An eight-connected porous metal–organic framework based on hetero pentanuclear clusters†
Yu-Hui
Luo
a,
Xiao-Yang
Yu
ab,
Jia-Jun
Yang
a and
Hong
Zhang
*a
aInstitute of Polyoxometalate Chemistry, Department of Chemistry, Northeast Normal University, Jilin 130024, Changchun, PR China. E-mail: zhangh@nenu.edu.cn
bCollege of Chemical and Pharmaceutical Engineering, Jilin Institute of Chemical Technology, Jilin, Jilin City, 132022, PR China
First published on 11th October 2013
Abstract
A new MOF based on hetero pentanuclear clusters, [(CH3)2NH2]1.66[Cd1.84Na0.66(BDC)3]·DMF·0.5EtOH (1), has been prepared. Iodine adsorption studies reveal that 1 can reversibly adsorb iodine. Furthermore, electrochemical impedance spectroscopy (EIS) of 1⊃I2 shows that it has a relatively high conductivity at room temperature.
Porous metal–organic frameworks (MOFs) have attracted great interest, owing to the variety of network structures1 and wide-ranging potential applications including gas storage, catalysis, nonlinear optics and drug delivery.2 In recent years, intensive research in this area has been directed toward the construction of high connectivity (>6) network structures because of their potential advantages such as enhancing the stability of the frameworks, and having permanent porosity for reversible gas adsorption.3 However, it remains challenging because of, at least partially, the limited coordination number of single metal centers and the steric hindrance of the organic ligands.4 One effective synthetic strategy to prepare high-connected frameworks is by using metal clusters, which can enhance the coordination number to produce new generations of porous MOFs, as building blocks.5 Among the reported metal clusters, metal carboxylate clusters are relatively more utilized because of the variety of coordination modes and the modifiable features of carboxylate ligands.6 Up to now, some metal carboxylate clusters, such as Zn4O(CO2)6, Cu2(CO2)4 and Fe3O(CO2)6, have been extensively used in the construction of MOFs.7 Also, beyond these classic building blocks, some new types of metal carboxylate clusters, such as Zn7O4(CO2)10, Co7(OH)3(CO2)12, Cd11(HCOO−)6(CO2)18 and so on, have been successfully employed to prepare porous materials with special properties.8 However, high-connected porous MOFs based on metal carboxylate clusters, especially heteronuclear metal carboxylate clusters, are relatively few. Considering the factors above, it is interesting but still challenging to construct high-connected porous MOFs with new types of metal carboxylate clusters. Recently, our group has focused on this field. In this work, we introduce sodium into the self-assembly system and successfully isolate a new eight-connected porous MOF, namely, [(CH3)2NH2]1.66[Cd1.84Na0.66(BDC)3]·DMF·0.5EtOH (1; H2BDC = 1,4-benzenedicarboxylic acid, DMF = N,N′-dimethylformamide), based on hetero pentanuclear metal carboxylate clusters. The 3D porous framework can be reduced to a 420·68 topology. To explore the functionality of this material, we have investigated the iodine adsorption properties of 1. Furthermore, electrochemical impedance spectroscopy (EIS) of the iodine-containing compound (1⊃I2) has been performed.
The solvent reaction of CdCl2·2.5H2O, H2BDC and NaCl (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3
3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 molar ratio) in a mixture of DMF and EtOH (1
1 molar ratio) in a mixture of DMF and EtOH (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 volume ratio) resulted in the formation of octahedral crystals of 1. The purity of 1 was confirmed by powder X-ray diffraction (PXRD). The formula of 1 was supported by elemental analysis (EA) and thermogravimetric analysis (TGA). The Cd
1 volume ratio) resulted in the formation of octahedral crystals of 1. The purity of 1 was confirmed by powder X-ray diffraction (PXRD). The formula of 1 was supported by elemental analysis (EA) and thermogravimetric analysis (TGA). The Cd![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Na ratio in the final structure was decided by inductively coupled plasma (ICP) analysis. It should be noted that NaCl plays an important role in the synthesis of 1: specifically, if more or less NaCl is added, the synthesized samples will become impure and difficult to purify by hand.
Na ratio in the final structure was decided by inductively coupled plasma (ICP) analysis. It should be noted that NaCl plays an important role in the synthesis of 1: specifically, if more or less NaCl is added, the synthesized samples will become impure and difficult to purify by hand.
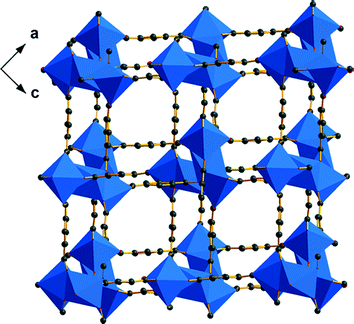
Single-crystal X-ray analysis9 reveals that the structure of 1 contains hetero pentanuclear metal carboxylate clusters (Fig. S1a†), which are formed by twelve carboxylate groups connecting metal ions. The positions of the Cd3 and Na3 ions are disordered, and the occupancy of Na3 has been determined to be 0.66 by ICP analysis. The 2-fold axis of the cluster lies on the Cd1 atom (Fig. S1b†). As shown in Fig. S1a,† the Cd1 atom is six coordinated by carboxylate oxygen atoms (Cd–O, 2.279(10)–2.575(13) Å) giving a distorted octahedral geometry. The Cd2 atom is seven coordinated by carboxylate oxygen atoms (Cd–O, 2.199(13)–2.600(14) Å) giving a distorted pentagonal bipyramid geometry. The disordered Cd3 and Na3 ions are both six coordinated into a distorted octahedral geometry with metal–oxygen bond lengths in the range 2.271(14)–2.495(15) Å. The BDC2− ligands in 1 display three types of bridging model: μ3-(η1:η1)-(syn-anti-μ2-η1:η1), μ4-(μ2-η2:η1)-(μ2-η2:η1) and μ5-(μ3-η2:η1)-(μ2-η2:η1) (Fig. S1c†). Due to the three coordination types of the BDC2− ligands, the metal carboxylate clusters are bridged into a 3D porous framework (Fig. 1). The total potential solvent-accessible volume of 1 is calculated to be 52.2% (8801.6 Å3 out of 16873.0 Å3 per unit cell volume) using the PLATON program.10
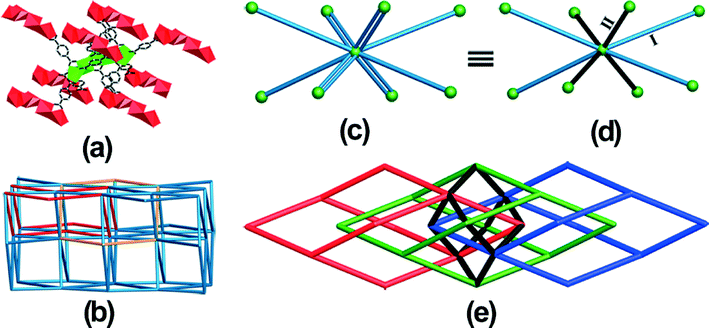
As shown in Fig. 2a, each cluster in 1 is linked to its eight nearest neighbors by twelve BDC2− ligands. From a topological point of view, the cluster can be defined as an eight-connected node, and 1 can be described as an eight-connected topology with the short Schläfli symbol of 420·68 (Fig. 2b).11 From the topological net of 1, we can see that the framework displays a self-penetrating network with the shortest six-membered rings interlocked (Fig. 2b). A further study on the structure indicates that the twelve BDC2− ligands around the pentanuclear cluster can be divided into two parts. As shown in Fig. 2c, the four single-rod linkers (type I) are formed by μ3-BDC2− ligands, and the four double-rod linkers (type II) are formed by μ4-BDC2− and μ5-BDC2− ligands. Interestingly, if we do not take type II linkers into consideration, 1 can be reduced into a three-folded diamondoid net (Fig. 2e, red, green and blue). Furthermore, when we only take type II linkers into consideration, 1 can be reduced into a single diamondoid net (Fig. 2e, black). Therefore, the eight-connected topological net of 1 is formed by a single diamondoid net connecting three-folded diamondoid nets. The topological net of 1 was also studied with new approaches widely used in the analysis of highly connected frameworks, as proposed by Hill et al.12 Thus, the eight-connected network of 1 can be described as being formed by zigzag chains connecting parallel (4,4) nets (Fig. S3†). In the topological net, the zigzag chains in the interlayer region bridge across the diagonals of the windows in the (4,4) net.
Thermogravimetric analysis (TGA) shows that 1 loses 11.7% of its total weight by heating to 130 °C, which perhaps corresponds with the loss of all the neutral guest molecules from the framework (calcd: 10.8%). Further heating results in the release of dimethylamine molecules and slow decomposition of the framework (Fig. S5†). PXRD measurements confirm that the as-synthesized sample of 1 is pure (Fig. S4†).
The FTIR spectrum for 1 was recorded at room temperature (Fig. S6†). The broad band generated by the stretch vibration of the OH group at around 3420 cm−1 can be attributed to the appearance of alcohol molecules. The band at around 1573 cm−1 is due to the νas(COO−) stretching vibration of the carboxylate groups, while a strong band at 1383 cm−1 corresponds to the νs(COO−) stretching vibration of the carboxylate groups.
An I2-loading experiment was carried out for 1. When the freshly prepared crystals of 1 (1.02 g) were immersed in a cyclohexane (100 ml) solution of I2 (0.05 mol L−1) for seven days, they turned a dark-brown in color. The resulting dark-brown crystals were thoroughly washed with fresh cyclohexane to remove I2 residue on the external surfaces of the crystals, and the mass increased by ca. 17.7 wt%, which indicates that 1 can accommodate about one I2 molecule per formula unit. The loading capacity for I2 has also been confirmed by ICP analysis according to the molar ratio of I2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
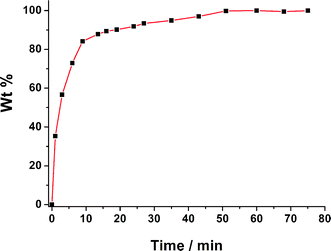
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Cd. Further studies indicated that 1⊃I2 could also release I2 into fresh EtOH. The kinetics of I2 delivery by 1⊃I2 was investigated by in situ UV–Vis spectroscopy at room temperature. Typically, several of the crystals were soaked in a colorimetric cell with 3 mL EtOH. The absorbance of I2 in EtOH increased linearly with time (Fig. 3 and S7†).13 The iodine release rate became slower after 9 min because the concentration of I2 in EtOH increased with time13b and the complete release of I2 from 1⊃I2 required about 51 min (Fig. 3). PXRD measurements for 1⊃I2, and 1 after releasing I2, demonstrate that I2 sorption by 1 is reversible (Fig. S4†). These results indicate that 1 is a good candidate material for iodine enrichment and detection.14
Cd. Further studies indicated that 1⊃I2 could also release I2 into fresh EtOH. The kinetics of I2 delivery by 1⊃I2 was investigated by in situ UV–Vis spectroscopy at room temperature. Typically, several of the crystals were soaked in a colorimetric cell with 3 mL EtOH. The absorbance of I2 in EtOH increased linearly with time (Fig. 3 and S7†).13 The iodine release rate became slower after 9 min because the concentration of I2 in EtOH increased with time13b and the complete release of I2 from 1⊃I2 required about 51 min (Fig. 3). PXRD measurements for 1⊃I2, and 1 after releasing I2, demonstrate that I2 sorption by 1 is reversible (Fig. S4†). These results indicate that 1 is a good candidate material for iodine enrichment and detection.14
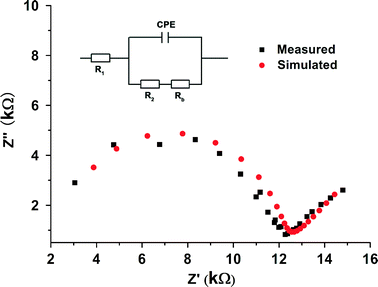
EIS of a pellet of 1⊃I2 with a diameter of 1.98 cm and thickness of 0.18 cm was performed at room temperature (Fig. 4). The total resistance (Rt) of the sample is equal to the sum of R1 and R2, the values of which are simulated to 2376 and 9670 Ω, respectively. Therefore, the calculated electrical conductivity values for σt, σ1 and σ2 are 4.85 × 10−6 S cm−1, 2.46 × 10−5 S cm−1 and 6.04 × 10−6 S cm−1, respectively. Notably, the electrical conductivity value of the sample is significantly greater than that of solid I2 (7.69 × 10−8 S cm−1).13a This may be attributed to the ordered dispersion of the I2 in the channels with the presence of high efficiency n → σ* charge-transfer (CT) between molecular I2 and the π-electron walls of 1⊃I2.13
In conclusion, we have successfully prepared an eight-connected self-interpenetrating porous MOF, [(CH3)2NH2]1.66[Cd1.84Na0.66(BDC)3]·DMF·0.5EtOH (1), by introducing sodium into the self-assembly system. Structural analysis reveals that 1 contains a new type of hetero pentanuclear metal carboxylate cluster. Iodine adsorption studies indicate that 1 is a good candidate material for iodine enrichment and detection. In addition, EIS of 1⊃I2 shows that it has a relatively high conductivity at room temperature. The successful synthesis of 1 provides useful information on the construction of new high connectivity network structures with special properties.
Notes and references
- (a) M. O'Keeffe and O. M. Yaghi, Chem. Rev., 2012, 112, 675 CrossRef PubMed; (b) H. Deng, S. Grunder, K. E. Cordova, C. Valente, H. Furukawa, M. Hmadeh, F. Gándara, A. C. Whalley, Z. Liu, S. Asahina, H. Kazumori, M. O'Keeffe, O. Terasaki, J. F. Stoddart and O. M. Yaghi, Science, 2012, 336, 1018 CrossRef CAS PubMed; (c) N. Stock and S. Biswas, Chem. Rev., 2012, 112, 933 CrossRef CAS PubMed.
- (a) Z. R. Herm, B. M. Wiers, J. A. Mason, J. M. van Baten, M. R. Hudson, P. Zajdel, C. M. Brown, N. Masciocchi, R. Krishna and J. R. Long, Science, 2013, 340, 960 CrossRef CAS PubMed; (b) F. Luo, M. S. Wang, M. B. Luo, G. M. Sun, Y. M. Song, P. X. Li and G. C. Guo, Chem. Commun., 2012, 48, 5989 RSC; (c) F. J. Ma, S. X. Liu, C. Y. Sun, D. D. Liang, G. J. Ren, F. Wei, Y. G. Chen and Z. M. Su, J. Am. Chem. Soc., 2011, 133, 4178 CrossRef CAS PubMed; (d) W. W. Zhou, J. T. Chen, G. Xu, M. S. Wang, J. P. Zou, X. F. Long, G. J. Wang, G. C. Guo and J. S. Huang, Chem. Commun., 2008, 2762 RSC; (e) M. J. Zhang, X. M. Jiang, L. J. Zhou and G. C. Guo, J. Mater. Chem. C, 2013, 1, 4754 RSC; (f) C. Y. Sun, X. L. Wang, C. Qin, J. L. Jin, Z. M. Su, P. Huang and K. Z. Shao, Chem.–Eur. J., 2013, 19, 3639 CrossRef CAS PubMed; (g) D. Zhao, S. Tan, D. Yuan, W. Lu, Y. H. Rezenom, H. Jiang, L. Q. Wang and H. C. Zhou, Adv. Mater., 2011, 23, 90 CrossRef CAS PubMed.
- (a) X. Gu, Z. H. Lu and Q. Xu, Chem. Commun., 2010, 46, 7400 RSC; (b) G. Ren, S. Liu, F. Ma, F. Wei, Q. Tang, Y. Yang, D. Liang, S. Li and Y. Chen, J. Mater. Chem., 2011, 21, 15909 RSC; (c) J. Jia, X. Lin, C. Wilson, A. J. Blake, N. R. Champness, P. Hubberstey, G. Walker, E. J. Cussen and M. Schroder, Chem. Commun., 2007, 840 RSC; (d) L. Hou, J. P. Zhang, X. M. Chen and S. W. Ng, Chem. Commun., 2008, 4019 RSC; (e) H. Chun, D. Kim, D. N. Dybtsev and K. Kim, Angew. Chem., Int. Ed., 2004, 43, 971 CrossRef CAS PubMed.
- (a) X. L. Wang, C. Qin, Y. Q. Lan, K. Z. Shao, Z. M. Su and E. B. Wang, Chem. Commun., 2009, 410 RSC; (b) D. L. Long, R. J. Hill, A. J. Blake, N. R. Champness, P. Hubberstey, D. M. Proserpio, C. Wilson and M. Schröder, Angew. Chem., 2004, 116, 1887 CrossRef.
- (a) S. T. Zheng, T. Wu, C. Chou, A. Fuhr, P. Y. Feng and X. H. Bu, J. Am. Chem. Soc., 2012, 134, 4517 CrossRef CAS PubMed; (b) Y. S. Wei, K. J. Chen, P. Q. Liao, B. Y. Zhu, R. B. Lin, H. L. Zhou, B. Y. Wang, W. Xue, J. P. Zhang and X. M. Chen, Chem. Sci., 2013, 4, 1539 RSC.
- (a) S. T. Zheng, C. Y. Mao, T. Wu, S. Lee, P. Y. Feng and X. H. Bu, J. Am. Chem. Soc., 2012, 134, 11936 CrossRef CAS PubMed; (b) G. Jiang, T. Wu, S. T. Zheng, X. Zhao, Q. Lin, X. Bu and P. Feng, Cryst. Growth Des., 2011, 11, 3713 CrossRef CAS.
- For example, (a) H. Li, M. Eddaoudi, M. O'Keeffe and O. M. Yaghi, Nature, 1999, 402, 276 CrossRef CAS; (b) H. K. Chae, D. Y. Siberio-Perez, J. Kim, Y. Go, M. Eddaoudi, A. J. Matzger, M. O'Keeffe and O. M. Yaghi, Nature, 2004, 427, 523 CrossRef CAS PubMed; (c) Y. Q. Lan, H. L. Jiang, S. L. Li and Q. Xu, Adv. Mater., 2011, 23, 5015 CrossRef CAS PubMed; (d) M. E. Braun, C. D. Steffek, J. Kim, P. G. Rasmussen and O. M. Yaghi, Chem. Commun., 2001, 2532 RSC; (e) G. Férey and A. K. Cheetham, Science, 1999, 283, 1125 CrossRef; (f) G. Férey, C. Mellot-Draznieks, C. Serre, F. Millange, J. Dutour, S. Surblé and I. Margiolaki, Science, 2005, 309, 2040 CrossRef PubMed.
- (a) S. S. Iremonger, R. Vaidhyanathan, R. K. Mah and G. K. H. Shimizu, Inorg. Chem., 2013, 52, 4124 CrossRef CAS PubMed; (b) X. N. Cheng, W. X. Zhang, Y. Y. Lin, Y. Z. Zheng and X. M. Chen, Adv. Mater., 2007, 19, 1494 CrossRef CAS; (c) Q. R. Fang, G. S. Zhu, Z. Jin, M. Xue, X. Wei, D. J. Wang and S. L. Qiu, Angew. Chem., Int. Ed., 2006, 45, 6126 CrossRef CAS PubMed.
- Crystal data for 1: C31.32H35.28Cd1.84Na0.66O13.5N2.66, Fw = 886.99 g mol−1, orthorhombic, Fdd2, a = 29.370(6) Å, b = 20.998(3) Å, c = 27.360(4) Å, V = 16
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 873(5) Å3, Z = 16, T = 293(2) K, R(int) = 0.0711, 10
873(5) Å3, Z = 16, T = 293(2) K, R(int) = 0.0711, 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 174 reflections collected, Dcal = 1.121 g cm−3, GOF = 0.991 for 5693 reflections with I > 2σ(I), R1 = 0.0746, wR2 = 0.1961 (I > 2 σ(I)).
174 reflections collected, Dcal = 1.121 g cm−3, GOF = 0.991 for 5693 reflections with I > 2σ(I), R1 = 0.0746, wR2 = 0.1961 (I > 2 σ(I)). - A. Spek, Acta Crystallogr., Sect. D: Biol. Crystallogr., 2009, 65, 148 CrossRef CAS PubMed.
- M. O'Keeffe and S. T. Hyde, Zeolites, 1997, 19, 370 CrossRef.
- R. J. Hill, D. L. Long, N. R. Champness, P. Hubberstey and M. Schröder, Acc. Chem. Res., 2005, 38, 335 CrossRef CAS PubMed.
- (a) Z. Yin, Q. X. Wang and M. H. Zeng, J. Am. Chem. Soc., 2012, 134, 4857 CrossRef CAS PubMed; (b) Y. C. He, J. Yang, G. C. Yang, W. Q. Kan and J. F. Ma, Chem. Commun., 2012, 48, 7859 RSC; (c) M. H. Zeng, Q. X. Wang, Y. X. Tan, S. Hu, H. X. Zhao, L. S. Long and M. Kurmoo, J. Am. Chem. Soc., 2010, 132, 2561 CrossRef CAS PubMed.
- (a) Z. J. Zhang, W. Shi, Z. Niu, H. H. Li, B. Zhao, P. Cheng, D. Z. Liao and S. P. Yan, Chem. Commun., 2011, 47, 6425 RSC; (b) Q. K. Liu, J. P. Ma and Y. B. Dong, Chem. Commun., 2011, 47, 7185 RSC; (c) L. Chen, K. Tan, Y. Q. Lan, S. L. Li, K. Z. Shao and Z. M. Su, Chem. Commun., 2012, 48, 5919 RSC.
Footnote |
| † Electronic supplementary information (ESI) available: X-ray crystallographic data in CIF format, structure diagrams, PXRD patterns, TGA curves, tables of selected bond lengths and angles. CCDC 926973. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c3ce41785a |
| This journal is © The Royal Society of Chemistry 2014 |