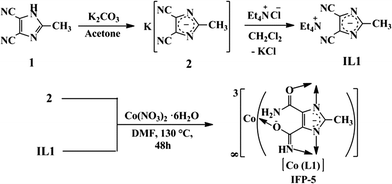
Synthesis of a Co(II)–imidazolate framework from an anionic linker precursor: gas-sorption and magnetic properties†
Suvendu Sekhar
Mondal
a,
Asamanjoy
Bhunia
b,
Serhiy
Demeshko
c,
Alexandra
Kelling
a,
Uwe
Schilde
a,
Christoph
Janiak
b and
Hans-Jürgen
Holdt
*a
aInstitut für Chemie, Anorganische Chemie, Universität Potsdam, Karl-Liebknecht-Straße 24-25, 14476 Potsdam, Germany. E-mail: holdt@uni-potsdam.de; Fax: +49 331 977 5055; Tel: +39 331 977 5180
bInstitut für Anorganische Chemie und Strukturchemie, Heinrich-Heine-Universität Düsseldorf, 40204 Düsseldorf, Germany
cInstitut für Anorganische Chemie, Georg-August-Universität Göttingen, Tammannstraße 4, 37077 Göttingen, Germany
First published on 25th October 2013
Abstract
A Co(II)–imidazolate-4-amide-5-imidate based MOF, IFP-5, is synthesized by using an imidazolate anion-based novel ionic liquid as a linker precursor under solvothermal conditions. IFP-5 shows significant amounts of gas (N2, CO2, CH4 and H2) uptake capacities. IFP-5 exhibits an independent high spin Co(II) centre and antiferromagnetic coupling.
Zeolitic imidazolate frameworks (ZIFs) are a subclass of metal azolate frameworks, which, in turn, are a subset of crystalline metal–organic frameworks (MOFs).1,2 They have many interesting characteristics, including permanently high porosity and exceptional chemical and thermal stability. These properties make ZIFs very promising for applications involving gas storage, gas separation, magnetic properties, catalysts and sensing.2–5
ZIFs are typically synthesized solvothermally, although preparatory procedures for ZIFs, involving mechanochemistry and aqueous media at room temperature and in ionic liquids (ILs), have already been established.6 Systematic investigations of isostructural frameworks of a particular deprotonated imidazole and/or its derivative as a linker and different metal based ZIF series may be limited.1,2,7 In order to derive an isoreticular series, recently, several examples of metal exchange (transmetalation) within MOFs8 and of solvent-assisted linker exchange9 within ZIFs have been reported. However, imidazolate anion-based ILs are synthesized,10 but the applications of ILs in the context of coordination chemistry were restricted.11 For the first time, we used an imidazolate anion-based IL as a precursor for MOF synthesis under solvothermal conditions. Here, we report a 2-methylimidazolate-4-amide-5-imidate linker (L1) based MOF, named as IFP-5 (co-centered, IFP = imidazolate framework potsdam), and its gas-sorption and magnetic properties.
IFP-5 ([Co(L1)]·0.5DMF) was synthesized from a solvothermal reaction of tetraethylammonium 4,5-dicyano-2-methylimidazolate (IL1) with Co(NO3)2·6H2O in N,N′-dimethylformamide (DMF; Scheme 1). We have already reported imidazolate-4-amide-5-imidate linker based Zn-frameworks, known as IFPs, from 2-substituted-4,5-dicyanoimidazole precursors under solvothermal conditions in DMF.12 To get a new, different metal centre based IFP from a neutral linker precursor, 1, we performed all types of reactions with different Co-salts, as mentioned in the synthetic strategies for preparing ZIFs (e.g., solvothermally in DMF, DMA and solvent mixtures and mechanochemically), but reactions did not yield crystalline materials.12b Hence, a 4,5-dicyano-2-methylimidazole linker (1) was modified to an ionic liquid precursor (IL1), wherein the linker is pre-ionized as a 4,5-dicyano-2-methylimidazolate anion. IFP-5 formed as a fine and pure crystalline material by using the IL precursor under solvothermal conditions in DMF (Fig. S1, ESI†). The anion (4,5-dicyano-2-methylimidazolate) of the ionic liquid acts as a source of the linker, while the counter cation (tetraethylammonium) is not involved in the coordination bonding, facilitating only structure growth. Implementation of the idea of utilization of the ionic liquid in the context of coordination polymers has been previously reported.13 2D/3D frameworks are formed by changing the anion of the ionic liquid with various polarities/hydrophobicities under ionothermal conditions.13,14 In case of synthesis of anionic zeolites or anionic metal–organic frameworks, ionic liquids act as solvents and templates wherein the cation of the ionic liquids participates in the frameworks.14 Moreover, the majority of porous MOFs including ZIFs feature charge-neutral frameworks.1–3 In our study, we used ionic liquid as a linker precursor; interestingly, the charge-neutral framework is formed. The original idea behind such synthetic approaches was to eliminate the competition between template–framework and solvent–framework interactions that are present in normal solvothermal reactions. However, the cation (tetraethylammonium) in ionic liquid is large and bulky, leading to the growth of hydrophobic character in the ionic liquid that alters the balance of chemistry, preventing favorable solvent–framework interactions and enhancing the formation of 3D framework.13d Moreover, potassium imidazolate salt (2) also yielded the powder material of IFP-5 which is not suitable for single X-ray crystallographic measurement. A suitable crystalline material of IFP-5 for single X-ray measurement is only formed by reacting Co(NO3)2·6H2O and IL1 under solvothermal conditions. Therefore, the cation of the ionic liquid for the synthesis of IFP-5 plays a vital role as a structure directing agent for the growth of crystals.
The structure of IFP-5 was determined by single-crystal X-ray crystallography and was found to be isostructural to Zn based IFP-1.12bIFP-5 crystallizes in the highly symmetric rhombohedral space group R![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) (see ESI† for details). The cobalt ion in the IFP-5 structure is penta-coordinated by the imidazolate–amidate–imidate linkers to form a distorted trigonal–bipyramidal geometry (Fig. 1a). The structure possesses 1D hexagonal channels running along 0, 0, z; 1/3, 2/3, z; and 2/3, 1/3, z. The methyl groups protrude into the open channels and determine their accessible diameter (Fig. 1b). By considering the van der Waals radii, the accessible diameter of the channels in IFP-5 was estimated to be 3.8 Å. Calculations with the PLATON toolkit indicated that IFP-5 contains 40.8% void space (see ESI†).
(see ESI† for details). The cobalt ion in the IFP-5 structure is penta-coordinated by the imidazolate–amidate–imidate linkers to form a distorted trigonal–bipyramidal geometry (Fig. 1a). The structure possesses 1D hexagonal channels running along 0, 0, z; 1/3, 2/3, z; and 2/3, 1/3, z. The methyl groups protrude into the open channels and determine their accessible diameter (Fig. 1b). By considering the van der Waals radii, the accessible diameter of the channels in IFP-5 was estimated to be 3.8 Å. Calculations with the PLATON toolkit indicated that IFP-5 contains 40.8% void space (see ESI†).
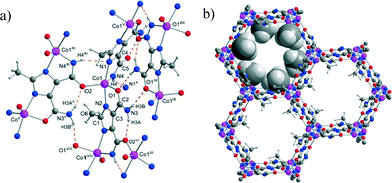 | ||
| Fig. 1 a) The crystal structure of IFP-5 showing the trigonal–bipyramidal coordination environment of CoII, the coordination mode of linker L1, and the hydrogen bonds (dotted lines). For the symmetry codes and the hydrogen bonds, see ESI.† b) Hexagonal channels in IFP-5 (the methyl substituent at the linker L1) are presented in a space filling mode (pink Co, blue N, red O, dark gray C, light gray H). | ||
Thermogravimetric analysis (TGA) of as-synthesized IFP-5 indicated a gradual weight-loss step of 13.7% (calculated 14.0%) at 25–310 °C corresponding to partial loss of guest species DMF, followed by the decomposition of framework (Fig. S10, ESI†). TGA trace shows that after removal of the solvent, IFP-5 is stable up to 300 °C. The material was activated at 200 °C and 10−3 mbar of pressure for 24 h. The powder X-ray diffraction pattern (PXRD) of the activated sample exhibited sharp diffraction peaks similar to those of the as-synthesized sample. Thus, the porous framework maintained the crystalline integrity even without solvent molecules (Fig. S5, ESI†).
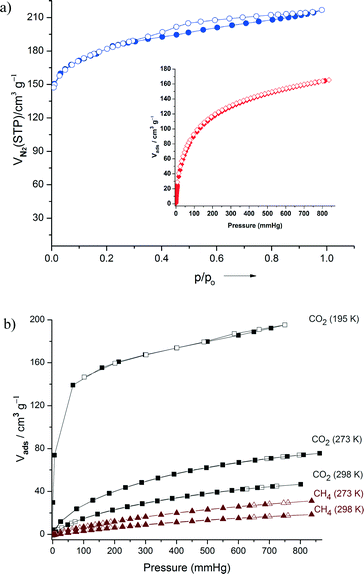
For gas sorption studies, the bulk material of IFP-5 was prepared from the ionic liquid precursor. The gas sorption isotherms were recorded for N2, H2, CH4, and CO2 gases at various temperatures at 1 bar. N2 sorption of IFP-5 at 77 K revealed a reversible type-I isotherm characteristic of microporous materials and of the H4 type hysteresis loop which is often associated with narrow slit-like pores (Fig. 2a).15 The observed hysteresis is similar to previously reported MOFs.16 The estimated Brunauer–Emmett–Teller (BET) surface area for IFP-5 was found to be 649 m2 g−1, and total pore volume was 0.30 cm3 g−1. At 77 K and 1 bar, the total H2 sorption capacity was 1.44 wt% for IFP-5 (Fig. 2a, inset). However, H2 uptake capacity is slightly higher than those of ZIFs (ZIF-8, 1.29 wt%;1c ZIF-11, 1.37 wt%1c). The CO2 sorption measurements at 195, 273 and 298 K show typical type-I isotherms with high uptake (Fig. 2b). At 195 K, a steep rise was observed at relatively low pressure in the adsorption branch of CO2, indicating the presence of a permanent micropore in IFP-5. The uptake of CO2 by IFP-5 at 298 K and 1 bar was 40 cm3 g−1. A similarly high uptake was found by Yaghi and co-workers for ZIF-69 and ZIF-82 (35.5 cm3 g−1, 1 bar, 298 K) which were synthesized by using imidazolates containing the functional groups Cl and CN.2 However, strong interactions are expected between the polar functional groups (amide and imidate) in IFP-5 and CO2, which have a significant quadrupole moment. To further understand the adsorption properties, the isosteric heats of adsorption were calculated from the CO2 adsorption isotherms at 273 K and 298 K (Fig. S12, ESI†). At zero loading, the Qst value (−ΔH) is 33.2 kJ mol−1 which is comparable with those for MOFs.3 Upon increasing the loading amount, the Qst value decreases rapidly to 27 kJ mol−1 which is still well above the heat of liquefaction of bulk CO2 at 17 kJ mol−1. The high Qst value can be attributed to the high polar framework and the pore size effect. The methane sorption capacity for IFP-5 at 273 K was estimated to be 29.6 cm3 g−1. The channel diameter of IFP-5 (3.8 Å) is slightly lower than that of Zn-based IFP-112b (4.2 Å); hence, the gas uptake capacity and BET surface area are slightly lower than those of IFP-1.12b Selectivity depends not only on the size of the gas components (kinetic diameter: CO2 3.3 Å, N2 3.6 Å and CH4 3.8 Å) but also on the polarizability of the surface and of the gas components.
Additionally, the chemical stability of IFP-5 was analyzed by suspending IFP-5 for 7 days in boiling methanol, benzene and water (Fig. S6–S8, ESI†), conditions that reflect extreme operational parameters of typical industrial chemical processes. After such extensive treatments, IFP-5 maintained its fully crystalline integrity in methanol and benzene as confirmed by powder X-ray diffraction, but in boiling water, the material irreversibly transformed to a so-far unknown crystalline phase after 24 h. It can be concluded that the polar water molecules attack the unsaturated pentacoordinated Co center of the IFP-5 structure.
The temperature dependent magnetic susceptibility for IFP-5 was measured in the range of 2 to 295 K at a magnetic field of 0.5 T. At 295 K, the measured value for the magnetic moment of IFP-5 is μeff = 4.18μB (Fig. S13, ESI†) via the spin only formula μeff = g(S(S + 1))1/2μb, where g for a free electron is 2.002 and S is the total spin of the Co(II) centers (half of the number of unpaired electrons); the value of μeff can be calculated for Co(II) high spin (S = 3/2) and Co(II) low spin (S = 1/2) complexes by neglecting spin–orbit interaction. The calculated values of μeff/μB are 3.87 for a Co(II) high spin center and 1.73 for a Co(II) low spin center. Therefore, IFP-5 with an experimental value of μeff = 4.18μB exhibits one independent high spin Co(II) center. A slightly higher experimental value of μeff/μB is consistent with the literature and caused by small spin–orbit couplings of 3d metal centers.4a Furthermore, the μeff/μBvs. T curve (Fig. S13, ESI†) shows a typical behavior of paramagnetic metal centers by decreasing μeff/μB with decreasing temperature up to a value of 1.45 (0.26 emu·K) at 2 K, suggesting antiferromagnetic coupling.4a The Curie–Weiss law,
| χM = C/(T − θ) |
Thus, it can be summarised that neutral imidazoles may not fabricate MOFs solvothermally in DMF. Therefore, in an alternative way, neutral imidazoles can be deprotonated in the presence of metal carbonate to form imidazolate salt. By choosing the suitable cation, ionic liquid (or organic salt) can be easily synthesized. Such imidazolate anion-based ionic liquid or metal imidazolate salt might be useful for the fabrication of stable MOFs solvothermally. The cation of the ionic liquid acts as a structure directing agent, and the pre-ionized imidazolate anion acts as a source of linker. Such modification of a linker to an ionic liquid can be useful for other triazoles and tetrazoles or their derivatives. Based on such a novel approach, we synthesized a MOF (IFP-5). Gas sorption properties of IFP-5 are comparable with and higher than those of IFP-1 and some reported ZIFs, respectively. IFP-5 shows an independent high spin Co(II) centre (μeff = 4.18μB) and antiferromagnetic coupling. Moreover, research extending this ionic liquid approach using other transition metal ions is in progress.
Acknowledgements
This work was financially supported by the Priority Program 1362 of the German Research Foundation on “Metal–Organic Frameworks.”Notes and references
- (a) A. Phan, C. J. Doonan, F. J. Uribe-Romo, C. B. Knobler, M. O'Keeffe and O. M. Yaghi, Acc. Chem. Res., 2010, 43, 58–67 CrossRef CAS PubMed; (b) R. Banerjee, A. Phan, B. Wang, C. Knobler, H. Furukawa, M. O'Keeffe and O. M. Yaghi, Science, 2008, 319, 939–943 CrossRef CAS PubMed; (c) K. S. Park, Z. Ni, A. P. Côté, J. Y. Choi, R. Huang, F. J. Uribe-Romo, H. K. Chae, M. O'Keeffe and O. M. Yaghi, Proc. Natl. Acad. Sci. U. S. A., 2006, 103, 10186–10191 CrossRef CAS PubMed.
- R. Banerjee, H. Furukawa, D. Britt, C. Knobler, M. O'Keeffe and O. M. Yaghi, J. Am. Chem. Soc., 2009, 131, 3875–3877 CrossRef CAS PubMed.
- K. Sumida, D. L. Rogow, J. A. Mason, T. M. McDonald, E. D. Bloch, Z. R. Herm, T. H. Bae and J. R. Long, Chem. Rev., 2012, 112, 724–781 CrossRef CAS PubMed.
- (a) P. Mahata, D. Sarma and S. Natarajan, J. Chem. Sci., 2010, 122, 19–35 CrossRef CAS PubMed; (b) P. W. Roesky, A. Bhunia, Y. Lan, A. K. Powell and S. Kureti, Chem. Commun., 2011, 47, 2035–2037 RSC.
- (a) C. Chizallet, S. Lazare, D. Bazer-Bachi, F. Bonnier, V. Lecocq, E. Soyer, A.-A. Quoineaud and N. Bats, J. Am. Chem. Soc., 2010, 132, 12365–12377 CrossRef CAS PubMed; (b) G. Lu and J. T. Hupp, J. Am. Chem. Soc., 2010, 132, 7832–7833 CrossRef CAS PubMed.
- (a) P. J. Beldon, L. Fábáin, R. S. Stein, A. Thirumurugan, A. K. Cheetham and T. Friscic, Angew. Chem., Int. Ed., 2010, 49, 9640–9643 CrossRef CAS PubMed; (b) Y. Pan, Y. Liu, G. Zeng, L. Zhao and Z. Lai, Chem. Commun., 2011, 47, 2071–2073 RSC; (c) S. Chen, J. Zhang, T. Wu, P. Feng and X. Bu, Dalton Trans., 2010, 39, 697–699 RSC.
- W. Morris, B. Leung, H. Furukawa, O. K. Yaghi, N. He, H. Hayashi, Y. Houndonougbo, M. Asta, B. B. Laird and O. M. Yaghi, J. Am. Chem. Soc., 2010, 132, 11006–11008 CrossRef CAS PubMed.
- Z. Zhang, L. Zhang, L. Wojtas, P. Nugent, M. Eddaoudi and M. J. Zaworotko, J. Am. Chem. Soc., 2012, 134, 924–927 CrossRef CAS PubMed.
- (a) O. Karagiaridi, W. Bury, A. A. Sarjeant, C. L. Stern, O. K. Farha and J. T. Hupp, Chem. Sci., 2012, 3, 3256–3260 RSC; (b) M. Kim, J. F. Cahill, Y. Su, K. A. Prather and S. M. Cohen, Chem. Sci., 2012, 3, 126–130 RSC.
- A. R. Katritzky, S. Singh, K. Kirichenko, M. Smiglak, J. D. Holbrey, W. M. Reichert, S. K. Spear and R. D. Rogers, Chem.–Eur. J., 2006, 12, 4630–4641 CrossRef CAS PubMed.
- (a) B. Mallick, H. Kierspel and A.-V. Mudring, J. Am. Chem. Soc., 2008, 130, 10068–10069 CrossRef CAS PubMed; (b) B. Mallick, B. Balke, C. Felser and A.-V. Mudring, Angew. Chem., Int. Ed., 2008, 47, 7635–7638 CrossRef CAS PubMed.
- (a) F. Debatin, K. Behrens, J. Weber, I. A. Baburin, A. Thomas, J. Schmidt, I. Senkovska, S. Kaskel, A. Kelling, N. Hedin, Z. Bacsik, S. Leoni, G. Seifert, C. Jäger, C. Günter, U. Schilde, A. Friedrich and H.-J. Holdt, Chem.–Eur. J., 2012, 18, 11630–11640 CrossRef CAS PubMed; (b) F. Debatin, A. Thomas, A. Kelling, N. Hedin, Z. Bacsik, I. Senkovska, S. Kaskel, M. Junginger, H. Müller, U. Schilde, C. Jäger, A. Friedrich and H.-J. Holdt, Angew. Chem., Int. Ed., 2010, 49, 1258–1262 CrossRef CAS PubMed; (c) S. S. Mondal, A. Bhunia, I. A. Baburin, C. Jäger, A. Kelling, U. Schilde, G. Seifert, C. Janiak and H.-J. Holdt, Chem. Commun., 2013, 49, 7599–7601 RSC; (d) S. S. Mondal, S. Dey, I. A. Baburin, A. Kelling, U. Schilde, G. Seifert, C. Janiak and H.-J. Holdt, CrystEngComm, 2013, 15, 9394–9399 RSC.
- (a) E. R. Parnham and R. E. Morris, Acc. Chem. Res., 2007, 40, 1005–1013 CrossRef CAS PubMed; (b) R. E. Morris and X. Bu, Nat. Chem., 2010, 2, 353–361 CrossRef CAS PubMed; (c) Z. Lin, D. S. Wragg, J. E. Warren and R. E. Morris, J. Am. Chem. Soc., 2007, 129, 10334–10335 CrossRef CAS PubMed; (d) R. E. Morris, Chem. Commun., 2009, 2990–2998 RSC.
- (a) Z. Lin, D. S. Wragg and R. E. Morris, Chem. Commun., 2006, 2021–2023 RSC; (b) D. N. Dybtsev, H. Chun and K. Kim, Chem. Commun., 2004, 1594–1595 RSC; (c) E. R. Parnham and R. E. Morris, J. Mater. Chem., 2006, 16, 3682–3684 RSC.
- K. S. W. Sing, D. H. Everett, R. A. Haul, L. Moscou, R. A. Pierotti, J. Rouquérol and T. Siemieniewska, Pure Appl. Chem., 1985, 57, 603–619 CrossRef CAS.
- (a) D. Liu, Z. Xie, L. Ma and W. Lin, Inorg. Chem., 2010, 49, 9107–9109 CrossRef CAS PubMed; (b) S.-M. Zhang, Z. Chang, T.-L. Hu and X.-H. Bu, Inorg. Chem., 2010, 49, 11581–11586 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Detailed experimental procedure, IR spectra, PXRD patterns, TGA traces, table of X-ray data of IFP-5, gas adsorption data. CCDC 942281. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c3ce42040j |
| This journal is © The Royal Society of Chemistry 2014 |