Constructing molecular polygons using halogen bonding and bifurcated N-oxides†
Christer B.
Aakeröy
*,
Tharanga K.
Wijethunga
and
John
Desper
Department of Chemistry, Kansas State University, Manhattan, KS 66506, USA. E-mail: aakeroy@ksu.edu
First published on 27th September 2013
Abstract
Bifurcated halogen bonds constructed from N-oxides and complementary halogen-bond donors provide the basis for a synthetic strategy for the deliberate assembly of molecular polygons in the solid state. The donor- and acceptor sites were located on different molecular components and a co-crystallization approach furnished the desired architectures.
N-oxides represent an interesting group of molecules that are highly polar due to the charge separation of the N–O bond.1 This molecular feature also leads to their high water solubility2 and lipophobicity.3 N-oxides have found applications as ligands in metal coordination complexes4 and they have been used as synthetic intermediates,5 biologically important compounds,6 drugs,7 protecting groups, auxiliary agents, photo-active compounds,8 oxidants and in catalysis.9
One of the significant structural characteristic of N-oxides is their ability to form bifurcated non-covalent bonds.10 Bifurcation can be defined as the participation of one atom in two different intermolecular interactions,11 and this is well known in many hydrogen-bonded systems where the N-oxide oxygen atom is interacting with two different hydrogen-bond donors simultaneously.12 An examination of known N-oxide crystal structures with bifurcated bond shows that most of the time the bifurcated angle (donor–O–donor) is close to 120°.
Heteroaromatic N-oxides have shown to interact effectively as electron donors towards perfluorocarbon iodides.13 Despite the many similarities between hydrogen bonding and halogen bonding, there are no reports on the deliberate use of bifurcated halogen bonds in combination with N-oxides for directed assembly of supramolecular architectures with predetermined topology and stoichiometries. In principle, such an undertaking should be possible as the halogen bond is mainly electrostatic in nature, and involves an electropositive σ-hole on a halogen atom and an electron-pair donor.14
Many elegant approaches for the synthesis of discrete polygons in the solid state have been presented using coordinate-covalent bonds as the primary synthetic tool,15 but relatively few efforts using purely organic building blocks are known.16 There are reports where halogen bonds have been used with nitroxides17 and ions as halogen bond linkers18 for polygon design to some extent. But halogen bonds have not been used in the deliberate construction of well-defined polygons in the solid state, where all interactions are rationalized in a well-defined synthetic strategy.
Herein we describe our attempts at synthesizing molecular polygons in the solid state while, at the same point, probing the relative effectiveness of bromo- and iodo-substituted halogen-bond donors in practical crystal engineering.
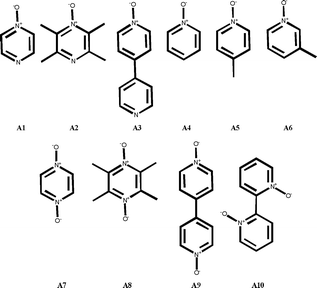
A total of ten N-oxides (A1–A10) were employed as potential halogen-bond acceptors (six mono-N-oxides and four bis-N-oxides), Fig. 1. The halogen-bond donors were selected such that the relative orientation of their donor sites would offer the appropriate geometric complementarity to the most commonly observed angle in bifurcated N-oxides in order to facilitate the assembly of the desired polygons.
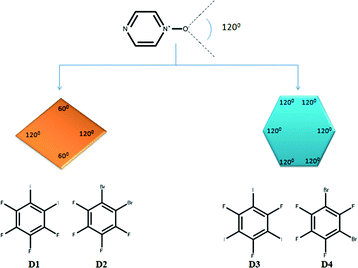
In order to build a rhomb, we needed a donor molecule with an approximate angle of 60° between the two donor sites to complement the inherent X⋯O⋯X bond angle of 120° in a bifurcated N-oxide acceptor site as this would complete the overall 360° requirement, Fig. 2. In order to make a regular hexagon, the donor moieties needed to be positioned at a 120° angle with respect to each other resulting in a total sum of 720° for the desired synthetic target, Fig. 2. We also postulated that a switch from a monotopic acceptor molecule to a bis-N-oxide, should change the outcome from discrete architectures to chains of polygons, Fig. 3.
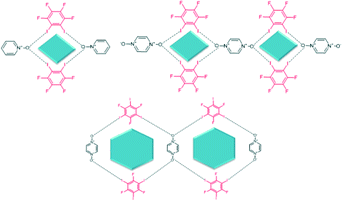
The synthetic procedure follows well-established solvent-assisted grinding protocols for co-crystallization reactions.19 Each resulting solid was analysed using IR spectroscopy in order to establish if the reaction resulted in a co-crystallization or simply a physical mixture of the two reactants (a re-crystallization). A total of forty grinding experiments were carried out and the desired outcome was (i) discrete tetrameric rhombi in reactions between 1,2-disubstituted donors and mono-N-oxide acceptors; (ii) chains of rhombi when 1,2-disubstituted donors and bis-N-oxides were employed; (iii); discrete hexagons in reactions between 1,3-disubstituted donors and mono-N-oxides and (iv) chains of hexagons when the 1,3-halogenated donors were allowed to react with bis-N-oxides, Fig. 3. 1,3,5-triiodo-2,4,6-trifluorobenzene was used instead of 1,3-diiodotetrafluorobenzene due to the fact that former has shown to act similarly to the 1,3-diiodo species.
All in all, 13 of the 40 experiments provided unambiguous IR spectroscopic evidence for co-crystal formation, resulting in a supramolecular yield of 32.5% (13/40). None of the bromo-substituted donors (which are less powerful halogen-bond donors) produced a co-crystal, resulting in an effective success rate for the iodo-based donors of 65% (13/20). Once a co-crystalline phase had been obtained, we attempted to grow crystals for single-crystal diffraction (a total of five structures were obtained); A4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) D3, A5
D3, A5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) D1, A8
D1, A8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) D1, A6
D1, A6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) D1 and A8
D1 and A8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) D3.
D3.
The structure determination of A4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
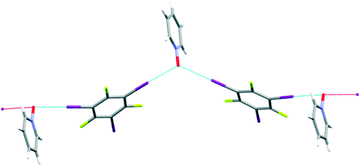
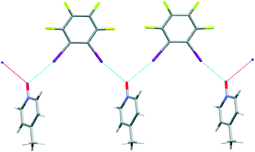
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) D3 established that the N-oxide was bifurcated (with an I–O–I angle of 117°) but instead of the desired discrete hexagons, an infinite polymer appeared, Fig. 4. The formation of a polymer is always a possibility and on this occasion our synthesis failed to meet the target.
D3 established that the N-oxide was bifurcated (with an I–O–I angle of 117°) but instead of the desired discrete hexagons, an infinite polymer appeared, Fig. 4. The formation of a polymer is always a possibility and on this occasion our synthesis failed to meet the target.
The crystal structure of A5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) D1 also shows bifurcation at the N-oxide moiety, this time with an I–O–I angle smaller than expected at 93°. Whether this small angle is preventing the formation of a rhomb, or is the result of other interactions is impossible to determine but, either way, a halogen-bonded chain is formed instead of the desired discrete polygon, Fig. 5.
D1 also shows bifurcation at the N-oxide moiety, this time with an I–O–I angle smaller than expected at 93°. Whether this small angle is preventing the formation of a rhomb, or is the result of other interactions is impossible to determine but, either way, a halogen-bonded chain is formed instead of the desired discrete polygon, Fig. 5.
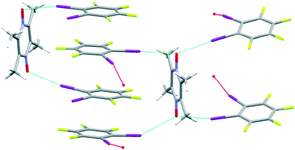
The crystal structure of A8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) D1 contains two crystallographically unique N-oxides, one with bifurcated halogen bonds and the other with a single halogen bond, Fig. 6.
D1 contains two crystallographically unique N-oxides, one with bifurcated halogen bonds and the other with a single halogen bond, Fig. 6.
The N-oxide entities with single halogen bonds interact with a nearby hydrogen atom forming a bifurcated hydrogen- and halogen bond combination with an angle of 88°. N-oxide entities with bifurcated halogen bonds have an I–O–I angle of 153° resulting in a chain of polygons albeit not with the intended topology and relative orientation of building blocks, Fig. 7. Neighbouring halogen-bond donors are stacked into columns with a distance of 0.337 nm hence stabilized by π–π interactions,20Fig. 6.
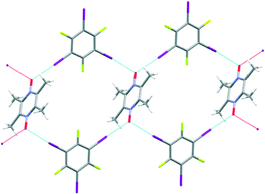
The first successful supramolecular synthesis in this study was realized in the crystal structure of A6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) D1. Bifurcation at the N-oxide (I–O–I, 105°) is present, and this time, the result is a discrete tetrameric rhomb, Fig. 8.
D1. Bifurcation at the N-oxide (I–O–I, 105°) is present, and this time, the result is a discrete tetrameric rhomb, Fig. 8.
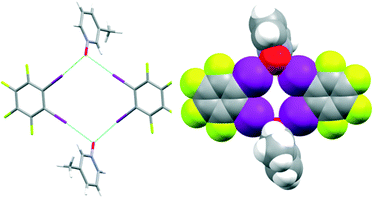 | ||
Fig. 8 Geometric complementarity leading to a discrete tetrameric rhomb in the crystal structure of A6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) D1. D1. | ||
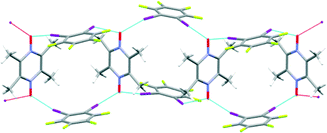
The second successful synthesis was achieved with the crystal structure A8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) D3. This time, the bifurcated I–O–I angle is close to 120°, and the complementary geometry furnished by the 1,3-substituted donor molecule affords the intended chain of hexagonal polygons, Fig. 9. Each hexagon offers a small interior ‘window’ approximately 0.72 × 0.92 nm, Fig. 10.
D3. This time, the bifurcated I–O–I angle is close to 120°, and the complementary geometry furnished by the 1,3-substituted donor molecule affords the intended chain of hexagonal polygons, Fig. 9. Each hexagon offers a small interior ‘window’ approximately 0.72 × 0.92 nm, Fig. 10.
All five co-crystal structures contained the desired bifurcated halogen bond to the N-oxide oxygen atom despite the fact that there have been only a small number of reports describing bifurcated halogen bonds,21 (most of which are theoretical studies).22 A survey of structures in the CSD produces over 800 hits containing at least one I⋯O halogen bond but less than 5% of those contain bifurcated bonds, but none of them include N-oxides as the acceptors even though there are some 30 structures in the CSD involving an N-oxide as a halogen-bond acceptor.
Our results also underscore that large difference in effective halogen-bond strength (for crystal engineering purposes) between iodo- and bromo based halogen-bond donors. The relatively ‘hard’ oxygen atom preferentially forms halogen bonds with the ‘softer’ iodine atom which also underscores that this type of interaction is dominated by electrostatics.
Conclusions
By using the geometric complementarity of two appropriate molecular building blocks it is possible to build polygons of specific dimensions using the strength and directionality of I⋯O halogen bonds. A key component of the synthetic strategy utilizes the propensity of the N-oxide moiety to form bifurcated intermolecular interactions with D⋯O⋯D angles close to 120°.Notes and references
- D. Ülkü, B. P. Huddle and J. C. Morrow, Acta Crystallogr., Sect. B: Struct. Crystallogr. Cryst. Chem., 1971, 27, 432 CrossRef.
- B. Verdejo, G. Gil-Ramirez and P. Ballester, J. Am. Chem. Soc., 2009, 131, 3178 CrossRef CAS PubMed.
- M. H. Abraham, L. Honcharova, S. A. Rocco, W. E. Acree and K. M. De Fina, New J. Chem., 2011, 35, 930 RSC.
- A. M. Atria, P. Cortes, M. T. Garland and R. Baggio, Acta Crystallogr., 2003, 59, 967 Search PubMed.
- J. P. Freeman and R. C. Grabiak, J. Org. Chem., 1976, 41, 3970 CrossRef CAS; J. P. Freeman and R. C. Grabiak, J. Org. Chem., 1976, 41, 2531 CrossRef; J. P. Freeman, J. A. Kassner and R. C. Grabiak, J. Org. Chem., 1975, 40, 3402 CrossRef.
- A. Monge, J. A. Palop, A. L. De Ceráin, V. Senador, F. J. Martinez-Crespo, Y. Sainz, S. Narro, E. Garcia, C. De Miguel, M. González, E. Hamilton, A. J. Barker, E. D. Clarke and D. T. Greenhowl, J. Med. Chem., 1995, 38, 1786 CrossRef CAS.
- S. Chopra, G. A. Koolpe, A. A. Tambo-ong, K. N. Matsuyama, K. J. Ryan, T. B. Tran, R. S. Doppalapudi, E. S. Riccio, L. V. Iyer, C. E. Green, B. Wan, S. G. Franzblau and P. B. Madrid, J. Med. Chem., 2012, 55, 6047 CrossRef CAS PubMed.
- A. Albini and M. Alpegiani, Chem. Rev., 1904, 84, 43 CrossRef.
- N. Takenaka, R. S. Sarangthem and B. Captain, Angew. Chem., 2008, 120, 9854 CrossRef.
- CSD ConQuest 1.15, Cambridge Crystallographic Data Centre, U. K., 2012 Search PubMed.
- R. Taylor, O. Kennard and W. Versichel, J. Am. Chem. Soc., 1984, 106, 244 CrossRef CAS.
- N. R. Goud, N. J. Babu and A. Nangia, Cryst. Growth Des., 2011, 11, 1930 Search PubMed; N. J. Babu, A. Nangia and L. S. Reddy, Mol. Pharmaceutics, 2007, 4, 417 CrossRef CAS PubMed; S. K. Koehn, N. L. Tran, S. Gronert and W. Wu, J. Am. Chem. Soc., 2010, 132, 390 CrossRef PubMed.
- M. T. Messina, P. Metrangolo, W. Panzeri, T. Pilati and G. Resnati, Tetrahedron, 2001, 57, 8543 CrossRef CAS.
- P. Metrangolo, F. Meyer, T. Pilati, G. Resnati and G. Terraneo, Angew. Chem., Int. Ed., 2008, 47, 6114 CrossRef CAS PubMed.
- T. Kawano, J. Kuwana, C.-X. Du and I. Ueda, Inorg. Chem., 2002, 41, 4078 CrossRef CAS PubMed; K. Kim, Chem. Soc. Rev., 2002, 31, 96 RSC.
- C. V. K. Sharma and A. Clearfield, J. Am. Chem. Soc., 2000, 122, 4394 CrossRef CAS.
- K. J. P. Davy, J. McMurtrie, L. Rintoul, P. V. Bernhardt and A. S. Micallef, CrystEngComm, 2011, 13, 5062 RSC.
- M. C. Pfrunder, A. S. Micallef, L. Rintoul, D. P. Arnold, K. J. P. Davy and J. McMurtrie, Cryst. Growth Des., 2012, 12, 714 CAS.
- S. L. James, C. J. Adams, C. Bolm, D. Braga, P. Collier, T. Friščić, F. Grepioni, K. D. M. Harris, G. Hyett, W. Jones, A. Krebs, J. Mack, L. Maini, A. G. Orpen, I. P. Parkin, W. C. Shearhouse, J. W. Steed and D. C. Waddell, Chem. Soc. Rev., 2012, 41, 413 RSC; C. B. Aakeröy, P. D. Chopade, C. Ganser, A. Rajbanshi and J. Desper, CrystEngComm, 2012, 14, 5845 RSC.
- H. W. Roesky and M. Andruh, Coord. Chem. Rev., 2003, 236, 91 CrossRef CAS; X. Mei, S. Liu and C. Wolf, Org. Lett., 2007, 9, 2729 CrossRef PubMed; J. K. Klosterman, Y. Yamauchi and M. Fujita, Chem. Soc. Rev., 2009, 38, 1714 RSC; C. A. Hunter, K. R. Lawson, J. Perkins and C. J. Urch, J. Chem. Soc., Perkin Trans. 2, 2001, 651 RSC.
- B. Ji, W. Wang, D. Deng and Y. Zhang, Cryst. Growth Des., 2011, 11, 3622 CAS.
- A. Bauzá, D. Quiñonero, A. Frontera and P. M. Deyà, Phys. Chem. Chem. Phys., 2011, 13, 20371 RSC.
Footnote |
| † Electronic supplementary information (ESI) available: Synthesis, characterization, details of the crystallographic work and cif files. CCDC 953680–953684. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c3ce41887a |
| This journal is © The Royal Society of Chemistry 2014 |