Induction of hepatocyte functional protein expression by submicron/nano-patterning substrates to mimic in vivo structures
Shimaa A.
Abdellatef
ab,
Akihiko
Ohi
c,
Toshihide
Nabatame
c and
Akiyoshi
Taniguchi
*ab
aCell-Materials Interaction Group, Biomaterials Unit, Nano-Life Field, International Center for Materials Nanoarchitectonics (MANA), National Institute for Materials Science, 1-1 Namiki, Tsukuba, Ibaraki 305-0044, Japan. E-mail: Taniguchi.Akiyoshi@nims.go.jp
bGraduate School of Advanced Science and Engineering, Waseda University, 3-4-1 Okubo, Shinjuku, Tokyo 169-8555, Japan
cMANA Foundry, International Center for Materials Nanoarchitectonics (MANA), National Institute for Materials Science, 1-1 Namiki, Tsukuba, Ibaraki 305-0044, Japan
First published on 11th November 2013
Abstract
To investigate the influence of bio-inspired metallic superficial topography on the cellular behaviour of a hepatocyte cell line, TiO2 nanopatterns with diversified shapes and heterotropic lateral dimensions were fabricated using electron beam lithography and atomic layer deposition. The dimensional uniformity and shape diversity of the nanopatterns were confirmed using scanning electron microscopy and atomic force microscopy. These topographical nanocues provide good tools for controlling and regulating multiple hepatocellular functions. The expressions of functional proteins such as albumin, transferrin and cytochrome P450 were tested as functional markers. In addition, the change in cellular orientation, cell alignment and native extracellular matrix (ECM) assembly induced by these well-defined nanotopographies were observed. Twelve hours after cell seeding, TiO2 nanogratings with a lateral dimension of 240 nm showed a higher degree of functional protein expression compared to other nanotopographical substrates and a flat surface. These findings suggest that the TiO2 surface resembles a hierarchically-extended collagen nanofibrillar surface and could be recognized by hepatocytes, allowing the proper cytoskeletal orientation and cellular integrity. This TiO2 nanopattern with a specific shape and dimension (240 nm) might therefore emulate ECM biophysical cues, and the intrinsic topography of TiO2 surfaces might evoke enhanced cellular responses. These unique surfaces could be further exploited for tissue engineering and bioreactor technology.
Introduction
The interaction between human cells and biomaterials has been an intense area of study in the last several decades. These studies have provided promising approaches for producing novel biomaterials with enhanced cell integration properties,1 and have participated in the generation of materials with implantable and bioartificial characteristics at both the research and clinical levels. Living cells in their native environment are embedded in complex, well-defined organic matrices that incorporate macromolecules to provide the extracellular matrix (ECM). The ECM is assembled into a three-dimensional mesh network containing precisely contoured nanostructures. The architecture of the ECM possesses the essential physical cues and biochemical factors that trigger and control specific reactions and optimize cellular behaviour.2 Collagen is one of the ECM molecules. It is a fibrous protein approximately 300 nm long and 1.5 nm wide with a triple helical structure.3 These helices are assembled into nanofibrillar networks that hierarchically extend for tens of micrometers in length and have diameters between 260 and 410 nm.4 It is believed that the micro/nano-texturing features of ECM provide the mechanotransductive cues that deeply influence cell morphology,5 migration,6 proliferation,7 and cytoskeleton organization.8 Consequently, manipulation of biomaterial superficial texturing and topographical features would provide an excellent means for controlling cellular functions by influencing physical cues that trigger various biological responses.9 These wide arrays of cellular responses are cell and topography-specific. Thus, these resulting interactions will be also affected by the size, geometry and shape of fabricated substrates.10Liver cells need a distinctive natural environment to maintain high levels of cellular function. The cellular function of hepatocyte cell lines usually decreases when cultured on two-dimensional cell culture surfaces. It is therefore very important to mimic ECM physical and chemical cues to maintain the preferred cellular in vivo architecture and optimum cellular function. Several techniques have been designed to mimic the innate ECM of liver cells, such as the use of co-culture systems,11 polymers,12 recombinant proteins13 and inorganic materials.14 In particular, the deposition of an ultrathin layer of inorganic material such as amorphous titanium oxide provides an excellent environment for the optimum functioning of hepatic cells.15 TiO2 thin films have the advantage of controllable thickness, as well as facile surface modelling by the simple incorporation of different organic moieties into the deposited inorganic film. Furthermore, modification or manipulation of the surface characteristics and topographical features of TiO2 based nanomaterials has been shown to change the biological behaviour of various cells in vivo and in vitro.16
Most previous research has focused on anisotropic nanotopography with equally spaced dimensions, without considering the impact of unequal alterations in the dimensional magnitude of topographical features on cellular functions.17 Furthermore, understanding the influence of different shapes and models of nanotopographical features would provide an excellent means for optimizing the geometry for desirable cellular behaviour in vitro. In this paper, we report the manipulation of amorphous TiO2 depressions and projections in nano and submicron size in order to mimic the dimensional and geometrical characteristics of the fibril structure of collagen. This manipulation was performed using electron beam lithography and atomic layer deposition. The diameter of the nano-scale depressions and projections were chosen to fit the dimensions of hierarchically-extended collagen nanofiber networks. The morphological and functional changes in the hepatocytes induced by the diversified nanofeatures were investigated; for example, the alignment and secretion of different functional proteins were observed using fluorescent immunostaining techniques. Our goal was to better understand superficial characteristics such as the optimum size and geometry of tangible nanocues in order to develop a model that mimics the unique features of ECM. Consequently, we optimized the performance of a liver cell line cultured in vitro by observing the expression of various hepatic functional proteins. These cell culture models could be further exploited in future research and clinical studies.
Materials and methods
Fabrication of nanopattern TiO2 substrates using electron beam lithography and atomic layer deposition
Cleaned Si(100) substrates were coated with ZEP520A resist (Nippon Zeon Co., Japan) and a thinner (anisole) at a ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 using a spin coater (Mikasa 1H-D7) at 6000 rpm. Prebaking was done at 180 °C for 3 min, followed by spin coating with a very thin layer of conductive material (10–20 nm) (Espacer; Syowa Denko Co., Japan) at 2000 rpm. The substrate was then irradiated with an e-beam (Elionix ELS-7500EX) with acceleration voltage of 50 kV and an I beam amperage of 220 pA. The precise size of the fabricated substrate resulting from each e-beam was confirmed using SEM. The substrate was then developed using H2O, n-amyl acetate and methyl isobutyl ketone (89%)/isopropyl alcohol (11%) (Wako Co., Japan) and dried with N2 gas. An etching step was performed using inductively coupled plasma-reactive ion etching at 50 W (sulphur hexafluoride 2.5 cc s−1 + methyl tetrafluoride 3.5 cc s−1) with a total pressure of 0.1 Pa for 101 s. Next, the resistance was removed using O2 plasma, DMAC (dimethyl acetamide) and SPM (H2SO4 + H2O2, 3
2 using a spin coater (Mikasa 1H-D7) at 6000 rpm. Prebaking was done at 180 °C for 3 min, followed by spin coating with a very thin layer of conductive material (10–20 nm) (Espacer; Syowa Denko Co., Japan) at 2000 rpm. The substrate was then irradiated with an e-beam (Elionix ELS-7500EX) with acceleration voltage of 50 kV and an I beam amperage of 220 pA. The precise size of the fabricated substrate resulting from each e-beam was confirmed using SEM. The substrate was then developed using H2O, n-amyl acetate and methyl isobutyl ketone (89%)/isopropyl alcohol (11%) (Wako Co., Japan) and dried with N2 gas. An etching step was performed using inductively coupled plasma-reactive ion etching at 50 W (sulphur hexafluoride 2.5 cc s−1 + methyl tetrafluoride 3.5 cc s−1) with a total pressure of 0.1 Pa for 101 s. Next, the resistance was removed using O2 plasma, DMAC (dimethyl acetamide) and SPM (H2SO4 + H2O2, 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), respectively. The next steps included coating with a photoresist (AZ-5214E), UV irradiation with a photomask, reversal baking at 120 °C, and flood exposure to UV. The substrate was then developed using HMD (hexamethyl disilazane) and 2.38% TMAH (tetramethyl ammonium hydroxide) (Wako Co. Japan) for 1 min, and then rinsed with H2O. Finally, atomic layer deposition was conducted (Picosun SUNALE R-150). The deposition pressure inside the chamber was 500 Pa at a temperature of 100 °C. The thickness of the TiO2 layer was controlled by the number of cycles: 70 cycles gave a thickness of 5 nm. The TiO2 precursor [tetra(dimethylamino)titanate] was pumped into the chamber, followed by argon gas to remove the undeposited precursor. Next, H2O vapour was pumped in to form the inorganic TiO2 layer from the organic precursor, and then argon gas was pumped in to remove residual H2O. Fabricated TiO2 nanopattern surfaces were characterized using a scanning electron microscope (Hitachi-S3000N) and an atomic force microscope (SIIL-trace). Further experiments were done using the fabricated substrates after dry heat sterilization of the substrate at 170 °C for 1 h.
1), respectively. The next steps included coating with a photoresist (AZ-5214E), UV irradiation with a photomask, reversal baking at 120 °C, and flood exposure to UV. The substrate was then developed using HMD (hexamethyl disilazane) and 2.38% TMAH (tetramethyl ammonium hydroxide) (Wako Co. Japan) for 1 min, and then rinsed with H2O. Finally, atomic layer deposition was conducted (Picosun SUNALE R-150). The deposition pressure inside the chamber was 500 Pa at a temperature of 100 °C. The thickness of the TiO2 layer was controlled by the number of cycles: 70 cycles gave a thickness of 5 nm. The TiO2 precursor [tetra(dimethylamino)titanate] was pumped into the chamber, followed by argon gas to remove the undeposited precursor. Next, H2O vapour was pumped in to form the inorganic TiO2 layer from the organic precursor, and then argon gas was pumped in to remove residual H2O. Fabricated TiO2 nanopattern surfaces were characterized using a scanning electron microscope (Hitachi-S3000N) and an atomic force microscope (SIIL-trace). Further experiments were done using the fabricated substrates after dry heat sterilization of the substrate at 170 °C for 1 h.
Hepatocyte cell culture
HepG2 cells were cultured in Dulbecco's MEM (Nacalai Tesque, Kyoto, Japan) with 10% heat inactivated FBS and supplemented with 100 U penicillin/100 μg streptomycin (Nacalai Tesque, Kyoto, Japan) per ml of medium. All cells were maintained at 37 °C in a 100% humidified atmosphere under 5% CO2. At 70–80% confluence, cells were trypsinized and seeded over the TiO2 nanopattern surface for 12 to 18 h. For the expression of ECM components, cells were cultured for 66 h; a fresh medium was added after 33 h.Immunostaining and fluorescence detection
HepG2 cells cultured on TiO2 nanopatterned substrates were fixed for 15 min in 4% p-formaldehyde (PFA) in PBS at 4 °C. Excess aldehyde was quenched using 0.1 M glycine for 5 min. Then, the cells were permeabilized in 1% Triton X-100 in PBS for 5 min, and then primary antibodies were added. The antibodies used: rabbit polyclonal anti-albumin antibody-C-terminal (diluted 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 25; Abcam, Cambridge, UK), rabbit polyclonal anti-transferrin antibody (diluted 1
25; Abcam, Cambridge, UK), rabbit polyclonal anti-transferrin antibody (diluted 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500; Abcam), mouse monoclonal anti-cytochrome P450 2C6 antibody (1 μg ml−1; Abcam), CD29 mouse anti-human mAb-ALexa Fluro® 488 conjugate for integrin β1 (diluted 1
500; Abcam), mouse monoclonal anti-cytochrome P450 2C6 antibody (1 μg ml−1; Abcam), CD29 mouse anti-human mAb-ALexa Fluro® 488 conjugate for integrin β1 (diluted 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100; Invitrogen Life Technologies, Eugene, Oregon, USA). These antibodies were incubated with the cells at 4 °C overnight. Then, secondary antibodies were added for 1 h. These secondary antibodies were Alexa Fluor® 568 goat anti-mouse IgG (H + L) (diluted 1
100; Invitrogen Life Technologies, Eugene, Oregon, USA). These antibodies were incubated with the cells at 4 °C overnight. Then, secondary antibodies were added for 1 h. These secondary antibodies were Alexa Fluor® 568 goat anti-mouse IgG (H + L) (diluted 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500; Invitrogen Life Technologies Eugene, Oregon, USA) and goat polyclonal anti-rabbit IgG – H&L-DyLight® 488 (diluted 1
500; Invitrogen Life Technologies Eugene, Oregon, USA) and goat polyclonal anti-rabbit IgG – H&L-DyLight® 488 (diluted 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500; Abcam). The cells were observed using an upright fluorescence microscope (Olympus BX51) equipped with an Olympus DP70 digital camera. DP Controller Ver. 3.1.1 was used to process the images. For quantitative comparison of albumin expression, we used the freeware image analysis software ImageJ, as previously reported.18 The cell area was determined by manual delineation of raw fluorescence images. A minimum of 12 cells were analysed from two independent experiments. Cytoskeletal F-actin was visualized by treating the cells with phalloidin – TRIC (Sigma-Aldrich, Germany) at 5 μg ml−1 for 15 min. Nuclei were stained using Hoechst 33342 at 5 μg ml−1 for 10 min.
500; Abcam). The cells were observed using an upright fluorescence microscope (Olympus BX51) equipped with an Olympus DP70 digital camera. DP Controller Ver. 3.1.1 was used to process the images. For quantitative comparison of albumin expression, we used the freeware image analysis software ImageJ, as previously reported.18 The cell area was determined by manual delineation of raw fluorescence images. A minimum of 12 cells were analysed from two independent experiments. Cytoskeletal F-actin was visualized by treating the cells with phalloidin – TRIC (Sigma-Aldrich, Germany) at 5 μg ml−1 for 15 min. Nuclei were stained using Hoechst 33342 at 5 μg ml−1 for 10 min.
To investigate ECM components, decellularization was performed as previously reported.19 Cells were cultured on TiO2 nanopattern surfaces for 66 h, and then incubated with 2 ml of distilled water for 1 h at 37 °C for cell lysis. A washing step by careful immersion in PBS was done 3 times. The deposited ECM was fixed using 4% PFA for 20 min, and then permeabilized using 0.5% Triton X-100 in PFA. Primary antibodies such as mouse monoclonal antifibronectin (diluted 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50; Santa Cruz Biotechnology) and mouse monoclonal anticollagen IV (diluted 1
50; Santa Cruz Biotechnology) and mouse monoclonal anticollagen IV (diluted 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50; Santa Cruz Biotechnology) were added for 30 min, and then secondary antibodies were added for 30 min. The secondary antibodies were goat anti-mouse IgG-TR (diluted 1
50; Santa Cruz Biotechnology) were added for 30 min, and then secondary antibodies were added for 30 min. The secondary antibodies were goat anti-mouse IgG-TR (diluted 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 250; Santa Cruz Biotechnology) and Alexa Fluor® 488 goat anti-mouse IgM (H + L) (diluted 1
250; Santa Cruz Biotechnology) and Alexa Fluor® 488 goat anti-mouse IgM (H + L) (diluted 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 250; Invitrogen Life Technologies). The effective removal of cells and cellular debris by this method was verified by fluorescent labelling of the decellularized TiO2 nanopattern surfaces with Hoechst 33342.
250; Invitrogen Life Technologies). The effective removal of cells and cellular debris by this method was verified by fluorescent labelling of the decellularized TiO2 nanopattern surfaces with Hoechst 33342.
Results and discussion
In this study we investigated the fabrication of TiO2-based biomaterials with specific dimensional and geometrical cues that stimulate specific cellular functions. These fabricated constructs mimic the native environment around cells. We used HepG2 cells to identify biomaterials that enhance hepatocellular functions. In general, HepG2 cells exhibit low cellular functions when cultured in flat dishes. Therefore, electron beam lithography was used to produce dimensionally well-defined TiO2 nano and submicro structures with various shapes. The influence of these shapes on the cellular behaviour of hepatocytes was determined.The shape, dimensions and topographical variation of the fabricated TiO2 nanopatterned substrates were characterized using SEM and AFM. Fig. 1 shows SEM and AFM three-dimensional figures for nanograting and nanorectangle patterns with total dimensions of 240 nm. These photographs show uniform dimensional spacing (in nanometers) and shape divergence between the TiO2 gratings and nanorectangles. For example, the TiO2 nanogratings are 90 nm wide and 150 nm apart (Fig. 1C), and each rectangle in the TiO2 nanorectangles also has a width of 90 nm and is separated from its neighbours by 150 nm (Fig. 1D). Thus, structural uniformity with shape and dimensional consistency were confirmed by these analyses.
One challenge in tissue engineering is the construction of a biomaterial that mimics the native in vivo micro/nanoenvironment while preserving cellular viability and functionality. Many reports have focused on the discrepancies in cellular responses, which are highly dependent on the type of human cell and the surface characteristics of the biomaterial.10,17
To address these challenges, we constructed various surfaces with different shapes and heterotropic dimensions. The multiple patterns of TiO2 mimic the nanocues provided by naturally occurring ECM components to control and regulate numerous hepatocyte functions. Thus, grating shapes and intermittent nanorectangles that could mimic hierarchically-extended collagen nanofibrillar structures were generated using electron beam lithography and atomic layer deposition. The distance between TiO2 nanogratings was 150, 220 or 320 nm, and the width of the nanogratings was either 90 or 120 nm (Fig. 1). The height of the nanofeatures (40 nm) was chosen to fit the size range of collagen fibrils20 found in cells in their native environment. An increase in the z scale dimension of protruding nanofeatures has been shown to impair cellular response in different cells.17,21
Influence on functional protein expression
The natural ECM around hepatocytes inherently stimulates the production of functional proteins such as albumin, transferrin, and cytochrome P-450. Therefore, to examine the effect of variations in TiO2 topography on the behaviour of HepG2 cells, and to identify which TiO2 nano-features closely mimic the natural ECM and support optimum cell behaviour, we observed the change in the production level of these functional proteins using fluorescence immunostaining techniques.Albumin
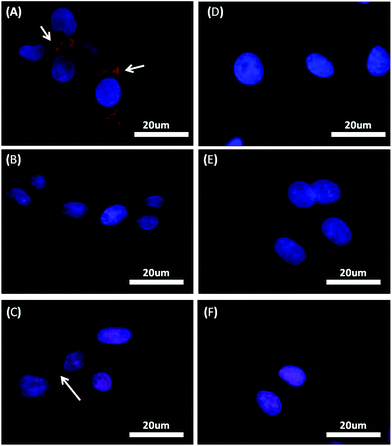
Fluorescence immunostaining of albumin was performed 12 h after seeding the HepG2 on various TiO2 substrates, followed by observation of green fluorescence using a fluorescence microscope (Fig. 2). Shape modulation and dimensional alteration of the TiO2 topography by the expansion and reduction of the distance between different sizes of TiO2 nanogratings greatly influenced the production of albumin (green fluorescence). Grating patterns with dimensions between 240 and 280 nm (Fig. 2A and B) and nanorectangles with 240 nm total dimension (Fig. 2E) exhibited higher albumin expression relative to HepG2 cells grown on flat surfaces (Fig. 2F). In particular, HepG2 cells cultured on a 240 nm TiO2 substrate (Fig. 2A) showed a greater increase in albumin production compared to cells cultured on other TiO2 nanograting substrates with different dimensions. A significant decrease in the production of albumin was observed upon alteration of geometry (rectangles instead of continuous lines) with the same dimensions (Fig. 1A and E).A quantitative comparison of albumin expression is presented in Fig. 2G, in which the calculated fluorescence intensities obtained from HepG2 cells cultured on TiO2 substrates were statistically compared to the fluorescence observed from cells cultured on flat surfaces. Cells grown on nanograting shapes with total dimensions of between 240 nm and 280 nm and nanorectangles of TiO2 substrates showed a significant increase in albumin expression relative to the control (p < 0.05). Continuous nanogratings with a dimension of 240 nm significantly stimulated the production of albumin compared to other TiO2 nanogratings with various dimensional spacings, and the other shape with the same dimensional spacing (p < 0.05) (Fig. 2G). Thus, the results suggest that the 240 nm TiO2 substrate with a continuous linear shape closely mimic the natural environment essential for the optimum production of albumin by HepG2 cells.
Transferrin
Production of the functional protein transferrin was determined from green fluorescence in the cells using an immunostaining technique. Fig. 3 shows fluorescence images of the formed transferrin upon culturing of HepG2 on TiO2 substrates. Transferrin production increased 12 h after seeding of HepG2 on the 240 nm linear nanopattern TiO2 substrate (Fig. 3A). Furthermore, seeding on the intermittent rectangle pattern resulted in only a slight increase in the production of transferrin compared to the control (Fig. 3F). Low transferrin expression was also observed in HepG2 cells cultured on TiO2 substrates with different winder interspacing between the nanogratings (Fig. 3B–D), and cells cultured on a flat surface also showed very low transferrin production (Fig. 3F). These results suggest that the level of transferrin is modulated by the simulation of natural ECM cues induced by 240 nm TiO2 substrates only, and that other size of nanofeatures decrease the level of transferrin production.Cytochrome P-450
The production of cytochrome P-450 2C6 was analysed after culturing HepG2 cells for various lengths of time on TiO2 nanopatterned substrates. Fig. 4 shows cytochrome P-450 as red fluorescence inside HepG2 cells cultured on TiO2 substrates after 18 h. The results indicate no significant increase in the production of cytochrome P-450 12 h after cell seeding (data not shown), but 18 h after seeding, cells cultured on TiO2 nanogratings with spacings of 240 nm and 310 nm (Fig. 4A and C) showed a significant increase in cytochrome P-450 compared to cells cultured on other topographical dimensional features and shapes, or on a flat surface (Fig. 4B, D, E and F). These results suggest that cytochrome P-450 levels are stimulated by topography with size 240 and 310 nm TiO2 substrate with continuously linear nanofeatures.Our results suggest several outcomes. First, the change in the planer area between the nanogratings of bio-inspired surfaces resulted in changes in hepatocellular functionality. Second, we demonstrate that the loss of collagen-like fibrillar structures by changing from a continuous linear shape to an intermittent shape or to rectangles considerably altered the cellular functionality of HepG2. Third, our findings suggest that the control of the physical nanocues associated with a certain dimension and shape that closely mimics the natural ECM could be critical for optimizing cellular behaviour.
We focused on determining the functional responses of HepG2 cells to a wide array of topographic features with various dimensions and shapes. HepG2 cells cultured on TiO2 nanopattern substrates were functionally more active compared to cells cultured on flat surfaces. The expression levels of albumin, transferrin and cytochrome P450 were tested as functional makers. Albumin, a liver-specific marker, was investigated in HepG2 cultured on different TiO2 substrates. TiO2 nanopatterns that closely mimicked the matrix configuration (total lateral dimension of 240 nm) showed higher albumin expression. Likewise, TiO2 substrate with this specific lateral dimension supported a higher level of two other functional proteins, cytochrome P-450 and transferrin.
Most of the previous research has focused on the study of total features dimensions (depth or width) ≥300 nm and up to microns,22,23 while a few research only tried to study the influence of smaller dimensional features or the role of diminished scale lateral features on different cell behaviours. Nanogratings total dimension equivalent to 210 nm was able to induce effective alteration in the alignment and orientation of glioma cells.24 Furthermore, osteoblast morphologically recognize surface topography starting from 75 nm with resulting alteration in the alignments and deposition of mineralized ECM components, while 150 nm features size confirmed to start the induction of osteoblast-specific genes expression25 (alkaline phosphatase, osteocalcin, and bone sialoprotein). Moreover, the expression of IL-1β and TNF-α from murine macrophage in vivo and in vitro was upregulated on nanogratings starting from 150 nm lateral dimension, on which these signalling proteins play a considerable role in the wound healing process.26 Such topography, features size <300 nm, in particular likely to highly mimics the variable distance between individual collagen fibrils since collagen fibrils size are varying in diameter 30–120 nm as previously reported.20 This allows the optimum cell–cell/cell–surface communication.
Previously, anisotropically dimensional nanostructures with gratings/interspace 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 were used, the increase in the lateral dimensions even up to micro-scale resulted in an enhancement in the cellular functions.17 However, our results suggested that an increase in the nanofeatures, gratings/interspace from ≃1
1 were used, the increase in the lateral dimensions even up to micro-scale resulted in an enhancement in the cellular functions.17 However, our results suggested that an increase in the nanofeatures, gratings/interspace from ≃1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 (90
2 (90![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 150 nm) to ≃1
150 nm) to ≃1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 (90
3 (90![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 220 nm) or ≃1
220 nm) or ≃1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 (90
4 (90![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 320 nm) has been associated with a decrease in production of some tested functional proteins (i.e. albumin). While a recent research reported that the increase in the interspace between nanogratings to 1
320 nm) has been associated with a decrease in production of some tested functional proteins (i.e. albumin). While a recent research reported that the increase in the interspace between nanogratings to 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 (300
5 (300![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1500 nm) would significantly enhance the differentiation of human neuronal stem cells with the increase in expression of specific neuronal biomarkers.23 These discrepancies in the behaviour of cells over topographical features could be reasoned by 2 facts: the cell type is a key element in cell–material interactions, as different cells behave diversely when cultured in the nano/submicro-scale nanofeatures.27 Secondly, the cells are sensitive to topographical alterations, as the same cells could behave differently by the modification in the feature specific dimensions despite fixing the lateral dimension and shape. For instance, a change in the alignment percentage of corneal epithelial cells is observed when altering the gratings/interspace dimension from 1
1500 nm) would significantly enhance the differentiation of human neuronal stem cells with the increase in expression of specific neuronal biomarkers.23 These discrepancies in the behaviour of cells over topographical features could be reasoned by 2 facts: the cell type is a key element in cell–material interactions, as different cells behave diversely when cultured in the nano/submicro-scale nanofeatures.27 Secondly, the cells are sensitive to topographical alterations, as the same cells could behave differently by the modification in the feature specific dimensions despite fixing the lateral dimension and shape. For instance, a change in the alignment percentage of corneal epithelial cells is observed when altering the gratings/interspace dimension from 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 (70
4 (70![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 330) to 1
330) to 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 (180
2 (180![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 220) for nanogratings with 400 nm lateral dimension.28,29 Consequently, the nanostructures lateral dimension (240 nm) likely supports the correct orientation and integrity of hepatocytes and allows optimum cellular behaviours.
220) for nanogratings with 400 nm lateral dimension.28,29 Consequently, the nanostructures lateral dimension (240 nm) likely supports the correct orientation and integrity of hepatocytes and allows optimum cellular behaviours.
The other geometry TiO2 surface investigated nanorectangles resulted in a decrease in the formation of albumin, transferrin and cytochrome P450 compared to cells attached to the continuous striated form of the same size. Thus, the higher the degree of resemblance between the superficial topographical cues on TiO2 substrates and natural ECM, the better the cytocompatibility and functionality of HepG2 grown on TiO2 nanopatterns.
Our results suggest that the topographical characteristics of nanocues of well-defined dimension and shape (240 nm with a striated appearance) can control and regulate multiple hepatocyte functions. While other dimensions and shapes resulted in increased production of hepatic proteins, such as the 310 nm dimension for gratings for cytochrome P-450 expression or nanorectangles for transferrin expression (all as compared to the control), the simultaneous maintenance of elevated levels of several hepatic functions relies on sophisticated signals. These entangled signals are organized to some extent by diversified physical and biochemical cues. The underlying mechanisms for this enhanced functionality are yet to be fully revealed and will be explored in the future. These studies will provide an in-depth understanding of the relationship between ECM-like topography and liver cell functionality, and thus will potentially be of great benefit for tissue engineering and its future applications.
Influence on the structure of hepatocytes
Modulation of TiO2 topography and changes in the shape of the TiO2 pattern while maintaining the critical dimension (240 nm) greatly influenced cellular orientation, cell alignment and elongation. These alterations in the cells, and its directional induced cellular orientations were examined by observing the rearrangement of actin filaments and integrin-mediated focal adhesion.Cell alignments and actin filament rearrangements
To investigate the effects on the cytoskeleton of simultaneously altering the shape of the substrate features while maintaining the dimensions, the assembly and reorganizations of actin filaments were observed by culturing HepG2 cells on heterotropic and dimensionally well-defined TiO2 nanograting/nanorectangle substrates. Fig. 5 shows the fluorescence staining of actin filaments, which indicates partial structural reorganization of hepatocyte cells to an orientation parallel to the TiO2 nanogratings. In contrast, rectangular nanostructures with the same dimension (240 nm) enhanced the formation of filopodia and lamellapodia (Fig. 5B) but provided a less organized cytoskeleton. These alterations in the cellular framework 12 h after cell seeding were compared to cells grown on flat surfaces. HepG2 cultured in TiO2 nanograting substrates displayed a fundamentally different morphology compared to cells cultured on nanorectangles or flat surfaces. On continuous nanogratings, around 60–70% of the cells are aligned in the longitudinal direction, parallel to the nanogratings, and exhibit enhanced cellular spreading. Cells cultured on intermittent rectangles or flat surfaces did not show this alignment. The aforementioned extension of actin filaments could result from the fixation of anchoring points in the planer area between the TiO2 gratings with the orientation of focal adhesion formation along the gratings.30 Alternatively, nanorectangles decreased the cellular spreading while guiding the formation of more filopodia compared to control. This suggests the extension of filopodia to explore the surrounding nanorectangles on which the focal adhesions’ assembly occurred.Integrin mediated focal adhesion
The number of integrin β1 mediated focal adhesions formed was compared for cells grown on different configurations of TiO2 nanopattern with a total dimension of 240 nm and was compared to a flat TiO2 substrate. Comparisons were done using immunofluorescence staining of a transmembrane protein characteristic for focal contacts, integrin β1. Fig. 6 shows the expression of integrin β1 clusters as green fluorescence upon culturing of HepG2 cells over TiO2 nanogratings and nanorectangles with 240 nm total dimensions and a flat surface substrate for 12 h. An increase in the number of integrin β1 formed was observed after seeding HepG2 cells on a continuous grating compared to seeding on nanorectangles or on a flat TiO2 substrate (Fig. 6A and C). Fewer integrin β1 clusters were expressed on the intermittent nanograting compared to the flat TiO2 substrate (Fig. 6B and C).While a diverse variety of cellular behaviours are influenced by integrins mediated signals, the recruitment and clustering of integrins usually occurs by a vast number of extracellular and intracellular stimuli for the anchoring and focal adhesion formation.31 Integrins are heterodimeric receptors that are responsible for transmembrane signal transduction from the surrounding native ECM to the cell. The extracellular domain of integrin binds to specific motifs in the ECM, while the cytoplasmic domain is associated with the actin cytoskeleton and other affiliated proteins. For example, integrin β1 receptors are likely involved in the maintenance and development of specific cell architectures. The multiplicity and functionality of integrin β1 expression in hepatic cells such as HepG2 has been previously reported.32 TiO2 substrate with a striated appearance altered the expression of integrin β1 more than the other substrates (nanorectangles and a flat surface), suggesting that the modification in physical traits of a substrate could be responsible for alteration in cellular behaviours. This is in correspondence with the previously reported research, in which integrin α2β1 receptors recognize collagen fibrils with a highly ordered architecture more than collagen monomers with only unique peptide motif consequently altered the cellular adhesion.33
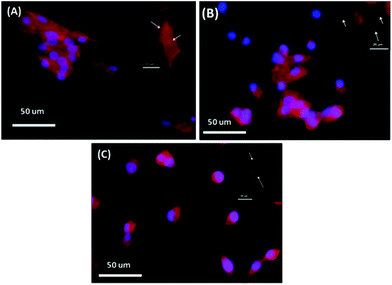
Alteration in natural extracellular matrix assembly
We propose that culturing HepG2 on TiO2 substrates will have a considerable impact on the formation and assembly of natural ECM components such as fibronectin and collagen IV. Thus, a decellularization procedure was performed using H2O, as previously reported, and then the change in the formation and degree of assembly of fibronectin and collagen IV was examined using an immunostaining technique. Fig. 7 shows the increase in the level of naturally assembled fibronectin and collagen IV after culturing and decellularization of HepG2 cells on 240 nm TiO2 substrate with different shapes relative to the control. The construction and assembly of two native ECM components, fibronectin and collagen IV, were considerably stimulated 66 h after seeding HepG2 cells on TiO2 substrate containing linear nanofeatures compared to cells seeded on rectangular nanostructures or on a flat surface (Fig. 7). These results suggest that TiO2 substrate with linear shape nanofeatures (total dimension of 240 nm) provided essential cues to increase the magnitude and assembly of naturally-produced ECM components.Our results suggest that both proteins were preferentially deposited and assembled in between the TiO2 gratings compared with nanorectangles with a total dimension of 240 nm or with flat surfaces. Thus, the striated appearance of the TiO2 surface, which resembles native collagen fibrils in shape and dimensions, might be responsible for the abundant and aligned assembly of ECM components. Furthermore, HepG2 cells recognized this possible bio-inspired relevance with native ECM and reacted with this prominent resemblance accordingly. Thus, the superficial traits of biomaterials are an important tool for manipulating and controlling the formation of highly organized ECM.
Conclusions
In summary, we fabricated a TiO2 substrate with specific superficial topographical nanofeatures of various sizes and shapes using electron beam lithography and atomic layer deposition. Our results suggest that the interactions of a mammalian hepatic cell line with nanogratings, which have heterotropic dimensions, may regulate cellular function. Additionally, we confirmed that alteration of the shape of nanofeatures while maintaining the same dimensional characteristics could be an effective tool to control cellular behaviour. For example, alteration of collagen fibrillar-like TiO2 structures from a continuous linear shape to intermittent rectangles lowered the cellular functionality of HepG2 cells. Finally, our findings suggest that physical nanocues, such as edge to edge spacing and feature models, must be controlled for optimum cellular behaviour. These findings could be utilized in the design of facile and reproducible surfaces with distinct functionalities. Identification of the best topographical dimensions and configurations for hepatocyte-based biomaterials might provide the basis for the fabrication of specialized surfaces for implantable bioreactors to treat liver failure, or the production of therapeutically valuable proteins.Notes and references
- A. S. G. Curtis, N. Gadegaard, M. J. Dalby, M. O. Riehle, C. D. W. Wilkinson and G. Aitchison, IEEE Trans Nanobiosci., 2004, 3, 61 CrossRef CAS; M. J. Dalby, N. Gadegaard1, R. Tare, A. Andar, M. O. Riehle, P. Herzyk, C. D. W. Wilkinson and R. O. C. Oreffo, Nat. Mater., 2007, 6, 997 CrossRef PubMed; M.-H. You, M. K. Kwak, D.-H Kim, K. Kim, A. Levchenko, D.-Y. Kim and K.-Y. Suh, Biomacromolecules, 2010, 11, 1856 CrossRef PubMed.
- M. E. Lukashev and Z. Werb, Trends Cell Biol., 1998, 8, 437 CrossRef CAS.
- E. Pamula, V. De Cupere, Y. F. Dufrene and P. G. Rouxhet, J. Colloid Interface Sci., 2004, 271, 80 CrossRef CAS PubMed.
- L. Bozec, G. van derHeijden and M. Horton, Biophys. J., 2007, 92, 70 CrossRef CAS PubMed.
- N. Wang and D. E. Ingber, Biophys. J., 1994, 66, 2181 CrossRef CAS.
- G. A. Abrams, S. L. Goodman, P. F. Nealy, M. Franco and C. J. Murphy, Cell Tissue Res., 2000, 299, 39 CrossRef CAS.
- L. K. Hornberger, S. Singhroy, T. Cavalle-Garrido, W. Tsang, F. Keeley and M. Rabinovitch, Circ. Res., 2000, 87, 508 CrossRef CAS.
- C. Wu and S. Dedhar, J. Cell Biol., 2001, 155, 505 CrossRef CAS PubMed.
- M. J. Paul Biggs, R. G. Ritchard and M. J. Dalby, Nanomedicine, 2010, 6, 619 CrossRef CAS PubMed; K. Kulangara, Y. Yang, J. Yang and K. W. Leong, Biomaterials, 2012, 33, 4998–5003 CrossRef PubMed.
- C.-H. Choi, S. H. Hagvallb, B. M. Wu, J. C. Y. Dunn, R. E. Beygui and C.-J. Kim, Biomaterials, 2007, 28, 1672 CrossRef CAS PubMed.
- G. Takayama, A. Taniguchi and T. Okano, Tissue Eng., 2007, 13, 159 CrossRef CAS PubMed.
- M. Bokhari, R. J. Carnachan, N. R. Cameron and S. A. Przyborski, J. Anat., 2007, 211, 567 CAS.
- Y. Nishida and A. Taniguchi, In Vitro Cell. Dev. Biol.: Anim., 2013, 49, 400 CrossRef CAS PubMed.
- A. Hoessa, N. Teuschera, A. Thormanna, H. Aurichb and A. Heilmanna, Acta Biomater., 2007, 3, 43 CrossRef PubMed.
- K. Nakazawa, S.-W. Lee, J. Fukuda, D.-H. Yang and T. Kunitake, J. Mater. Sci. Mater. Med., 2006, 17, 359 CrossRef CAS PubMed.
- K. Anselme, Biomaterials, 2000, 21, 667 CrossRef CAS; K. Kubo, N. Tsukimura, F. Iwasa, T. Ueno and L. Saruwatari, et al. , Biomaterials, 2009, 30, 5319 CrossRef PubMed.
- P.-Y. Wang, J. Yu, J.-H. Lin and W.-B. Tsai, Acta Biomater., 2011, 7, 3285 CrossRef CAS PubMed; K. E. Myrna, R. Mendonsa, P. Russell, S. A. Pot, S. J. Liliensiek, J. V. Jester, P. F. Nealey, D. Brown and C. J. Murphy, Invest. Ophthalmol. Vis. Sci., 2012, 811 Search PubMed; W.-B. Tsai and J.-H. Lin, Acta Biomater., 2009, 5, 1442 CrossRef PubMed; J. A. Wood, I. Ly, D. L. Borjesson, P. F. Nealey, P. Russell and C. J. Murphy, Exp. Cell Res., 2012, 318, 2438 CrossRef PubMed.
- A. Burgess, S. Vigneron, E. Brioudes, J.-C. Labbé, T. Lorca and A. Castro, Proc. Natl. Acad. Sci. U. S. A., 2010, 107, 12564 CrossRef CAS PubMed.
- H. Herrema, D. Czajkowska, D. Théard, J. M. van der Wouden, D. Kalicharan, B. Zolghadr, D. Hoekstra and S. C. D. van IJzendoorn, Mol. Biol. Cell, 2006, 17, 3291 CrossRef CAS PubMed.
- B. Zhu, Q. Lu, J. Yin, J. Hu and Z. Wang, Tissue Eng., 2005, 11, 825 CrossRef CAS PubMed; S. Perumal, O. Antipova and J. P. Orgel, Proc. Natl. Acad. Sci. U. S. A., 2008, 105, 2824 CrossRef PubMed.
- S. A. Biela, Y. i. Su, J. P. Spatz and R. Kemkemer, Acta Biomater., 2009, 5, 2460 CrossRef CAS PubMed.
- F. Pan, M. Zhang, G. Wu, Y. Lai, B. Greber, H. R. Schöler and L. Chi, Biomaterials, 2013, 34, 8131 CrossRef CAS PubMed; K. Kulangara, Y. Yang, J. Yang and K. W. Leong, Biomaterials, 2012, 33, 4998 CrossRef PubMed.
- K. Yang, K. Jung, E. Ko, J. Kim, K. I. Park, J. Kim and S.-W. Cho, ACS Appl. Mater. Interfaces, 2013 DOI:10.1021/am402156f.
- B. Zhu, Q. Zhang, Q. Lu, Y. Xu, J. Yin, J. Hu and Z. Wang, Biomaterials, 2004, 25, 4215 CrossRef CAS PubMed.
- E. Lamers, X. F. Walboomers, M. Domanski, J. te Riet, F. C. M. J. M. van Delft, R. Luttge, L. A. J. A. Winnubst, H. J. G. E. Gardeniers and J. A. Jansen, Biomaterials, 2010, 31, 3307 CrossRef CAS PubMed.
- E. Lamers, X. F. Walboomers, M. Domanski, L. Prodanov, J. Melis, R. Luttge, L. Winnubst, J. M. Anderson, H. J. G. E. Gardeniers and J. A. Jansen, Nanomedicine, 2012, 8, 308 CrossRef CAS PubMed.
- S. A. Biela, Y. i. Su, J. P. Spatz and R. Kemkemer, Acta Biomater., 2009, 5, 2460 CrossRef CAS PubMed.
- S. A. Fraser, Y.-H. Ting, K. S. Mallon, A. E. Wendt, C. J. Murphy and P. F. Nealey, J. Biomed. Mater. Res. A, 2007, 87A, 725 Search PubMed.
- A. I. Teixeira, G. A. Abrams, P. J. Bertics, C. J. Murphy and P. F. Nealey, J. Cell Sci., 2003, 116, 1881 CrossRef CAS PubMed.
- W. A. Loesberg, J. te Riet, F. C. M. J. M. van Delft, P. Schön, C. G. Figdor, S. Speller, J. J. W. A. van Loon, X. F. Walboomers and J. A. Jansen, Biomaterials, 2007, 28, 3944 CrossRef CAS PubMed.
- A. Luque, M. Gomez, W. Puzon, Y. Takada, F. Sanchez-Madrid and C. Cabanas, J. Biol. Chem., 1996, 271, 11067 CrossRef CAS PubMed; M. Gomez, A. Luque, M. A. del Pozo, N. Hogg, F. Sanchez-Madrid and C. Cabanas, Eur. J. Immunol., 1997, 27, 8 CrossRef PubMed.
- A. Matsumotu, S. Arao and M. Otsuki, Hepatology, 1999, 29, 68 CrossRef PubMed.
- J. Jokinen, E. Dadu, P. Nykvist, J. kapyla, D. J. White, J. Ivaska, P. Vehvilainen, H. Reunanent, H. Larjava, L. Hakkinen and J. Heino, J. Biol. Chem., 2007, 279, 31956 CrossRef PubMed.
| This journal is © The Royal Society of Chemistry 2014 |