Reversible gelation of cells using self-assembling hydrophobically-modified biopolymers: towards self-assembly of tissue
Vishal
Javvaji†
a,
Matthew B.
Dowling†
b,
Hyuntaek
Oh
a,
Ian M.
White
b and
Srinivasa R.
Raghavan
*ab
aDepartment of Chemical and Biomolecular Engineering, University of Maryland, College Park, MD 20742-2111, USA. E-mail: sraghava@umd.edu
bFischell Department of Bioengineering, University of Maryland, College Park, MD 20742-2111, USA
First published on 5th March 2014
Abstract
Polymer hydrogels have long been used to hold and culture biological cells within their three-dimensional (3-D) matrices. Typically, in such cases, the cells are passively entrapped in a mesh of polymer chains. Here, we demonstrate an alternate approach where cells serve as active structural elements (crosslinks) within a polymer gel network. The polymers used in this context are hydrophobically modified (hm) derivatives of common biopolymers such as chitosan and alginate. We show that hm-polymers rapidly transform a liquid suspension of cells into an elastic gel. In contrast, the native biopolymers (without hydrophobes) do not cause such gelation. Gelation occurs because the hydrophobes on the polymer get embedded within the hydrophobic interiors of cell bilayer membranes. The polymer chains thus connect the cells into a 3-D sample-spanning network, with the cells serving as the junctions in the network. We demonstrate that a variety of cell types, including blood cells, endothelial cells, and breast cancer cells can be gelled by this approach. Cells gelled by hm-alginate are shown to remain viable within the network. Also, since the crosslinking mechanism is based on hydrophobic interactions, we show that the addition of supramolecules with hydrophobic binding pockets can reverse the gelation and release the cells. Cell-gels can be employed as injectable biomaterials since the connections in the network are susceptible to shear, but recover rapidly once shear is stopped. The overall approach provides a simple route towards the directed assembly of cell clusters and potentially to living tissue.
Introduction
Biological cells are the building blocks of all organisms. Most cells in complex organisms, however, do not exist as discrete entities—instead, they are usually assembled into functional higher-order structures, viz. tissues and organs.1–4 The connection of cells into tissue varies with tissue type. For example, cells could be close-packed via adhesive interactions, or they could be embedded within the extracellular matrix (ECM), which is a fibrous polymeric material that forms a gel-like network around the cells.1 In all cases, the resulting tissue is a squishy, gel-like material within which the cells are immobilized.Inspired by the natural assembly of complex biological tissues from cells and ECM, scientists have begun to explore whether cells can be assembled ex vivo to create tissue mimics.4–7 A recent review by Taguchi discusses the growing area of “cellular assembly” and describes various approaches in this regard.4 It is worth emphasizing that cellular assembly is at the heart of biomaterial and tissue engineering.6,7 The standard approach in tissue engineering is to culture cells within a polymeric hydrogel. In this case, the cells are passively entrapped as discrete structures in a three-dimensional (3-D) network of polymer chains.6,7 Direct connection or contact between adjacent cells occurs only at very high cell densities.
Rather than the above typical case of passively embedding cells in a polymer network, an alternative can be envisioned. That is, a network can be formed in which the cells serve as active structural elements (nodes or junctions or crosslinks), with the various nodes being connected (bridged) by polymer chains.8–11 In such a scenario, each polymer chain is attached simultaneously to two or more adjacent cells and together these elements build a sample-spanning 3-D network. One example of such cellular assembly was shown by Mooney et al.8 who synthesized a derivative of the polysaccharide alginate with grafted cell-adhesive peptides (containing the RGD, i.e., arginine-glycine-aspartic acid, motif). The polymer chains became bound to cells bearing RGD receptors via their RGD domains, and the net result was the formation of a network of cells bridged by polymer chains.
More recently, we and others have suggested that cell networking (gelling) can be induced in a simpler manner by hydrophobic interactions.9–11 In particular, we synthesized a hydrophobically modified (hm) derivative of the cationic aminopolysaccharide chitosan and combined this polymer with human blood.11 We showed that hm-chitosan was able to convert a liquid suspension of blood cells into an elastic gel. To explain this gelation, we hypothesized that the polymer chains inserted their hydrophobes into the lipid bilayers of blood cells (due to their mutual hydrophobic affinity).11–13 In turn, this resulted in a network with the cells acting as junction points between polymer chains (see schematic in Fig. 1b). In contrast to hm-chitosan, native chitosan (with no hydrophobes) did not cause such gelation.11
Based on the above findings, we hypothesized that hydrophobic interactions could be employed in a generic manner for gelation of cells, i.e. it would neither require specific cells nor a specific polymer backbone (as long as hydrophobes are grafted to the backbone). The present study seeks to test this hypothesis, i.e., whether this gelation mechanism can be extended to a variety of cells and to different hm-polymers. Towards this end, we have studied several kinds of cells, including human or bovine blood, endothelial cells, and breast cancer cells. We have used two different hm-polymers: the aforementioned hm-chitosan as well as a hydrophobically modified derivative of alginate (hm-alginate). We chose alginate because it is a biocompatible and bioresorbable polymer that is widely used in tissue engineering and also as an implantable biomaterial.14 The chemical structures of the hm-chitosan and hm-alginate employed here are shown in Fig. 1a.
Our results, as shown below, confirm the cell-gelling abilities of hm-polymers. Moreover, in the case of hm-alginate, we have assayed the viability of gelled cells and we find the cells to be mostly viable. We also demonstrate a unique aspect of hm-polymer-mediated gelation of cells, which is that such gelation can be readily reversed by introducing the sugar-based supramolecule α-cyclodextrin (α-CD).11 This is possible because the hydrophobic interactions that mediate gelation are weak, physical bonds. α-CD has a hydrophobic binding pocket that sequesters the hydrophobes present along the polymer and thereby eliminates the interaction between polymer chains and cells.11,15 Overall, cell-gelling (networking) by hm-polymers is shown to be a simple, benign process, with its reversibility being an added benefit. This approach could have wide utility, e.g., in biomedical applications such as wound healing, tissue sealing, and for the injectable delivery of cells. In the long term, it could also provide a route towards the directed assembly of cell clusters and tissues.
Experimental section
Materials
The polymers chitosan and alginate were obtained from Sigma-Aldrich. Chitosan (product number 448877) was of medium molecular weight (190![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000–310
000–310![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000) and its degree of deacetylation was 80%. Sodium alginate (product number A2033, from brown algae) had a molecular weight of 80
000) and its degree of deacetylation was 80%. Sodium alginate (product number A2033, from brown algae) had a molecular weight of 80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000–120
000–120![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000. The following chemicals were also purchased from Sigma-Aldrich: n-octylamine, n-dodecyl aldehyde, and N-(3-dimethylamino-propyl)-N′-ethylcarbodiimide hydrochloride (EDC). The supra-molecule α-cyclodextrin (α-CD) was obtained from TCI. Heparinized bovine blood was purchased from Lampire. Human umbilical vein endothelial cells (HUVEC) (product number: C2517A) were purchased from Lonza. Breast cancer cells (MCF7) were purchased from ATCC. For MCF7 cell culture, Dulbecco's modified eagle medium (DMEM) containing high glucose was purchased from Fisher Scientific, and this was supplemented with 10% fetal bovine serum (FBS) and 1% penicillin-streptomycin. For HUVEC cell culture, endothelial growth media (EGM-2) and supplement EGM-2 bulletkit were purchased from Lonza. Live/Dead® assay kit for mammalian cells was purchased from Invitrogen. All chemicals and materials were used as received without further purification.
000. The following chemicals were also purchased from Sigma-Aldrich: n-octylamine, n-dodecyl aldehyde, and N-(3-dimethylamino-propyl)-N′-ethylcarbodiimide hydrochloride (EDC). The supra-molecule α-cyclodextrin (α-CD) was obtained from TCI. Heparinized bovine blood was purchased from Lampire. Human umbilical vein endothelial cells (HUVEC) (product number: C2517A) were purchased from Lonza. Breast cancer cells (MCF7) were purchased from ATCC. For MCF7 cell culture, Dulbecco's modified eagle medium (DMEM) containing high glucose was purchased from Fisher Scientific, and this was supplemented with 10% fetal bovine serum (FBS) and 1% penicillin-streptomycin. For HUVEC cell culture, endothelial growth media (EGM-2) and supplement EGM-2 bulletkit were purchased from Lonza. Live/Dead® assay kit for mammalian cells was purchased from Invitrogen. All chemicals and materials were used as received without further purification.
Hydrophobically modified chitosan (hm-chitosan) synthesis
To make hm-chitosan with C12 hydrophobes, chitosan was reacted with n-dodecyl aldehyde following procedures described in the literature16,17 and in our earlier studies.11,18 The degree of hydro-phobic substitution follows the reaction stoichiometry18 and was fixed at 6 mol% of the available amine groups on the chitosan.Hydrophobically modified alginate (hm-alginate) synthesis
hm-Alginate with C8 hydrophobes was synthesized by an amidation reaction with n-octylamine using EDC as the coupling reagent. The synthesis procedure followed that described by Nystrom et al.19,20 The product was precipitated by adding acetone and separated by vacuum filtration. This purification step was repeated 5 times. The final product was recovered by vacuum drying at room temperature. The degree of hydrophobic modification was determined by 1H-NMR as described previously.201H NMR spectra were taken on a Bruker AVANCE 500 MHZ spectrometer. Spectra were referenced to the 3-trimethylsilylpropionic acid sodium salt-d4. The calculated degree of hydrophobic modification was 25 mol% of uronic acid residues in the alginate chains.Cell culture and cell gelation
For culture of MCF7 cells, high-glucose DMEM media supplemented with 5 μL mL−1 of penicillin-streptomycin and 10% fetal bovine serum (FBS) was used. For HUVEC cells, EGM-2 media was first completed with EGM-2 bulletkit (containing hydrocortisone, gentamicin-amphotericin-B, fetal bovine serum, growth factors, ascorbic acid, and heparin). Both cells were cultured separately in T75 flasks in a 37 °C incubator with 5% CO2. Cells were subcultured every 5–7 days by trypsinization with 0.25%/0.02% trypsin/EDTA. To prepare cells for gelation experiments, confluent cells were harvested from the T75 flask, centrifuged to a pellet and re-suspended in 0.5 mL of cell growth media or acetate-buffered saline (ABS). Cell gelation was studied by adding stock solutions of the corresponding polymer to the above cell suspension, followed by gentle mixing. In the case of chitosan and hm-chitosan, the polymers were dissolved in 0.15 M acetic acid. In the case of alginate and hm-alginate, the polymers were dissolved in saline solution (0.9 wt% NaCl) for osmotic balance.Rheological studies
A TA Instruments AR2000 stress-controlled rheometer was used to perform steady and dynamic rheological experiments. All experiments were done at 25 °C using a cone-and-plate geometry (40 mm diameter and 2° cone angle). A solvent trap was used to minimize drying of the sample during measurements. Dynamic frequency spectra were conducted in the linear viscoelastic regime of the samples, as determined by prior dynamic strain sweeps.Cyclodextrin gel reversal
A stock solution of 10 wt% α-CD was prepared in deionized (DI) water and a small volume (200 μL) of this solution was added to 2 mL of the cell-containing gel, followed by vortex mixing. Adding just a small volume of the α-CD solution ensured that the gel was negligibly diluted in the process.Live-dead assay
A solution containing 4 μM of live (calcien-AM) and dead (ethidium homodimer) assay reagent was prepared in PBS. To stain the cells, 10 μL of this solution was added to the cell-gel, incubated at room temperature for 15 min and then imaged on a confocal microscope (Leica SP5 X). For imaging calcein-AM, the excitation was done at 495 nm and emitted light was recorded using a 505–554 nm band-pass filter. For imaging ethidium homodimer, the excitation was done at 556 nm and imaging was done with a 568–700 nm band pass filter. Cells were imaged within successive optical slices of 1.3 μm thickness to visualize live cells (stained green) and dead cells (stained red) along different planes of the sample. Projections of these images along the XY plane (top view) and XZ plane (cross-sectional view) were then constructed using the Leica Application Suite. All images were obtained within 1 h of sample preparation.Results and discussion
Cell gelation with hm-chitosan
Previously, we have demonstrated that hm-chitosan was capable of gelling the cells in whole human blood.11 Here, we show that the same polymer is able to gel other biological cells as well. Specifically, we began with a suspension of HUVEC cells in ABS and to this we added a solution of hm-chitosan (with C12 hydrophobes). The concentration of cells in the final sample was 1.35 × 105 cells mL−1 while the concentration of polymer was 0.5 wt% (i.e., about 5 mg mL−1). The sample immediately transformed into a self-supporting gel, as shown by Photo 1 in Fig. 2. We observe from the photograph that the gel is able to hold its weight upon vial inversion, which shows that it has a substantial yield stress.21 Note also that the cell-gel remains a homogeneous, transparent material with no evidence of turbidity, precipitation, or phase separation. For comparison, an identical sample of HUVEC cells was combined with 0.5% of the native chitosan (lacking any hydrophobes). In this case, the sample remained a freely flowing liquid, as shown by Photo 2 in Fig. 2. | ||
| Fig. 2 HUVEC cell gelation by hm-chitosan. A mixture of HUVEC cells and hm-chitosan (0.5 wt%) forms a homogeneous, self-supporting gel that holds its weight in the inverted vial (Photo 1). In contrast, a mixture of the same cells and chitosan (0.5 wt%) is a freely flowing liquid (Photo 2). The plot shows dynamic rheological data for the elastic modulus G′ (red circles) and the viscous modulus G′′ (green triangles) as functions of the frequency ω. The hm-chitosan/HUVEC sample (closed symbols) shows an elastic, gel-like response, consistent with Photo 1. On the other hand, the chitosan/HUVEC sample (open symbols) responds like a viscous solution, consistent with Photo 2. The schematics indicate that the hm-chitosan connects cells into a network (as in Fig. 1b) whereas the chitosan does not. | ||
To quantify the rheological differences between the above two samples, dynamic rheological measurements were performed. The data plotted in Fig. 2 are for the elastic (G′) and viscous (G′′) moduli as functions of the angular frequency ω. For the hm-chitosan/HUVEC sample, G′ exceeds G′′ over the entire frequency range and both moduli are weakly dependent on frequency. This is the rheological signature of a gel-like sample.22 The frequency-independent plateau in G′ at low frequencies is indicative of a sample-spanning network. On the other hand, for the chitosan/HUVEC sample, G′′ exceeds G′ over the frequency range and both moduli exhibit a strong dependence on frequency. This rheological response is indicative of a viscous or liquid-like sample.22 Overall, the rheological data support the visual observations. We should point out that a solution of 0.5% hm-chitosan is not a gel on its own,18i.e., the gel is formed only when the cells are combined with hm-chitosan. Based on our previous work, the likely mechanism for gelation is that the hydrophobes from hm-chitosan chains insert into the bilayers of HUVEC cells, resulting in the cells being bridged by polymer chains into a 3-D network.11,18 A schematic of such a network was shown earlier in Fig. 1 and is indicated again in Fig. 2. Our observations with gelling two different types (and sizes) of cells, i.e., blood cells11 and HUVECs, suggests that hm-chitosan can act as a generic gelator for a variety of cell types.
Cell gelation with hm-alginate
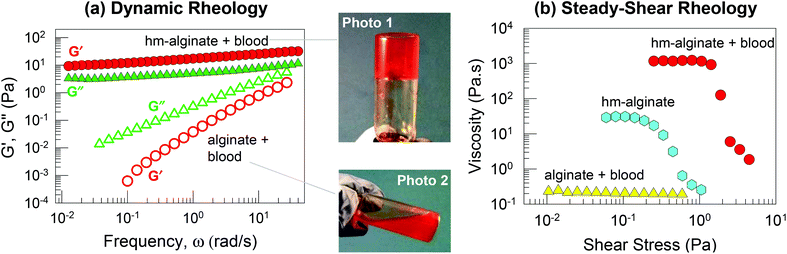
Next, we explored cell gelation using hm-alginate (having C8 hydrophobes). One advantage of alginate derivatives including hm-alginate is that they can be dissolved in water at physiological pH whereas chitosan derivatives are soluble only under mildly acidic conditions. Our initial studies were done with heparinized bovine blood; note that heparin deactivates thrombin and thereby ensures that the blood will not undergo the natural clotting process. To this blood, we added a solution of hm-alginate so that the concentration of polymer in the overall sample was 0.93 wt%. For comparison, a second sample was made by combining the same blood with an identical concentration of the parent alginate polymer. The mixture of hm-alginate and blood quickly formed a gel that supported its weight in the inverted vial, as shown by Photo 1 in Fig. 3. In contrast, the mixture of alginate and blood remained a freely flowing liquid (similar to the blood alone), as indicated by Photo 2 in Fig. 3.Rheological data confirm the differences between the above samples. Fig. 3a presents data from dynamic rheology. The hm-alginate/blood sample shows the rheological signature of a weak gel, as noted earlier in Fig. 3: i.e., G′ > G′′ over the frequency range, with both moduli showing negligible variation with frequency. On the other hand, the alginate/blood sample exhibits a viscous response: both moduli vary sharply with frequency and G′′ > G′ at all frequencies. Next, Fig. 3b presents data from steady-shear rheology on the same samples. Here, the apparent viscosity is plotted as a function of shear stress. The alginate/blood sample displays a constant viscosity of ∼0.2 Pa s−1 (Newtonian response). In contrast, the hm-alginate/blood sample shows a shear-thinning (non-Newtonian) response, with a high viscosity at low shear-stresses followed by a decrease in viscosity at higher shear stresses. The zero-shear viscosity, i.e., the viscosity in the low-shear limit, is ∼1000 Pa s−1, which is four orders of magnitude higher than that of the alginate/blood sample. The steep drop in viscosity around a stress of 2 Pa is indicative of a yield stress in the material, i.e., the sample flows negligibly at stresses below this value, consistent with its ability to hold its weight under vial inversion. Also, it should be noted that 0.93 wt% hm-alginate alone (without blood) is not a gel; rather its rheological profile (Fig. 3b) is indicative of a viscous liquid with moderate shear-thinning.20,23 The above data show that gelation of blood can be induced by hm-alginate (but not native alginate). Once again, this implies a cell network bridged by hm-alginate chains, with the driving force being the affinity between the hydrophobes on the polymer and cellular bilayers (Fig. 1).
To demonstrate the ability of hm-alginate to gel a variety of cells, we tested two cell types, HUVECs and mammalian breast cancer cells (MCF7). Hm-alginate was able to gel both types of cells and results are shown here for MCF7. Initially, a freely flowing suspension of MCF7 cells was placed in a tube and to this a solution of hm-alginate in PBS was added. The concentration of cells in the overall mixture was 1.15 × 107 cells mL−1 while the concentration of polymer was 0.93 wt%. Upon addition of hm-alginate, the cell suspension was transformed into a gel that did not flow in the inverted tube, as can be seen from the photograph in Fig. 4a. When this gel was observed under optical microscopy, clusters of cells were evident (Fig. 4b) and it appears that the cells are connected into a 3-D network, consistent with the schematic in Fig. 1. In contrast, a mixture of MCF7 cells with the native alginate (at identical concentrations of cells and polymer) remained a freely flowing liquid. Dynamic rheological data (Fig. 4c) again confirm the differences between MCF7 samples containing hm–alginate and alginate. The former shows the signature of a gel (G′ > G′′, both nearly independent of frequency) whereas the latter shows the response of a viscous liquid (G′′ > G′, both varying with frequency). Thus, our results confirm the ability of hm-alginate to act as a generic gelator for various cells, similar to hm-chitosan.
The above results can be generalized in several ways. On the one hand, we expect other hydrophobically modified biopolymers to also be capable of inducing cell gelation by an identical mechanism.9,10 More generally, such gelation should be possible for any structures covered with a lipid membrane (bilayer), since hydrophobic moieties have a propensity to embed in these membranes.12,13 Vesicles and liposomes are self-assembled containers having a bilayer membrane enclosing an aqueous core. Previously, we have induced gelation of vesicles using either hm-chitosan18,24–26 or hm-alginate.27 Hydrophobic interactions were shown to be the key factor in these cases as well; specifically, gelation did not occur if the polymer chains did not bear hydrophobes. Additional support for such a mechanism has been provided using techniques such as small-angle neutron scattering (SANS)18,24 and cryo-transmission electron microscopy (cryo-TEM).25,26 One should also note that gelation requires a critical density of nodes (i.e., vesicles or cells) as well as connectors (i.e., polymer chains) in the sample.13,18 The effect of vesicle and hm-chitosan concentrations on gelation has been reported before in the form of a “phase map” that distinguishes regions corresponding to elastic gels from regions corresponding to viscoelastic solutions.18 We expect similar phase maps to exist for the case of cells as well. One additional factor with cells is that the cell morphology (size and shape) varies from one type of cell to another, and so the results will be unique to each cell type.
Reversal of hm-alginate-induced cell gelation
A distinct advantage of cell (or vesicle) gelation by hm-polymers is that it is based on weak, non-covalent interactions and hence can be reversed by addition of species with hydrophobic binding pockets.15 Reversal of gelation has been shown in previous studies with blood11 as well as with vesicles.26 We demonstrate this now for the case of MCF7 cells gelled by hm-alginate. To the gel from Fig. 4, we add the supramolecule α-cyclodextrin (α-CD) at a concentration of 0.91 wt% (9.4 mM). As shown in Fig. 5, the gel (Photo 1) is immediately transformed into a freely flowing liquid (Photo 2). This result is corroborated by dynamic rheology (Fig. 5b). The initial sample has a gel-like response whereas, after addition of α-CD, the sample shows a viscous response (G′′ > G′, both varying with frequency). The mechanism for the reversal of gelation is shown by the schematics in Fig. 5a and b. Initially, the hydrophobes (red tails) along the polymer chains are embedded in cell membranes. When the α-CD is added, the hydrophobic tails instead become sequestered within the hydrophobic binding pockets of α-CD molecules (shown in orange).11,15 The polymer chains are thus prevented from interacting with the cell membranes. In turn, because the cells are no longer bridged by polymer chains, the gel is converted to a liquid, i.e., the cells are released. Note that the ability to reverse gelation through α-CD further substantiates the fact that hydrophobic interactions are responsible for the gelation in the first place.11 Similar results on reversal of gelation were observed in all the cases mentioned above, i.e., regardless of cell type or the type of hm-polymer. We should also note that the size of the binding pocket in α-CD is such that it effectively binds the single-tailed hydrophobes on our hm-polymers; however, it is not large enough to bind the twin-tails on lipids that constitute cell membranes.15 Thus, the use of α-CD to reverse cell-gels constitutes a simple, benign, and biocompatible approach.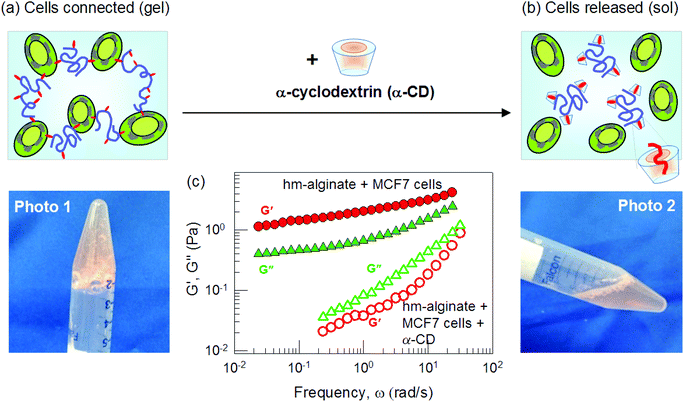 | ||
| Fig. 5 Reversal of hm-alginate-induced cell gelation by α-cyclodextrin (α-CD). A cell-gel of MCF7 cells and hm-alginate (0.93 wt%) is shown in Photo 1 (same as in Fig. 4). When 0.91 wt% α-CD is added, the sample is converted into a flowing liquid (Photo 2). These observations are supported by dynamic rheological data in (c) for the elastic modulus G′ (red circles) and the viscous modulus G′′ (green triangles) as functions of the frequency ω. The initial sample (filled symbols) shows a gel-like response whereas upon addition of α-CD (open symbols), the response is that of a viscous sol. The schematics in (a) and (b) illustrate the mechanism for this reversal. The α-CD supramolecule has a barrel shape with an inner hydrophobic pocket. When added to the cell-gel, these supramolecules sequester the hydrophobic tails present on the polymer chains. In turn, the connections between the cells are eliminated and thereby the gel is converted to a liquid. | ||
Viability of cells in cell-gels
A key remaining question is the fate of the cells within a cell gel, i.e., do the cells continue to remain viable even as they serve as the nodes in a 3-D polymer-bridged network? To assess the effect of gelation on cell viability, we performed live-dead assays on hm-alginate-MCF7 cell-gels (composition identical to that in Fig. 5 and 6). Calcein-AM was used as the live stain and ethidium homodimer as the dead stain. Following exposure of cells to these stains, confocal microscopy was used to image the resulting fluorescence after an incubation time of 1 h (Fig. 6). Live cells are indicated by their green fluorescence due to conversion of calcein AM to calcein by intracellular esterases. Conversely, red fluorescence is indicative of dead cells due to permeation of ethidium through their membranes followed by intercalation of this dye within nucleic acids. Confocal microscope images at different optical sections of the gel reflect a superposition of green and red fluorescence. Fig. 6a shows a projection of multiple optical sections captured at different heights of the cell gel. Fig. 6b shows a projection of the same images on the XZ plane. We find that the vast majority of the cells are stained green whereas only a few cells are stained red. This demonstrates that gelation of MCF7 cells by hm-alginate is benign to the cells over the period examined. The images also indicate the presence of a 3-D network of cells throughout the sample. Although more extensive studies on cell viability (over longer timescales) need to be conducted, our initial results from this live-dead assay do suggest that cell gelation by hm-polymers is a benign process. The ability to form gels of cells in this manner by self-assembly (and also to reverse the gelation, on demand) could prove to be useful in biomedical engineering. For example, cell-gels could serve as injectable biomaterials. The shear-thinning property of cell-gels (see Fig. 4) should allow them to be injectable by a syringe. Once the gel is ejected from the tip of the syringe needle, we have found that it reforms and reverts to its initial gel state within seconds, as confirmed by rheological studies (data not shown). This property ensures that an injected gel will remain localized at the site of injection.Conclusions
We have shown that a variety of cells can be gelled by hm-biopolymers (hm-chitosan and hm-alginate). These hydrophilic biopolymers with hydrophobic grafts attached along their backbone have the ability to transform freely flowing cell suspensions into elastic gels. The gelling mechanism is based on the insertion of hydrophobes on the polymers into the lipid-rich cellular membrane, which results in the bridging of cells into a physical 3-D network. Such cell gelation occurs without the need of an external crosslinking reagent because the functional components (cells) also serve as active structural components (nodes or junction points) in the network. An additional and unique advantage of cell gelation with hm-polymers is that the cells can be detached from the gel by introduction of the supramolecule α-CD, i.e., without the use of enzymes or harsh oxidizing or reducing agents. Furthermore, the process of cell-gelation with hm-alginate is benign to cells, as demonstrated by a live-dead cell assay. Owing to the generality of hydrophobe–cell–membrane interactions, we expect that the gelation mechanism demonstrated here can be extended to other hydrophobically modified biopolymers and also to gel a variety of cell types. The use of hm-polymers for cell-gelation may find several significant biological applications, e.g. in 3-D cell culture, injectable cell gels (for cellular therapy), tissue sealants and hemostatic materials, and in tissue engineering.Acknowledgements
This work was partially funded by grants from the TEDCO MII program and from NSF. We acknowledge Prof. John Fisher (Bioengineering) and Dr Amy Beaven (Imaging Core Facility) for assistance with optical and confocal microscopy. We also acknowledge undergraduate students Feili Huang and Mark Keibler for assistance with some of the experiments.References
- B. Alberts, Molecular Biology of the Cell, Garland Publishers, 2002 Search PubMed.
- G. M. Whitesides and B. Grzybowski, Self-assembly at all scales, Science, 2002, 295, 2418–2421 CrossRef CAS PubMed.
- G. F. Payne, E. Kim, Y. Cheng, H.-C. Wu, R. Ghodssi, G. W. Rubloff, S. R. Raghavan, J. N. Culver and W. E. Bentley, Accessing biology's toolbox for the mesoscale biofabrication of soft matter, Soft Matter, 2013, 9, 6019–6032 RSC.
- T. Taguchi, Assembly of cells and vesicles for organ engineering, Sci. Technol. Adv. Mater., 2011, 12 Search PubMed.
- J. Yang, M. Yamato, C. Kohno, A. Nishimoto, H. Sekine, F. Fukai and T. Okano, Cell sheet engineering: Recreating tissues without biodegradable scaffolds, Biomaterials, 2005, 26, 6415–6422 CrossRef CAS PubMed.
- R. Langer and J. P. Vacanti, Tissue engineering, Science, 1993, 260, 920–926 CAS.
- B. O. Palsson and S. N. Bhatia, Tissue Engineering, Pearson Prentice Hall, 2004 Search PubMed.
- K. Y. Lee, H. J. Kong, R. G. Larson and D. J. Mooney, Hydrogel formation via cell crosslinking, Adv. Mater., 2003, 15, 1828–1832 CrossRef CAS.
- W. Meier, J. Hotz and S. GuntherAusborn, Vesicle and cell networks: Interconnecting cells by synthetic polymers, Langmuir, 1996, 12, 5028–5032 CrossRef CAS.
- M. Ito and T. Taguchi, Enhanced insulin secretion of physically crosslinked pancreatic beta-cells by using a poly(ethylene glycol) derivative with oleyl groups, Acta Biomater., 2009, 5, 2945–2952 CrossRef CAS PubMed.
- M. B. Dowling, R. Kumar, M. A. Keibler, J. R. Hess, G. V. Bochicchio and S. R. Raghavan, A self-assembling hydrophobically modified chitosan capable of reversible hemostatic action, Biomaterials, 2011, 32, 3351–3357 CrossRef CAS PubMed.
- C. Tribet and F. Vial, Flexible macromolecules attached to lipid bilayers: Impact on fluidity, curvature, permeability and stability of the membranes, Soft Matter, 2008, 4, 68–81 RSC.
- F. E. Antunes, E. F. Marques, M. G. Miguel and B. Lindman, Polymer-vesicle association, Adv. Colloid Interface Sci., 2009, 147–48, 18–35 CrossRef PubMed.
- K. Y. Lee and D. J. Mooney, Alginate: Properties and biomedical applications, Prog. Polym. Sci., 2012, 37, 106–126 CrossRef CAS PubMed.
- R. Kumar and S. R. Raghavan, Thermothickening in solutions of telechelic associating polymers and cyclodextrins, Langmuir, 2010, 26, 56–62 CrossRef CAS PubMed.
- J. Desbrieres, C. Martinez and M. Rinaudo, Hydrophobic derivatives of chitosan: Characterization and rheological behaviour, Int. J. Biol. Macromol., 1996, 19, 21–28 CrossRef CAS.
- M. Larsson, W. C. Huang, M. H. Hsiao, Y. J. Wang, M. Nyden, S. H. Chiou and D. M. Liu, Biomedical applications and colloidal properties of amphiphilically modified chitosan hybrids, Prog. Polym. Sci., 2013, 38, 1307–1328 CrossRef CAS PubMed.
- J. H. Lee, J. P. Gustin, T. H. Chen, G. F. Payne and S. R. Raghavan, Vesicle-biopolymer gels: Networks of surfactant vesicles connected by associating biopolymers, Langmuir, 2005, 21, 26–33 CrossRef CAS PubMed.
- C. Galant, A. L. Kjoniksen, G. T. M. Nguyen, K. D. Knudsen and B. Nystrom, Altering associations in aqueous solutions of a hydrophobically modified alginate in the presence of beta-cyclodextrin monomers, J. Phys. Chem. B, 2006, 110, 190–195 CrossRef CAS PubMed.
- H. T. Bu, A. L. Kjoniksen, K. D. Knudsen and B. Nystrom, Effects of surfactant and temperature on rheological and structural properties of semidilute aqueous solutions of unmodified and hydrophobically modified alginate, Langmuir, 2005, 21, 10923–10930 CrossRef CAS PubMed.
- S. R. Raghavan and B. H. Cipriano, Gel formation: Phase diagrams using tabletop rheology and calorimetry, in Molecular Gels, ed. R. G. Weiss and P. Terech, Springer, Dordrecht, 2005, pp. 233–244 Search PubMed.
- R. G. Larson, The Structure and Rheology of Complex Fluids, Oxford University Press, Oxford, 1999 Search PubMed.
- S. Choudhary and S. R. Bhatia, Rheology and nanostructure of hydrophobically modified alginate (HMA) gels and solutions, Carbohydr. Polym., 2012, 87, 524–530 CrossRef CAS PubMed.
- J. H. Lee, V. Agarwal, A. Bose, G. F. Payne and S. R. Raghavan, Transition from unilamellar to bilamellar vesicles induced by an amphiphilic biopolymer, Phys. Rev. Lett., 2006, 96, 048102 CrossRef.
- J. H. Lee, H. Oh, U. Baxa, S. R. Raghavan and R. Blumenthal, Biopolymer-connected liposome networks as injectable biomaterials capable of sustained local drug delivery, Biomacromolecules, 2012, 13, 3388–3394 CrossRef CAS PubMed.
- Y. J. Chen, V. Javvaji, I. C. MacIntire and S. R. Raghavan, Gelation of vesicles and nanoparticles using water-soluble hydrophobically modified chitosan, Langmuir, 2013, 29, 15302–15308 CrossRef CAS PubMed.
- H. Oh, V. Javvaji, N. A. Yaraghi, L. Abezgauz, D. Danino and S. R. Raghavan, Light-induced transformation of vesicles to micelles and vesicle-gels to sols, Soft Matter, 2013, 9, 11576–11584 RSC.
Footnote |
| † Equal contribution. |
| This journal is © The Royal Society of Chemistry 2014 |