New magnetic nanocomposites of ZrO2–Al2O3–Fe3O4 as green solid acid catalysts in organic reactions
Anqi
Wang
,
Xiang
Liu
*,
Zhongxing
Su
and
Huanwang
Jing
*
State Key Laboratory of Applied Organic Chemistry, College of Chemistry and Chemical Engineering, Lanzhou University, 222 South Tianshui Road, Gansu, Lanzhou, 730000, PR China. E-mail: liuxiang@lzu.edu.cn; hwjing@lzu.edu.cn; Fax: +86 0931 8912582; Tel: +86 0931 8912585
First published on 25th September 2013
Abstract
A series of magnetic solid acid nano-catalysts were designed and prepared through a facile co-precipitate approach. The original nanocomposites ZrO2–Al2O3–Fe3O4 were characterized by means of ICP-AES, BET, XRD, TEM, HRTEM, VSM, FT-IR, NH3-TPD and TG. Their catalytic behaviours were investigated via esterification, the synthesis of bis-indolylmethanes, Hantzsch reaction, Biginelli reaction and Pechmann reaction. In all of these organic reactions, the corresponding products were obtained in moderate to excellent yields. The optimal catalyst was ZAF-16/16, which retained catalytic activity after several recycles.
Introduction
Acid-catalyzed organic reactions are topics of overwhelming interest in view of vast applications in the chemical industry. Conventional liquid inorganic acids, such as H2SO4, HCl, H3PO4 and HF have been developed and applied to various organic syntheses. However, these homogeneous acids are not suitable for industrial processes due to separation problems and environmental issues. In the last few decades, many types of solid acids have emerged as potential alternate catalysts to the mineral acids, including sulfated metal oxides, zeolites, heteropolyacids, metal phosphates, inorganic oxides, etc.1–5 The preparation and use of solid acid catalysts are active research fields for esterification,6,7 isomerization,8,9 acylation,10,11 alkylation,12,13 nitration,14,15 as well as other important organic transformations.16–21 It is well known that the solid superacid catalysts of sulfated metal oxides (SO42−–MxOy) offer new opportunities for developing environmentally benign and friendly processes in organic syntheses. However, the SO42−–MxOy catalysts suffer from the disadvantage of deactivation in practical applications, possibly due to sulfur reduction during the reaction process, or the formation of coke on the surface of catalysts.22–24 Therefore, a more stable solid acid catalyst capable of resisting deactivation needs to be developed.In general, the traditional heterogeneous catalyst separation methods, such as filtration or centrifugation, become tedious and hamper complete separation of the catalysts. Consequently, a great amount of research work has been devoted to the development of readily separable heterogeneous catalysts for organic reactions. Magnetic nanoparticles (MNPs) are one of the most widely studied materials in multi-disciplinary research, covering magnetic resonance imaging (MRI),25 magnetic storage media,26 biotechnology,27 ferrofluids,28etc. Among magnetic materials, Fe3O4 nanoparticles have been used as a versatile support for a variety of heterogeneous catalysts in diverse classes of organic transformations.29
Based on the above fundamental understandings, we designed and prepared a series of green magnetic solid acid nano-catalysts ZrO2–Al2O3–Fe3O4 (ZAF) that have been applied in various organic reactions. Esterification of carboxylic acids with alcohols are classical chemical reactions. The synthesis of n-butyl acetate was selected as a probe reaction to evaluate the catalytic activities of the prepared magnetic solid acid nano-catalysts, ZAF. Heterocyclic compounds constitute a paramount group of natural products with various pharmaceutical properties and biological activities, as well as other applications. Recently, numerous methods describing the preparation of bis-indolylmethanes (BIMs) have been reported in the literature employing protic acids and Lewis acids catalysts.21,30,31 The Hantzsch reaction, which provides 1,4-dihydropyridines (1,4-DHPs),32–34 and the Biginelli synthesis, which generates 3,4-dihydropyrimidinones (3,4-DHPMs),35–37 are two remarkable multicomponent reactions that could be catalyzed by acidic catalysts. Moreover, the acid-catalyzed Pechmann reaction is a method commonly used for synthesizing coumarin derivatives.19,38,39 Herein, the prepared ZrO2–Al2O3–Fe3O4 nanocomposites acted as efficient and versatile catalysts in these reactions, and the excellent results were disclosed in this paper.
Results and discussion
Characterization of catalysts
The ICP-AES data indicated that only small differences between the actual and controlled molar ratio of Zr–Al–Fe3O4 were observed in ZAF catalysts (Table 1, entries 1–11). Using N2 adsorption technology, the specific surface areas of the prepared magnetic solid acid nano-catalysts were obtained and are listed in Table 1. It could be seen that the composition of ZAF catalysts had an obvious impact on the specific surface areas (SBET). There was an appreciable variation in SBET with the increase of ZrO2–Al2O3 contents, represented by the SBET of ZAF-20/20 being much higher than that of ZAF-2/2 (Table 1, entry 6 vs. 1). On the other hand, addition of alumina component to the catalysts resulted in an increased trend of the specific surface areas (Table 1, entries 5, 7–11). The catalyst ZAF-16/0 showed lower specific surface area (92 m2 g−1), while the catalyst ZAF-16/32 revealed the highest specific surface area (199 m2 g−1). Thus, the SBET increased along with the increased amount of alumina component. Additionally, the specific surface area of catalyst ZAF-16/16 declined sharply after the catalyst was calcined at temperatures from 400 to 700 °C, and the SBET of all the calcined catalysts were reduced below 50 m2 g−1 (Table 1, entries 12–15).| Entry | Catalyst | Zr![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Al Al![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Fe3O4 (molar ratio) Fe3O4 (molar ratio) |
S BET | |
|---|---|---|---|---|
| Controlled | Measured a | (m2 g−1) | ||
| a Determined by ICP-AES analysis. | ||||
| 1 | ZAF-2/2 | 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.9 1.9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
48 |
| 2 | ZAF-4/4 | 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
3.9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3.6 3.6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
57 |
| 3 | ZAF-8/8 | 8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8 8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
7.8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7.6 7.6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
95 |
| 4 | ZAF-12/12 | 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 12 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
11.8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 11.4 11.4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
141 |
| 5 | ZAF-16/16 | 16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 16 16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
15.2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 14.8 14.8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
165 |
| 6 | ZAF-20/20 | 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
18.2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 17.4 17.4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
167 |
| 7 | ZAF-16/0 | 16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
15.8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
92 |
| 8 | ZAF-16/8 | 16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8 8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
15.4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7.9 7.9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
144 |
| 9 | ZAF-16/12 | 16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 12 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
15.1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10.8 10.8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
157 |
| 10 | ZAF-16/24 | 16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 24 24![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
14.7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20.8 20.8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
187 |
| 11 | ZAF-16/32 | 16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 32 32![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
14.9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 29.1 29.1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
199 |
| 12 | ZAF-16/16 (400) | — | — | 46 |
| 13 | ZAF-16/16 (500) | — | — | 26 |
| 14 | ZAF-16/16 (600) | — | — | 23 |
| 15 | ZAF-16/16 (700) | — | — | 16 |
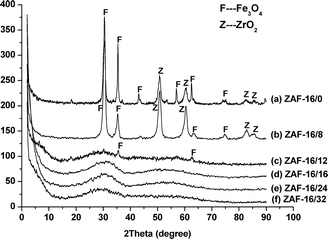
The XRD patterns of amine-functionalized Fe3O4 MNPs are shown in Fig. 1a. The diffraction peaks at 18.2°, 30.0°, 35.4°, 37.0°, 43.0°, 53.3°, 56.9°, 62.5° and 73.9° could be ascribed to reflections from (111), (220), (311), (222), (400), (422), (511), (440) and (533) planes of Fe3O4, respectively. In contrast, the diffraction peaks of both Fe3O4 and ZrO2 were observed in Fig. 1b–d. Besides the corresponding peaks of Fe3O4, the 2θ values at 50.7°, 60.5°, 82.8° and 85.8° were attributed to the characteristic reflections from (202), (311), (133) and (042) planes of tetragonal ZrO2, respectively. It was notable that the separate diffraction peaks of Al2O3 were absent in the XRD profiles. This might suggest that the alumina component had homogeneously dispersed into the structure of ZrO2–Al2O3, forming the uniform bi-component of ZrO2–Al2O3 nanocomposites that was coated on the surface of the Fe3O4 MNPs. However, samples with higher ZrO2–Al2O3 contents, namely, the catalysts ZAF-12/12, ZAF-16/16 and ZAF-20/20, revealed no evident diffraction peaks except a broadened peak around 30° (Fig. 1e–g). It was concluded that the content of ZrO2–Al2O3 would deeply affect the crystalline phase of ZAF catalysts.
 | ||
| Fig. 1 XRD patterns of amine-functionalized Fe3O4 and ZAF catalysts with different contents of ZrO2–Al2O3. | ||
Fig. 2 shows that the crystalline phase of the nanocomposites changed with increasing alumina content in ZAF catalysts. It could be observed from Fig. 2a that the diffraction peaks of both Fe3O4 and tetragonal ZrO2 were detected in ZAF-16/0 where there was not any alumina component. Otherwise, the 2θ values of ZAF-16/8 were unaltered compared to ZAF-16/0 except the peak intensity of tetragonal ZrO2 grew slightly and that of Fe3O4 declined (Fig. 2b). But when the alumina content further increased, the separate diffraction peaks of Fe3O4 and tetragonal ZrO2 gradually diminished while the amorphous phase replaced the crystalline phase (Fig. 2c–f).
The effect of calcination temperature on the crystalline structure of the catalyst ZAF-16/16 is shown in Fig. 3. It could be seen that the ZAF-16/16 (400) and ZAF-16/16 (500) kept an almost constant structure, which was similar to the uncalcined catalyst ZAF-16/16 (Fig. 3a and b). However, further increase in calcination temperature (600 and 700 °C) resulted in an appearance of some characteristic reflections of Fe3O4 and tetragonal ZrO2 (Fig. 3c and d).
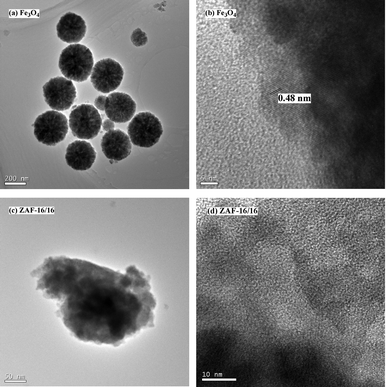
The morphology and fine structure of amine-functionalized Fe3O4 MNPs and catalyst ZAF-16/16 were examined by TEM and HRTEM. It was clear to see in Fig. 4a that the Fe3O4 MNPs had a size range of 200–300 nm. Fig. 4b indicated that the interplanar distance was 0.48 nm, attributed to the (111) lattice planes of Fe3O4. Additionally, Fig. 4c confirmed the presence of a ZrO2–Al2O3 coating. Compared with Fe3O4 MNPs, there was a conspicuous increase in the particle size of catalyst ZAF-16/16. Meanwhile, the detailed structural information of ZAF-16/16 was amorphous, which was also evidenced by HRTEM (Fig. 4d). These observations were in good agreement with the XRD results (Fig. 1a and f).
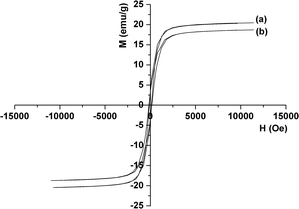
The magnetic properties of as-synthesized Fe3O4 and ZAF-16/16 were measured at room temperature. As shown in Fig. 5a, the saturation magnetization value of Fe3O4 was 20.5 emu g−1. After being coated by the ZrO2–Al2O3 layer, the saturation magnetization value of ZAF-16/16 was 18.7 emu g−1 (Fig. 5b). The slight decrease in magnetic saturation for ZAF-16/16 could be explained by the presence of a surface coating of ZrO2–Al2O3 nanocomposites.
Fig. 6a presents the strong IR band at 577 cm−1 characteristic of the Fe–O vibrations, while the transmissions around 1623, 1480 and 872 cm−1 were well matched with that from free 1,6-hexanediamine, indicating the existence of –NH2 group on the amine-functionalized Fe3O4.40 The IR spectrum for catalyst ZAF-16/16 without pyridine adsorption is illustrated in Fig. 6b. It was clear to see that the vibration peak of Zr–O band was at 712 cm−1,41 and the characteristic band of 1620 cm−1 could be ascribed to –NH2 originating from the Fe3O4 surface. The IR spectrum of pyridine adsorbed on magnetic solid acid nano-catalyst ZAF-16/16 in Fig. 6c shows characteristic bands in the range of 1400–1650 cm−1. Namely, the bands at 1456 and 1542 cm−1 could be assigned to Lewis and Brønsted acid sites, respectively. Moreover, an additional broad and intense band at 3403 cm−1 corresponding to stretching vibrations of hydroxyl groups and absorbed water accompanied by the 1627 cm−1 band were observed.42,43
 | ||
| Fig. 6 FT-IR spectra of (a) Fe3O4 MNPs, (b) ZAF-16/16 without pyridine adsorption, (c) ZAF-16/16 with pyridine adsorption. | ||
Temperature-programmed desorption of ammonia (NH3-TPD) was used to compare the acidic characteristics of the catalysts ZAF-16/16 and ZAF-16/0. Fig. 7 showed that each catalyst exhibited a broad NH3 desorption peak starting from 100 °C and extending beyond 800 °C. It was observed in Fig. 7a and b that there were three distinct desorption peaks at the range of 100–300 °C, 300–400 °C and 400–700 °C, which demonstrated that both of the two catalysts contained weak, moderate and strong acid sites.44 The amounts of acid sites on the catalysts and the peak temperature of the TPD profiles are also listed in Table 2. The data showed that the total amount of acid sites on ZAF-16/16 (2.77 mmol g−1) was higher than that of ZAF-16/0 (1.61 mmol g−1). This phenomenon could be interpreted as the acid strength of catalyst ZAF-16/16 being much stronger, indicating that the addition of alumina component led to the increase in catalyst acidity.
| Entry | Catalyst | NH3 adsorption (mmol g−1) | |||
|---|---|---|---|---|---|
| 100–300 °C | 300–400 °C | 400–700 °C | Total | ||
| 1 | ZAF-16/16 | 1.15 | 0.28 | 1.34 | 2.77 |
| 2 | ZAF-16/0 | 0.16 | 0.32 | 1.13 | 1.61 |
The thermal properties of the catalysts ZAF-16/16 and ZAF-16/0 were determined by TG analyses and the results are illustrated in Fig. 8. Generally, the weight loss of the catalyst could be explained by the following reasons: the gradient that appeared at a lower temperature below 200 °C was ascribed to desorption of water adsorbed on the surface of catalysts, whereas the gradient that emerged above 200 °C corresponded to the release of crystal water and the chemical structure water of Zr(OH)4. The TG curves revealed that the total mass loss of ZAF-16/16 (38.2%) was higher than that of ZAF-16/0 (22.4%). We inferred that the addition of alumina component was beneficial to enlarge the surface area of catalyst ZAF-16/16, which resulted in more hydroxyl groups existing in ZAF-16/16.
Esterification
Esterification is a fundamental organic reaction which can be catalyzed by both homogeneous and heterogeneous acidic catalysts. n-Butyl acetate is widely used as a solvent and in plasticizers, perfumes and flavors, and also as an organic feedstock and intermediate.45,46 The synthesis of n-butyl acetate was chosen as a model reaction to examine the catalytic performance of the prepared magnetic solid acid nano-catalysts and the results are listed in Table 3. It could be seen that the reaction was quite difficult to take place in the absence of catalyst (Table 3, entry 1). However, when the esterification was catalyzed by the series of ZrO2–Al2O3–Fe3O4 catalysts the n-butyl acetate was formed as a single product (Table 3, entries 2–11). The catalysts with various contents of ZrO2–Al2O3 revealed different activities during the reaction process, and the catalytic activity of ZAF-16/16 was much higher compared to the other catalysts (Table 3, entry 6). On the other hand, the calcination temperatures had a bearing on the catalytic activity of ZAF-16/16. The catalyst ZAF-16/16 after calcination at temperatures from 400–700 °C displayed lower activities than the initial precipitated catalyst, and the conversion of n-butyl alcohol decreased sharply with increasing calcination temperatures (Table 3, entries 12–15). It was worthy to mention that the catalyst ZrO2–Al2O3, which did not contain a magnetic component, was less effective than ZAF-16/16 (Table 3, entry 16 vs. 6); meanwhile, a trace amount of n-butyl acetate was formed when the reaction was catalyzed by Fe3O4 MNPs (Table 3, entry 17). We could infer that the Fe3O4 MNPs not only acted as a magnetic component for separation, but also participated in the catalysis. The control experiments showed that the conversions were obviously declined when ZrO2–Fe3O4 (ZAF-16/0), Al2O3–Fe3O4 (ZAF-0/16), ZrO2 and Al2O3 acted as catalysts in the esterification (Table 3, entries 18–21), which verified the synergy effect in the tri-component nanocomposites of ZrO2–Al2O3–Fe3O4 catalysts. Consequently, the original precipitated catalyst of ZAF-16/16 was determined as the optimal catalyst for the next catalytic reactions. Compared to some solid acid catalysts that have been reported in the literature, the optimal catalyst ZAF-16/16 revealed higher activity in a shorter reaction time and at a lower temperature (Table 3, entry 6 vs. entries 22–27). We also carried out the reaction using prepared ZAF-16/16 as the catalyst under the same reaction conditions of literature studies and the results showed that the ZAF-16/16 was more active than the reported solid acid catalysts (Table 3, entries 22–27, data in parentheses). According to the literature, the esterification mechanism involved both Lewis and Brønsted acid sites.48| Entry | Catalyst | Conv.b (%) | Entry | Catalyst | Conv. (%) |
|---|---|---|---|---|---|
| a Reaction conditions: catalysts 0.50 g, acetic acid 0.15 mol, n-butyl alcohol 0.10 mol, cyclohexane 25 mL, reflux, 1.5 hours. b Determined by GC with FID detector, n-butyl acetate selectivity > 99%. c See ref. 6. d See ref. 47. e See ref. 48. f The numbers in parentheses represented the results of control experiments that catalyzed by ZAF-16/16 under the identical reaction conditions in references. | |||||
| 1 | No trace | 15 | ZAF-16/16 (700) | 39.2 | |
| 2 | ZAF-2/2 | 21.2 | 16 | ZA-16/16 | 85.1 |
| 3 | ZAF-4/4 | 32.5 | 17 | Fe3O4 | 2.4 |
| 4 | ZAF-8/8 | 75.9 | 18 | ZAF-16/0 | 22.5 |
| 5 | ZAF-12/12 | 84.4 | 19 | ZAF-0/16 | 17.1 |
| 6 | ZAF-16/16 | 90.0 | 20 | ZrO2 | 20.2 |
| 7 | ZAF-20/20 | 86.2 | 21 | Al2O3 | 14.8 |
| 8 | ZAF-16/8 | 27.7 | 22c | SO42−/ZrO2 | 63 (91.4)f |
| 9 | ZAF-16/12 | 51.1 | 23c | SiO2–Al2O3 | 34 (91.4)f |
| 10 | ZAF-16/24 | 40.7 | 24d | Al2O3 | 32.2 (90.4)f |
| 11 | ZAF-16/32 | 26.2 | 25d | V2O5–Al2O3 | 62.6 (90.4)f |
| 12 | ZAF-16/16 (400) | 80.4 | 26e | ZrO2–WO3 | 58 (88.5)f |
| 13 | ZAF-16/16 (500) | 68.9 | 27e | SO42−/ZrO2–WO3 | 86 (88.5)f |
| 14 | ZAF-16/16 (600) | 45.0 | |||
Synthesis of bis-indolylmethanes
Bis-indolymethane derivatives have been reported to be isolated from natural products, which display important biological activity. Typical approaches involve the reaction of indole with aldehydes under Lewis and protic acid catalytic systems, and the plausible mechanism of BIMs synthesis revealed that the substrate aldehydes could be activated by both Lewis and Brønsted acid sites on the catalysts.49–51 In this work, the magnetic solid acid nano-catalyst ZAF-16/16 revealed an excellent catalytic efficiency in the synthesis of BIMs. From the results in Table 4, it could be seen that benzaldehyde reacted smoothly with indole to produce BIMs in excellent yield (98.4%) under refluxing in methanol, while the blank experiment showed that the reaction could barely occur in the absence of catalyst (Table 4, entries 2 vs. 1). In addition, aromatic aldehydes with electron-donating as well as electron-withdrawing substituents also gave good yields (Table 4, entries 7–23). However, under identical reaction conditions, n-butyl aldehyde did not give any product until the reaction time was doubled to 3 hours (Table 4, entry 24). The reaction of benzaldehyde and indole was chosen as a model to examine the recyclability of catalyst ZAF-16/16. The recycling results showed that the magnetic solid acid nano-catalyst ZAF-16/16 could retain almost its initial activity up to five reaction cycles. Meanwhile, the loss of the nano-catalyst during the separation process was negligible (the total loss was about 1% in weight).| Entry | R | Yieldb (%) | Entry | R | Yield (%) |
|---|---|---|---|---|---|
| a Reaction conditions: catalyst ZAF-16/16 0.20 g, aldehyde 1.25 mmol, indole 2.5 mmol, methanol 10 mL, reflux, 1.5 hours. b Isolated yield. c In the absence of catalyst. d See ref. 21. e Recycled ZAF-16/16 was used. f Reaction time was 3 hours. | |||||
| 1c | Ph | Trace | 13 | 3,4-(CH3)2C6H3 | 73.5 |
| 2 | Ph | 98.4 | 14 | 2-ClC6H4 | 98.5 |
| 85d | |||||
| 3e | Ph | 97.5 | 15 | 3-ClC6H4 | 83.0 |
| 4e | Ph | 98.0 | 16 | 4-ClC6H4 | 91.5 |
| 5e | Ph | 97.6 | 17 | 2,4-Cl2C6H3 | 84.9 |
| 6e | Ph | 97.1 | 18 | 2-BrC6H4 | 59.0 |
| 7 | 2-CH3OC6H4 | 64.6 | 19 | 2-NO2C6H4 | 53.4 |
| 8 | 3-CH3OC6H4 | 92.7 | 20 | 3-NO2C6H4 | 97.4 |
| 9 | 4-CH3OC6H4 | 64.6 | 21 | 4-NO2C6H4 | 98.1 |
| 10 | 2,5-(CH3O)2C6H3 | 58.7 | 22 | 2-HOC6H4 | 88.9 |
| 11 | 3-CH3C6H4 | 62.0 | 23 | Ph–CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH CH |
93.4 |
| 12 | 4-CH3C6H4 | 92.1 | 24f | C3H7 | 51.4 |
Hantzsch reaction
Multicomponent reactions (MCRs) are one of the most powerful emerging synthetic tools for the creation of molecular structure complexity and diversity.52,53 The Hantzsch reaction, which affords 1,4-dihydropyridines as products, is one of the earliest and best known MCRs. In recent decades, considerable attention has been paid to the synthesis of 1,4-DHPs owing to their pivotal pharmaceutical and biological activities.54 We have introduced the catalyst ZAF-16/16 into the Hantzsch reaction and the results are summarized in Table 5. In all cases, the corresponding products were obtained in the presence of a catalytic amount of ZAF-16/16. Compared with the substituted aromatic aldehydes, the unsubstituted benzaldehyde achieved the highest yield (94.5%). Moreover, the aryl group substituted with different groups and the same group located at different positions on the aromatic ring did not present much effect on the formation of the final products and afforded the target products in good yields (Table 5, entries 3–17). We also carried out the reaction without any catalyst but the product was isolated in poor yield (8.5%). Based on the proposed mechanism of Hantzsch reaction reported in the literature, the synthesis of 1,4-DHPs involved a protocol of Lewis-acid catalyzed cyclocondensation.55| Entry | R | Yieldb (%) | Entry | R | Yield (%) |
|---|---|---|---|---|---|
| a Reaction conditions: catalyst ZAF-16/16 0.05 g, aldehyde 5 mmol, ethyl acetoacetate 10 mmol, ammonium acetate 10 mmol, ethanol 10 mL, reflux, 1.5 hours. b Isolated yield. c In the absence of catalyst. d See ref. 55. | |||||
| 1c | Ph | 8.5 | 10 | 3-ClC6H4 | 78.3 |
| 2 | Ph | 94.5 95d | 11 | 4-ClC6H4 | 77.3 |
| 3 | 2-CH3OC6H4 | 77.7 | 12 | 2,4-Cl2C6H3 | 82.7 |
| 4 | 4-CH3OC6H4 | 73.6 | 13 | 2-NO2C6H4 | 77.7 |
| 5 | 2,5-(CH3O)2C6H3 | 83.3 | 14 | 3-NO2C6H4 | 53.8 |
| 6 | 3-CH3C6H4 | 83.8 | 15 | 4-NO2C6H4 | 80.3 |
| 7 | 4-CH3C6H4 | 85.4 | 16 | 2-HOC6H4 | 65.8 |
| 8 | 3,4-(CH3)2C6H3 | 60.0 | 17 | Ph–CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH CH |
64.0 |
| 9 | 2-ClC6H4 | 75.5 | |||
Biginelli reaction
More than one century ago, the Italian chemist Pietro Biginelli reported a one-pot cyclocondensation reaction between aldehydes, β-ketoesters and urea to generate 3,4-dihydropyrimidinones under strongly acidic conditions. Since then, the development of efficient and versatile catalytic systems for the Biginelli reaction has become an active ongoing research area.35–37 To further extend the application scopes of the as-prepared magnetic solid acid nano-catalysts, the optimal catalyst ZAF-16/16 was also applied in the Biginelli reaction. On the basis of the results in Table 6, the reaction hardly proceeded in the absence of catalyst (Table 6, entry 1). Furthermore, the solvents played an important role in the synthesis of 3,4-DHPMs. Several classical solvents were chosen as the reaction media for comparison (Table 6, entry 2). It was found that the best result was obtained in ethylene glycol. The reaction was completed within 5 hours and the expected product was isolated in 82.4% yield. A class of aromatic aldehydes were reacted with ethyl acetoacetate and urea under the same conditions. In all cases, the aromatic aldehydes with either electron-donating or electron-withdrawing groups participated in the condensation reaction readily. The nature and the position of substitution on the aromatic ring did not have much effect on the reaction (Table 6, entries 3–19). To our delight, the reaction also proceeded smoothly when thiourea was used in place of urea to afford the corresponding 3,4-dihydropyrimidinthiones in moderate yields (Table 6, entries 20–35). The mechanism of the Biginelli reaction showed that the Brønsted acid sites on the solid acid catalysts played an important role in this typical procedure.1| Entry | R | X | Yieldb (%) | Entry | R | X | Yield (%) |
|---|---|---|---|---|---|---|---|
| a Reaction conditions: catalyst ZAF-16/16 0.20 g, aldehyde 5 mmol, ethyl acetoacetate 5 mmol, urea or thiourea 7.5 mmol, ethylene glycol 10 mL, 140 °C, 5 hours. b Isolated yield. c In the absence of catalyst. d See ref. 36. e Methanol 10 mL, reflux. f Ethanol 10 mL, reflux. g Acetonitrile 10 mL, reflux. h Tetrahydrofuran 10 mL, reflux. i N,N-Dimethylformamide (DMF) 10 mL, 140 °C. j N-Methyl-2-pyrrolidone (NMP) 10 mL, 140 °C. k Mixed solvents of dioxane 5.7 mL and i-propanol 4.3 mL, reflux. | |||||||
| 1c | Ph | O | Trace | 19 | Ph–CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH CH |
O | 81.6 |
| 82.4 | |||||||
| 80d | |||||||
| 11.9e | |||||||
| 13.7f | |||||||
| 2 | Ph | O | 8.2g | 20 | Ph | S | 63.4 |
| 19.5h | |||||||
| 25.4i | |||||||
| 37.1j | |||||||
| 35.5k | |||||||
| 3 | 2-CH3OC6H4 | O | 54.6 | 21 | 2-CH3OC6H4 | S | 46.2 |
| 4 | 3-CH3OC6H4 | O | 51.9 | 22 | 3-CH3OC6H4 | S | 49.3 |
| 5 | 4-CH3OC6H4 | O | 68.2 | 23 | 4-CH3OC6H4 | S | 57.1 |
| 6 | 2,5-(CH3O)2C6H3 | O | 75.6 | 24 | 2,5-(CH3O)2C6H3 | S | 45.3 |
| 7 | 3-CH3C6H4 | O | 53.7 | 25 | 3-CH3C6H4 | S | 44.1 |
| 8 | 4-CH3C6H4 | O | 80.4 | 26 | 4-CH3C6H4 | S | 59.0 |
| 9 | 3,4-(CH3)2C6H3 | O | 71.5 | 27 | 3,4-(CH3)2C6H3 | S | 54.7 |
| 10 | 2-ClC6H4 | O | 40.1 | 28 | 2-ClC6H4 | S | 35.5 |
| 11 | 3-ClC6H4 | O | 67.0 | 29 | 3-ClC6H4 | S | 40.9 |
| 12 | 4-ClC6H4 | O | 66.6 | 30 | 4-ClC6H4 | S | 54.8 |
| 13 | 2,4-Cl2C6H3 | O | 68.5 | 31 | 2,4-Cl2C6H3 | S | 43.0 |
| 14 | 2-BrC6H4 | O | 64.6 | 32 | 2-NO2C6H4 | S | 34.5 |
| 15 | 2-NO2C6H4 | O | 78.7 | 33 | 3-NO2C6H4 | S | 48.9 |
| 16 | 3-NO2C6H4 | O | 79.7 | 34 | 2,4-(NO2)2C6H3 | S | 37.2 |
| 17 | 4-NO2C6H4 | O | 75.4 | 35 | Ph–CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH CH |
S | 47.1 |
| 18 | 2,4-(NO2)2C6H3 | O | 36.8 | ||||
Pechmann reaction
Coumarins are widely distributed in the families of rutaceae and umbelliferae, and were obtained from these plants by virtue of various extraction methods in earlier years. Chemically, several protocols have been developed for the syntheses of coumarins, including Pechmann, Perkin, Knoevenagel, Wittig, Reformatsky and Claisen reactions.19,56–58 Among these, the acid-catalyzed Pechmann reaction is simple and commonly used for synthesizing 7-hydroxyl-4-methyl coumarin from ethyl acetoacetate and m-dihydroxybenzene. Accordingly, the optimal catalyst ZAF-16/16 was applied in the Pechmann reaction instead of the conventional liquid acid catalyst. When toluene was used as a water-carrying agent, a 65.1% isolated yield of 7-hydroxyl-4-methyl coumarin was accomplished after 4 hours (Scheme 1). As previously reported in the literature, the Pechmann reaction is catalyzed by the Brønsted acid site of the catalyst. A possible mechanism for the formation of 7-hydroxyl-4-methyl coumarin is the chemisorption of the carbonyl group of ethyl acetoacetate on the Brønsted acid site of the catalyst.59Conclusions
In summary, we have designed and synthesized a series of magnetic separable nanocomposites ZrO2–Al2O3–Fe3O4 that are used as green solid acid catalysts in various organic reactions. Both Lewis and Brønsted acid sites are present on the ZAF catalysts. The catalytic activities of all the prepared catalysts were evaluated via the esterification reaction between acetic acid and n-butyl alcohol. The maximum activity was found over the catalyst ZAF-16/16, which gave a 90.0% yield of n-butyl acetate. In addition, the optimal catalyst ZAF-16/16 was also used to catalyze several organic reactions, such as the synthesis of bis-indolylmethanes, Hantzsch reaction, Biginelli reaction and Pechmann reaction, facilitating the formation of diverse pharmacological and biological activities heterocyclic compounds. In these organic reactions, the target products were obtained in moderate to good yields. Meanwhile, simple magnetic removal and recycling of ZAF-16/16 is shown to proceed without obvious loss of either catalyst and activity.Experimental section
Materials
All reagents were obtained from commercial sources and used as received without further purification.Preparation of magnetic Fe3O4 nanoparticles
The amine-functionalized magnetic nanoparticles were prepared via the versatile solvothermal reaction reported by Li.40Preparation of magnetic solid acid catalysts
A series of magnetic solid acid nano-catalysts ZrO2–Al2O3–Fe3O4 were prepared via a simple co-precipitation method at room temperature. The model catalyst was labelled as ZAF-16/16, which means the feeding mole ratios of ZrOCl2·8H2O and AlCl3·6H2O were 16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 16. A typical procedure is described as follows: Fe3O4 nanoparticles (1 mmol, 0.2315 g) were dispersed into deionized water (20 mL) with ultrasound for 30 min, then ZrOCl2·8H2O (16 mmol, 5.1562 g) and AlCl3·6H2O (16 mmol, 3.8629 g) were dissolved in deionized water (60 mL) to obtain a clear solution that was added into the above system in one portion. After that, ammonia hydroxide (NH3·H2O) was added dropwise with vigorous mechanical stirring until the pH value of the solution reached 9 and began to precipitate. After continuous stirring for 8 hours at room temperature, the precipitate was filtered and washed with deionized water until no chloride ions could be detected. Finally, the precipitate was dried at 100 °C in a vacuum. To verify the effect of calcination temperature on the catalytic performance of the magnetic solid acid nano-catalysts, the model catalyst ZAF-16/16 was also calcined in a muffle furnace for 3 hours at temperatures from 400 to 700 °C. These calcined catalysts were named as ZAF-16/16 (400–700) where the numbers in parentheses indicate the different calcination temperatures.
16. A typical procedure is described as follows: Fe3O4 nanoparticles (1 mmol, 0.2315 g) were dispersed into deionized water (20 mL) with ultrasound for 30 min, then ZrOCl2·8H2O (16 mmol, 5.1562 g) and AlCl3·6H2O (16 mmol, 3.8629 g) were dissolved in deionized water (60 mL) to obtain a clear solution that was added into the above system in one portion. After that, ammonia hydroxide (NH3·H2O) was added dropwise with vigorous mechanical stirring until the pH value of the solution reached 9 and began to precipitate. After continuous stirring for 8 hours at room temperature, the precipitate was filtered and washed with deionized water until no chloride ions could be detected. Finally, the precipitate was dried at 100 °C in a vacuum. To verify the effect of calcination temperature on the catalytic performance of the magnetic solid acid nano-catalysts, the model catalyst ZAF-16/16 was also calcined in a muffle furnace for 3 hours at temperatures from 400 to 700 °C. These calcined catalysts were named as ZAF-16/16 (400–700) where the numbers in parentheses indicate the different calcination temperatures.
Catalysts characterization
ICP-AES was performed on an IRIS Advantage ER/S instrument (American TJA Company) for determining the composition of the catalysts. The specific surface areas of the obtained catalysts were determined on a ChemiSorb 2750 apparatus by N2 adsorption at 77 K. Powder X-ray diffraction (XRD) patterns were recorded on X'Pert PRO (Holland PANalytical Company), in the range of 2° ≤ 2θ ≤ 90° at a scanning rate of 8° min−1, using Cu Kα radiation (λ = 1.5406 Å). TEM and HRTEM images were collected on a field emission transmission electron microscope (Tecnai-G2-F30). Magnetic hysteresis loops were conducted on a vibrating sample magnetometer (Lake Shore 7304) at room temperature. FT-IR spectra were recorded on a Nicolet Fourier-transform infrared spectrometer (NEXUS 670) in the range of 400–4000 cm−1 with a resolution of 4 cm−1. The pretreatment of catalyst that was adsorbed with pyridine is described as follows: the catalyst sheet was evacuated at 300 °C for 2 hours under a vacuum of 10−2 Pa, then cooled to room temperature. After pyridine adsorption for 30 min and evacuation at 200 °C for 1 hour, the IR spectrum was recorded. NH3-TPD measurements were carried out on a TP-5080 instrument. The sample (100 mg) was swept by an Ar flow (20 mL min−1) at 450 °C for 1 hour and then exposed to NH3 at room temperature for 1 hour. After adsorption of NH3 on the sample, the carrier gas Ar (20 mL min−1) was allowed to flow over the sample at 120 °C. Once a stable baseline of NH3 was obtained, the sample was heated from room temperature to 850 °C at a rate of 20 °C min−1. Thermal gravimetric (TG) analyses were measured on a Linseis STA PT 1600 thermoanalyzer. The analyses were carried out in N2 atmosphere from room temperature to 800 °C with a heating rate of 10 °C min−1.General procedure for the esterification
The reaction was performed in a three-neck flask equipped with a condenser and water separator. A mixture of n-butanol (0.10 mol, 9.2 mL), acetic acid (0.15 mol, 8.6 mL), and nano-catalysts ZrO2–Al2O3–Fe3O4 (0.50 g) was heated at reflux in cyclohexane (25 mL) for 1.5 hours. After completion of the reaction (no water-drops appeared in the water separator), the catalysts could be recovered by an external magnet. The reaction mixture was analyzed by GC to give the conversion of substrate n-butanol and the selectivity of target product n-butyl acetate.General procedure for the synthesis of bis-indolylmethanes
A mixture of aldehyde (1.25 mmol), indole (2.5 mmol, 0.2929 g) and nano-catalyst ZAF-16/16 (0.20 g) was heated at reflux in methanol (10 mL) for the appropriate time. After completion of the reaction (monitored by TLC), the mixture was cooled to room temperature. The nano-catalyst ZAF-16/16 could be separated by an external magnet. Then the reaction mixture was poured into ice water (20 mL). The resulting solid precipitate was filtered and recrystallized from methanol and water to afford pure products. To examine the reusability of the catalyst, the used catalyst ZAF-16/16 was washed with acetone and dried at 80 °C for the next cycle.General procedure for the Hantzsch reaction
A mixture of aldehyde (5 mmol), ethyl acetoacetate (10 mmol, 1.26 mL), ammonium acetate (10 mmol, 0.7708 g) and nano-catalyst ZAF-16/16 (0.05 g) was heated at reflux in ethanol (10 mL) for the appropriate time. After completion of the reaction (monitored by TLC), the mixture was cooled to room temperature. The nano-catalyst ZAF-16/16 could be recovered by an external magnet. The solvent was evaporated under reduced pressure to yield the crude product, which was then purified by recrystallization from ethanol and water to provide the pure product.General procedure for the Biginelli reaction
A mixture of aldehyde (5 mmol), ethyl acetoacetate (5 mmol, 0.63 mL), urea (7.5 mmol, 0.4505 g) or thiourea (7.5 mmol, 0.5709 g) and nano-catalyst ZAF-16/16 (0.20 g) was heated at 140 °C in ethylene glycol (10 mL) for the appropriate time. After completion of the reaction (monitored by TLC), the mixture was cooled to room temperature. The nano-catalyst ZAF-16/16 could be removed by an external magnet. Then the reaction mixture was poured into ice water (20 mL). The resulting solid precipitate was filtered and recrystallized from ethanol and water to give pure products.General procedure for the Pechmann reaction
The reaction was performed in a three-neck flask equipped with a condenser and water separator. A mixture of m-dihydroxybenzene (5 mmol, 0.5506 g), ethyl acetoacetate (5 mmol, 0.63 mL) and nano-catalyst ZAF-16/16 (1.00 g) was heated at reflux in toluene (25 mL). After completion of the reaction (no water-drops appeared in the water separator), the mixture was cooled to room temperature, and hot methanol (40 mL) was added to dissolve all the products. The nano-catalyst ZAF-16/16 could be separated by an external magnet. Subsequently, the solvent was evaporated under reduced pressure to yield the crude product, which was then purified by recrystallization from methanol and water to furnish the pure product.Acknowledgements
This work was financially supported by the National Natural Science Foundation of China (NSFC 21173106).Notes and references
- B. M. Reddy and M. K. Patil, Chem. Rev., 2009, 109, 2185–2208 CrossRef CAS PubMed.
- K. Arata, Green Chem., 2009, 11, 1719–1728 RSC.
- T. Okuhara, Chem. Rev., 2002, 102, 3641–3666 CrossRef CAS PubMed.
- J. H. Clark, Acc. Chem. Res., 2002, 35, 791–797 CrossRef CAS PubMed.
- A. Corma, Chem. Rev., 1995, 95, 559–614 CrossRef CAS.
- T. A. Peters, N. E. Benes, A. Holmen and J. T. F. Keurentjes, Appl. Catal., A, 2006, 297, 182–188 CrossRef CAS PubMed.
- D. R. Fernandes, A. S. Rocha, E. F. Mai, C. J. A. Mota and V. T. da Silva, Appl. Catal., A, 2012, 425–426, 199–204 CrossRef CAS PubMed.
- N. Soultanidis, W. Zhou, A. C. Psarras, A. J. Gonzalez, E. F. Lliopoulou, C. J. Kiely, I. E. Wachs and M. S. Wong, J. Am. Chem. Soc., 2010, 132, 13462–13471 CrossRef CAS PubMed.
- M. K. Yadav, C. D. Chudasama and R. V. Jasra, J. Mol. Catal. A: Chem., 2004, 216, 51–59 CrossRef CAS PubMed.
- V. N. Sheemol, B. Tyagi and R. V. Jasra, J. Mol. Catal. A: Chem., 2004, 215, 201–208 CrossRef CAS PubMed.
- C. Khatri, D. Jain and A. Rani, Fuel, 2010, 89, 3853–3859 CrossRef CAS PubMed.
- N. Katada, J. Endo, K. Notsu, N. Yasunobu, N. Naito and M. Niwa, J. Phys. Chem. B, 2000, 104, 10321–10328 CrossRef CAS.
- G. D. Yadav and M. S. M. Mujeebur Rahuman, Appl. Catal., A, 2003, 253, 113–123 CrossRef CAS.
- V. N. Sheemol, B. Tyagi and R. V. Jasra, J. Mol. Catal. A: Chem., 2006, 252, 194–201 CrossRef CAS PubMed.
- G. D. Yadav and J. J. Nair, Catal. Lett., 1999, 62, 49–52 CrossRef CAS.
- S. Wang, S. Matsumura and K. Toshima, Tetrahedron Lett., 2007, 48, 6449–6452 CrossRef CAS PubMed.
- H. P. Yan, Y. Yang, D. M. Tong, X. Xiang and C. W. Hu, Catal. Commun., 2009, 10, 1558–1563 CrossRef CAS PubMed.
- B. M. Reddy, M. K. Patil, K. N. Rao and G. K. Reddy, J. Mol. Catal. A: Chem., 2006, 258, 302–307 CrossRef CAS PubMed.
- B. Tyagi, M. K. Mishra and R. V. Jasra, J. Mol. Catal. A: Chem., 2007, 276, 47–56 CrossRef CAS PubMed.
- R. Fazaeli, S. Tangestaninejad, H. Aliyan and M. Moghadam, Appl. Catal., A, 2006, 309, 44–51 CrossRef CAS PubMed.
- B. M. Reddy, P. M. Sreekanth and P. Lakshmanan, J. Mol. Catal. A: Chem., 2005, 237, 93–100 CrossRef CAS PubMed.
- X. R. Chen, C. L. Chen, N. P. Xu and C. Y. Mou, Catal. Today, 2004, 93–95, 129–134 CrossRef CAS PubMed.
- C. Zhang, T. Liu, H. J. Wang, F. Wang and X. Y. Pan, Chem. Eng. J., 2011, 174, 236–241 CrossRef CAS PubMed.
- E. Liu, A. J. Locke, R. L. Frost and W. N. Martens, J. Mol. Catal. A: Chem., 2012, 353–354, 95–105 CrossRef CAS PubMed.
- J. Q. Wan, W. Cai, X. X. Meng and E. Z. Liu, Chem. Commun., 2007, 5004–5006 RSC.
- D. E. Speliotis, J. Magn. Magn. Mater., 1999, 193, 29–35 CrossRef CAS.
- T. Neuberger, B. Schöpf, H. Hofmann, M. Hofmann and B. von Rechenberg, J. Magn. Magn. Mater., 2005, 293, 483–496 CrossRef CAS PubMed.
- S. H. Sun and H. Zeng, J. Am. Chem. Soc., 2002, 124, 8204–8205 CrossRef CAS PubMed.
- M. B. Gawande, P. S. Branco and R. S. Varma, Chem. Soc. Rev., 2013, 42, 3371–3393 RSC.
- R. Nagarajan and P. T. Perumal, Chem. Lett., 2004, 33, 288–289 CrossRef CAS.
- V. T. Kamble, K. R. Kadam, N. S. Joshi and D. B. Muley, Catal. Commun., 2007, 8, 498–502 CrossRef CAS PubMed.
- U. Eisner and J. Kuthan, Chem. Rev., 1972, 72, 1–42 CrossRef CAS.
- D. M. Stout, Chem. Rev., 1982, 82, 223–243 CrossRef CAS.
- J. P. Wan and Y. Y. Liu, RSC Adv., 2012, 2, 9763–9777 RSC.
- Z. D. Aron and L. E. Overman, Chem. Commun., 2004, 253–265 RSC.
- V. R. Rani, N. Srinivas, M. R. Kishan, S. J. Kulkarni and K. V. Raghavan, Green Chem., 2001, 3, 305–306 RSC.
- A. R. Gholap, K. Venkatesan, T. Daniel, R. J. Lahoti and K. V. Srinivasan, Green Chem., 2004, 6, 147–150 RSC.
- M. C. Laufer, H. Hausmann and W. F. Hölderich, J. Catal., 2003, 218, 315–320 CrossRef CAS.
- S. Palaniappan and R. C. Shekhar, J. Mol. Catal. A: Chem., 2004, 209, 117–124 CrossRef CAS PubMed.
- L. Y. Wang, J. Bao, L. Wang, F. Zhang and Y. D. Li, Chem.–Eur. J., 2006, 12, 6341–6347 CrossRef CAS PubMed.
- M. Sharbatdaran, L. J. Foruzin, F. Farzaneh and M. M. Larijani, C. R. Chim., 2013, 16, 176–182 CrossRef CAS PubMed.
- W. H. Chen, H. H. Ko, A. Sakthivel, S. J. Huang, S. H. Liu, A. Y. Lo, T. C. Tsai and S. B. Liu, Catal. Today, 2006, 116, 111–120 CrossRef CAS PubMed.
- F. Babou, G. Coudurier and J. C. Vedrine, J. Catal., 1995, 152, 341–349 CrossRef CAS.
- R. F. Nie, H. Lei, S. Y. Pan, L. N. Wang, J. H. Fei and Z. Y. Hou, Fuel, 2012, 96, 419–425 CrossRef CAS PubMed.
- S. R. Kirumakki, N. Nagaraju and S. Narayanan, Appl. Catal., A, 2004, 273, 1–9 CrossRef CAS PubMed.
- F. F. Bamoharram, M. M. Heravi, M. Roshani, M. Jahangir and A. Gharib, Appl. Catal., A, 2006, 302, 42–47 CrossRef CAS PubMed.
- G. Mitran, É. Makó, Á. Rédey and I. C. Marcu, C. R. Chim., 2012, 15, 793–798 CrossRef CAS PubMed.
- E. A. El-Sharkawy and S. S. Al-Shihry, Mater. Lett., 2004, 58, 2122–2127 CrossRef CAS PubMed.
- C. L. Zhang and Z. Q. Du, Chin. Chem. Lett., 2009, 20, 1411–1414 CrossRef CAS PubMed.
- J. Azizian, F. Teimouri and M. R. Mohammadizadeh, Catal. Commun., 2007, 8, 1117–1121 CrossRef CAS PubMed.
- M. Karthik, A. K. Tripathi, N. M. Gupta, M. Palanichamy and V. Murugesan, Catal. Commun., 2004, 5, 371–375 CrossRef CAS PubMed.
- P. Slobbe, E. Ruijter and R. V. A. Orru, Med. Chem. Commun., 2012, 3, 1189–1218 RSC.
- M. J. Climent, A. Corma and S. Iborra, RSC Adv., 2012, 2, 16–58 CAS.
- A. Debache, W. Ghalem, R. Boulcina, A. Belfaitah, S. Rhouati and B. Carboni, Tetrahedron Lett., 2009, 50, 5248–5250 CrossRef CAS PubMed.
- N. Koukabi, E. Kolvari, A. Khazaei, M. A. Zolfigol, B. S. Shaghasemi and H. R. Khavasi, Chem. Commun., 2011, 47, 9230–9232 RSC.
- A. M. Song, X. B. Wang and K. S. Lam, Tetrahedron Lett., 2003, 44, 1755–1758 CrossRef CAS.
- H. Valizadeh, A. Shockravi and H. Gholipur, J. Heterocycl. Chem., 2007, 44, 867–870 CrossRef CAS.
- N. Cairns, L. M. Harwood and D. P. Astles, J. Chem. Soc., Perkin Trans. 1, 1994, 3101–3107 RSC.
- S. Sudha, K. Venkatachalam, S. V. Priya and J. H. Mabel, J. Mol. Catal. A: Chem., 2008, 291, 22–29 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2014 |