A sildenafil cocrystal based on acetylsalicylic acid exhibits an enhanced intrinsic dissolution rate†
Miroslav
Žegarac
a,
Edislav
Lekšić
a,
Primož
Šket
bc,
Janez
Plavec
bc,
Maja
Devčić Bogdanović
a,
Dejan-Krešimir
Bučar
*d,
Miljenko
Dumić
e and
Ernest
Meštrović
*a
aPLIVA Croatia, TAPI R&D, Prilaz Baruna Filipovića 25, HR-10000 Zagreb, Croatia. E-mail: Ernest.Mestrovic@pliva.com
bSlovenian NMR Center, National Institute of Chemistry, Hajdrihova 19, SI-1000 Ljubljana, Slovenia
cEN-FIST Center of Excellence, SI-1000 Ljubljana, Slovenia
dDepartment of Chemistry, University College London, 20 Gordon Street, London WC1H 0AJ, UK. E-mail: d.bucar@ucl.ac.uk
eDepartment of Biotechnology, University of Rijeka, R. Matejčić 2, HR-51000 Rijeka, Croatia
First published on 9th October 2013
Abstract
Cocrystallisation of sildenafil with acetylsalicylic acid yields a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 sildenafil–acetylsalicylic-acid cocrystal, as well as a 1
1 sildenafil–acetylsalicylic-acid cocrystal, as well as a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 cocrystal salt involving a sildenafil cation, acetylsalicylate and salicylic acid. The 1
1 cocrystal salt involving a sildenafil cation, acetylsalicylate and salicylic acid. The 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 acetylsalicylic-acid-based cocrystal exhibits an enhanced intrinsic dissolution rate, as compared to sildenafil citrate (the active ingredient of Viagra®).
1 acetylsalicylic-acid-based cocrystal exhibits an enhanced intrinsic dissolution rate, as compared to sildenafil citrate (the active ingredient of Viagra®).
Combining multiple drugs into a single oral dosage form (such as a tablet) has become a popular strategy in drug development.1 Such combined forms boost clinical effectiveness and improve patient adherence by simplifying disease management.2,3 Drug combinations also reduce administrative and production costs by minimising expenses related to tablet packaging and drug prescription.1,4 An alternative to combining two or more solid drugs into one tablet is the use of single crystalline solids, such as cocrystals and salts, which are comprised of two or more active pharmaceutical ingredients (APIs). Relatively little attention has been devoted to the development of such multi-API cocrystals, although their potential has been recognised by solid-state scientists.5–11
Here, we describe a part of a study that explores the applicability of cocrystallisation in the development of combination drugs, wherein we used sildenafil (sil, Scheme 1) as model compound. Sil is a selective inhibitor of type-5 cGMP-specific phosphodiesterase (PDE5). It is used in the treatment of angina, hypertension, congestive heart failure, atherosclerosis and a range of other disorders, but is particularly useful for the treatment of male erectile dysfunction, as it facilitates improved smooth muscle relaxation and increased blood flow in the human corpus cavernosum.12Pfizer in 1998 marketed sil as a citrate salt under the name Viagra® as the first effective oral drug for the treatment of sexual dysfunctions. Viagra® soon became a highly popular13 blockbuster drug.14 Since then, a surge in the demand for fast-acting therapies for erectile dysfunction has driven efforts to identify sil forms that exhibit improved pharmacokinetic properties.15,16
The demand for optimized sil forms, as well as the fact that sil is contraindicated in men suffering from cardiovascular diseases,12,17 suggests a need for sil forms that 1) have improved intrinsic dissolution rates and 2) display a protective cardiac effect that could benefit patients who suffer from or are at heightened risk of cardiovascular diseases. Accordingly, we embarked on a study of solid multi-component sil forms (i.e. cocrystals and salts) that include acetylsalicylic acid (asa). Asa is a highly popular analgesic, and its antiplatelet activity makes it a commonly prescribed long-term preventative agent for combating heart attacks and strokes.18 Our investigation resulted in the discovery of a (sil)·(asa) cocrystal (1) and a cocrystal salt containing sil, asa and salicylic acid (sa), (sil)+·(asa)−·(sa) (2). Cocrystal 1 exhibits an intrinsic dissolution rate (IDR) that is about 75% higher than the IDR of sil citrate (the marketed form of sil), thus indicating that 1 could be useful in the formulation of faster-acting sil tablets.
A solid form of 1 was prepared via solution crystallisation. Specifically, 0.025 mol of sil was dissolved in 15 mL of hot 2-propanol. An equimolar amount of asa was dissolved in about 5 mL of acetonitrile and added to the sil solution. The resulting solution was refluxed and stirred for two hours, and then cooled down to 15 °C. A white precipitate was subsequently formed and immediately separated via vacuum filtration to be identified as the sil base. The remaining solution was left to evaporate for two days at room temperature to yield very small crystals of 1 that were barely suitable for single crystal X-ray diffraction studies.
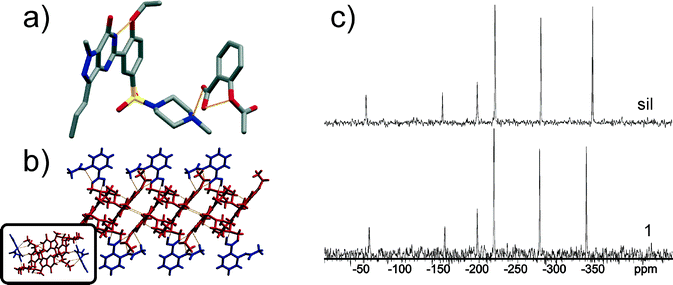
Compound 1 crystallises in the triclinic space group P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) with one molecule of sil and asa in the asymmetric unit.‡ In the crystal structure of 1, sil exhibits a similar conformation to that seen in the crystal structure of sil citrate hydrate (CCDC reference code FEDTEO).19 Specifically, the piperazine moiety in 1 displays a gauche conformation, whereby the methyl and sulfonyl groups are positioned equatorially. Furthermore, the ethoxy and propyl groups exhibit a trans conformation, while the pyrazolopyrimidine and ethoxybenzene moieties are nearly planar due to an intramolecular N–H(pyrimidone)⋯O(ethoxy) hydrogen bond (d(N⋯O) ~ 2.62 Å). The other cocrystal component in 1, asa, displays the same conformation as its crystal structure (CCDC reference code ACSALA).20 The carboxylic group of asa is coplanar with the benzene moiety, while the acetoxy group is positioned perpendicularly to the o-phenylene moiety. Sil and asa are held together by an intermolecular O–H(carboxyl)⋯N(piperazine) hydrogen bond (d(O⋯N) ~ 2.59 Å) (Fig. 1a). The sil:asa assemblies are stacked in the solid state and held together by a series of C–H⋯O and C–H⋯π forces (Fig. 1b). Finally, the sil:asa stacks form a three-dimensional structure sustained by C–H⋯O forces.
with one molecule of sil and asa in the asymmetric unit.‡ In the crystal structure of 1, sil exhibits a similar conformation to that seen in the crystal structure of sil citrate hydrate (CCDC reference code FEDTEO).19 Specifically, the piperazine moiety in 1 displays a gauche conformation, whereby the methyl and sulfonyl groups are positioned equatorially. Furthermore, the ethoxy and propyl groups exhibit a trans conformation, while the pyrazolopyrimidine and ethoxybenzene moieties are nearly planar due to an intramolecular N–H(pyrimidone)⋯O(ethoxy) hydrogen bond (d(N⋯O) ~ 2.62 Å). The other cocrystal component in 1, asa, displays the same conformation as its crystal structure (CCDC reference code ACSALA).20 The carboxylic group of asa is coplanar with the benzene moiety, while the acetoxy group is positioned perpendicularly to the o-phenylene moiety. Sil and asa are held together by an intermolecular O–H(carboxyl)⋯N(piperazine) hydrogen bond (d(O⋯N) ~ 2.59 Å) (Fig. 1a). The sil:asa assemblies are stacked in the solid state and held together by a series of C–H⋯O and C–H⋯π forces (Fig. 1b). Finally, the sil:asa stacks form a three-dimensional structure sustained by C–H⋯O forces.
The pKa values21 corresponding to the most basic N-atom in sil (pKa(sil) = 6.03) and the most acidic proton in asa (pKa(asa) = 3.48) were not sufficient (ΔpKa = 2.55) to predict whether compound 1 would crystallise as a salt or as a cocrystal.22 ATR-IR spectroscopy and 1H, 13C and 15N CP-MAS NMR were therefore utilised to determine whether a proton transfer from the carboxylic group of asa to the piperazine moiety in sil has occurred. The lack of a strong N+–H band at 2700–2250 cm−1 in the ATR-IR spectrum of 1 strongly suggests that no proton transfer has occurred in 1 (see ESI†). The extent of the proton transfer in 1 was finally determined using cross polarisation magic-angle spinning (CP-MAS) NMR spectroscopy: a 15N CP-MAS NMR spectrum of 1 showed that the signal belonging to the most basic nitrogen atom of the piperazine moiety (N▲ in Scheme 1) in sil exhibits a very similar chemical shift (δ1 −339 ppm) to that of the N▲-atom found in pure sil (δsil −347 ppm, Fig. 1c), suggesting its unprotonated state.23 In addition, the 13C CP-MAS NMR spectrum of 1 revealed a signal that corresponds to the carboxyl group (rather than the carboxylate group) of asa (see ESI†), thus confirming that solid 1 is indeed a cocrystal.
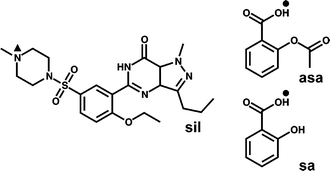 | ||
| Scheme 1 Chemical structures of sil, asa and sa. The symbol ▲ designates the most basic N-atom in sil, while ● labels the most acidic protons in asa and sa. | ||
It is important to note that the second sil solid materialises when the crystallisation of cocrystal 1 is attempted with very diluted solutions and over longer periods of time. In particular, attempts to grow larger single crystals of 1 that were suitable for the collection of high-quality diffraction data unexpectedly resulted in the discovery of 2. The mother liquor obtained during the preparation of a second batch of 1 (through fast precipitation) was left to evaporate at a slow rate over more than one week. The solution yielded a powder that was identified as a solid form of 2 by powder X-ray diffraction (PXRD), HPLC, differential-scanning calorimetry (DSC) and both ATR-IR and 13C and 15N CP-MAS NMR spectroscopies (see ESI†). Whereas PXRD experiments (Fig. 2a) and thermal analyses (see ESI†) indicated the formation of a new crystalline phase, HPLC experiments revealed that the solid consists of sil, asa and sa in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio (see ESI†). The presence of sa in the solid was attributed to the hydrolysis/solvolysis of asa in solution by residual water and/or 2-propanol during the crystallisation attempt. The ATR-IR spectrum of the solid displayed a N+–H band at 2474 cm−1 corresponding to a protonated piperazine moiety in sil, whereas the presence of a deprotonated carboxyl group (i.e. carboxylate group) could not be confirmed or dismissed based on the ATR-IR data (see ESI†).
1 ratio (see ESI†). The presence of sa in the solid was attributed to the hydrolysis/solvolysis of asa in solution by residual water and/or 2-propanol during the crystallisation attempt. The ATR-IR spectrum of the solid displayed a N+–H band at 2474 cm−1 corresponding to a protonated piperazine moiety in sil, whereas the presence of a deprotonated carboxyl group (i.e. carboxylate group) could not be confirmed or dismissed based on the ATR-IR data (see ESI†).
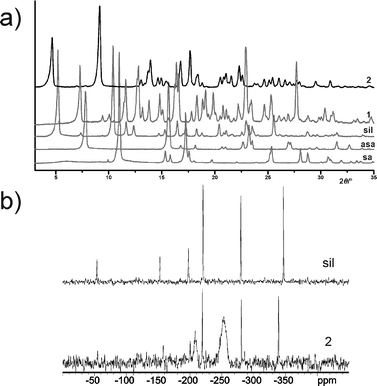 | ||
| Fig. 2 PXRD pattern of 2, as compared to the patterns of 1, sil, asa and sa (shown in a). The 15N CP-MAS NMR spectra of sil and 2 are shown in b). | ||
Attempts to grow crystals of 2 that were suitable for single crystal X-ray diffraction studies failed. CP-MAS NMR spectroscopy was, therefore, used to gain further insight into the composition of solid 2. The 13C CP-MAS NMR spectrum of 2 (see ESI†) revealed the presence of an acetylsalicylate anion and a neutral salicylic acid molecule, as well as a sildenafil cation with a protonated piperazine moiety. The 15N CP-MAS NMR spectra of 2 display two broad signals at δ −253 ppm and δ −208 ppm, in addition to a set of six sharp signals at positions similar to those found in sil and cocrystal 1, thus indicating that solid 2 contains a sil cation with two partially protonated N-atoms sites. While the broad signal observed at δ −253 ppm could be assigned to the protonated N▲ atom (Fig. 2b), the signal appearing at δ −208 could not be ascribed with certainty to any N-atom in the sil cation.
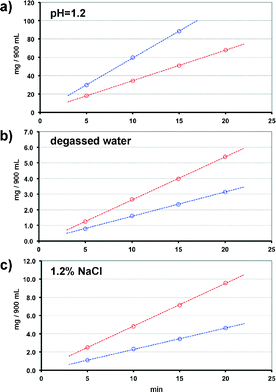
Sa is a metabolite of asa that also exhibits anti-inflammatory effects, but without the antiplatelet activity of the latter.24Sa's lack of antiplatelet activity reduced our interest solely to the asa-based cocrystal 1. To evaluate the pharmacokinetic properties of 1, we measured the intrinsic dissolution rates (IDR) of 1. The IDR is directly related to the onset and duration of the drug's action25,26 and is defined as the dissolution rate of a pure substance under the conditions of constant surface area at constant temperature, stirring rates and solution pH.25 The IDRs were measured using 100 mg of sample and 900 mL of different media, viz. degassed water, degassed water with 1.2% NaCl and buffer at a pH of 1.2 (see ESI†). The results showed that compound 1 exhibits IDRs of 11.34 mg min−1 cm−2 under acidic conditions (pH 1.2), 0.31 mg min−1 cm−2 in degassed water and 0.47 mg min−1 cm−2 in 1.2% NaCl aqueous solution. Notably, sil citrate (i.e. the active ingredient of Viagra®) displays a significantly lower IDR under acidic conditions, i.e. 6.64 mg min−1 cm−2. An approximately 75% increase in the IDR of 1 at a pH of 1.2 indicates that sil should be absorbed at significantly higher rates in the gastrointestinal tract (pH ~ 1.5) when compared to the marketed sil citrate, thus leading to improved bioavailability (Fig. 3).
The utility of cocrystal 1 as a combination drug for the treatment of erectile dysfunction in patients suffering from cardiovascular diseases has, thus far, not been established. It is, however, reasonable to expect that patients who are in need of treatment for erectile dysfunction and are also enduring daily asa therapy could potentially benefit from a combination drug based on sil and asa. The possibility of using cocrystal 1 as combination drug is also supported by the fact that a clinical trial revealed that combined use of sil and asa shows no pharmacokinetic interaction between them, and that no additional effects of sil on bleeding times have been observed.17
In conclusion, we have described a cocrystal involving sil and asa, which effectively combines a popular treatment for erectile dysfunction with a widely-recommended treatment and prophylactic agent for cardiovascular diseases. The cocrystal exhibits an IDR that improved by 75% in comparison to the poorly-to-moderately soluble sil citrate (the active ingredient of the blockbuster drug Viagra®). The enhanced dissolution rate marks the cocrystal as a possible candidate for the formulation of fast-acting sil-based dosage forms. The cocrystal also displays a potential dual therapeutic effect owing to asa's antiplatelet activity, which suggests a potential use for the cocrystal in the treatment of erectile dysfunction in patients suffering from cardiac diseases. The intriguing pharmacokinetic properties of the multi-drug cocrystal emphasise the utility of cocrystals in the development of combination drugs.
Acknowledgements
MZ, EL, MDB, MD and EM acknowledge PLIVA for financial support. PŠ and JP acknowledge the Slovenian Research Agency for funding [ARRS, P1-0242]. DKB thanks University College London for support and Dr Arundhuti Sen for useful discussions.Notes and references
- F. Simon, Nat. Rev. Drug Discovery, 2006, 5, 881–882 CrossRef CAS PubMed
.
- E. Vermeire, H. Hearnshaw, P. Van Royen and J. Denekens, J. Clin. Pharm. Ther., 2001, 26, 331–342 CrossRef CAS
.
- F. Pan, M. Chernew and A. M. Fendrick, J. Gen. Intern. Med., 2008, 23, 611–614 CrossRef PubMed
.
- A. I. Wertheimer and A. Morrison, Pharmacol. Ther., 2002, 27, 44–49 Search PubMed
.
- S. Aitipamula, P. S. Chow and R. B. H. Tan, CrystEngComm, 2009, 11, 1823–1827 RSC
.
- A. O. L. Évora, R. A. E. Castro, T. M. R. Maria, M. T. S. Rosado, M. Ramos Silva, A. Matos Beja, J. Canotilho and M. E. S. Eusébio, Cryst. Growth Des., 2011, 11, 4780–4788 Search PubMed
.
- S. Cherukuvada and A. Nangia, CrystEngComm, 2012, 14, 2579–2588 RSC
.
- P. Grobelny, A. Mukherjee and G. R. Desiraju, CrystEngComm, 2011, 13, 4358–4364 RSC
.
- P. M. Bhatt, Y. Azim, T. S. Thakur and G. R. Desiraju, Cryst. Growth Des., 2009, 9, 951–957 CAS
.
- H. G. Lee, G. G. Z. Zhang and D. R. Flanagan, J. Pharm. Sci., 2011, 100, 1736–1744 CrossRef CAS PubMed
.
- M. L. Cheney, D. R. Weyna, N. Shan, M. Hanna, L. Wojtas and M. J. Zaworotko, J. Pharm. Sci., 2011, 100, 2172–2181 CrossRef CAS PubMed
.
- See the United States product insert (USPI) for Viagra®, http://www.pfizer.com/files/products/uspi_viagra.pdf
.
- M. Loe, Sex Cult., 2001, 5, 97–125 CrossRef PubMed
.
- A. Keith, Health Aff., 2000, 19, 147–157 CrossRef CAS
.
- S.-Y. Jung, Y.-G. Seo, G. Kim, J. Woo, C. Yong and H.-G. Choi, Arch. Pharmacal Res., 2011, 34, 451–454 CrossRef CAS PubMed
.
- M. M. Al Omari, M. B. Zughul, J. E. D. Davies and A. A. Badwan, J. Inclusion Phenom. Macrocyclic Chem., 2007, 57, 379–384 CrossRef CAS PubMed
.
- M. D. Cheitlin, J. A. M. Hutter, R. G. Brindis, P. Ganz, S. Kaul, J. R. O. Russell, R. M. Zusman, J. S. Forrester, P. S. Douglas, D. P. Faxon, J. D. Fisher, R. J. Gibbons, J. L. Halperin, J. Adolph, M. Hutter, J. S. Hochman, S. Kaul, W. S. Weintraub, J. William, L. Winters and M. J. Wolk, Circulation, 1999, 99, 168–177 CrossRef CAS
.
- D. Pawar, S. Shahani and S. Maroli, Hong Kong Med. J., 1998, 4, 415–418 Search PubMed
.
- H. S. Yathirajan, B. Nagaraj, P. Nagaraja and M. Bolte, Acta Crystallogr., Sect. E: Struct. Rep. Online, 2005, 61, o489–o491 CAS
.
- P. J. Wheatley, J. Chem. Soc., 1964, 6036–6048 RSC
.
- Calculated using Advanced Chemistry Development (ACD/Labs) Software V11.02 (1994–2013 ACD/Labs).
- A. J. Cruz-Cabeza, CrystEngComm, 2012, 14, 6362–6365 RSC
.
- I. Wawer, M. Pisklak and Z. Chilmonczyk, J. Pharm. Biomed. Anal., 2005, 38, 865–870 CrossRef CAS PubMed
.
- R. Amann and B. A. Peskar, Eur. J. Pharmacol., 2002, 447, 1–9 CrossRef CAS
.
-
Developing Solid Oral Dosage Forms: Pharmaceutical Theory & Practice:
Pharmaceutical Theory and Practice, ed. Y. Qiu, Y. Chen, G. G. Z. Zhang, L. Liu and W. Porter, Academic Press Inc., London, 2009 Search PubMed
.
- M. G. I. Issa and H. G. Ferraz, Dissolution Technol., 2011, 18, 6–13 CAS
.
Footnotes |
| † Electronic supplementary information (ESI) available: Synthetic procedures, HPLC traces, TG and DSC thermograms, IR and CP-MAS NMR spectra, and crystallographic data. CCDC 952210. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c3ce42013b |
‡ Crystal data for 1 (CCDC 952210): (C22H30N6O4S)·(C9H8O4), Mr = 654.74 g mol−1, triclinic, P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) , a = 9.6707(7) Å, b = 12.3070(7) Å, c = 14.5432(12) Å, α = 85.266(2)°, β = 74.549(5)°, γ = 82.829(6)°, V = 1653.1(2) Å3, T = 298(2) K, Z = 2, μ(MoKα) = 0.156 mm−1, 5899 reflections measured, 4911 independent reflections (Rint = 0.2930), R1(obs) = 0.1221, wR1(obs) = 0.3076, R2(all) = 0.1858, wR2(all) = 0.3751, S = 1.153. , a = 9.6707(7) Å, b = 12.3070(7) Å, c = 14.5432(12) Å, α = 85.266(2)°, β = 74.549(5)°, γ = 82.829(6)°, V = 1653.1(2) Å3, T = 298(2) K, Z = 2, μ(MoKα) = 0.156 mm−1, 5899 reflections measured, 4911 independent reflections (Rint = 0.2930), R1(obs) = 0.1221, wR1(obs) = 0.3076, R2(all) = 0.1858, wR2(all) = 0.3751, S = 1.153. |
| This journal is © The Royal Society of Chemistry 2014 |