Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Amino-alcohol cyclization: selective synthesis of lactams and cyclic amines from amino-alcohols†‡
Dennis
Pingen
and
Dieter
Vogt
*
Industrial Chemistry, School of Chemistry, University of Edinburgh, King's Buildings, West Mains Road, Edinburgh, EH9 3JJ, Scotland, UK. E-mail: D.Vogt@ed.ac.uk
First published on 17th September 2013
Abstract
By employing an amination catalyst, previously used in the direct synthesis of amines from alcohol with ammonia, n-amino-alcohols could be selectively cyclized to either the amide or the amine. By the addition of water, the amine could be produced as the major product whereas adding a sacrificial ketone as a hydrogen acceptor resulted in the amide as the major product. Without an additive a mixture of both the amine and the amide was observed. N-substituted amino-alcohols solely gave cyclic amines under these conditions. From 2-(n-alkanol) anilines the cyclic amines were produced, where the n-propanol derivative selectively formed quinoline as the major product.
Introduction
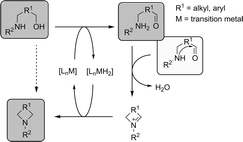
Amines and amides are versatile building blocks for intermediates and fine chemicals. Much interest has been addressed to the direct catalytic synthesis of these types of compounds via alcohols and amines.1–3 The selective conversion of alcohols with amines would provide sustainable and efficient routes to those building blocks. Cyclic amines and amides are very important structural features in pharmaceutical chemistry.4 In syntheses of both natural products and medicinal compounds, alcohol and amine moieties are often combined to form new cyclic compounds. However, the alcohol group is often derivatized to create a leaving group. Therefore it is highly desirable to develop catalysts that are able to directly perform these reactions without the need for protective groups.The group of Milstein recently developed a catalytic system containing an acridine based diphosphine and RuHCl(CO)(PPh3)3 which was able to convert primary alcohols to primary amines with ammonia.5 However, secondary amines remained untouched. Shortly after that, our group and simultaneously the group of Beller7 reported a highly selective Ru catalyst system, which was able to convert secondary alcohols with ammonia to primary amines. This transformation follows the concept of ‘Hydrogen Shuttling’, in which an alcohol is first dehydrogenated to the corresponding carbonyl compound that then undergoes condensation with an amine to form an imine, which is subsequently hydrogenated to the amine (Scheme 1).
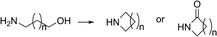
Exploring the potential of this catalytic system gave rise to intramolecular reactions between amines and alcohols. Examples in which amino-alcohols are cyclized go back to the late 1940's, where Woods and Sanders reported the cyclization of 5-amino-1-pentanol.8 In the early 1980's, a few examples are known in which amino-alcohols are cyclized to the corresponding cyclic amines by applying RuH2(PPh3)4 as a catalyst.9 Furthermore, Bartók reported RuCl2(PPh3)4 as a catalyst for N-substituted amino-alcohol cyclization (Scheme 2).10
Later, Murahashi showed the cyclization of 1,4- and 1,5-amino-alcohols to cyclic amides by applying a hydrogen acceptor. However, only moderate yields and selectivities were obtained.11 Van Koten's group performed reactions of diols with aniline employing Ru-pincer complexes. Here it was found that the cyclization of the amino-alcohol was slow and incomplete, giving mainly mono-alkylated products.12 In recent years, more examples have been developed aiming at the cyclic amide,13 although the reactions often required significant amounts of base.14–17
Results and discussion
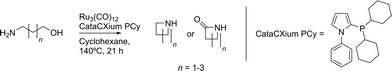
As Ru3(CO)12/CataCXium® PCy was shown to be a very effective catalytic system for alcohol amination,6 we decided to use this system for the cyclization of amino-alcohols (Scheme 3). The full conversion of 5-amino-1-pentanol was achieved giving only 2 different products: piperidine and piperidone. Table 1 summarizes the results of the cyclization of several commercially available α,ω-amino-alcohols. The ratio of amine versus amide formation shows a distinct dependence on the ring-size of the product formed.| Amino-alcohol | Conversionb | Amine selectivityb (%) | Amide selectivityb (%) | Other (%) |
|---|---|---|---|---|
a 1 mmol substrate, 0.5 mol% Ru3(CO)12, 3 mol% CataCXium® PCy (Ru![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P = 1 P = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2), 0.6 mL cyclohexane, 140 °C, 21 h.
b Conversions and selectivities determined by GC, based on amino-alcohol consumption and amine/amide production.
c Cyclic imine, intermediate towards cyclic amine.
d Most likely polymers or oligomers. 2), 0.6 mL cyclohexane, 140 °C, 21 h.
b Conversions and selectivities determined by GC, based on amino-alcohol consumption and amine/amide production.
c Cyclic imine, intermediate towards cyclic amine.
d Most likely polymers or oligomers.
|
||||
| 5-Amino-1-pentanol | 100 | 69.5 | 30.5 | 0 |
| 6-Amino-1-hexanol | 67.7 | 35.4 | 37.3 | 27.2c |
| 4-Amino-1-butanol | 94.5 | 88 | 0 | 12d |
| 3-Amino-1-propanol | 6 | 0 | 0 | 6c |
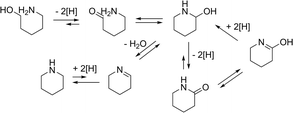
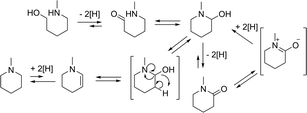
Considering the equilibria involved in the reaction, it can be rationalized how both the cyclic amine as well as the amide are formed (Scheme 4). Two important steps determine the products: the loss of hydrogen or the loss of water. For the amide formation the mechanism of ‘Hydrogen Shuttling’ is interrupted by the loss of hydrogen.
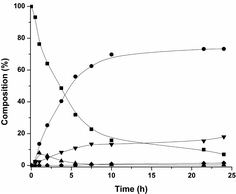
Some of the intermediates shown in Scheme 4 can indeed be observed during in the reaction (Fig. 1). Only low amounts of the intermediate hemi-aminal are observed, as this is a fairly unstable intermediate, though sufficiently stable in solution for GC and GC-MS analyses. The cyclic imine is more stable and can indeed be observed. The imine builds up quickly in the beginning and then decreases to form the cyclic amine, which is the most favoured product in the reaction.
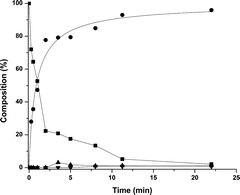
Following the substrate pathway, it is expected that the addition of water would shift the equilibrium towards the amide (Scheme 4). Remarkably and in contrast with the results obtained by Murahashi, the addition of water resulted in a higher selectivity towards the cyclic amine (Fig. 2). Table 2 shows that for all of the substrates tested, the cyclic amine yield and selectivity goes up when water is added; even obtaining complete selectivity for 5-amino-1-pentanol. For the substrates resulting in more strained cyclic products, the selectivity towards the amine also increased.
| Amino-alcohol | Conversionb (%) | Amine selectivityb (%) | Amide selectivityb (%) | Other (%) |
|---|---|---|---|---|
a 1 mmol substrate, 10 mmol H2O, 0.5 mol% Ru3(CO)12, 3 mol% CataCXium® PCy (Ru![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P = 1 P = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2), 0.6 mL cyclohexane, 140 °C, 21 h.
b Conversions and selectivities determined by GC, based on amino-alcohol consumption and amine/amide production.
c Most likely polymers or oligomers.
d 1 mmol phenol added instead of water. 2), 0.6 mL cyclohexane, 140 °C, 21 h.
b Conversions and selectivities determined by GC, based on amino-alcohol consumption and amine/amide production.
c Most likely polymers or oligomers.
d 1 mmol phenol added instead of water.
|
||||
| 5-Amino-1-pentanol | 100 | 100 | 0 | 0 |
| 5-Amino-1-pentanold | 80 | 100 | 0 | 0 |
| 6-Amino-1-hexanol | 78.5 | 81 | 19 | 0 |
| 4-Amino-1-butanol | 100 | 61.3 | 0 | 38.7c |
| 3-Amino-1-propanol | 18.6 | 68 | 0 | 30c |
This interesting result might be due to the use of the very apolar, aprotic solvent; water can act as a weak acid. Water might facilitate the dehydration step by hydrogen bonding to the intermediate cyclic half-aminal, as depicted in Fig 3. If this is true, replacing water for a fairly acidic non-reactive alcohol should give a similar effect. However, most acids will poison the catalyst or react with the substrates; only a few possibilities are allowed. Replacing water by phenol, indeed gave complete selectivity for the cyclic amine. However, in this case, the conversion was somewhat lower at 80% (Table 2, entry 2). Additionally, performing the reaction in a hydrogen atmosphere produces solely the amine in full conversion. In that case, the resulting imine is consumed even more rapidly. The beneficial effect of water has been reported earlier for a related reaction; the metal-catalyzed reductive amination of ketones but no explanation was given.18
To direct the reaction towards the cyclic amide, several ketones were tested as hydrogen acceptors. Although the intramolecular condensation of the intermediate aldehyde with the amine is highly favoured, it appeared to be important to use a slightly bulky ketone to prevent any intermolecular competition. Still the ketone has to be reactive enough to act as a hydrogen acceptor. The complete selectivity to the cyclic amide was achieved using propiophenone with 5-amino-1-pentanol as the substrate (Table 3). No condensation was observed in this case.
| Amino-alcohol | Additiveb | Conversionc (%) | Amine selectivityc (%) | Amide selectivityc (%) | Other (%) |
|---|---|---|---|---|---|
a 1 mmol substrate, 0.5 mol% Ru3(CO)12, 3 mol% CataCXium® PCy (Ru![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P = 1 P = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2), 0.6 mL cyclohexane, 140 °C, 21 h.
b 10 mmol for H2O, 2 mmol for ketone.
c Conversions and selectivities determined by GC, based on amino-alcohol consumption and amine/amide production.
d Condensation products.
e Most likely polymers or oligomers.
f Totals of the hemi-aminal and amide. 2), 0.6 mL cyclohexane, 140 °C, 21 h.
b 10 mmol for H2O, 2 mmol for ketone.
c Conversions and selectivities determined by GC, based on amino-alcohol consumption and amine/amide production.
d Condensation products.
e Most likely polymers or oligomers.
f Totals of the hemi-aminal and amide.
|
|||||
| 5-Amino-1-pentanol | Water | 100 | 100 | 0 | 0 |
| 5-Amino-1-pentanol | Propiophenone | 100 | 0 | 100 | 0 |
| 6-Amino-1-pentanol | Water | 78.5 | 81 | 19 | 0 |
| 6-Amino-1-pentanol | Cyclohexanone | 100 | 15 | 71.3 | 13.7d |
| 4-Amino-1-butanol | Water | 100 | 61.3 | 0 | 38.7e |
| 4-Amino-1-butanol | Propiophenone | 91.6 | 38.3 | 61.7f | 0 |
| 3-Amino-1-propanol | Water | 18.6 | 68 | 0 | 30e |
| 3-Amino-1-propanol | Propiophenone | 100 | 0 | 0 | 100e |
The amide formation appears to be fairly sensitive with regard to the substrate as well as to the hydrogen accepting ketone. E.g. propiophenone gave good results for 5-amino-1-pentanol but for 6-amino-1-hexanol the selectivity was not influenced much. The slightly less sterically demanding cyclohexanone (Table 3, entry 4) gave the highest selectivity to the cyclic amide. Variations such as the addition of molecular sieves or other (less) bulky ketones were applied but did not result in better selectivities.19 In most of the cases, only the cyclic amine was observed. Lowering the reaction temperature resulted in intermolecular condensation reactions and no cyclic products were observed.
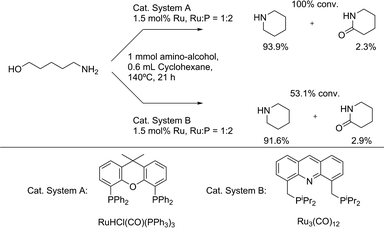
In addition, two other excellent catalytic systems used in the direct amination of secondary alcohols were employed, in order to find if these could be steered as well. The RuHCl(CO)(PPh3)3/Xantphos7b system (‘A’) produced only the cyclic amine, though with a high conversion (100%) and yield (94%), whereas the recently published Ru3(CO)12/acridine diphosphine20 (‘B’) only gave around 50% conversion yet with a high amine selectivity (Scheme 5). However, for both catalytic systems, a ketone additive did not result in any lactam formation. This emphasizes the uniqueness of the Ru3(CO)12/CataCXium® PCy combination.
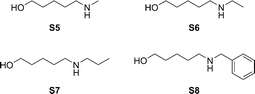
The scope of this transformation was further explored towards the cyclization of secondary amino-alcohols (Fig 4).
Table 4 shows the results of the cyclization of substrates S5–S8. However, in this case no amides were formed. Only substrate S5 gave a small amount of amide. In all other cases, the cyclic amine was produced with a full selectivity. The additions of several ketones did not affect the selectivity at all.
| Amino-alcohol | Conversionb (%) | Amine selectivityb (%) | Amide selectivityb (%) | Other (%) |
|---|---|---|---|---|
a 1 mmol substrate, 10 mmol H2O, 0.5 mol% Ru3(CO)12, 3 mol% CataCXium® PCy (Ru![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P = 1 P = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2), 0.6 mL cyclohexane, 140 °C, 21 h.
b Conversion determined by GC, based on the consumption of amino-alcohol and amine/amide production.
c Hemi-aminal.
d 10 mmol H2O added.
e 2 mmol propiophenone added. 2), 0.6 mL cyclohexane, 140 °C, 21 h.
b Conversion determined by GC, based on the consumption of amino-alcohol and amine/amide production.
c Hemi-aminal.
d 10 mmol H2O added.
e 2 mmol propiophenone added.
|
||||
| S5d | 100 | 100 | 0 | 0 |
| S5e | 96.1 | 43.3 | 29.4 | 23.4c |
| S5 | 100 | 58.1 | 0 | 41.9c |
| S6d | 100 | 95 | 5 | 0 |
| S6e | 100 | 100 | 0 | 0 |
| S6 | 100 | 100 | 0 | 0 |
| S7d | 70 | 100 | 0 | 0 |
| S7e | 88.3 | 100 | 0 | 0 |
| S7 | 100 | 100 | 0 | 0 |
| S8 | 71.9 | 100 | 0 | 0 |
The secondary imine, formed as an intermediate, would be present as an unstable zwitterionic iminium ion, which could not be observed. This would suggest that from the hemi-aminal, the amide is formed more easily. Though imine formation can occur, rapid isomerization to the enamine would be more likely, as this is much more stable compared to a zwitterionic species (Scheme 6).
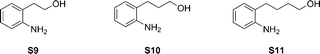
An important class of molecules are the benz-annulated N-heterocycles. Aniline-derived amino-alcohols were synthesized with different n-alkanol groups (Fig 5).
The lower conversions for these substrates might be due to the lower nucleophilicity of aniline. However, a high selectivity for the cyclic amines was observed, as was already seen for the α,ω-amino-alcohols and the N-substituted amino-alcohols.
A recent publication by Andersson et al. showed the efficient Ir-catalyzed cyclization of 2-(3-propanol)aniline and 2-(2-ethanol)aniline.21 They successfully synthesized indoles and tetrahydroquinolines in high yields. Another recent report by Cho et al. described a Ru-catalyzed synthesis of quinoline from aniline and tripropanolamine.22 Employing our new procedure, indoline can be produced in a high yield. Unfortunately no selectivity could be induced here to steer the reaction to the corresponding lactam. The amine was the only product although employing a propyl spacer resulted in the aromatization of the resulting amine product (Table 5). This is not surprising as this six-membered ring only needs to loose hydrogen from the imine to form the aromatic product (Scheme 7). Using propiophenone or cyclohexanone as the H-acceptor, the quinolone formation could be improved.
| Amino-alcohol | Additiveb | Conversion (%) c | Amine selectivity (%) c | Amide selectivity (%) c | Other (%) |
|---|---|---|---|---|---|
a 1 mmol substrate, 0.5 mol% Ru3(CO)12, 3 mol% CataCXium® PCy (Ru![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P = 1 P = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2), 0.6 mL cyclohexane, 140°C, 21 h.
b 10 mmol for H2O, 2 mmol for ketone.
c Conversions and selectivities determined by GC, based on amino-alcohol consumption and amine/amide production.
d Quinoline. 2), 0.6 mL cyclohexane, 140°C, 21 h.
b 10 mmol for H2O, 2 mmol for ketone.
c Conversions and selectivities determined by GC, based on amino-alcohol consumption and amine/amide production.
d Quinoline.
|
|||||
| S9 | None | 50.4 | 100 | 0 | 0 |
| S9 | Water | 36.4 | 100 | 0 | 0 |
| S9 | Propiophenone | 71.9 | 100 | 0 | 0 |
| S9 | Cyclohexanone | 100 | 97.9 | 2.1 | 0 |
| S10 | Propiophenone | 77.9 | 19.1 | 0 | 58.8d |
| S10 | Water | 65.9 | 47.0 | 0 | 18.9d |
| S10 | Cyclohexanone | 100 | 21.2 | 0 | 78.8d |
| S11 | Water | 18.8 | 100 | 0 | 0 |
| S11 | Propiophenone | 14.7 | 14.7 | 0 | 0 |
Conclusions
A simple Ru catalyst system derived from Ru3(CO)12 and CataCXium® PCy was employed in the efficient cyclization of amino-alcohols. By the addition of water or a ketone as the hydrogen acceptor, the reaction could be steered to either cyclic amines or cyclic amides, respectively, giving moderate to high yields and selectivities. To the best of our knowledge, the selectivities are the highest reported for α,ω-amino-alcohols. The cyclizations of N-substituted amino-alcohols and 2-(n-alkanol) anilines are very promising for the synthesis of N-heterocyclic amines and quinoline derivatives. This route enables various transformations in a single step, which otherwise would require multiple steps and the use of activating and protecting groups.Experimental section
Chemicals and were purchased from Sigma-Aldrich and Acros, Ru3(CO)12 was purchased from Strem and all chemicals were used as received. Substrates S5–S8 were synthesized according to a literature procedure.23 The 2-(n-alkanol) anilines S9–S11 were synthesized according a literature procedure.24 The synthesis and catalysis reactions were performed under an inert Ar atmosphere using standard Schlenk techniques. The product distribution and yield analyses were performed on a Shimadzu GC-17 A instrument with an Ultra 2 column (25 m, 0.2 mm id). GC-MS analyses were conducted on a Shimadzu GCMS-QP2010 SE with a DB-1 MS column (10 m, 0.1 mm id). Amino-alcohol cyclizations were performed in a 10 mL stainless steel autoclave. The reaction profiles were recorded using a homemade 75 mL stainless steel autoclave equipped with a manometer and a sampling unit for 50 μL samples. Samples were subjected directly to GC without further workup.The procedures for the cyclization of amino-alcohols: using RuHCl(CO)(PPh3)3/Xantphos: α,ω-amino-alcohol (1 mmol) was weighed into a 10 mL stainless steel autoclave applying a blanket of Ar. RuHCl(CO)(PPh3)3 (1.5 mol%, 0.015 mmol, 14.3 mg) and Xantphos (1.5 mol%, 0.015 mmol, 8.7 mg) were added followed by cyclohexane (0.6 mL). For the reactions using water as an additive, degassed H2O (10 mmol) was added and the autoclave was closed tightly and heated in an oil bath for the appropriate time. The reactions using ketone as the additive were performed using dried, degassed ketone (2 mmol). The reaction mixture was subjected to GC and GC-MS analyses.
Using Ru3(CO)12/acridine diphosphine: α,ω-amino-alcohol (1 mmol) was weighed into a 10 mL stainless steel autoclave applying a blanket of Ar. Ru3(CO)12 (0.5 mol%, 0.005 mmol, 3.2 mg) and acridine diphosphine (1.5 mol%, 0.015 mmol, 6.6 mg) were added followed by cyclohexane (0.6 mL). For the reactions using water as an additive, degassed H2O (10 mmol) was added and the autoclave was closed tightly and heated in an oil bath for the appropriate time. Reactions using ketone as the additive were performed using dried, degassed ketone (2 mmol). The reaction mixture was subjected to GC and GC-MS analyses.
Using Ru3(CO)12/CataCXium® PCy: α,ω-amino-alcohol (1 mmol) was weighed into a 10 mL stainless steel autoclave applying a blanket of Ar. Ru3(CO)12 (0.5 mol%, 0.005 mmol, 3.2 mg) and CataCXium® PCy (3 mol%, 0.03 mmol, 10.2 mg) were added followed by cyclohexane (0.6 mL). For the reactions using water as an additive, degassed H2O (10 mmol) was added and the autoclave was closed tightly and heated in an oil bath for the appropriate time. The reactions using ketone as the additive were performed using dried, degassed ketone (2 mmol). The reaction mixture was subjected directly to GC and GC-MS analyses without further workup.
Fig 1 and 2 were produced via a modified procedure: in an Ar-purged Schlenk tube, Ru3(CO)12 (0.5 mol%, 0.075 mmol, 48 mg) and CataCXium® PCy (3 mol%, 0.45 mmol, 153 mg) was dissolved in 9 mL cyclohexane. To this α,ω-amino-alcohol was added. The mixture was then transferred to a 75 mL stainless steel autoclave purged with Ar. The autoclave was closed tightly and heated to 140 °C using a heating mantle. Samples were taken at t = 0.5, 1, 2, 3.75, 5.5, 7.5 10, 21 and 24 h for Fig. 1 and at t = 0.5, 1, 2, 3.75, 5, 7, 12 and 24 h for Fig. 2.
Acknowledgements
We would like to thank Ton Staring for his technical assistance. This research has been funded by the Netherlands Ministry of Economic Affairs and the Netherlands Ministry of Education, Culture, and Sciences within the framework of the CatchBio program.Notes and references
- C. Gunanathan and D. Milstein, Science, 2007, 317, 790–792 CrossRef CAS PubMed.
- S. C. Ghosh, S. Muthaiah, Y. Zhang, X. Xu and S. H. Hong, Adv. Synth. Catal., 2009, 351, 2643–2649 CrossRef CAS.
- T. Zweifel, J.-V. Naubron and H. Grützmacher, Angew. Chem., Int. Ed., 2009, 48, 559–563 CrossRef CAS PubMed.
- (a) R. P. Tangallapally, R. Yendapally, R. E. Lee, A. J. M. Lenaerts and R. E. Lee, J. Med. Chem., 2005, 48, 8261–8269 CrossRef CAS PubMed; (b) E. Busto, V. Gotor-Fernández and V. Gotor, Chem. Rev., 2011, 111, 3998–4035 CrossRef CAS PubMed.
- C. Gunanathan and D. Milstein, Angew. Chem., Int. Ed., 2008, 47, 8661 CrossRef CAS PubMed.
- D. Pingen, C. Müller and D. Vogt, Angew. Chem., Int. Ed., 2010, 49, 8130–8133 CrossRef CAS PubMed.
- (a) S. Imm, S. Bähn, L. Neubert, H. Neumann and M. Beller, Angew. Chem., Int. Ed., 2010, 49, 8126–8129 CrossRef CAS PubMed; (b) S. Imm, S. Bähn, M. Zhang, L. Neubert, H. Neumann, F. Klasovsky, J. Pfeffer, T. Haas and M. Beller, Angew. Chem., Int. Ed., 2011, 50, 7599–7603 CrossRef CAS PubMed.
- G. F. Woods and H. Sanders, J. Am. Chem. Soc., 1946, 68, 2111–2112 CrossRef CAS.
- S.-I. Murahashi, K. Kondo and T. Hakata, Tetrahedron Lett., 1982, 23, 229–232 CrossRef CAS.
- K. Felföldi, M. S. Klyavlin and M. Bartók, J. Organomet. Chem., 1989, 362, 193–195 CrossRef.
- T. Naota and S.-I. Murahashi, Synlett, 1991, 1991, 693–694 Search PubMed.
- R.A.T.M Abbenhuis, J. Boersma and G. van Koten, J. Org. Chem., 1998, 63, 4282–4290 CrossRef CAS.
- J. H. Dam, G. Osztrovszky, L. U. Nordstrøm and R. Madsen, Chem.–Eur. J., 2010, 16, 6820–6827 CrossRef CAS PubMed.
- L. U. Nordstrom, H. Vogt and R. Madsen, J. Am. Chem. Soc., 2008, 130, 17672–17673 CrossRef CAS PubMed.
- A. Nova, D. Balcells, N. D. Schley, G. E. Dobereiner, R. H. Crabtree and O. Eisenstein, Organometallics, 2010, 29, 6548–6558 CrossRef CAS.
- K.-I. Fujita, Y. Takahashi, M. Owaki, K. Yamamoto and R. Yamaguchi, Org. Lett., 2004, 6, 2785–2788 CrossRef CAS PubMed.
- S. Muthaiah, S. C. Ghosh, J.-E. Jee, C. Chen, J. Zhang and S. H. Hong, J. Org. Chem., 2010, 75, 3002–3006 CrossRef CAS PubMed.
- (a) R. Kadyrov and T. Riermeier, Angew. Chem., Int. Ed., 2003, 42, 5472–5474 CrossRef CAS PubMed; (b) T. E. Müller, K.C. Hultzsch, M. Yus, F. Faubelo and M. Tada, Chem. Rev., 2008, 108, 3795–3892 CrossRef PubMed; (c) S. Sato, T. Sakamoto, E. Miyazawa and Y. Kikugawa, Tetrahedron, 2004, 60, 7899 CrossRef CAS PubMed; (d) T. Gross, A. M. Seayad, M. Ahmed and M. Beller, Org. Lett., 2002, 4, 2055 CrossRef CAS PubMed; (e) M. Kitamura, D. Lee, S. Hayashi, S. Tanaka and M. Yoshimura, J. Org. Chem., 2002, 67, 8685–8687 CrossRef CAS PubMed.
- For the different conditions and additives applied, see the ESI † .
- D. Pingen and D. Vogt, ChemCatChem, 2013 DOI:10.1002/cctc.201300407.
- J.-Q. Li and P. G. Andersson, Chem. Commun., 2013, 49, 6131–6133 RSC.
- C. S. Cho, D. T. Kim, T.-J. Kim and S. C. Shim, Bull. Korean Chem. Soc., 2003, 24, 1026–1028 CrossRef CAS.
- R. V. Hoffman and J. M. Salvador, J. Chem. Soc., Perkin Trans. 1, 1375–1380 Search PubMed.
- N. T. Patil, H. Wu and Y. Yamamoto, J. Org. Chem., 2007, 72, 6577–6579 CrossRef CAS PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c3cy00513e |
| ‡ Celebrating 300 years of Chemistry at Edinburgh |
| This journal is © The Royal Society of Chemistry 2014 |