Solvent micro-evaporation and concentration gradient synergistically induced crystallization of poly(L-lactide) and ring banded supra-structures with radial periodic variation of thickness
Shaoyong
Huang
a,
Hongfei
Li
*b,
Huiying
Wen
b,
Donghong
Yu
c,
Shichun
Jiang
*d,
Gao
Li
a,
Xuesi
Chen
a and
Lijia
An
*b
aKey Laboratory of Polymer Eco-materials, Changchun 130022, PR China
bState Key Laboratory of Polymer Physics and Chemistry, Changchun Institute of Applied Chemistry, Chinese Academy of Sciences, Changchun 130022, PR China. E-mail: hfli@ciac.ac.cn; ljan@ciac.ac.cn
cDepartment of Biotechnology, Chemistry and Environmental Engineering, Aalborg University, DK-9000, Aalborg, Denmark
dSchool of Materials Science and Engineering, Tianjin University, Tianjin 300072, PR China. E-mail: scjiang@tju.edu.cn
First published on 24th October 2013
Abstract
The crystalline morphology and structure of poly(L-lactide) (PLLA) in a PLLA film–chloroform system were investigated by means of wide angle X-ray diffraction (WAXD), polarized optical microscopy (POM) and atomic force microscopy (AFM). Birefringent and nonbirefringent ring banded supra-structures with radial periodic variation of thickness were obtained, which were induced by micro-evaporation of solvents and concentration gradient of PLLA. The ring banded morphologies consisted of multilayer lamellar crystals, which is a manifestation of alternating ridge and valley bands of periodic variation of thicknesses along the radial direction. The formation of the ring banded supra-structures is associated with diffusion and crystal growth induced periodic variation of concentration gradient, which is attributed to diffusion-related rhythmic growth and the competition between diffusion of polymer segments and growth of crystal lamellae. The mechanism of such banded-ring formation was explored on the basis of rhythmic growth resulting from non-linear diffusion.
Introduction
Ring banded supra-structures have been observed in crystallization of homopolymers, block copolymers, and polymer blends grown from melts.1–12 It is generally believed that the formation of ring banded supra-structures results from periodic twisting of crystal lamellae along the radial growth direction.13–16 However, the formation of ring banded supra-structures remains one of the major challenges in the field of crystalline polymer morphology. Keith and Padden showed that lamellar twisting was related to some features of the lamellae themselves that introduced imbalanced surface stresses on their surface;14 Toda et al. linked lamellae twisting with repetition of isochiral screw dislocations in growing lamellae;17 Schultz assumed self-induced compositional or mechanical fields generated near the advancing crystal front;18 Kyu et al. considered that lamellar twisting might not be the only reason for the formation of ring banded structures, and it might be a consequence of a rhythmic crystal growth resulting from non-linear diffusion.19,20 Nonbirefringent ring banded spherulites grown from melts, which are different from classical birefringent ring banded spherulites, were observed in isotactic polystyrene (iPS) and poly(bisphenol A hexane ether).21,22 Nonbirefringent ring banded structures are composed of multilayer lamellae, and the formation is associated with rhythmic crystal growth. Under a slow solvent evaporation rate, Wang et al. obtained rhythmic growth-induced nonbirefringent ring banded spherulites in poly(ε-caprolactone) (PCL) casting films.23,24 The ring banded spherulites result from alternating ridge and valley bands of periodic variation of thicknesses, rather than lamellar twisting. Application properties such as electric conductivity are related to the morphology.25PLLA is a biodegradable semi-crystalline polymer widely investigated from a biomedical application and an environmentally degradable plastic view point. Its application and properties are strongly related to its crystal structure and morphology.26–30 Crystal structure and morphology are determined not only by thermodynamics but also by kinetics of crystallization. To achieve a better understanding of the crystallization process and the resulting final structure of polymers, a model system was employed by controlling kinetic factors. The formation of crystal structure and morphology is determined by two competitive factors: the migration of polymer chains to the crystal growth front and the rate of crystal growth, which can be modulated in solution crystallization by controlling rate of solvent evaporation. Thus, the development of crystalline structure and morphology can also be modulated in this way.31
In this paper, we investigated the crystalline morphology and structure of PLLA in a PLLA–chloroform system by controlling the kinetic process. Birefringent and nonbirefringent ring banded supra-structures were obtained by solvent micro-evaporation. The ring banded supra-structures are different from the classical ring banded spherulites grown from melts. The morphologies and structures were studied by wide angle X-ray diffraction (WAXD), polarized optical microscopy (POM) and atomic force microscopy (AFM). The development of the morphologies was explored on the basis of a rhythmic growth mechanism.
Experimental
Materials
PLLA was purchased from Aldrich. Gel permeation chromatography (GPC, Waters 410) determined that its number averaged molecular weight (Mn) and polydispersity index (Mw/Mn) are 34![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 g mol−1 and 1.39, respectively. The melting point determined by differential scanning calorimetry (DSC-7, Perkin-Elmer) is 170.2 °C measured at a heating rate of 10 °C min−1.
100 g mol−1 and 1.39, respectively. The melting point determined by differential scanning calorimetry (DSC-7, Perkin-Elmer) is 170.2 °C measured at a heating rate of 10 °C min−1.
Sample preparation
PLLA films were prepared by spin-casting a PLLA–chloroform solution with concentrations of 0.8% and 0.3% (wt%) on silicon wafers at speeds of 0, 500, and 1000 rpm for 30 seconds. Film thickness could be modulated by varying solution concentration and spinning speed. Thicknesses of the films ranging from several microns to tens of microns were generally evaluated by AFM height phase.After solvent evaporation, the films were dried in vacuum for 24 hours and then quenched to 30 °C from complete melts at 200 °C, leading to disordered PLLA in films. Each disordered PLLA film was sealed in a cylinder which was placed in a thermostatic oven at 30 °C under atmospheric pressure. The sample preparation has been shown in a previous work.32
Measurements
The polarized optical microscopic (POM) observations of the samples were performed using a Leica DMLP (Leica, DMLP) microscope equipped with a Panasonic CCD camera. The AFM measurements were carried out with a SPA-300HV AFM equipped with a SPI 3800N controller (Seiko Instruments Industry Co., Ltd.). A 150 μm scanner and SiN4-cantilevers were applied for tapping mode. The WAXD data were collected using X-ray diffraction (Rigaku, D/max 2500V PC X-ray scattering) with Cu-Kα radiation (λ = 1.54 Å). The 2θ scanning rate is 3° min−1 from 10° to 35°. Selected voltage and current were 50 kV and 250 mA, respectively.Results and discussion
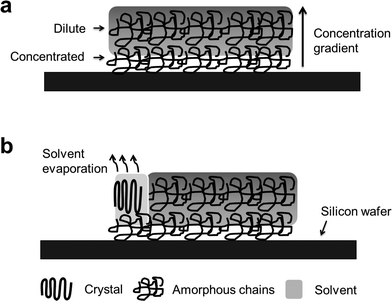
The crystallization process of PLLA can be divided into three stages: solvent adsorption, surface gel formation, and crystallization. Chloroform is a good solvent to PLLA, and small molecules of chloroform were continuously adsorbed into PLLA samples. Surface gel formation and even local PLLA–chloroform solution were obtained, due to adsorption of chloroform into the surface of the PLLA film, diffusion of PLLA segments and hydrodynamic flow, as shown in Fig. 1(a). A concentration gradient of PLLA chains was generated, because the amount of solvent adsorbed into each layer of PLLA decreased along the direction perpendicular to the substrate, which is associated with the amount of solvent adsorbed and the migration of PLLA segments.Nucleation of PLLA occurred because of concentration fluctuation of PLLA which was associated with the variation of concentration gradient and the non-linear kinetics of solvent evaporation. Then, crystal lamellae of PLLA grew around nuclei from the PLLA–chloroform system, which was driven by solvent evaporation, as shown in Fig. 1(b). Meanwhile, nucleation and crystal growth depleted the disordered PLLA segments, and then the adjacent PLLA segments diffused to the growth point, resulting in a concentration gradient of PLLA chains along the radial direction. The temperature and pressure in the thermostatic oven were constant during the whole process, and the phase equilibrium of the solvent was maintained. Consequently, the concentration of amorphous PLLA phases in the whole system was kept approximately constant. The decrease in the concentration of PLLA phases due to crystal growth can be compensated by the depletion of the amount of solvent associated with slow evaporation. After crystallization, the samples were taken out for further measurements.
Birefringent ring banded spherulites
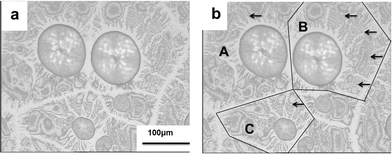
As shown in Fig. 1, self-assembly of PLLA chains in the PLLA–chloroform system induced by slow evaporation of chloroform and variation of concentration gradient of PLLA resulted in disorder to order structural transition and even crystallization of PLLA. As presented in Fig. 2, birefringent ring banded spherulites of PLLA were formed in films of tens of microns in thickness, which are similar to the ring banded spherulites grown from the melts of PLLA blends and copolymers, and PLLA homopolymer at high crystallization temperatures.3,4,7,26,33–35 It indicated that the solvent in the PLLA–chloroform system had a similar influence on the crystalline morphology of PLLA as melted amorphous components in blends or copolymers. In PLLA films, mobility of polymer segments and crystal lamellae twisting became easier due to the addition of chloroform.36,37 As a result, birefringent ring banded spherulites could be formed at 30 °C which was driven by solvent evaporation.Solvent evaporation and the variation of concentration gradient in the system result in fluctuation of local concentration of PLLA chains, and then nuclei are obtained for further crystal growth. The diameter (D) of the ring banded spherulites increases from ca. 50 μm to ca. 100 μm when film thickness decreases from ca. 25 μm to ca. 15 μm, due to the decrease in the number of nuclei per unit volume. For melt crystallization of PLLA blends or copolymers, large ring banded spherulites with diameters ranging from 300 μm to 1500 μm could be formed within 1 hour.3–5,7,26,34 The widths of bright and dark bands grown from melts are ~50 μm and ~30 μm, respectively.3–5,7,26,32,35 The alternating bright and dark bands in spherulites grown from melt are associated with lamellae twisting in crystal packing. The widths of the bands increased on addition of amorphous components in blends or copolymers, because the amorphous phases, e.g. PEG, PEO, PHB, are dispersed among crystal lamellae of PLLA. The widths of the dark bands of ring banded spherulites induced by solvent evaporation are ~5.0 μm, which are much smaller than those of ring banded spherulites grown from melts. Moreover, the dark bands in Fig. 2 are discontinuous, which are different from those of classic ring banded spherulites.
The widths of the bands of ring banded spherulites induced by solvent evaporation are much smaller than those grown from melts. Several factors might be responsible for the differences between the two kinds of ring banded spherulites. First, the crystal growth rate is much lower in the PLLA–solvent system than that in melt crystallization. Second, the temperature (30 °C) is much lower, while the crystallization temperatures in melt crystallization are often 100 °C and above.26–31 Third, the driving force of solvent evaporation for crystal growth is much weaker than that for super-cooling in melt crystallization, and the imbalance of stress of crystal lamellae was strengthened due to the concentration gradient of PLLA. Fourth, solvent molecules are excluded from folded chains of PLLA, and the bands only consisted of PLLA folded chains, without PEO and PEG components. Moreover, the formation of the ring banded morphology may take a new growth mechanism, which is different from the lamellar twisting of classic ring banded spherulite.11,17,18 The birefringent ring banded morphology in Fig. 2 might have originated from a rhythmic crystal growth resulting from non-linear diffusion.20
Fig. 3 shows the one dimensional WAXD patterns of thick PLLA films (tens of microns) induced by chloroform evaporation at 30 °C for 30 days. Typical reflections for the α-crystal of PLLA, including (2 0 0)/(1 1 0) and (2 0 3) reflections,4–6,27–29 were pronounced, which indicated that the morphologies in Fig. 2 were stacked with α-crystals of PLLA. The nucleation and crystal growth of PLLA were induced by the variation of concentration gradient and solvent evaporation, and the crystallization process has been mentioned in the experimental part. The intensities of crystalline peaks are quite weak compared to those of amorphous peaks, indicating that the lamellar packing is not as compact as that of the PLLA crystal structure grown from melt and solution, and also the overall crystallization rate (G) is much slower than those crystallized from melt and solution, due to the much different temperatures used for these two systems.7,24,29,35–38 The relatively slow G could be ascribed to the adsorption of solvent molecules into PLLA films, the long inducement time of nucleation and the weak driving force for crystal growth, from a kinetics points of view. These have been confirmed by the small number of spherulites per unit volume and the small size of spherulite as revealed in Fig. 2.
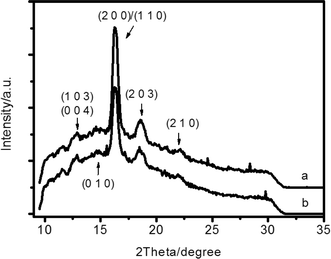 | ||
| Fig. 3 WAXD patterns of PLLA ring banded spherulites collected at different angles in films with thicknesses of (a) 25.0 ± 5.0 μm and (b) 15.0 ± 3.0 μm. | ||
Concentric ring banded morphologies
The crystallization, crystalline structure and morphology of polymers grown from solution are predominated by migration of polymer chains and crystal growth velocity, which are associated with the concentration of polymers.22–24,38–41 Furthermore, rhythmic crystal growth mode is closely related with concentration gradient and its variation during the growth process, which is also associated with the concentration of polymer in solution or melt.20 In our experiment, the concentration gradient of PLLA in the whole system can be modulated by changing the thickness of the PLLA film. Different morphologies of nonbirefringent ring banded supra-structures were observed in thin films with thickness of ca. 10 μm as shown in Fig. 4.The sizes of the supra-structures in Fig. 4 ranged from 200 to 400 μm, which were much larger than those of birefringent ring banded spherulites in Fig. 2. Moreover, as shown in Fig. 4, all of the birefringent spherulites were in the center, and the nonbirefringent concentric ring bands developed around the birefringent spherulites. The boundaries of the concentric supra-structures are straight as suggested in Fig. 4(b), and the whole micrograph of Fig. 4(b) was divided into four parts on the basis of the straight boundary lines, and three parts were labeled as A, B, and C. Each part includes a birefringent spherulite in the center and nonbirefringent concentric ring bands around. The concentric ring bands in the outer part are discontinuous in each band, caused by non-linear diffusion-induced rhythmic growth associated with variation of concentration gradient of PLLA chains and fluctuation of concentration of polymer chains. The development of the crystal structures and the concentric ring banded morphologies are predominated by two competitive kinetic processes, the diffusion of polymer chains towards the growth front and the rate of growth.22–24,42,43
As labeled by arrows in Fig. 4(b), concentric ring bands were observed. The thickness of the film was 10.0 ± 3.0 μm, which was thinner than those in Fig. 2, indicating that the amount of PLLA chains per volume for each nucleus was smaller than those in Fig. 2. Consequently, it was difficult to form continuous spherulitic morphologies, due to insufficient polymer chains for further crystal growth. A new morphology of spherulitic supra-structure in the center and concentric ring bands in the outer part was then generated as shown in Fig. 4, which could grow with rhythmic growth mode.20,42 The spherulites in the center were similar to those formed in thicker films in Fig. 2, indicating that the crystal growth started in the center, because there were sufficient polymer chains for crystal growth in the initial stage. The concentric ring bands in the outer part were discontinuous, due to variation of concentration gradient formed by diffusion, crystal growth and fluctuation of polymer chains.22–24 The thickness of the boundary lines between the two supra-structures, which were labeled by lines in Fig. 4(b), was the thinnest, because the amount of polymer chains per unit volume was the smallest due to the diffusion of polymer chains to the growth fronts.
Fig. 5 shows the X-ray diffraction pattern of the PLLA film corresponding to that in Fig. 4. As shown in Fig. 4, concentric ring banded supra-structures were obtained under micro-evaporation. The typical reflections of the PLLA crystal, including (1 0 3)/(0 0 4) and (2 0 0)/(1 1 0) reflections, indicated that the ordered supra-structures were multilayer ensembles of PLLA crystals. However, the reflection peaks were much weaker, which suggested that the packing of the crystal structure was quite loose, and the degree of crystallization was very low, which was less than 10%.
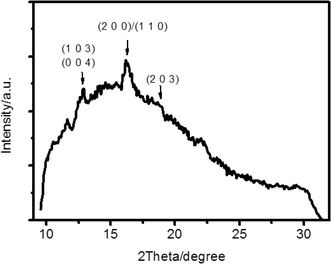 | ||
| Fig. 5 WAXD pattern of PLLA concentric ring banded supra-structures grown from solution under solvent micro-evaporation. The film thickness of the PLLA film is 10.0 ± 3.0 μm. | ||
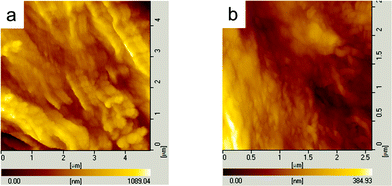
The nonbirefringent concentric ring bands shown in Fig. 4 were further investigated by AFM. The typical AFM topography images have been reported in our recent work.32 Alternating ridge and valley bands are evident. Lamellar twisting of birefringent ring banded spherulites was not observed, which was the reason for the nonbirefringent optical property.13–17 As presented in Fig. 4, in each selected domain, besides a birefringent spherulite in the center, nonbirefringent ring bands further grew around it. In the first ridge band, the packing of lamellar crystals takes an edge-on mode combined with the flat-on mode due to sufficient supply of polymer chains for growth fronts, and then screw dislocations and lamellae tilt may take place. As a result, the concentric ring banded structure may probably take a similar initiation mode for crystal growth as that for the birefringent spherulite in Fig. 2 and 4.22,23
Fig. 6 clearly reveals the morphological details of the outer ring banded structures. From Fig. 6(a), it can be seen that lamellar crystals grow with the flat-on mode in the valley bands. Instead, tilted and even edge-on lamellae can be seen in proximity to the ridges in Fig. 6(b), which suggests a similar initiation mode for new layers of lamellar crystals in valleys and ridges in the ring banded morphologies. The packing of crystal lamellae takes a flat-on mode, which is different from the growth and packing of crystal lamellae in spherulites. As a result, the bands formed are nonbirefringent.
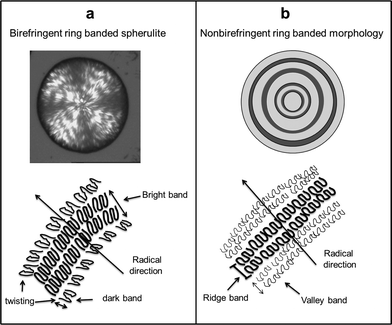
The two kinds of ring banded morphologies with different optical properties and their correlations with lamellar orientations are illustrated in Fig. 7.15,24,42 The micro-structure of alternating bright and dark bands in birefringent ring banded spherulites is demonstrated in Fig. 7(a). In such spherulites, lamellar growth and packing take flat-on and edge-on modes. The orientation of lamellar crystal changes gradually in the radial direction by lamellar twisting, and the thickness of the bright band includes two different parts, the radial orientation part and the twisting part as shown in Fig. 7(a). While in nonbirefringent ring banded morphology, lamellar growth and packing predominately take a flat-on mode. As a result, the ridge band includes only the radial orientation part as proven in Fig. 7(b). Moreover, the orientation of lamellae in dark bands is perpendicular to that in bright ones, and thus alternating bright and dark optical properties occur in birefringent ring banded spherulites in Fig. 7(a). However, both ridge and valley bands of the concentric ring banded morphology as shown in Fig. 7(b) are arranged by lamellae in a radial direction. As a result, it is nonbirefringent, and alternating ridge and valley bands with different thicknesses are the origin of the concentric ring bands.
The nonbirefringent concentric ring banded morphology is different from classical ring banded spherulites, and its formation is attributed to a nonlinear diffusion-induced rhythmic growth process.20,24,43 The concentric ring banded structures reflect the competition between diffusion flux of polymer chains in solution (J) and the crystal growth velocity (V). The competition can be illustrated by the amount of polymer chains per unit volume diffused to the crystal growth front (δ), where δ = J/V.24,42 The development of alternating ridge and valley bands results from periodic changes of δ along the growth direction. The periodic imbalance of supply (J) and demand (V) of polymer chains results in alternating ring banded structures with periodic variation of thicknesses. When δ is sufficient, a thick ridge band is generated. When δ is insufficient, a thin valley band is formed.
The influence of fluctuation of polymer concentration on the development of structure and morphology should be considered, when the amount of polymer chains diffused into a new growth front was not large enough. In solutions formed in films of tens of microns in Fig. 2 and 4, polymer chains are sufficient for crystal growth in the initial stage of crystallization. The diffusion flux of polymer chains (J) increases a little due to a slight increase in concentration gradient.24,43 As a result, the thicknesses of ridge and valley bands are kept nearly constant. In solutions formed in films of several microns, the influence of fluctuation of polymer concentration is evitable. The diffusion flux of polymer chains (J) increases with the increase in concentration gradient. The growth velocity (V) can be considered as invariable during the growth process. Therefore, the thickness and the band spacing of the initial ridge bands are larger than those of the outer ridge bands.
Rhythmic growth mechanism of ring banded morphologies
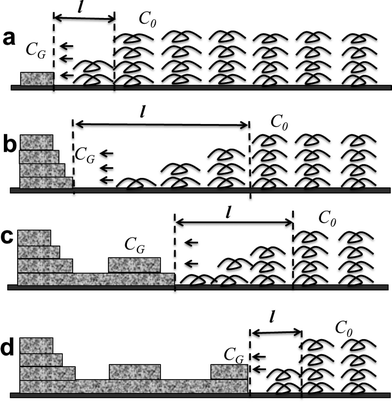
On the basis of the experimental results and the analysis, the birefringent and nonbirefringent concentric ring banded morphologies of PLLA with radial periodic variation of thicknesses could be attributed to a periodic diffusion-induced rhythmic growth mechanism. For the rhythmic growth mechanism, the formation of the concentric ring banded structures is associated with the amount of polymer chains per unit volume diffused to the growth front δ, which is determined by the diffusion flux of polymer chains in solution (J) and growth velocity at the growth front (V).23,24,43,44The variation of concentration is balanced via the decrease of polymer chains by crystal growth and the decrease of solvent by evaporation. Generally, the concentration of the whole solution (C0) is kept approximately constant during the whole process. The growth velocity is faster, and the depletion of polymer chains due to crystal growth cannot be balanced by diffusion of polymer chains adjacent to the growth front. Consequently, the concentration of polymer chains at the growth front (CG) sharply decreases. The development of the structure and morphology is predominated by diffusion of polymer chains and periodic variation of concentration gradient (G). We denote with l the diffusion length of polymer chains to the growth front along the radial growth direction. Accordingly, the concentration gradient G along the growth direction can be calculated as G = (C0 − CG)/l. Then, the diffusion flux of polymer chains (J) from the solution to the growth front is obtained according to Fick's first diffusion law, J = DG = D(C0 − CG)/l, where D is the diffusion coefficient of polymer chains in solution. Thus, the amount of polymer chains per unit volume diffused to the growth front δ can be calculated by δ = J/V = D(C0 − CG)/lV.
Then, the development of the concentric ring banded morphologies with periodic variation of thickness can be illustrated by schematics in Fig. 8. In the initial growth stage of the ridge band, the concentration at the growth front sharply decreases to zero, due to consumption of polymer chains by fast crystal growth. At the same time, the diffusion length l reaches its minimum, as shown in Fig. 8(a). Thus, the concentration gradient G reaches the maximum, G = C0/lmin. As has been previously discussed, the values of diffusion coefficient D and crystal growth velocity V are kept nearly constant during the whole growth process. Therefore, the amount of polymer chains per unit volume diffused to the growth front, δ = J/V = DC0/lminV, is the largest. Consequently, there are sufficient polymer chains at the growth front for further crystal growth. The ridge band in the concentric ring banded morphologies is formed as crystal lamellae growing upward and forward as shown in Fig. 8(b).
The consumption of polymer chains results in rapid decrease of δ. In order to supply more polymer chains, polymer chains from neighboring regions need to cover longer distance to the growth front, which makes l increase. Meanwhile, the increase in diffusion length (l) reduces the concentration gradient along the growth direction (G). Then, the amount of polymer chains is not enough for further crystal growth with constant thickness, and the lamellar crystals in the ridge band cannot grow any longer upward. As a result, the thickness of the ridge band reaches its maximum. As diffusion length (l) increases and concentration gradient (G) decreases, δ becomes smaller and smaller. The amount of polymer chains for further crystal growth becomes fewer and fewer, which cannot supply sufficient polymer chains for further growth with constant thickness, and the thickness of multilayers packed with lamellar crystals rapidly decreases. When concentration gradient G reaches its minimum, growth of most crystal lamellae terminates, and the layers of crystal lamellae stop thickening. Consequently, the valley band is obtained as shown in Fig. 8(c).
The decrease in thickness in the valley band reduces the consumption of polymer chains. The diffusion of polymer chains from neighboring regions gradually balanced the consumption of polymer chains, and the concentration gradient (G) restores to the initial stage. As concentration gradient (G) gradually increases and diffusion length (l) decreases, the amount of polymer chains diffused to the new growth front (δ) becomes sufficient for fast crystal growth. The thickness of the crystal layers increases, and another ridge band will be formed, as shown in Fig. 8(d).
Accordingly, the concentric ring banded spherulitic morphologies with alternating ridge and valley structures in the radial growth direction are produced through a repetition of the rhythmic growth process. The periodic variation of concentration gradient is responsible for the periodic variation of thickness in the concentric ring banded morphologies. The concentration gradient is much smaller than that in melt; thus, the diffusion length (l) of polymer chains in solution is much larger than that in melt. Consequently, the crystallization rate is much slower than that in melt, and the values of thicknesses of ridges and valleys, and band spacing are smaller than those in ring banded spherulites obtained in melt crystallization.
Conclusions
Concentric ring banded morphologies of PLLA grown from PLLA–chloroform systems were obtained via solvent micro-evaporation. These concentric ring banded morphologies are different from classical ring banded spherulites, which took a rhythmic growth mechanism due to the concentration gradient formed by diffusion and growth. In films of tens of microns in thickness, birefringent ring banded spherulites could be formed, and alternating bright and dark bands resulting from lamellar twisting are responsible for the radial periodic variation of thickness. In films of several microns in thickness, nonbirefringent concentric ring banded morphologies could be seen, and alternating ridges and valleys with different thicknesses are the reason for the radial periodic variation of thickness. Both birefringent and nonbirefringent forms take a similar initiation mode for the growth of lamellar crystals but take a different growth mechanism and packing mode of lamellar crystals.The development of the concentric ring banded morphologies is predominated by a periodic competition between diffusion and growth induced rhythmic growth mechanism, which is associated with periodic variation of concentration gradient in the growth direction. The parameters can be characterized as in the following equation: δ = J/V = D(C0 − CG)/lV. Alternating ridge and valley bands in the ring banded morphologies grow step by step from the center to the outside with periodic variation of concentration gradient.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (51103151, 51173130, 51073157, 51233004, 51021003, and 51073155).References
- A. Keller, J. Polym. Sci., 1955, 17, 351–364 CrossRef CAS.
- H. D. Keith, Macromolecules, 1982, 15, 114–122 CrossRef CAS.
- D. Maillard and R. E. Prud'homme, Macromolecules, 2008, 41, 1705–1712 CrossRef CAS.
- J. R. Sun, Z. K. Hong, L. X. Yang, Z. H. Tang, X. S. Chen and X. B. Jing, Polymer, 2004, 45, 5969–5977 CrossRef CAS PubMed.
- S. Y. Huang, S. C. Jiang, L. J. An and X. S. Chen, J. Polym. Sci., Part B: Polym. Phys., 2008, 46, 1400–1411 CrossRef CAS.
- C. L. He, J. R. Sun, C. Deng, T. Zhao, M. X. Deng, X. S. Chen and X. B. Jing, Biomacromolecules, 2004, 5, 2042–2047 CrossRef CAS PubMed.
- J. Xu, B. H. Guo, J. J. Zhou, L. Li, J. Wu and M. Kowalczuk, Polymer, 2005, 46, 9176–9185 CrossRef CAS PubMed.
- K. L. Singfield and G. R. Brown, Macromolecules, 1995, 28, 1290–1297 CrossRef CAS.
- M. Byun, S. W. Hong, L. Zhu and Z. Q. Lin, Langmuir, 2008, 24, 3525–3531 CrossRef CAS PubMed.
- Z. G. Wang, X. H. Wang, D. H. Yu and B. Z. Jiang, Polymer, 1997, 38, 5897–5901 CrossRef CAS.
- Z. G. Wang, L. J. An, B. Z. Jiang and X. H. Wang, J. Polym. Sci., Part B: Polym. Phys., 1999, 37, 2682–2691 CrossRef CAS.
- F. F. Xue, X. S. Chen, L. J. An, S. S. Funari and S. C. Jiang, Chin. J. Polym. Sci., 2013, 31(9), 1260–1270 CrossRef CAS PubMed.
- A. Keller, Nature, 1952, 31, 913–914 CrossRef.
- H. D. Keith and F. J. Padden, Polymer, 1984, 25, 28–42 CrossRef CAS.
- B. Lotz and S. Z. D. Cheng, Polymer, 2005, 46, 577–610 CrossRef CAS PubMed.
- R. M. Ho, K. Z. Ke and M. Chen, Macromolecules, 2000, 33, 7529–7537 CrossRef CAS.
- A. Toda, T. Arita and M. Hikosaka, Polymer, 2001, 42, 2223–2233 CrossRef CAS.
- J. M. Schultz, Polymer, 2003, 44, 433–441 CrossRef CAS.
- X. H. Wang, Z. G. Wang, W. Jiang, C. H. Liu, X. H. Zhang and B. Z. Jiang, Macromol. Rapid Commun., 1998, 19(2), 131–133 Search PubMed.
- T. Kyu, H. W. Chiu, A. J. Guenther, Y. Okabe, H. Saito and T. Inoue, Phys. Rev. Lett., 1999, 83, 2749–2752 CrossRef CAS.
- Y. X. Duan, Y. Jiang, S. D. Jiang, L. Li, S. K. Yan and J. M. Schultz, Macromolecules, 2004, 37, 9283–9286 CrossRef CAS.
- Y. Wang, C. M. Chan, L. Li and K. M. Ng, Langmuir, 2006, 22, 7384–7390 CrossRef CAS PubMed.
- Z. B. Wang, Z. J. Hu, Y. Z. Chen, Y. M. Gong, H. Y. Huang and T. B. He, Macromolecules, 2007, 40, 4381–4385 CrossRef CAS.
- Z. B. Wang, G. C. Alfonso, Z. J. Hu, J. Zhang and T. B. He, Macromolecules, 2008, 41, 7584–7595 CrossRef CAS.
- Z. H. Xu, Y. Q. Zhang, Z. G. Wang, N. Sun and H. Li, ACS Appl. Mater. Interfaces, 2011, 3(12), 4858–4864 CAS.
- M. Yasuniwa, S. Tsubakihara, K. Iura, Y. Ono, Y. Dan and K. Takahashi, Polymer, 2006, 47, 7554–7563 CrossRef CAS PubMed.
- S. Y. Huang, H. F. Li, S. C. Jiang, X. S. Chen and L. J. An, Polymer, 2011, 52, 3478–3487 CrossRef CAS PubMed.
- J. M. Zhang, K. Tashiro, H. Tsuji and A. J. Domb, Macromolecules, 2008, 41, 1352–1357 CrossRef CAS.
- J. M. Zhang, Y. X. Duan, H. Sato, H. Tsuji, I. Noda, S. K. Yan and Y. Ozaki, Macromolecules, 2005, 38, 8012–8021 CrossRef CAS.
- Y. Kikkawa, H. Abe, T. Iwata, Y. Iuoue and Y. Doi, Biomacromolecules, 2002, 3, 350–356 CrossRef CAS PubMed.
- H. Z. Song, Y. H. Niu, J. Yu, J. Zhang, Z. G. Wang and J. S. He, Soft Matter, 2013, 9, 3013–3020 RSC.
- S. Y. Huang, H. F. Li, Y. R. Shang, D. H. Yu, G. Li, S. C. Jiang, X. S. Chen and L. J. An, RSC Adv., 2013, 3, 13705–13711 RSC.
- H. Tsuji, Y. Tezuka, S. K. Saha, M. Suzuki and S. Itsuno, Polymer, 2005, 46, 4917–4927 CrossRef CAS PubMed.
- Y. Q. Zhang and Z. G. Wang, Chin. J. Polym. Sci., 2013, 31(9), 1276–1283 CrossRef CAS.
- Y. Yuryev, P. Wood-Adams, M. C. Heuzey, C. Dubois and J. Brisson, Polymer, 2008, 49, 2306–2320 CrossRef CAS PubMed.
- Y. Q. Zhang, H. J. Xu, J. J. Yang, S. Y. Chen, Y. S. Ding and Z. G. Wang, J. Phys. Chem. C, 2013, 117, 5882–5893 CAS.
- Y. Q. Zhang, Z. K. Wang, F. Jiang, J. Bai and Z. G. Wang, Soft Matter, 2013, 9, 5771–5778 RSC.
- G. Wegner, Macromol. Chem. Phys., 2003, 204, 347–357 CrossRef CAS.
- Z. Hu, F. Zhang, H. Y. Huang, M. Zhang and T. B. He, Macromolecules, 2004, 37, 3310–3318 CrossRef CAS.
- G. Reiter and J. U. Sommer, Phys. Rev. Lett., 1998, 80, 3771–3774 CrossRef CAS.
- G. Reiter and J. U. Sommer, J. Chem. Phys., 2000, 112, 4376–4383 CrossRef CAS.
- S. Nurkhamidah, E. M. Woo and K. Tashiro, Macromolecules, 2012, 45, 7313–7316 CrossRef CAS.
- H. K. Henisch, Crystals in Gels and Leisegang Rings, Cambridge University Press, Cambridge, UK, 1988 Search PubMed.
- Y. X. Duan, Y. Zhang, S. K. Yan and J. M. Schultz, Polymer, 2005, 46, 9015–9021 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2014 |