig. 4 were essentially affected only by the shapes of the particles. Considering that the photocatalytic activity per unit surface area in the case of A-1 was superior to that for A-2 (Table 1), these results reveal that the photocatalytic activity of the {021} facets is higher than that of the {110} facets. In a catalytic reaction involving photocatalyst particles, the carriers photogenerated in the particles need to react efficiently with the reactants adsorbed on the surfaces of the photocatalyst particles; however, electron–hole recombination takes place readily in the particles because of the short carrier diffusion distance (i.e., owing to the low mobility of the carriers).1,30 Therefore, the surface properties of the photocatalyst particles often affect their photocatalytic activity.31–34 In general, it is considered that the surface hydroxyl group (M–OH, M = metal) plays an important role in photocatalytic reactions. The surface hydroxyl groups accept holes generated under irradiation to form hydroxyl radicals and thus prevent electron–hole recombination.31–33,35 Therefore, it is expected that the presence of a greater number of hydroxyl groups results in a higher reaction rate. It has been reported that, in goethite, the density of Fe–OH sites on the {021} facets (3.4 sites per nm2) is higher than that on the {110} facets (2.8 sites per nm2).24,36 Thus, the high photocatalytic activity of the {021} facets seen in this work can be correlated to the presence of a greater number of hydroxyl species on the surfaces of the facets.
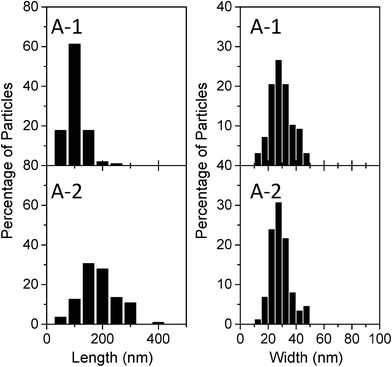 | ||
| Fig. 6 Distributions of the sizes of A-1 and A-2 particles, as determined from SEM images. The number of particles measured per sample type was 100. | ||
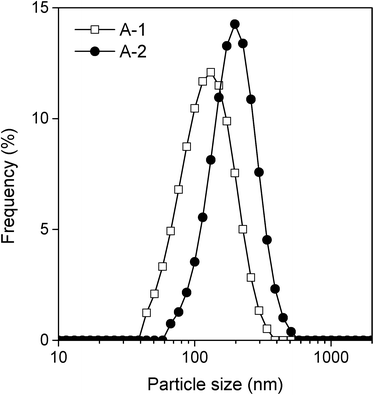 | ||
| Fig. 7 Distributions of the sizes of A-1 and A-2 particles in a 50 mM aqueous solution of acetaldehyde after 5 h of irradiation, as measured by the dynamic light-scattering method. | ||
In the photocatalytic decomposition of organic compounds by iron oxides and iron oxyhydroxides, molecular oxygen works as an electron acceptor for the reduction reaction.7 It is considered that the lattice surface of iron (≡Fe(III), ≡ denotes the lattice surface of iron oxides) acts as a reduction site.12 Two types of hole scavengers, i.e. OH− and the organic compound being decomposed, are considered to participate in the oxidation reactions, with the Fe–OH site acting as an oxidation site for both scavengers.12,37 Therefore, it can be deduced that {021} facets with a high density of Fe–OH sites may have high reactivity for oxidation reactions.
The correlation of photocatalytic activities between {110} facets and {021} facets also explains the difference in the photocatalytic activities of B-1 and B-2 (Fig. 5 and Table 1). As mentioned above, the particles of samples B-1 and B-2 have pancake-like and array-like structures, respectively. Since the relative surface area of the {021} facets is greater in B-1, the photocatalytic activity per unit surface area in the case of B-1 is superior to that in the case of B-2 (Table 1). The photocatalytic activity of B-1 was similar to that of A-2, even though the specific surface area of the B-1 particles was lower than that of the A-2 particles. These results suggest that shape-selective synthesis is important for producing highly active goethite photocatalyst particles as well as for obtaining particles with a high specific surface area.
Conclusions
In this study, the photocatalytic activities of goethite particles with different morphologies (rod-like, pancake-like, and array-like) were examined through the decomposition of acetaldehyde under irradiation. The activity of each particle type depended on its shape as well as on its specific surface area. A detailed investigation of the particle shapes revealed that the {021} facets of goethite have a higher photocatalytic activity than do the {110} facets. The short rod-like and pancake-like particles of goethite, owing to the high relative surface area of {021} facets, exhibited higher photocatalytic activities than did the long rod-like and array-like particles. These results indicated that the shape-selective synthesis of goethite that takes into account the high-activity facets will lead to a more active photocatalyst.Acknowledgements
This work was supported by a Grant-in-Aid for Young Scientists (B) (no. 24760556) from the Japan Society for the Promotion of Science (JSPS).References
- M. P. Dare-Edwards, J. B. Goodenough, A. Hamnett and P. R. Trevellick, J. Chem. Soc., Faraday Trans. 1, 1983, 79, 2027–2041 RSC.
- W. Feng and D. Nansheng, Chemosphere, 2000, 41, 1137–1147 CrossRef CAS.
- S. Kakuta and T. Abe, J. Mater. Sci., 2009, 44, 2890–2898 CrossRef CAS PubMed.
- S. Kakuta, T. Okayama, M. Kato, A. Oda and T. Abe, Catal. Sci. Technol., 2011, 1, 1671–1676 CAS.
- J. K. Leland and A. J. Bard, J. Phys. Chem., 1987, 91, 5076–5083 CrossRef CAS.
- H. Li, T. Wang, Y. Zhang, L. Jiang, C. Shu and C. Wang, J. Nanosci. Nanotechnol., 2012, 12, 1910–1918 CrossRef CAS PubMed.
- N. Murakami, T. Matsuo, T. Tsubota and T. Ohno, Catal. Commun., 2011, 12, 341–344 CrossRef CAS PubMed.
- M. C. Pereira, E. M. Garcia, A. Cândido da Silva, E. Lorençon, J. D. Ardisson, E. Murad, J. D. Fabris, T. Matencio, T. de Castro Ramalho and M. V. J. Rocha, J. Mater. Chem., 2011, 21, 10280–10282 RSC.
- K. Sivula, F. Le Formal and M. Grätzel, ChemSusChem, 2011, 4, 432–449 CrossRef CAS PubMed.
- S. Yamanaka, T. Doi, S. Sako and M. Hattori, Mater. Res. Bull., 1984, 19, 161–168 CrossRef CAS.
- D. M. Sherman, Geochim. Cosmochim. Acta, 2005, 69, 3249–3255 CrossRef CAS PubMed.
- W. Du, Y. Xu and Y. Wang, Langmuir, 2008, 24, 175–181 CrossRef CAS PubMed.
- H. Li, W. Li, Y. Zhang, T. Wang, B. Wang, W. Xu, L. Jiang, W. Song, C. Shu and C. Wang, J. Mater. Chem., 2011, 21, 7878–7881 RSC.
- I. A. Shkrob, T. M. Marin, A. Adhikary and M. D. Sevilla, J. Phys. Chem. C, 2011, 115, 3393–3403 CAS.
- X. Zhou, H. Yang, C. Wang, X. Mao, Y. Wang, Y. Yang and G. Liu, J. Phys. Chem. C, 2010, 114, 17051–17061 CAS.
- J.-Y. Ho and M. H. Huang, J. Phys. Chem. C, 2009, 113, 14159–14164 CAS.
- X.-Q. Gong and A. Selloni, J. Phys. Chem. B, 2005, 109, 19560–19562 CrossRef CAS PubMed.
- Q. Wu, M. Liu, Z. Wu, Y. Li and L. Piao, J. Phys. Chem. C, 2012, 116, 26800–26804 CAS.
- X. Zhao, W. Jin, J. Cai, J. Ye, Z. Li, Y. Ma, J. Xie and L. Qi, Adv. Funct. Mater., 2011, 21, 3554–3563 CrossRef CAS.
- H. Xu, S. Ouyang, P. Li, T. Kako and J. Ye, ACS Appl. Mater. Interfaces, 2013, 5, 1348–54 CAS.
- F. Gilbert, P. Refait, F. Lévêque, C. Remazeilles and E. Conforto, J. Phys. Chem. Solids, 2008, 69, 2124–2130 CrossRef CAS PubMed.
- A. V. Tolchev, D. G. Kleschov, R. R. Bagautdinova and V. Y. Pervushin, Mater. Chem. Phys., 2002, 74, 336–339 CrossRef CAS.
- F. Geng, Z. Zhao, J. Geng, H. Cong and H.-M. Cheng, Mater. Lett., 2007, 61, 4794–4796 CrossRef CAS PubMed.
- J. R. Rustad and A. R. Felmy, Geochim. Cosmochim. Acta, 2005, 69, 1405–1411 CrossRef CAS PubMed.
- K. Iwasaki, T. Itoh and T. Yamamura, Mater. Trans., 2001, 42, 1629–1637 CrossRef CAS.
- V. Barrón, N. Gálvez, M. F. Hochella Jr. and J. Torrent, Am. Mineral., 1997, 82, 1091–1100 Search PubMed.
- C.-H. Kuo, C.-H. Chen and M. H. Huang, Adv. Funct. Mater., 2007, 17, 3773–3780 CrossRef CAS.
- H. Yoneyama, S. Haga and S. Yamanaka, J. Phys. Chem., 1989, 93, 4833–4837 CrossRef CAS.
- H. Yoneyama, Res. Chem. Intermed., 1991, 15, 101–111 CrossRef CAS PubMed.
- J. H. Kennedy, J. Electrochem. Soc., 1978, 125, 709–714 CrossRef CAS PubMed.
- A. H. Boonstra and C. A. H. A. Mutsaers, J. Phys. Chem., 1975, 79, 1694–1698 CrossRef CAS.
- S. Sato, Langmuir, 1988, 4, 1156–1159 CrossRef CAS.
- D. Lawless, N. Serpone and D. Meisel, J. Phys. Chem., 1991, 95, 5166–5170 CrossRef CAS.
- J. Zhu, J. Yang, Z.-F. Bian, J. Ren, Y.-M. Liu, Y. Cao, H.-X. Li, H.-Y. He and K.-N. Fan, Appl. Catal., B, 2007, 76, 82–91 CrossRef CAS PubMed.
- K. Nagaveni, M. S. Hegde, N. Ravishankar, G. N. Subbanna and G. Madras, Langmuir, 2004, 20, 2900–2907 CrossRef CAS.
- J. R. Rustad, A. R. Felmy and B. P. Hay, Geochim. Cosmochim. Acta, 1996, 60, 1563–1576 CrossRef CAS.
- Q. Lan, H. Liu, F. Li, F. Zeng and C. Liu, Chem. Eng. J., 2011, 168, 1209–1216 CrossRef CAS PubMed.
- K. Momma and F. Izumi, J. Appl. Crystallogr., 2011, 44, 1272–1276 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2014 |