A peptide functionalized poly(ethylene glycol) (PEG) hydrogel for investigating the influence of biochemical and biophysical matrix properties on tumor cell migration†
Samir P.
Singh
a,
Michael P.
Schwartz
b,
Justin Y.
Lee
a,
Benjamin D.
Fairbanks‡
a and
Kristi S.
Anseth
*ac
aDepartment of Chemical and Biological Engineering, University of Colorado, Boulder, CO, USA. E-mail: kristi.anseth@colorado.edu; Fax: +1 303-735-0095; Tel: +1 303-492-7471
bDepartment of Orthopedics and Rehabilitation, University of Wisconsin-Madison, Madison, WI, USA
cHoward Hughes Medical Institute, University of Colorado, Boulder, CO, USA
First published on 22nd April 2014
Abstract
To address the challenges associated with defined control over matrix properties in 3D cell culture systems, we employed a peptide functionalized poly(ethylene glycol) (PEG) hydrogel matrix in which mechanical modulus and adhesive properties were tuned. An HT-1080 human fibrosarcoma cell line was chosen as a model for probing matrix influences on tumor cell migration using the PEG hydrogel platform. HT-1080 speed varied with a complex dependence on both matrix modulus and Cys-Arg-Gly-Asp-Ser (CRGDS) adhesion ligand concentration, with regimes in which motility increased, decreased, or was minimally altered being observed. We further investigated cell motility by forming matrix interfaces that mimic aspects of tissue boundaries that might be encountered during invasion by taking advantage of the spatial control of the thiol–ene photochemistry to form patterned regions of low and high cross-linking densities. HT-1080s in 100 Pa regions of patterned PEG hydrogels tended to reverse direction or aggregate at the interface when they encountered a 360 Pa boundary. In contrast, HT-1080s were apparently unimpeded when migrating from the stiff to the soft regions of PEG peptide hydrogels, which may indicate that cells are capable of “reverse durotaxis” within at least some matrix regimes. Taken together, our results identified matrix regimes in which HT-1080 motility was both positively and negatively influenced by cell adhesion or matrix modulus.
Introduction
Metastasizing tumor cells migrate away from the primary tumor, undergo lymphatic or haemotogenous spreading, and implant within a tissue distant from the primary tumor.1–3 Thus, a migratory tumor cell experiences a spatially varied myriad of microenvironmental cues as cells move between tissue compartments. Tissue density and biochemical composition, as well as interfaces between tumors and surrounding tissue, are important factors during tumor progression. Investigating how these properties contribute to invasion is difficult when using traditional 3D platforms such as collagen or Matrigel, which contain endogenous signaling sites, and thus directly couple biomechanical and biochemical properties. Further, naturally-derived culture materials offer less control over material properties, as they suffer from batch-to-batch variability, are often ill-defined,4 and depend on extraction techniques5 or environmental conditions during gel formation.6 For instance, mechanical properties of collagen vary widely depending on the conditions used to form gels.6–8 It has been reported that a maximum storage modulus <200 Pa is achieved when formed at standard incubation temperature for cell culture (37 °C), even when using a higher range of densities possible for commercially available solutions.6 Therefore, there is growing motivation to develop new strategies to complement studies using naturally-derived culture materials with platforms that provide stricter control over individual extracellular matrix (ECM) properties.To circumvent limitations associated with naturally derived materials, synthetic bioinert hydrogels have been employed for cellular studies. Synthetic PEG hydrogels have been formed by a variety of methods, including free radical polymerization,9 Michael type addition,10 and “click chemistries”.11–13 Synthetic hydrogels have also been functionalized to model critical properties of the extracellular matrix. Matrix metalloproteinase (MMP) degradable peptide cross-linkers allow for matrix remodeling and adhesion peptides for cell attachment, making hydrogels suitable for studies with numerous cell types.10,13–26 Photoinitiated polymerization strategies also provide researchers with temporal and spatial control suitable for mechanical and/or chemical patterning within discrete regions of PEG hydrogels11–13,22,27–29 or in situ changes in matrix mechanics,30–33 a property that spontaneous polymerization chemistries such as Michael type addition do not possess. Therefore, while naturally derived materials capture complex features of the ECM, synthetic hydrogels provide a complementary tool for investigating a wide range of questions important for regenerative medicine and disease due to more precise control over biochemical and biophysical matrix properties.
We recently developed a 3D culture platform13 using a thiol–ene “click” chemistry34 to form peptide functionalized poly(ethylene glycol) hydrogels, which is flexible towards incorporation of any biomolecule containing a thiol group and has been used to investigate a variety of biological questions.13–19,21,35,36 Peptide functionalized PEG hydrogels formed through thiol–ene photopolymerizations provide several advantages as a 3D culture platform, including (1) homogeneous network formation, through a step-growth mechanism, (2) cytocompatible polymerization conditions, (3) facile biomolecule incorporation, and (4) the ability to spatially control the reaction through standard lithographic processes.13 The goal of this work was to demonstrate the utility of peptide-functionalized PEG hydrogels for investigating ECM influences on tumor cell migration. We observed that both adhesion and matrix modulus positively and negatively contribute to cell migration, where a required minimum threshold of adhesion is required for cell motility and a maximum matrix density exists in which cells cannot migrate. Finally, when cells encountered a mechanical interface in which regions of varied modulus were photopatterned within the same hydrogel, they were able to penetrate from the rigid to compliant regions, but not vice versa, suggesting a “reverse durotaxis” mechanism.
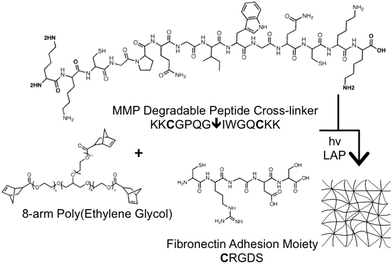
Results
We aimed to demonstrate the benefits of synthetic control provided by peptide functionalized poly(ethylene glycol) (PEG) hydrogels to systematically investigate matrix influences on cell function. PEG hydrogels were formed through photoinitiated coupling of thiol groups within cysteine-containing peptides and alkene groups of norbornene-terminated 8-arm poly(ethylene glycol) (PEG) monomers. The hydrogels were formulated to include a matrix metalloproteinase (MMP)-degradable37 cross-linking peptide flanked with cysteines and a pendant RGD (arginine-glycine-aspartic acid)-containing adhesion peptide terminated with a cysteine.38,39 The thiol–ene reaction proceeds through a radical mediated step growth polymerization upon exposure to 365 nm light in the presence of a photoinitiator (lithium phenyl-2,4,6-trimethylbenzoylphosphinate, or LAP) (Fig. 1). Migration is restricted to proteolytic mechanisms when cells are encapsulated in peptide-functionalized PEG hydrogels due to a mesh size on the order of 10 s of nm.13–15 Therefore, peptide-functionalized PEG hydrogels are well-suited for investigating proteolytic migration modes and provide a homogenous scaffold in which modulus and adhesion can be investigated while minimizing confounding effects due to porosity or fibril structure.For synthetic PEG hydrogels, storage modulus (G′) directly correlates with cross-linking density (ρx), which is calculated based on experimental variables of mechanical properties including mass swelling ratio (q) and G′ (Fig. 2B). Using an 8-arm (40 kDa) PEG backbone, G′ can be manipulated through varying the –ene concentration, while maintaining a constant thiol![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ene ratio. Thus, G′ can be tuned to a large range of values based on the concentration of functional groups. For example, hydrogels were formed with storage moduli of ∼1–40 kPa by simply changing the total –ene concentration from 5–50 mM (Fig. 2A). Alternatively, G′ can be modulated by varying the network cross-linking density while maintaining a constant 8-arm PEG backbone molecule concentration (i.e., constant –ene concentration) and changing the stoichiometric ratio of thiol relative to norbornene groups. Using a 6 mM –ene concentration, the amount of MMP sensitive cross-linker was varied to control the matrix modulus. Rheological measurements determined that the stoichiometric ratio of 0.50
ene ratio. Thus, G′ can be tuned to a large range of values based on the concentration of functional groups. For example, hydrogels were formed with storage moduli of ∼1–40 kPa by simply changing the total –ene concentration from 5–50 mM (Fig. 2A). Alternatively, G′ can be modulated by varying the network cross-linking density while maintaining a constant 8-arm PEG backbone molecule concentration (i.e., constant –ene concentration) and changing the stoichiometric ratio of thiol relative to norbornene groups. Using a 6 mM –ene concentration, the amount of MMP sensitive cross-linker was varied to control the matrix modulus. Rheological measurements determined that the stoichiometric ratio of 0.50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.0 (thiol
1.0 (thiol![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ene) resulted in hydrogels with G′ = 60 Pa. Increasing this ratio to 0.55
ene) resulted in hydrogels with G′ = 60 Pa. Increasing this ratio to 0.55![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.0 or 0.60
1.0 or 0.60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.0 resulted in a more cross-linked hydrogel with G′ = 120 Pa and 250 Pa, respectively, a range that is often studied in collagen and which has been reported for soft tissues in vivo (Fig. 2B and C).40
1.0 resulted in a more cross-linked hydrogel with G′ = 120 Pa and 250 Pa, respectively, a range that is often studied in collagen and which has been reported for soft tissues in vivo (Fig. 2B and C).40
A particular advantage of tuning matrix modulus by changing cross-linking density through altering the thiol![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ene ratio, while maintaining a constant –ene concentration is that the norbornene arms not utilized in cross-linking can be functionalized with pendant biomolecules at defined concentrations without substantially altering mechanical properties (Fig. 2B). Due to the importance of adhesion for cell migration,41–44 PEG hydrogels were functionalized with peptides containing a fibronectin derived adhesion ligand (CRGDS).39 A non-bioactive scrambled peptide (CRDGS) was also incorporated to maintain a constant total pendant peptide concentration (CRGDS + CRDGS), thus limiting non-specific effects due to the presence of charged chemical functional groups.45,46 Importantly, hydrogels could be tuned for a wide range of adhesion properties by altering CRGDS concentration (0.0–1.9 mM CRGDS, adjusted concentration, see Methods) without substantially altering matrix modulus (Fig. 2C). Therefore, synthetic ECM formed through thiol–ene chemistries provide a 3D cell culture platform that is well suited for investigating matrix influences on cell function by enabling systematic control over critical matrix properties, such as adhesion and modulus, while minimizing other confounding factors.
ene ratio, while maintaining a constant –ene concentration is that the norbornene arms not utilized in cross-linking can be functionalized with pendant biomolecules at defined concentrations without substantially altering mechanical properties (Fig. 2B). Due to the importance of adhesion for cell migration,41–44 PEG hydrogels were functionalized with peptides containing a fibronectin derived adhesion ligand (CRGDS).39 A non-bioactive scrambled peptide (CRDGS) was also incorporated to maintain a constant total pendant peptide concentration (CRGDS + CRDGS), thus limiting non-specific effects due to the presence of charged chemical functional groups.45,46 Importantly, hydrogels could be tuned for a wide range of adhesion properties by altering CRGDS concentration (0.0–1.9 mM CRGDS, adjusted concentration, see Methods) without substantially altering matrix modulus (Fig. 2C). Therefore, synthetic ECM formed through thiol–ene chemistries provide a 3D cell culture platform that is well suited for investigating matrix influences on cell function by enabling systematic control over critical matrix properties, such as adhesion and modulus, while minimizing other confounding factors.
We validated the use of PEG hydrogels formed via the thiol–ene chemistry by investigating the role for extracellular matrix properties on migration for HT-1080 fibrosarcoma cells (HT-1080s), a widely studied model cell type for investigating tumor cell motility.5,14,15,22,35,47–52 We compared measures of cell motility and morphology for HT-1080s embedded in either MMP-degradable PEG hydrogels or collagen, which is a common naturally derived material used to investigate HT-1080 migration.5,47,48,51,52 HT-1080s adopted similar morphologies and migrated in collagen (3.5 mg mL−1) similarly to PEG hydrogels (e.g., 60 Pa, 620 μM RGD), with a comparable fraction of migrating cells (87 ± 5% in collagen vs. 82 ± 4% in PEG hydrogels) and cell speed (39 ± 1 μm h−1 in collagen vs. 35 ± 1 μm h−1 in PEG hydrogels) observed for both materials (Fig. 3). Thus, synthetic PEG hydrogels can be tailored to recapitulate biologically relevant cell function observed in collagen, a common naturally-derived matrix for 3D cell culture.
Next, we investigated the role of adhesion on HT-1080 motility by systematically varying the concentration of a cell adhesion ligand (CRGDS)39 within PEG hydrogels formed at different moduli. HT-1080 motility was dependent on adhesion, as cell migration was minimal for PEG hydrogels in the absence of active adhesion ligand (0 mM RGD), but increased in hydrogels when RGD was incorporated, even at a very low concentration of ∼75 μM (13%–37% cells migrating more than one cell length in 60–260 Pa hydrogels). The fraction of migrating HT-1080s and average cell speed further increased for each of the 60–260 Pa conditions when the RGD concentration was increased to intermediate concentrations (∼75 to ∼750 μM; Fig. 4A). However, the trend in motility diverged for HT-1080s in PEG gels of varying moduli for higher RGD concentrations (∼1500–1900 μM). Specifically, HT-1080s migrated with decreased cell speed for the 120 Pa condition, resulting in a pronounced biphasic relationship between the fraction of migrating cells and cell speed as a function of adhesion (Fig. 4A). In contrast, HT-1080 motility was only weakly biphasic with RGD concentration for the 60 Pa condition, while migration speed continued to increase for the 260 Pa condition (Fig. 4A). Notably, migration was substantially reduced for all concentrations of RGD for hydrogels with higher moduli (540 Pa), with <6% cells migrating regardless of modulus (Fig. 4B), which demonstrates that there is a limiting matrix density where motility is inhibited within the range of RGD concentrations investigated.
 | ||
| Fig. 4 Modulus and adhesion both influence cell migration. (A) Cells migrate in hydrogels of starting at low RGD concentrations. As adhesion is increased many more cells migrate at intermediate RGD concentrations, a point where migration is insensitive to modulus. At higher concentrations of RGD, modulus plays a dominant role. Cells in compliant (60 Pa) and rigid (260 Pa) hydrogels exhibit high levels of faction migrating, where intermediate moduli (120 Pa) revert back to lower levels. At low RGD concentrations, minimal speed is observed, while at high levels of RGD, cells migrate faster in rigid matrices (260 Pa) and display a biphasic response in softer matrices (60/120 Pa). □ 60 Pa; ○ 120 Pa; ◊ 260 Pa. (B) Contour plots of fraction of migratory cells, speed, and distance to origin depend on contributions of each modulus and stiffness. Cells become non-migratory at low adhesion levels and high modulus. Cells were tracked for 6 hours, DTO ∼ time1/2. Quantified migration was determined by analyzing migrating cells. Experimental values for cell migration speed and distance to origin were determined as an average for all cells (9 total hydrogels: 3 hydrogels per ea. triplicate experiments) (n ≥ 175 per condition; for table of statistical significance, see ESI Fig. S1†). Percent migration values were determined as an average of each of the 9 hydrogels imaged (n = 9). All errors reported as SEM. | ||
An important advantage of thiol–ene photopolymerization is that hydrogels can be spatially and temporally patterned using standard lithographic methods to confine light exposure (and the subsequent reaction) to specific regions within the hydrogel (Fig. 5).11,13 We first formed hydrogels without patterning to demonstrate our approach in which off stoichiometric ratios of monomer functional groups (SH![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) norbornene) was used, thereby leaving unreacted pendant norbornene groups throughout the bulk hydrogel for further modification. Soluble MMP cross-linker and initiator were then swelled into the partially cross-linked bulk hydrogel and exposed to light a second time to controllably increase modulus. The resulting gel modulus increased with exposure time, thereby providing precise control over mechanical properties within the range defined by the partially and fully cross-linked thiol–ene hydrogels (temporal control, Fig. 5A).
norbornene) was used, thereby leaving unreacted pendant norbornene groups throughout the bulk hydrogel for further modification. Soluble MMP cross-linker and initiator were then swelled into the partially cross-linked bulk hydrogel and exposed to light a second time to controllably increase modulus. The resulting gel modulus increased with exposure time, thereby providing precise control over mechanical properties within the range defined by the partially and fully cross-linked thiol–ene hydrogels (temporal control, Fig. 5A).
Patterned regions of higher modulus were also formed in the hydrogel by using a photomask to couple soluble MMP cross-linker with unreacted norbornene groups in pre-defined regions, which was illustrated using a fluorescently tagged cross-linking peptide to form well-defined stripes of varying cross-linking density within the thiol–ene hydrogel (Fig. 5B). We encapsulated primary human dermal fibroblasts (hDF) in thiol–ene hydrogels with alternating stripes of lower and higher modulus (100 Pa (soft)–360 Pa (stiff); 200 μm width) to demonstrate the capacity to alter cell function within the same hydrogel. hDFs were more spread in the 100 Pa regions compared to 360 Pa regions, demonstrating that influences of ECM density on biological function can be investigated within the same material by in situ photopatterning (Fig. 5C).
To investigate how a locally changing ECM in the tumor microenvironment might affect tumor cell motility, HT-1080s were encapsulated in hydrogels with a 12 mM –ene concentration and a thiol![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ene ratio of 0.28
ene ratio of 0.28![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (100 Pa; 1600 μM RGD). Then, 200 μm lines of higher modulus (360 Pa; 2000 μM RGD) were formed through in situ stiffening (final thiol
1 (100 Pa; 1600 μM RGD). Then, 200 μm lines of higher modulus (360 Pa; 2000 μM RGD) were formed through in situ stiffening (final thiol![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ene, 0.35
ene, 0.35![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). As was observed for 560 Pa thiol–ene hydrogels, HT-1080s were less motile in the 360 Pa region, which lends further support for the negative correlation between quantified migration and matrix modulus above a threshold cross-linking density (Fig. 6). Interestingly, cells encapsulated in the soft region were unable to penetrate the stiff regions and instead migrated backward once encountering the interface or aggregated along the interface, while cells starting in the stiff regions were less motile, but were not inhibited from crossing into the softer region (Fig. 7, ESI movies S2–3†). Taken together, PEG hydrogels can be formed with regions of distinct biochemical and/or biophysical properties, which provides a synthetically controlled technique for investigating cell function at matrix boundaries.
1). As was observed for 560 Pa thiol–ene hydrogels, HT-1080s were less motile in the 360 Pa region, which lends further support for the negative correlation between quantified migration and matrix modulus above a threshold cross-linking density (Fig. 6). Interestingly, cells encapsulated in the soft region were unable to penetrate the stiff regions and instead migrated backward once encountering the interface or aggregated along the interface, while cells starting in the stiff regions were less motile, but were not inhibited from crossing into the softer region (Fig. 7, ESI movies S2–3†). Taken together, PEG hydrogels can be formed with regions of distinct biochemical and/or biophysical properties, which provides a synthetically controlled technique for investigating cell function at matrix boundaries.
Discussion
The 3D microenvironment plays an important role during tumor migration and invasion,49,53–57 with adhesion and matrix modulus each implicated in invasion.54,58–64 For example, changes in extracellular matrix (ECM) stiffness have been implicated playing a role in promoting tumor cell migration and invasion.54,58–64 However, the role for stiffness in promoting invasion and metastasis is not clear since in vivo extracellular matrix changes that are associated with tumor progression include multiple matrix components, while biochemical (e.g., adhesion ligand density) and biomechanical properties (e.g., modulus) are coupled for naturally derived materials such as collagen or Matrigel. Collectively, these results highlight the complexities of biophysical/biochemical influences on tumor cell function and identify a need for culture materials that provide systematic control over matrix properties.Here, we investigated tumor cell motility using PEG hydrogels formed through “thiol–ene” photopolymerization to couple thiol groups with alkenes (Fig. 1). This step growth mechanism34 provides strict control over polymer structure and minimizes variability common to other naturally derived and synthetic platforms, and are well-suited for 3-dimensional (3D) cell culture.13–19,21,36 PEG hydrogels based on thiol–ene chemistry can be tuned within a wide range of moduli corresponding to many in vivo tissues60,65 by varying monomer concentration or cross-linking density (Fig. 2). The RGD adhesion ligand concentration was changed with minimal influences on the final gel modulus by varying the ratio of the adhesion peptide and scramble sequence (Fig. 2). Finally, we demonstrated that distinct regions of alternating modulus density could be formed to investigate the influence of changing matrix boundaries on tumor cell migration (Fig. 5–7, ESI movies S2–3†). Thus, peptide-functionalized PEG hydrogels provide a tool that is well-suited for deconstructing biochemical and biophysical influences on tumor cell function by enabling systematic investigation of individual matrix properties, in this case cross-linking density and cell adhesion ligand concentration.
During metastasis, tumor cells invade surrounding tissue and must migrate through several barriers to successfully take residence in distant secondary sites.1–3 We investigated HT-1080 migration at 3D matrix boundaries by forming regions of alternating modulus within PEG hydrogels. We note that creating regions of alternating modulus does lead to interfacial boundaries which may have complex affects on hydrogel properties such as nutrient diffusion, but do not believe that this is a barrier to cellular motility, especially as hydrogels were equilibrated in wells with a sink of media for 24 hours prior to migration studies. HT-1080s tended to reverse direction rather than migrating from the lower into the higher modulus region of 3D patterned PEG hydrogels. Our results contrast 2D64 and 3D66 migration models that predict cells will preferentially migrate from compliant to rigid regions of the matrix, a process called “durotaxis”.64 The term durotaxis refers to cellular migration across mechanical interfaces,64 although it is often associated with gradients. In 2D culture, migrating cells have been characterized by increased cell speed and decreased persistence, increased spreading, and preferential aggregation on higher modulus compared to lower modulus regions of patterned hydrogels.67,68 In the case of 3D durotaxis, fibroblasts preferentially aggregated in the stiffer region of non-uniformly compressed collagen matrix,66 but a biochemical gradient cannot be ruled out since endogenous signals (such as adhesion sites) are also enriched with increasing fibril density. Previously, PEG hydrogels with varying moduli were used to demonstrate that HT-1080s invade a 21.5 kPa matrix, while minimal invasion was observed for a 42 kPa formulation, which is qualitatively consistent with our results here.22 However, the barrier presented by the higher stiffness boundary for HT-1080s in softer patterned regions was not due to the inability of cells to migrate, for example due to higher cross-linking, as cells were motile within 360 Pa regions of PEG hydrogels. We further demonstrated that there exists a threshold modulus (560 Pa), above which HT-1080 motility is prevented (Fig. 4B), which is consistent with a previous study that demonstrated glioma migration was blocked in collagen-agarose gels when agarose reached a critical density.63 Therefore, while durotaxis may be the driving force for 3D motility within certain matrix regimes, our results demonstrate that there may be a limiting upper boundary in matrix density that prevents migration in the direction of increasing stiffness, but which is below the critical density that prevents migration when cells are directly encapsulated (∼560 Pa). This upper range of motility may be due to the inability of cells to proteolytically degrade the surrounding matrix on the time scales measured. Importantly, HT-1080s were apparently unimpeded when migrating from the higher to the lower modulus regions of the matrix (ESI movies S2–3†), which suggests that some regimes promote “reverse durotaxis”, which has been predicted computationally,69 but to the best of our knowledge has not been demonstrated experimentally.
Finally, we note that HT-1080s are an aggressive tumorigenic cell type,70–72 which may play an important role in our observations here. Transformation alters cell adhesion properties,73–80 cytoskeletal structure,81–86 and may induce a more deformable phenotype.87,88 Notably, several migration models implicate strengthening of adhesions in stiffer regions as a driving force for durotaxis.53,61,89–91 However, durotaxis has been investigated primarily for strongly adherent cell types,64,66–68 whereas HT-1080s migrate with minimal adhesion-dependence and do not require leading protrusions to generate traction, but instead express many features of cortical-contractility driven motility from the rear and adopt a distinct phenotype compared to primary adherent cell types.14,15,35 Further, it was recently reported that tumorigenic human osteosarcoma cell line (U2OS) were relatively insensitive to a stiffness gradient,92 which is consistent with tumor cells having altered capacity for durotaxis. Therefore, while we cannot rule out matrix regimes in which a wider variety of cell types would be capable of migrating from stiff to soft regions, we speculate that aggressive tumorigenic cell types such as HT-1080s may be capable of reverse durotaxis in 3D matrices due to a weakly adherent phenotype and rear driven migration.
Methods
Cell culture
HT-1080s (ATCC) were cultured in Minimum Essential Medium (AlphaMEM Lonza) supplemented with 10% Fetal Bovine Serum (Gibco) and 1% penicillin/streptomycin (Gibco). All experiments were performed with HT-1080s passaged less than 12 times. Primary neonatal human fibroblasts were a generous gift from Prof. R. Rivkah Isseroff (University of California-Davis, Department of Dermatology),93 were cultured in Dubellco's Modified Eagle's Medium (DMEM, Gibco) supplemented 10% Fetal Bovine Serum (Gibco) and 1% penicillin/streptomycin (Gibco).8-Arm 40k MW PEG norbornene synthesis
8-Arm PEG norbornene was synthesized as previously described, except that 8-arm PEG MW 40k was substituted for 4-arm PEG MW 20k.13 All reagents were purchased from Sigma-Aldrich unless otherwise noted. All equivalents are with respect to PEG terminal hydroxyls. All glassware was flame dried and kept under an argon blanket. 8-Arm, hydroxyl terminated PEG MW ∼ 40![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 (JenKemUSA), 5 eq. pyridine, 0.5 eq. 4-(dimethylamino)pyridine (DMAP, Fluka) were dissolved anhydrous dichloromethane (DCM) to an round bottom flask. In a separate round bottom flask, DCM, 5 eq. N,N′-dicyclohexylcarbodiimide (DCC, Fluka), and 10 eq. 5-norbornene-2-carboxylic acid were added and allowed to react for 30 min. Following the reaction, the PEG, DMAP, pyridine solution was added and the mixture was allowed to react overnight, at which point was filtered. The filtrate was discarded and the norbornene functionalized PEG solution was dissolved in chloroform and subsequently first washed with a 0.05 M glycine buffer and then with brine. PEG was precipitated with cold diethyl ether containing 10% hexanes and vacuum dried. Proton NMR confirmed >95% conversion and purity.
000 (JenKemUSA), 5 eq. pyridine, 0.5 eq. 4-(dimethylamino)pyridine (DMAP, Fluka) were dissolved anhydrous dichloromethane (DCM) to an round bottom flask. In a separate round bottom flask, DCM, 5 eq. N,N′-dicyclohexylcarbodiimide (DCC, Fluka), and 10 eq. 5-norbornene-2-carboxylic acid were added and allowed to react for 30 min. Following the reaction, the PEG, DMAP, pyridine solution was added and the mixture was allowed to react overnight, at which point was filtered. The filtrate was discarded and the norbornene functionalized PEG solution was dissolved in chloroform and subsequently first washed with a 0.05 M glycine buffer and then with brine. PEG was precipitated with cold diethyl ether containing 10% hexanes and vacuum dried. Proton NMR confirmed >95% conversion and purity.
Peptide synthesis
Standard Fmoc protected amino acids were purchased from Anaspec. All peptide sequences were synthesized using solid phase Fmoc chemistry on Rink Amide Resin with a Tribute Peptide Synthesizer Protein Technologies). Fmoc cleavage was performed using 20%/2% pyridine (Aldrich)/1,8-diazobicyclo[5.4.0]undec-7-ene (DBU, Aldrich) in dimethylformamide. Amino acid coupling was performed using 1 eq. (with respect to amino acid) HBTU (Anaspec)/0.04 M N-methylmorpholine in N-methylpyrrolidone (NMP, Applied Biosystems). Peptides were deprotected and cleaved from the resin using 95%/2.5%/2.5% trifluoroacetic acid (TFA, Aldrich)–water–triisopropylsilane (TIPS, Aldrich) at room temperature for 4 hours. Peptides were precipitated into ice-cold diethyl ether and washed 4 times in diethyl ether, pelleted, and dried. A 1.3% linear gradient starting at 5%/95% acetonitrile (ACN)–water was used to purify the peptides on a reversed phase HPLC C18 column (Waters Delta Prep 4000). Peptide purity was confirmed via matrix-assisted laser desorption ionization time-of-flight mass spectroscopy (Applied Biosystem DE Voyager).Monomer solution preparation
8-Arm 40 kDa norbornene functionalized PEG was dissolved to a 10 wt% solution in phosphate buffered saline (PBS, Gibco). The adhesive peptide, CRGDS, and adhesive peptide scramble, CRDGS were added to the desired concentrations (pre-swelling). Lithium phenyl-2,4,6-trimethylbenzoylphosphinate (LAP, synthesized as previously described)94 was also added and the solution was exposed to 365 nm light to allow for coupling of ligands to PEG. The MMP-degradable peptide KKCGGPQG↓IWGQGCKK was then added to achieve the desired cross-linking density and the solution was diluted with PBS to yield final concentrations of, PEG (3 wt%), LAP (0.05 wt%). The monomer solutions were aliquoted and stored at −80 °C.Rheometry
Rheology on hydrogels swollen in PBS for 24 h at 37 °C was performed on an Ares 4400 rheometer (TA Instruments). A parallel plate geometry with a gap of 1 mm (10% compression to alleviate slippage) was employed. Strain sweeps were performed to ensure operation in the linear range and a frequency sweep (0.1–100 rad s−1) at a constant strain of 20% was used for modulus measurements. For hydrogels formed in situ, incomplete hydrogels were first formed by reacting monomers in off-stoichiometric conditions (excess norbornene). Hydrogels were then placed in an MMP degradable peptide solution so that the final thiol concentration would be equal to the norbornene concentration. Swollen hydrogels were tested as above, except that the hydrogels were exposed to 365 nm light after 30 seconds of strain of 20% at a frequency of 10 rad s−1.Mass swelling ratio (q)
Polymerized hydrogels were allowed to equilibrate in water overnight. Hydrogels were patted dry with a Kimwipe and weighed. Hydrogel were then flash frozen in liquid nitrogen and lyophilized overnight to remove water. The dried hydrogels were subsequently weighed and the mass swelling ratio (q) was calculated via the following equation:| q = Mass Wet Hydrogel/Mass Dry Hydrogel. |
Equilibrium RGD concentration
While initial concentrations of RGD for the starting monomers were equivalent, differences in swelling between hydrogels of different moduli led to slight changes in the final RGD concentrations (Fig. 2C). Therefore, all RGD concentration values are an adjusted value based on the post-swelling, equilibrium volume, which was determined as follows:where qf = mass swelling ratio after cure; q = mass swelling ratio after equilibrium; mr = mass of cured gel, md = mass of dry gel, ms = mass of swollen gel, Vs = volume fraction of polymer in swollen gel, Vr = volume fraction of polymer in cured gel, Qf = volume swelling ratio after cure, ρp = PEG density (1.12 g cm−3), ρsol = sol fraction density (1.0 g cm−3), [RGD]i = initial RGD concentration, and [RGD]equ = equilibrium RGD concentration.
Cell encapsulation
Cells were trypsinized, suspended, and counted with a hemocytometer. 20k cells were pelleted in a 15 mL conical vial and the supernatant removed. Cells were resuspended in 100 μL of monomer solution and 30 μL of this solution was pipetted into a 1 mL syringe with a cut off tip. Polymerization occurred via exposure of the cell suspension to a 365 nm light source at 10 mW cm−2 for 2 min (XX-40 light source, UVP).Patterning hydrogels
CRGDS, LAP, and norbornene functionalized 8-arm 40 kDa PEG were combined and diluted with PBS (Gibco) to yield final concentrations of 4 mM, 127 μM, and 20 mM, respectively. This solution was exposed to 365 nm light at 10 mW cm−2 to allow pre-coupling of CRGDS to the PEG backbone. The MMP sensitive peptide cross-linker, additional LAP, and PBS was added to this solution to yield final concentrations of 3.36 mM and 127 μM, respectively. Cells were encapsulated utilizing the procedure outlined above. These PEG hydrogels were then soaked in 200 μL of a 4.83 mM solution of the MMP degradable cross-linker, LAP (127 μM) and PBS for one hour in an incubator at 37 °C, 5% CO2. The hydrogels were placed on a quartz photomask containing alternating 200 μm wide parallel lines. The samples were again exposed to a beam collimated light (365 nm, 10 mW cm−2) to generate regions of higher modulus. Samples were placed in an incubator at 37 °C, 5% CO2 for 24 hours prior to performing migration experiments.Migration experiments
Migration experiments and image analysis were performed as previously described.15 Time-lapse microscopy was used to track cells in synthetic ECM for 6 hours (15 minute increments) and analyzed using MetaMorph. We note that matrix connectivity, permeability, and elasticity are coupled within PEG hydrogels. Therefore, increased crosslinking density leads to higher G′ and decreased permeability, which may influence nutrient exchange within the PEG hydrogel. However, previous studies demonstrated that changes in permeability were minimal within the range described for PEG hydrogels here. Further, HT-1080 migration was investigated after 24 hours of swelling, which was previously shown to be a sufficient time scale for nutrient equilibrium to be reached in PEG hydrogels with cross-linking densities similar to those reported here.95Data analysis
Migration was determined for cells in the middle 500 μm of an ∼1000 μm thick PEG hydrogel disk formed as described above. Cells that interacted or divided were not included in analysis. The fraction of migrating cells represents the average of individual hydrogels where a migrating cell was defined as having migrated a distance greater than one cell length (defined as 44 μm)15 any time during tracking (6 hours). Reported values for experiments in synthetic ECM represent a 3D correction for 2D minimum intensity z-projections used for tracking (multiplied by a factor of √3/2, migration was assumed to be isotropic). Experimental values for cell migration speed and distance to origin were determined as an average for all cells (9 total hydrogels: 3 hydrogels per ea. triplicate experiments). All statistical analysis was performed using a two-tailed student's t-test. All error bars represent standard error of the mean (SEM).3D immunohistochemistry in synthetic ECM
Cells were stained with Alexa Fluor 546 Phalloidin (Life Technologies A22283). A modification of the instructions for focal adhesion staining kit (Millipore, FAK100) was used to allow for diffusion into the synthetic ECM (8-arm PEG hydrogel). All steps were performed at room temperature except antibody incubations (4 °C). Specifically, longer incubation times included fixing (30 min), permeabilization (30 min), blocking (1 h), washing steps (15 min ea−1. for all steps, 30 min ea−1. after antibody incubations), and antibody incubations (overnight, 4 °C).Conclusions
In this work, we utilized a peptide functionalized PEG hydrogel to examine the influence of matrix adhesion at varying moduli on HT-1080 migration. HT-1080 motility was characterized by a complex trend in which positive and negative matrix effects on motility were observed. Our results add new insight into the role of extracellular matrix properties and their influence on tumor cell migration by identifying trends that have not previously been appreciated, including “reverse durotaxis”, where HT-1080s migrated from rigid to compliant patterned regions. Notably, tumors have been characterized by substantially higher moduli than both normal tissue and the adjacent ECM,91 which suggests that invading tumor cells may initially be exposed to a reverse stiffness gradient. While further work will be required to identify the extent to which “reverse durotaxis” influences migration and invasion, our results provide new insight into 3D tumor cell motility by taking advantage of synthetic control over biochemical and biophysical matrix properties while minimizing confounding factors. Importantly, synthetic strategies to form patterned regions within 3D matrices offer an important tool for investigating several unresolved questions in tumor progression, such as the effects of tissue interfaces on tumor invasion and motility.Acknowledgements
The authors would like to acknowledge HHMI and the National Institutes of Health (RO1 CA132633-02) for funding this work. S. P. Singh would like to acknowledge the biophysics training program (NIH grant T32 GM-065103) for fellowship support.References
- D. Hanahan and R. A. Weinberg, Cell, 2011, 144, 646–674 CrossRef CAS PubMed.
- B. Vogelstein and K. W. Kinzler, Nat. Med., 2004, 10, 789–799 CrossRef CAS PubMed.
- D. Hanahan and R. A. Weinberg, Cell, 2000, 100, 57–70 CrossRef CAS.
- C. S. Hughes, L. M. Postovit and G. A. Lajoie, Proteomics, 2010, 10, 1886–1890 CrossRef CAS PubMed.
- F. Sabeh, R. Shimizu-Hirota and S. J. Weiss, J. Cell Biol., 2009, 185, 11–19 CrossRef CAS PubMed.
- Y. L. Yang, L. M. Leone and L. J. Kaufman, Biophys. J., 2009, 97, 2051–2060 CrossRef CAS PubMed.
- G. Beier and J. Engel, Biochemistry, 1966, 5, 2744–2755 CrossRef CAS.
- K. Pietrucha, Int. J. Biol. Macromol., 2005, 36, 299–304 CrossRef CAS PubMed.
- J. Elisseeff, K. Anseth, D. Sims, W. McIntosh, M. Randolph and R. Langer, Proc. Natl. Acad. Sci. U. S. A., 1999, 96, 3104–3107 CrossRef CAS.
- M. P. Lutolf, J. L. Lauer-Fields, H. G. Schmoekel, A. T. Metters, F. E. Weber, G. B. Fields and J. A. Hubbell, Proc. Natl. Acad. Sci. U. S. A., 2003, 100, 5413–5418 CrossRef CAS PubMed.
- C. A. DeForest, B. D. Polizzotti and K. S. Anseth, Nat. Mater., 2009, 8, 659–664 CrossRef CAS PubMed.
- B. D. Polizzotti, B. D. Fairbanks and K. S. Anseth, Biomacromolecules, 2008, 9, 1084–1087 CrossRef CAS PubMed.
- B. D. Fairbanks, M. P. Schwartz, A. E. Halevi, C. R. Nuttelman, C. N. Bowman and K. S. Anseth, Adv. Mater., 2009, 21, 5005–5010 CrossRef CAS.
- M. P. Schwartz, R. E. Rogers, S. P. Singh, J. Y. Lee, S. G. Loveland, J. T. Koepsel, E. S. Witze, S. I. Montanez-Sauri, K. E. Sung, E. Y. Tokuda, Y. Sharma, L. M. Everhart, E. H. Nguyen, M. H. Zaman, D. J. Beebe, N. G. Ahn, W. L. Murphy and K. S. Anseth, PLoS One, 2013, 8, e81689 Search PubMed.
- M. P. Schwartz, B. D. Fairbanks, R. E. Rogers, R. Rangarajan, M. H. Zaman and K. S. Anseth, Integr. Biol., 2010, 2, 32–40 RSC.
- S. B. Anderson, C. C. Lin, D. V. Kuntzler and K. S. Anseth, Biomaterials, 2011, 32, 3564–3574 CrossRef CAS PubMed.
- J. A. Benton, B. D. Fairbanks and K. S. Anseth, Biomaterials, 2009, 30, 6593–6603 CrossRef CAS PubMed.
- P. D. Mariner, E. Johannesen and K. S. Anseth, J. Tissue Eng. Regen. Med., 2012, 6, 314–324 CrossRef CAS PubMed.
- E. H. Nguyen, M. R. Zanotelli, M. P. Schwartz and W. L. Murphy, Biomaterials, 2014, 35, 2149–2161 CrossRef CAS PubMed.
- A. Raza, C. S. Ki and C. C. Lin, Biomaterials, 2013, 34, 5117–5127 CrossRef CAS PubMed.
- C.-C. Lin, A. Raza and H. Shih, Biomaterials, 2011, 32, 9685–9695 CrossRef CAS PubMed.
- J. C. Hoffmann and J. L. West, Integr. Biol., 2013, 5, 817–827 RSC.
- J. J. Moon, J. E. Saik, R. A. Poche, J. E. Leslie-Barbick, S. H. Lee, A. A. Smith, M. E. Dickinson and J. L. West, Biomaterials, 2010, 31, 3840–3847 CrossRef CAS PubMed.
- G. P. Raeber, M. P. Lutolf and J. A. Hubbell, Biophys. J., 2005, 89, 1374–1388 CrossRef CAS PubMed.
- B. K. Mann, A. S. Gobin, A. T. Tsai, R. H. Schmedlen and J. L. West, Biomaterials, 2001, 22, 3045–3051 CrossRef CAS.
- J. L. West and J. A. Hubbell, Macromolecules, 1999, 32, 241–244 CrossRef CAS.
- M. S. Hahn, J. S. Miller and J. L. West, Adv. Mater., 2006, 18, 2679–2684 CrossRef CAS.
- M. S. Hahn, J. S. Miller and J. L. West, Adv. Mater., 2005, 17, 2939–2942 CrossRef CAS.
- S. A. DeLong, A. S. Gobin and J. L. West, J. Controlled Release, 2005, 109, 139–148 CrossRef CAS PubMed.
- A. M. Kloxin, J. A. Benton and K. S. Anseth, Biomaterials, 2010, 31, 1–8 CrossRef CAS PubMed.
- A. M. Kloxin, A. M. Kasko, C. N. Salinas and K. S. Anseth, Science, 2009, 324, 59–63 CrossRef CAS PubMed.
- C. S. Ki, H. Shih and C. C. Lin, Polymer, 2013, 54, 2115–2122 CrossRef CAS PubMed.
- H. Wang, S. M. Haeger, A. M. Kloxin, L. A. Leinwand and K. S. Anseth, PLoS One, 2012, 7 Search PubMed.
- C. E. Hoyle and C. N. Bowman, Angew. Chem., Int. Ed., 2010, 49, 1540–1573 CrossRef CAS PubMed.
- T. D. Hansen, J. T. Koepsel, N. N. Le, E. H. Nguyen, S. Zorn, M. Parlato, S. G. Loveland, M. P. Schwartz and W. L. Murphy, Biomater. Sci., 2014, 2, 745–756 RSC.
- H. Shih and C. C. Lin, Biomacromolecules, 2012, 13, 2003–2012 CrossRef CAS PubMed.
- H. Nagase and G. B. Fields, Biopolymers, 1996, 40, 399–416 CrossRef CAS.
- E. Ruoslahti, Annu. Rev. Cell Dev. Biol., 1996, 12, 697–715 CrossRef CAS PubMed.
- M. D. Pierschbacher and E. Ruoslahti, Nature, 1984, 309, 30–33 CrossRef CAS.
- P. G. Agache, C. Monneur, J. L. Leveque and J. Rigal, Arch. Dermatol. Res., 1980, 269, 221–232 CrossRef CAS.
- A. Huttenlocher and A. R. Horwitz, Cold Spring Harbor Perspect. Biol., 2011, 3 Search PubMed.
- A. J. Ridley, M. A. Schwartz, K. Burridge, R. A. Firtel, M. H. Ginsberg, G. Borisy, J. T. Parsons and A. R. Horwitz, Science, 2003, 302, 1704–1709 CrossRef CAS PubMed.
- D. A. Lauffenburger and A. F. Horwitz, Cell, 1996, 84, 359–369 CrossRef CAS.
- A. Huttenlocher, R. R. Sandborg and A. F. Horwitz, Curr. Opin. Cell Biol., 1995, 7, 697–706 CrossRef CAS.
- N. R. Gandavarapu, P. D. Mariner, M. P. Schwartz and K. S. Anseth, Acta Biomater., 2013, 9, 4525–4534 CrossRef CAS PubMed.
- D. S. W. Benoit, M. P. Schwartz, A. R. Durney and K. S. Anseth, Nat. Mater., 2008, 7, 816–823 CrossRef CAS PubMed.
- S. I. Fraley, Y. F. Feng, R. Krishnamurthy, D. H. Kim, A. Celedon, G. D. Longmore and D. Wirtz, Nat. Cell Biol., 2010, 12, 598-U169 CrossRef PubMed.
- D. Yamazaki, S. Kurisu and T. Takenawa, Oncogene, 2009, 28, 1570–1583 CrossRef CAS PubMed.
- M. H. Zaman, L. M. Trapani, A. Siemeski, D. MacKellar, H. Y. Gong, R. D. Kamm, A. Wells, D. A. Lauffenburger and P. Matsudaira, Proc. Natl. Acad. Sci. U. S. A., 2006, 103, 10889–10894 CrossRef CAS PubMed.
- V. Niggli, M. Schmid and A. Nievergelt, Biochem. Biophys. Res. Commun., 2006, 343, 602–608 CrossRef CAS PubMed.
- N. O. Carragher, S. M. Walker, L. S. A. Carragher, F. Harris, T. K. Sawyer, V. G. Brunton, B. W. Ozanne and M. C. Frame, Oncogene, 2006, 25, 5726–5740 CrossRef CAS PubMed.
- K. Wolf, I. Mazo, H. Leung, K. Engelke, U. H. von Andrian, E. I. Deryugina, A. Y. Strongin, E. B. Brocker and P. Friedl, J. Cell Biol., 2003, 160, 267–277 CrossRef CAS PubMed.
- P. P. Provenzano, D. R. Inman, K. W. Eliceiri and P. J. Keely, Oncogene, 2009, 28, 4326–4343 CrossRef CAS PubMed.
- M. J. Paszek, N. Zahir, K. R. Johnson, J. N. Lakins, G. I. Rozenberg, A. Gefen, C. A. Reinhart-King, S. S. Margulies, M. Dembo, D. Boettiger, D. A. Hammer and V. M. Weaver, Cancer Cell, 2005, 8, 241–254 CrossRef CAS PubMed.
- V. M. Weaver, O. W. Petersen, F. Wang, C. A. Larabell, P. Briand, C. Damsky and M. J. Bissell, J. Cell Biol., 1997, 137, 231–245 CrossRef CAS.
- L. A. Liotta, Cancer Res., 1986, 46, 1–7 CAS.
- M. J. Bissell, H. G. Hall and G. Parry, J. Theor. Biol., 1982, 99, 31–68 CrossRef CAS.
- M. W. Conklin, J. C. Eickhoff, K. M. Riching, C. A. Pehlke, K. W. Eliceiri, P. P. Provenzano, A. Friedl and P. J. Keely, Am. J. Pathol., 2011, 178, 1221–1232 CrossRef PubMed.
- P. P. Provenzano, D. R. Inman, K. W. Eliceiri, J. G. Knittel, L. Yan, C. T. Rueden, J. G. White and P. J. Keely, BMC Med., 2008, 6 Search PubMed.
- H. M. Yu, J. K. Mouw and V. M. Weaver, Trends Cell Biol., 2011, 21, 47–56 CrossRef PubMed.
- P. P. Provenzano and P. J. Keely, J. Cell Sci., 2011, 124, 1195–1205 CrossRef CAS PubMed.
- T. R. Cox and J. T. Erler, Dis. Models Mech., 2011, 4, 165–178 CrossRef CAS PubMed.
- T. A. Ulrich, A. Jain, K. Tanner, J. L. MacKay and S. Kumar, Biomaterials, 2010, 31, 1875–1884 CrossRef CAS PubMed.
- C. M. Lo, H. B. Wang, M. Dembo and Y. L. Wang, Biophys. J., 2000, 79, 144–152 CrossRef CAS.
- J. Z. Gasiorowski, C. J. Murphy and P. F. Nealey, in Annual Review of Biomedical Engineering, ed. M. L. Yarmush, Annual Reviews, Palo Alto, 2013, vol. 15, pp. 155–176 Search PubMed.
- E. Hadjipanayi, V. Mudera and R. A. Brown, Cell Motil. Cytoskeleton, 2009, 66, 121–128 CrossRef CAS PubMed.
- J. Y. Wong, A. Velasco, P. Rajagopalan and Q. Pham, Langmuir, 2003, 19, 1908–1913 CrossRef CAS.
- D. S. Gray, J. Tien and C. S. Chen, J. Biomed. Mater. Res., Part A, 2003, 66A, 605–614 CrossRef CAS PubMed.
- Y. Ni and M. Y. M. Chiang, Soft Matter, 2007, 3, 1285–1292 RSC.
- S. Rasheed, N. Wa, E. M. Toth, P. Arnstein and M. B. Gardner, Cancer, 1974, 33, 1027–1033 CrossRef CAS.
- S. Gupta, S. Stuffrein, R. Plattner, M. Tencati, C. Gray, Y. E. Whang and E. J. Stanbridge, Mol. Cell. Biol., 2001, 21, 5846–5856 CrossRef CAS.
- S. Gupta, R. Plattner, C. J. Der and E. J. Stanbridge, Mol. Cell. Biol., 2000, 20, 9294–9306 CrossRef CAS.
- R. Hirst, A. Horwitz, C. Buck and L. Rohrschneider, Proc. Natl. Acad. Sci. U. S. A., 1986, 83, 6470–6474 CrossRef CAS.
- T. Volk, B. Geiger and A. Raz, Cancer Res., 1984, 44, 811–824 CAS.
- E. Roos, Biochim. Biophys. Acta, 1983, 738, 263–284 Search PubMed.
- A. Raz and B. Geiger, Cancer Res., 1982, 42, 5183–5190 CAS.
- B. M. Steinberg, K. Smith, M. Colozzo and R. Pollack, J. Cell Biol., 1980, 87, 304–308 CrossRef CAS.
- M. Cottlerfox, W. Ryd, B. Hagmar and C. H. Fox, Int. J. Cancer, 1980, 26, 689–694 CrossRef CAS.
- J. Bubenik, P. Perlmann, E. M. Fenyo, T. Jandlova, E. Suhajova and M. Malkovsky, Int. J. Cancer, 1979, 23, 392–396 CrossRef CAS.
- D. R. Coman, Cancer Res., 1944, 4, 625–629 Search PubMed.
- G. Pawlak and D. M. Helfman, Curr. Opin. Genet. Dev., 2001, 11, 41–47 CrossRef CAS.
- E. Friedman, M. Verderame, S. Winawer and R. Pollack, Cancer Res., 1984, 44, 3040–3050 CAS.
- C. B. Boschek, B. M. Jockusch, R. R. Friis, R. Back, E. Grundmann and H. Bauer, Cell, 1981, 24, 175–184 CrossRef CAS.
- T. David-Pfeuty and S. J. Singer, Proc. Natl. Acad. Sci. U. S. A., 1980, 77, 6687–6691 CrossRef CAS.
- E. Wang and A. R. Goldberg, Proc. Natl. Acad. Sci. U. S. A., 1976, 73, 4065–4069 CrossRef CAS.
- R. Pollack, M. Osborn and K. Weber, Proc. Natl. Acad. Sci. U. S. A., 1975, 72, 994–998 CrossRef CAS.
- D. Wirtz, K. Konstantopoulos and P. C. Searson, Nat. Rev. Cancer, 2011, 11, 512–522 CrossRef CAS PubMed.
- T. Ochalek, F. J. Nordt, K. Tullberg and M. M. Burger, Cancer Res., 1988, 48, 5124–5128 CAS.
- K. A. Lazopoulos and D. Stamenovic, J. Biomech., 2008, 41, 1289–1294 CrossRef PubMed.
- I. V. Dokukina and M. E. Gracheva, Biophys. J., 2010, 98, 2794–2803 CrossRef CAS PubMed.
- K. R. Levental, H. M. Yu, L. Kass, J. N. Lakins, M. Egeblad, J. T. Erler, S. F. T. Fong, K. Csiszar, A. Giaccia, W. Weninger, M. Yamauchi, D. L. Gasser and V. M. Weaver, Cell, 2009, 139, 891–906 CrossRef CAS PubMed.
- J. S. Martinez, A. M. Lehaf, J. B. Schlenoff and T. C. S. Keller, Biomacromolecules, 2013, 14, 1311–1320 CrossRef CAS PubMed.
- A. L. Sillman, D. M. Quang, B. Farboud, K. S. Fang, R. Nuccitelli and R. R. Isseroff, Exp. Dermatol., 2003, 12, 396–402 CrossRef CAS.
- B. D. Fairbanks, M. P. Schwartz, C. N. Bowman and K. S. Anseth, Biomaterials, 2009, 30, 6702–6707 CrossRef CAS PubMed.
- L. M. Weber, C. G. Lopez and K. S. Anseth, J. Biomed. Mater. Res., Part A, 2009, 90A, 720–729 CrossRef CAS PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available: Table of statistical significance for Fig. 4 (S1) and “reverse durotaxis” movies (S2–3). See DOI: 10.1039/c4bm00022f |
| ‡ Current affiliation: CSIRO Materials Science and Engineering, Clayton, Victoria, Australia. |
| This journal is © The Royal Society of Chemistry 2014 |