Osteogenic differentiation of human mesenchymal stem cells on α5 integrin binding peptide hydrogels is dependent on substrate elasticity†
Navakanth R.
Gandavarapu
a,
Daniel L.
Alge
b and
Kristi S.
Anseth
*ab
aDepartment of Chemical and Biological Engineering and the BioFrontiers Institute, University of Colorado Boulder, Boulder, Colorado – 80309, USA
bHoward Hughes Medical Institute, University of Colorado Boulder, Boulder, Colorado – 80309, USA. E-mail: Kristi.anseth@colorado.edu
First published on 18th November 2013
Abstract
The extracellular matrix plays a crucial role in controlling human mesenchymal stem cell (hMSC) biology including differentiation, and α5β1 integrin signaling plays an important role during osteogenic differentiation of hMSCs. Here, peptide-functionalized hydrogels were used to examine the role of α5β1 integrin signaling in inducing osteogenic differentiation in hMSCs. Further, the role of substrate elasticity was also studied. A thiol–ene chemistry was used to functionalize poly(ethylene glycol) hydrogels with a pendant peptide moiety, c(RRETAWA), as previous studies have shown that RRETAWA containing peptides bind to the α5β1 integrin with very high specificity. Notably, hMSC attachment to c(RRETAWA)-functionalized hydrogels was found to occur primarily through α5 integrins, as the number of attached cells was significantly reduced to ∼20% upon blocking the α5 integrin during culture. To investigate the interplay between stiffness and c(RRETAWA) concentration, hydrogels were formulated with Young's moduli of ∼2 kPa (soft) and ∼25 kPa (stiff) and c(RRETAWA) concentrations of 0.1 mM and 1 mM. Stiff substrates led to ∼3.5 fold higher hMSC attachment and ∼3 fold higher cell area in comparison to soft substrates. hMSCs formed robust and larger focal adhesions on stiff substrates at 1 mM c(RRETAWA) compared to soft substrates. Alkaline phosphatase (ALP) activity in hMSCs cultured on stiff gels at 0.1 mM and 1 mM c(RRETAWA) was increased 2.5 and 3.5 fold, respectively after 14 days in growth media. hMSCs did not show an increase in ALP activity when cultured on soft gels. Further, gene expression of osteogenic related genes, core binding factor-1, osteopontin and Collagen-1a at day 14 in hMSCs cultured on stiff gels at 1 mM c(RRETAWA) were increased 10, 7 and 4 fold, respectively, while on soft gels, gene expression was at basal levels. Collectively, these results demonstrate that the combination of high substrate stiffness and α5β1 integrin signaling stimulated by c(RRETAWA) is sufficient to induce osteogenic differentiation of hMSCs without requiring the addition of soluble factors.
Introduction
In vitro osteogenic differentiation of human mesenchymal stem cells (hMSCs) is often achieved by dosing with soluble cues (e.g., dexamethasone, β-glycerol phosphate) and/or by insoluble cues presented by the extracellular matrix (ECM). Significant progress has been made in understanding the role of soluble cues and the signaling path binding to cell receptors. For example, upon dosing with dexamethasone, a synthetic glucocorticoid, hMSCs upregulate integrin expression1–3 and signal expression of core binding factor-1 (CBFA1), which drives osteogenic differentiation. In addition, researchers are beginning to understand the complementary role of insoluble cues on the differentiation program of hMSCs. Integrin signaling is one of the important mechanisms originating from the ECM, and several studies have implicated its role in maintaining the survival,4 differentiation2,5 and migration6,7 of hMSCs. For example, α5β1 integrin has been shown to play an important role in hMSC migration and osteogenic differentiation, while upregulating αVβ3 integrin negatively regulates osteogenic differentiation.Binding of extracellular ligands to integrins initiates intracellular biochemical signaling pathways. However, the dynamics of this signaling is highly dependent on the biophysical properties of the underlying substrate, which directly affects binding and engagement. In addition, several studies have demonstrated that the biophysical properties of the ECM microenvironment directly influence the differentiation program of hMSCs via ROCK/Rho pathways that converge to upregulate expression of several genes.8,9 For example, hMSCs cultured on hydrogels with a high substrate elasticity (e.g., ∼25 kPa) upregulated expression of the osteogenic related gene CBFA1 via Rho/ROCK dependent pathways without the need for any soluble cues.8 Nevertheless, because integrins act as pivot points through which cells sense the mechanical properties of the underlying substrate, understanding the interplay between substrate elasticity and integrin signaling is critical for the design of cell-instructive biomaterials capable of directing hMSC fate and function.
Several integrins have been implicated in the osteogenic differentiation of hMSCs.2,5,10,11 Studies blocking integrin function via antibodies or siRNA knockdown have demonstrated that α5β1,2,5 α2β1,4 and α3β110 integrins promote osteogenesis, while αVβ35,12 integrin lowers ALP activity and reduces matrix mineralization. Recent studies by Hamidouche et al.2,3 have demonstrated that dexamethasone induced osteogenic differentiation in hMSCs is mediated via α5β1 integrin, as evidenced by abrogation of ALP activity and osteogenic gene expression upon silencing α5β1 integrin expression with siRNA. Also, hMSCs underwent osteogenesis upon forced induction of α5β1 integrin without the need for dexamethasone,2 further demonstrating the importance of the α5β1 integrin signaling pathway in osteogenic differentiation in hMSCs. Further, fibronectin fragments, which allowed for specific interaction with α5β1 integrin, upregulated ALP activity and osteogenic gene expression, demonstrating that signaling through the α5β1 integrin specifically promotes osteogenic differentiation in hMSCs.5
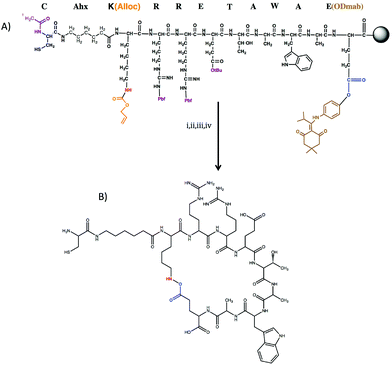
Owing to the importance of α5β1 integrin signaling, we sought to further understand the interplay between α5β1 integrin signaling and substrate elasticity in osteogenic differentiation of hMSCs. To realize this goal, we synthesized a cyclic RRETAWA peptide (i.e., c(RRETAWA)), which is fully synthetic and is not derived from an extracellular matrix protein. The selection of this peptide was inspired by previous studies showing that the cyclic peptide CRRETAWAC binds α5β1 integrin with very high affinity and specificity (IC50 for α5β1 integrin binding is 0.01 μM and for αVβ3 integrin is >1000 μM)13 due to the interaction of the Arg–Arg–Glu motif with the β1 integrin subunit and also the strong hydrophobic interaction of Trp residue with the α5 subunit.14–16 In our peptide, the key RRETAWA sequence was cyclized via on-resin lactam bridge formation between the Lys and Glu residues flanking the RRETAWA. A Cys residue was also included at the N-terminus to allow for facile incorporation into synthetic thiol–ene PEG hydrogels, which are readily tuned to vary the presentation of biochemical cues as well as substrate elasticity.17–19
In this study, hMSCs were seeded onto c(RRETAWA) functionalized hydrogels and cultured in 2D. Prior to experimentation, the ability of soluble peptide to induce hMSC osteogenic differentiation was assessed, and the specificity of the peptide ligand for α5β1 integrin was evaluated by integrin blocking experiments. The interplay between the level of α5 integrin priming and substrate elasticity in driving osteogenic differentiation of hMSCs was then evaluated in 2D culture by quantitatively characterizing cell attachment, focal adhesion formation, ALP activity, and osteogenic gene expression. Our results demonstrate that both substrate stiffness and ligand concentration play a significant role in the interaction of hMSCs with the peptide-functionalized gels. Stiffer substrates allowed hMSCs to interact with the peptides much more strongly, as evidenced by their ability to form well developed focal adhesions, even at lower peptide concentrations, while softer substrates required a higher ligand concentration to form stable focal adhesions. Our results also suggest that priming of α5 integrin alone is not sufficient for inducing osteogenic differentiation in hMSCs and a higher substrate elasticity is required.
Materials and methods
All materials were purchased from Sigma unless otherwise mentioned.Cell culture
hMSCs were isolated from bone marrow (Lonza) and cultured in growth media (low glucose Dulbecco's Modified Eagle Medium (DMEM, Gibco) supplemented with 10% fetal bovine serum (Gibco), 1% Penicillin/Streptomycin (Gibco), and 0.2% Fungizone (Gibco)). hMSCs at passage three were used in all the studies.Synthesis of c(RRETAWA) and CRDGS
Peptide sequences CAhxK(Alloc)RRETAWAE(ODmab), and CRDGS were synthesized on rink amide resin through standard Fmoc solid-phase synthesis method using 2-(1H-benzotriazole-1-yl)-1,1,3,3-tetramethyluronium hexafluorophosphate (HBTU) activation on a Tribute solid-phase peptide synthesizer. The N-terminus amine of the H-CAhxK(Alloc)RRETAWAE(ODmab) was acetyl capped (resulting in Ac-CAhxK(Alloc)RRETAWAE(ODmab) peptide sequence) and on-resin cyclization was performed as described below.The peptide sequence, Ac-CAhxK(Alloc)RRETAWAE(ODmab), was treated with 2% hydrazine in DMF (3×) to cleave ODmab protecting group. The resin was then thoroughly washed in DMF, followed by washing in DCM and allowed to swell for overnight. The Alloc deprotection was carried out by treating resin with a solution of 0.1 eq. Tetrakis(triphenylphosphine)palladium(0) (Pd(PPh3)4, Sigma) and 24 eq. phenyl silane (C6H8Si, Sigma) in DCM for 10 min and washed in DCM (4×). The Alloc deprotection step was repeated three times to ensure complete deprotection. Alloc deprotection of amines was verified by a positive Kaiser test. The resin was washed and swelled in DMF for overnight. On resin cyclization of the peptide was conducted in 1 eq. of HBTU in DMF for overnight. A negative Kaiser test was used to monitor the disappearance of amines due to cyclization of the peptide.
Both peptides were cleaved from the resin with a trifluoroacetic acid–triisopropyl silane–water–phenol (94![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.5
2.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.5
2.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) mixture and precipitated and washed (2×) in cold ethyl ether. Precipitated peptide was dessicated overnight. The c(RRETAWA) peptide was purified using HPLC, lyophilized and stored at −20 °C until further use. Purified fractions of the target peptide were identified using matrix assisted laser desorption/ionization time of flight mass spectroscopy (MALDI-TOF MS, predicted m/z: 1384.90 found m/z: 1384.87).
1) mixture and precipitated and washed (2×) in cold ethyl ether. Precipitated peptide was dessicated overnight. The c(RRETAWA) peptide was purified using HPLC, lyophilized and stored at −20 °C until further use. Purified fractions of the target peptide were identified using matrix assisted laser desorption/ionization time of flight mass spectroscopy (MALDI-TOF MS, predicted m/z: 1384.90 found m/z: 1384.87).
Synthesis of poly(ethylene glycol)-norbornene (PEG-N)
Multiarm poly(ethylene glycol) (8 arm Mn = 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 Da and 4 arm Mn 20
000 Da and 4 arm Mn 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 Da) norbornene was synthesized as described previously. Briefly, poly(ethylene glycol) amine (JenKem, USA) was dissolved in DCM and reacted with 2 eq. norbornene carboxylic acid at 50 °C for overnight in the presence of 0.5 eq. 4-dimethylaminopyridine (DMAP) and 1 eq. N,N′-diisopropylcarbodiimide (DIC). The reaction mixture was then precipitated slowly in cold ethyl ether dropwise. The product was dessicated for overnight and dialysed against water using a 2000 Da molecular weight cutoff membrane for 24 h. The product was lyophilized and stored at −20 °C until further use. Proton NMR was used to verify successful functionalization and >90% of amine groups were found to be functionalized with norbornene.
000 Da) norbornene was synthesized as described previously. Briefly, poly(ethylene glycol) amine (JenKem, USA) was dissolved in DCM and reacted with 2 eq. norbornene carboxylic acid at 50 °C for overnight in the presence of 0.5 eq. 4-dimethylaminopyridine (DMAP) and 1 eq. N,N′-diisopropylcarbodiimide (DIC). The reaction mixture was then precipitated slowly in cold ethyl ether dropwise. The product was dessicated for overnight and dialysed against water using a 2000 Da molecular weight cutoff membrane for 24 h. The product was lyophilized and stored at −20 °C until further use. Proton NMR was used to verify successful functionalization and >90% of amine groups were found to be functionalized with norbornene.
Preparation of thiolated glass coverslips
Heat treated glass cover slips were dipped in a solution of (3-mercaptopropyl)triethyoxy silane in ethanol for 5–10 min. Coated glass cover slips were then cleaned with ethanol and water (2×) to remove any excess silane and dried in air. Air dried cover slips were then baked at 80 °C for at least 1 h and stored at 4 °C until further use.Preparation of peptide functionalized hydrogels
For the preparation of stiff hydrogels, eight-armed PEG-N (MW: 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 Da), poly(ethylene glycol) dithiol (PEG-diSH) (Sigma, MW: 2000) and 1 mM of pendant monothiol peptides were dissolved in sterile PBS to yield final monomer solutions of 80 mM [ene], 79 mM [crosslinker thiol] and 1 mM [monothiol peptide]. For preparation of soft hydrogel, four-armed PEG-N (MW: 20
000 Da), poly(ethylene glycol) dithiol (PEG-diSH) (Sigma, MW: 2000) and 1 mM of pendant monothiol peptides were dissolved in sterile PBS to yield final monomer solutions of 80 mM [ene], 79 mM [crosslinker thiol] and 1 mM [monothiol peptide]. For preparation of soft hydrogel, four-armed PEG-N (MW: 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000), PEG-diSH (MW: 2000) and 1 mM pendant monothiol peptides were dissolved in sterile PBS to yield a final monomer solution with 16 mM [ene], 15 mM [crosslinker thiol] and 1 mM [monothiol peptides]. To these monomer solutions, the photoinitiator lithium phenyl-2,4,6-trimethylbenzoylphosphinate (LAP) was added at a final concentration of 0.05 wt% (1.7 mM). The 1 mM pendant monothiol peptide mixture consisted of either 0.1 mM of c(RRETAWA) and 0.9 mM CRDGS or 1 mM of c(RRETAWA). 120 μm thick hydrogels were formed on thiolated glass coverslips by exposing the monomer solution to ∼10 mW cm−2 of 365 nm light for 5 min. Gels were swelled in PBS for at least 48 h and sterilized under germicidal UV light for 12 h before cell seeding.
000), PEG-diSH (MW: 2000) and 1 mM pendant monothiol peptides were dissolved in sterile PBS to yield a final monomer solution with 16 mM [ene], 15 mM [crosslinker thiol] and 1 mM [monothiol peptides]. To these monomer solutions, the photoinitiator lithium phenyl-2,4,6-trimethylbenzoylphosphinate (LAP) was added at a final concentration of 0.05 wt% (1.7 mM). The 1 mM pendant monothiol peptide mixture consisted of either 0.1 mM of c(RRETAWA) and 0.9 mM CRDGS or 1 mM of c(RRETAWA). 120 μm thick hydrogels were formed on thiolated glass coverslips by exposing the monomer solution to ∼10 mW cm−2 of 365 nm light for 5 min. Gels were swelled in PBS for at least 48 h and sterilized under germicidal UV light for 12 h before cell seeding.
Integrin blocking experiments
hMSCs were incubated with antibody solutions containing antibodies against α5 (Abcam), αVβ3 (Abcam) integrins or IgG (Isotype control, Sigma, M5284) in serum-free media for 30 min and seeded onto 1 mM c(RRETAWA) stiff gels (see Table 1 for description of stiff gels) in serum free media at 5 × 103 cells per cm2 density. Integrin blocked hMSCs were allowed to attach to the gels for 12 h, washed with PBS to remove loosely attached cells and stained with calcein AM for live cells. Stained hMSCs were imaged using an epifluorescence microscope, and the number of attached cells was counted manually using ImageJ software.| PEG-N MW | Functionality | In-text description | [Ene]/mM | c(RRETAWA)/mM | CRDGS/mM | [Linker peptide thiol]/mM | Young's modulus, E/kPa |
|---|---|---|---|---|---|---|---|
| 20 kDa | 4 | Soft | 16 | 0.1 | 0.9 | 15 | ∼2.5 |
| 20 kDa | 4 | Soft | 16 | 1 | 0 | 15 | ∼2.7 |
| 10 kDa | 8 | Stiff | 80 | 0.1 | 0.9 | 79 | ∼22 |
| 10 kDa | 8 | Stiff | 80 | 1 | 0 | 79 | ∼25 |
Rheological characterization of peptide hydrogels
Gels were formed between two glass slides separated by a 1 mm gasket and allowed to swell in PBS for 48 h. 8 mm cylindrical disks were then cut out, and their shear modulus was measured by conducting frequency sweep from 1–100 rad s−1 at 3% strain using a rheometer (Discovery DH-3, TA Instruments). The strain rate chosen was in the linear visco-elastic regime. The results are reported as average measurements of three independent gels. The Young's modulus (E) of each gel was then calculated from the shear modulus (G) using the following equation from rubber elasticity theory,20| E = 2(1 + ν)G | (1) |
Focal adhesion staining and determination of cell area, aspect ratio and focal adhesion area
Focal adhesion staining was performed on hMSCs attached to gels (5 × 103 cells per cm2 seeding density) using rhodamine phalloidin (Molecular Probes, R415, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200 dilution) for f-actin and mouse anti-vinculin antibody (Abcam). This cell density was chosen to avoid excessive cell–cell contact after hMSC attachment to gels. Cells were counterstained with 4,6-diamidino-2-phenylindole (DAPI, Invitrogen, 300 nM) for nuclei and stored in PBS at 4 °C until imaged with a Zeiss confocal microscope (LSM710). To calculate hMSC shape parameters, fluorescent images were converted to binary images and analyzed using the built-in ‘analyze particles’ macro in ImageJ that automatically generates an average area per cell and aspect ratio. A total of nine images per condition were analyzed. To quantify focal adhesion size, fluorescent images were processed using ImageJ for background normalization using a FFT bandpass filter, converted to binary images, and morphometric analysis of focal adhesions were conducted by an analyze particles plug-in. A total of nine images per condition were analyzed.
200 dilution) for f-actin and mouse anti-vinculin antibody (Abcam). This cell density was chosen to avoid excessive cell–cell contact after hMSC attachment to gels. Cells were counterstained with 4,6-diamidino-2-phenylindole (DAPI, Invitrogen, 300 nM) for nuclei and stored in PBS at 4 °C until imaged with a Zeiss confocal microscope (LSM710). To calculate hMSC shape parameters, fluorescent images were converted to binary images and analyzed using the built-in ‘analyze particles’ macro in ImageJ that automatically generates an average area per cell and aspect ratio. A total of nine images per condition were analyzed. To quantify focal adhesion size, fluorescent images were processed using ImageJ for background normalization using a FFT bandpass filter, converted to binary images, and morphometric analysis of focal adhesions were conducted by an analyze particles plug-in. A total of nine images per condition were analyzed.
Alkaline phosphatase (ALP) production of hMSCs on peptide functionalized gels
ALP production was measured using an assay based on the change in absorbance of o-nitrophenol (ALP substrate, Invitrogen), as it is enzymatically cleaved by ALP. Cells were removed from culture, washed in PBS, and lysed in 50 μl RIPA buffer (Invitrogen) for 15 min with gentle shaking. The sample solutions were diluted with 50 μl of PBS. 50 μl of the sample solution was then mixed with 50 μl of ALP substrate, and the absorbance of the solution at 405 nm was measured at 1-minute intervals ten times with a plate reader (Biotek). ALP activity levels were calculated as the slope of the increase in absorbance at 405 nm with time. DNA content in the cell lysate was also measured using the Picogreen assay for dsDNA (Invitrogen). ALP activity levels were normalized to total DNA content (μg) and reported relative to day 1 as normalized ALP activity.Gene expression of hMSCs cultured on peptide functionalized gels
Expression of osteogenic related genes in hMSCs cultured on c(RRETAWA) functionalized gels was analyzed using reverse-transcription polymerase chain reaction (RT-PCR). At day 14, gels were removed from culture and washed three times in PBS. RNA was isolated using TRI reagent (Sigma) according to standard manufacturer's protocols. The obtained RNA pellet was re-suspended in nuclease free water, treated with DNAse (Bio-Rad), washed with phenol–chloroform solution and was precipitated in isopropanol. Obtained RNA was re-suspended in 20 μl nuclease free water and quantified by measuring absorbance at 280 nm using a NanoDrop spectrophotometer (Thermo Scientific). Samples whose 280 nm to 260 nm absorbance ratio was greater than 1.85 were considered pure and were used for reverse transcription step.Reverse transcription was performed using the iScript cDNA Synthesis Kit (Bio-Rad). A total of 15 ng RNA was used for the single-stranded cDNA synthesis. The reverse transcription reaction tube was incubated at 25 °C for 5 min, at 42 °C for 30 min, and terminated at 85 °C for 5 min. PCR was conducted using an iCycler Real-Time PCR machine (Bio-Rad). Primers and probes used in this study are summarized in Table S1.† The following PCR parameters were utilized: 95 °C for 90 s followed by 45 cycles of 95 °C for 30 s and 55 °C for 60 s. Threshold cycle (CT) analysis was used to quantify PCR products, normalized to GAPDH and relative to expression of hMSCs at day 1 cultured on TCPS in growth media.
Statistical analysis
Data collected throughout this study are represented as mean ± standard error of at least three samples. For comparison between stiff, soft gels the data set were analyzed using 2-way ANOVA analysis with Tukey tests for comparison. In all studies, p-values less than 0.05 were considered significant.Results
Synthesis and characterization of α5 binding peptide
Kouvinen et al.13 have shown that cyclic CRRETAWAC peptide binds to α5 integrin with high specificity and selectivity. To create a peptide that could be incorporated into a thiol–ene hydrogel network, this peptide sequence was modified slightly. Briefly, the key RRETAWA sequence13,14 was flanked by lysine(Alloc) and glutamic acid(ODmab) residues at the N- and C-terminal ends, respectively. As the Alloc and ODmab groups can be selectively removed on resin, this design allowed for cyclization via lactam bridge formation between the side chain amine of lysine and the side chain carboxylic acid of glutamic acid. To tether the peptide into the network during gel formation, a cysteine residue was introduced at the N-terminus. To facilitate cell binding, an alkyl spacer, amino-hexanoic acid (Ahx), was included in the peptide sequence, resulting in Cys-Ahx-Lys(Alloc)-Arg-Arg-Glu-Thr-Ala-Trp-Ala-Glu(ODmab). On-resin cyclization of the peptide was conducted as detailed in Section 2.2 and verified via MALDI TOF mass spectroscopy. Fig. 1 details the peptide design and synthesis scheme for the c(RRETAWA).To assess the effect of c(RRETAWA) on osteogenic differentiation of hMSCs, the peptide was first dissolved in growth media and delivered to hMSCs cultured on tissue culture polystyrene (TCPS) at a dosage of either 7.35 or 73.5 μM. Fig. S1† shows that the normalized ALP activity of hMSCs was significantly upregulated at day 7 and 14 compared to the negative control. Also, increasing the dosage 10 fold (i.e., from 10 μg ml−1 to 100 μg ml−1) significantly increased ALP activity, indicating that the effect was dosage dependent. ALP activity results demonstrate that priming α5 integrin via the c(RRETAWA) peptide induced ALP production indicating osteogenic differentiation in hMSCs. We next sought to incorporate the c(RRETAWA) into a gel formulation, and characterize hMSC interactions with and response to the immobilized peptide.
hMSCs bind to c(RRETAWA) gels via α5 integrin
hMSC binding to c(RRETAWA) functionalized gels was assessed by studying their attachment to c(RRETAWA) functionalized PEG gels in the presence and absence of antibodies blocking the α5 integrin. As shown in Fig. 2A, the attachment of hMSCs to c(RRETAWA) gels was reduced to ∼20% of control upon blocking the α5 integrin before seeding, suggesting that hMSCs are primarily utilizing α5 integrin in binding to these gels. To support this further, hMSCs were blocked with an antibody for αVβ3 integrin, and their attachment to the c(RRETAWA) gels was tested. As shown in Fig. 2A, ∼85% of the hMSCs remained attached to the gels when αVβ3 was blocked, demonstrating that the αVβ3 integrin is less important in cell binding to the c(RRETAWA) surfaces.Finally, Fig. 2B–D shows immunostaining for α5 integrin (green) and f-actin cytoskeleton (red) in hMSCs seeded onto c(RRETAWA). These images clearly demonstrate localization of α5 integrin, as dash shaped structures at the tips of the actin fibers, further supporting that hMSCs are binding to the c(RRETAWA) gels via α5 integrin.
Effect of substrate elasticity and peptide concentration on hMSC adhesion to c(RRETAWA) gels
hMSCs have been shown to be sensitive to substrate elasticity, and several studies have implicated the role of mechanotransduction in determining the differentiation pathway of hMSCs.9,21–23 In this study, we were interested in examining the role that substrate elasticity might play in hMSC interactions with c(RRETAWA) functionalized gels, and ultimately, how this could influence hMSC fate. Studies by Engler et al.8 have shown that substrates with a Young's modulus between 25–40 kPa promote osteogenic differentiation while those with ∼0.1–1 kPa are too soft to promote osteogenic differentiation in hMSCs. Hence, we formulated soft hydrogels with a Young's modulus that is not conducive for hMSC osteogenic differentiation (i.e., ∼2 kPa) and stiff gels with a modulus that should promote hMSC osteogenic differentiation (i.e., ∼25 kPa). These gels were functionalized with either 0.1 or 1 mM c(RRETAWA). Table 1 lists the specific monomer formulations used to form both the soft and stiff gels, along with their corresponding Young's modulus measured by rheology. As a negative control, the non-cell adhesive scrambled peptide, CRDGS, was included in the monomer formulations to maintain the total thiol![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ene functional group ratio at 1
ene functional group ratio at 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and keep the total pendant peptide concentration constant.
1 and keep the total pendant peptide concentration constant.
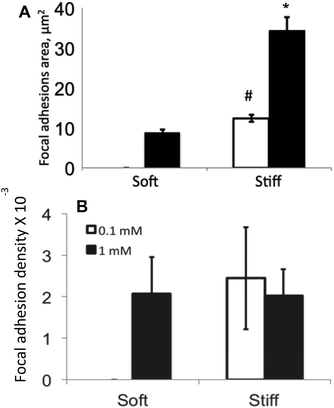
Initially, we assessed the effect of substrate elasticity and ligand concentration on hMSC adhesion by measuring the cell attachment density, cell shape parameters (i.e., area and aspect ratio) and focal adhesion formation. Fig. 3A shows hMSC attachment density to soft and stiff c(RRETAWA) gels as a function of peptide concentration. In general, hMSC attachment was significantly higher on stiffer substrates as compared to the softer ones, irrespective of ligand concentration. Also, at both of the substrate elasticities studied, a higher peptide concentration allowed greater hMSCs attachment, and interestingly, this difference was largest on the stiff gels. Fig. 3B and C summarizes the influence of material properties on cell area and the aspect ratio of attached hMSCs, respectively. A significantly higher cell area on stiff gels compared to soft gels demonstrates that higher stiffness encouraged higher spreading of hMSCs; however, the aspect ratio of the attached cells was relatively unchanged, regardless of the gel properties. At a ligand concentration of 1 mM, the cell area increased 3 fold from ∼5000 μm2 on soft to ∼15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 μm2 on stiff gels. Interestingly, increasing the ligand concentration on stiff gels significantly increased the cell spreading area without affecting the aspect ratio. Only a marginal effect of ligand concentration on aspect ratio was observed on soft gels.
000 μm2 on stiff gels. Interestingly, increasing the ligand concentration on stiff gels significantly increased the cell spreading area without affecting the aspect ratio. Only a marginal effect of ligand concentration on aspect ratio was observed on soft gels.
To assess the nature of the focal adhesions formed in hMSCs cultured on these gels, immunostaining for vinculin (green) and f-actin formation (red) was conducted, and the resulting images are shown in Fig. 4. These images clearly demonstrate that, on stiffer gels, hMSCs form prominent focal adhesions and display well developed actin cytoskeleton as compared to softer gels. On soft gels, hMSCs formed prominent focal adhesions and developed actin fibers only at the 1 mM c(RRETAWA) concentration. At lower concentrations, hMSC did not develop an organized cytoskeleton, as evidenced by the diffuse vinculin and f-actin staining, suggesting no focal adhesion formation. On stiff gels, hMSCs formed well-developed actin fibers and prominent dash shaped focal adhesions at both the 0.1 and 1 mM peptide concentrations. Qualitatively, there was no difference in actin fiber organization on stiff gels as a function of adhesive peptide concentration.
Upon binding to their corresponding ligands, integrins present on the cell surface undergo clustering and form dash shaped focal adhesions, which can act as a scaffold for recruitment of several signaling proteins including focal adhesion kinase (FAK).24 Given the importance of FAK signaling in osteogenic differentiation of hMSCs demonstrated by previous studies,11 we quantified the area of focal adhesions by immunostaining for vinculin in hMSCs on these various gel formulations. As shown in Fig. 5, hMSCs developed larger focal adhesions on stiff gels compared to soft gels. Specifically, on the stiff gels, the focal adhesions were ∼3 fold larger at 1 mM as compared to 0.1 mM, indicating that the interaction of hMSCs with the material microenvironment depends on both the ligand concentration and substrate mechanical properties. Similar results were also observed on soft gels, where hMSCs developed small, but prominent, focal adhesions, but only at the higher ligand concentration. We next quantified the focal adhesion density to determine the fraction of cell area occupied by focal adhesions. As shown in Fig. 5B, the focal adhesion density was found to be similar on stiff gels on both the ligand density studied. Interestingly, the focal adhesion density on soft gel at 1 mM c(RRETAWA) was only slightly lower than that on stiff gel.
Osteogenic differentiation of hMSCs on c(RRETAWA) functionalized gels
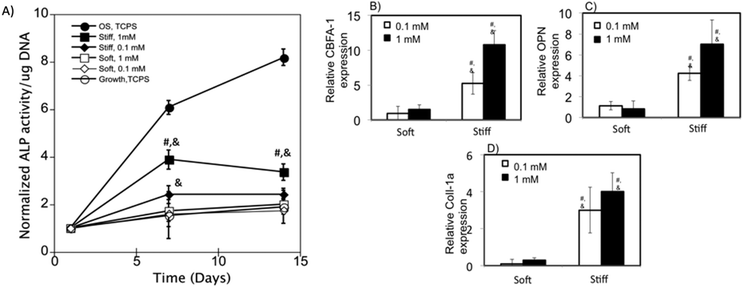
To test the osteogenic differentiation of hMSCs on c(RRETAWA) functionalized gels, we measured ALP activity of hMSCs on stiff and soft gels at both the 0.1 and 1 mM peptide concentrations cultured in growth media. Fig. 6A shows the normalized ALP activity of hMSCs relative to day 1 for cells cultured on these gels in growth media without any osteogenic supplements. ALP activity of hMSCs on TCPS grown in growth media and osteogenic media is also included as negative and positive controls respectively. The ALP activity of hMSCs at day 7 and 14 grown on soft gels was found to be similar to the negative control irrespective of the ligand concentration, indicating that osteogenesis was not induced in hMSCs on softer gels. hMSCs cultured on stiff gels significantly upregulated ALP activity ∼4 fold at day 7 and ∼3.5 fold at day 14 on 1 mM c(RRETAWA) gels. Interestingly, ALP activity on 0.1 mM stiff gels was increased only slightly to around ∼2.5 by day 7 and day 14, but was significantly higher than the negative control.To further characterize osteogenic differentiation of hMSCs cultured on these materials, gene expression of CBFA1, osteopontin (OPN), and Coll-1a genes was also measured at day 14 using RT-PCR. As shown in Fig. 6B–D, expressions of the genes associated with osteogenesis were significantly upregulated on stiff gels compared to hMSCs on TCPS culture in growth media at both the peptide concentrations. Specifically, gene expression of CBFA1, OPN and Coll-1a was increased by 10, 7, and 4 fold, respectively, on 1 mM c(RRETAWA) gels and by 5, 4, and 3 fold, respectively, on 0.1 mM c(RRETAWA) gels when hMSCs were cultured in growth media, indicating a dose dependent upregulation of the genes. On soft gels, expression of the osteogenic genes was at basal levels at both the peptide concentrations studied, indicating that hMSCs did not undergo significant osteogenesis on soft gels, which was consistent with the ALP activity measurements. Taken together, our results indicate that the combination of high substrate stiffness and α5β1 integrin signaling stimulated by c(RRETAWA) is sufficient to induce osteogenic differentiation of hMSCs without requiring the addition of soluble factors.
Discussion
α5β1 is an important integrin that binds specifically to fibronectin and has been previously implicated in hMSC osteogenic differentiation.2,5 Expression of α5β1 is upregulated in dexamethasone induced hMSC, and several pathways that converge to upregulate osteogenic related genes have been shown to be dependent on α5β1 integrin activation.2 Previous studies have shown that α5β1 binds synergistically to both the RGD and PHSRN peptide motifs found in fibronectin.25 Interestingly, other fibronectin binding integrins, such as αVβ3, do not require this synergistic interaction with PHSRN. Hence, stimulation of α5β1 integrin cannot be accomplished via RGD peptide motifs alone, although the RGD peptide supports hMSC attachment.26 To overcome this problem of specificity, researchers have previously used engineered fibronectin fragments that retain the domain conformation to bind and activate α5β1.5 However, use of these fragments poses significant challenges to systematically study the interplay between substrate elasticity and the level of integrin binding, as often the non-covalent coating efficiency of these fragments on culture substrates is highly dependent on chemical composition and elasticity of the underlying substratum, thus coupling the substrate elasticity and ligand concentration.27–29 In this study, we utilized a thiol–ene chemistry to present a small peptide motif that has been previously shown to be capable of binding preferentially to α5β1 integrin to study how substrate elasticity influences osteogenic differentiation of hMSC induced by α5β1 integrin signaling.The non-natural peptide motif RRETAWA was used as a ligand to facilitate α5β1 binding, as it has been previously found to bind α5β1 integrin with high specificity.13,15,16 In this study, we synthesized a c(RRETAWA) peptide that allowed its covalent conjugation into PEG hydrogels via cysteine (Fig. 1). First, we tested the effect of α5β1 integrin binding on osteogenic differentiation of hMSCs by delivering the cyclic peptide in its soluble form. Dosing hMSCs with 7.35 and 73.5 μM of c(RRETAWA) (corresponding to 10 and 100 μg ml−1 respectively) increased ALP activity significantly at day 7 and 14 (Fig. S1†), suggesting that this peptide cue enhances hMSCs osteogenic differentiation.
Ultimately, we were interested in studying the effect of substrate elasticity on α5β1 induced osteogenic differentiation of hMSCs. Thus, the peptide was covalently tethered to a PEG hydrogel to form c(RRETAWA) functionalized cell culture substrates. Thiol–ene photopolymerizations have been recently established as a versatile and robust scheme for the synthesis of peptide containing step-growth PEG hydrogels.19 This synthesis scheme allows for facile functionalization of PEG hydrogels with cysteine containing biochemical moieties without any requirement of post-synthetic modification and also provides the spatiotemporal control of photochemistry. Using this scheme, our group has previously reported PEG gels whose biochemical functionalization and elasticity can be tuned via controlling the ratio of reactive functionalities (i.e., [thiol]![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [ene] ratio), as well as PEG macromer molecular weight and functionality.18 In this study, we used an 8 arm PEG macromer (10 kDa) and a 4 arm PEG macromer (20 kDa) to form hydrogels with elastic moduli of ∼25 kPa and ∼2 kPa, respectively. These hydrogels were formed using stoichiometric reaction conditions and functionalized with either 0.1 mM or 1 mM of the c(RRETAWA) peptide. To maintain the on-stoichiometry conditions, a scrambled CRDGS peptide was included in the monomer formulations. CRDGS peptide motif is well known to not support cell adhesion30 and hence should not compete or interfere with the binding to the c(RRETAWA) peptide.
[ene] ratio), as well as PEG macromer molecular weight and functionality.18 In this study, we used an 8 arm PEG macromer (10 kDa) and a 4 arm PEG macromer (20 kDa) to form hydrogels with elastic moduli of ∼25 kPa and ∼2 kPa, respectively. These hydrogels were formed using stoichiometric reaction conditions and functionalized with either 0.1 mM or 1 mM of the c(RRETAWA) peptide. To maintain the on-stoichiometry conditions, a scrambled CRDGS peptide was included in the monomer formulations. CRDGS peptide motif is well known to not support cell adhesion30 and hence should not compete or interfere with the binding to the c(RRETAWA) peptide.
Binding of hMSCs to these gels through α5β1 integrin was verified via integrin blocking experiments (Fig. 2). Blocking of α5 integrin with antibodies resulted in ∼80% reduction of hMSC attachment to c(RRETAWA) gels. Although we did not block the β subunit, prior research has shown that the Arg–Arg–Glu motif of RRETAWA peptides interacts strongly with the β1 integrin subunit. Thus, the drastic reduction in cell adhesion with α5 integrin blocking strongly suggests that hMSCs interact with c(RRETAWA) functionalized hydrogels through the α5β1 integrin. We also monitored attachment levels upon blocking αVβ3 integrin on hMSCs. αVβ3 blocking only resulted in a slight reduction in hMSC attachment (∼15%), suggesting a minor role for this integrin in hMSC attachment to c(RRETAWA) functionalized hydrogels. These results agree well with the work of Koivunen et al. showing high affinity and specificity of CRRETAWAC for α5β1 (IC50 = 0.01 μM) compared to αVβ3 (IC50 > 1000 μM).13 Immunostaining for α5 integrin was performed to verify visually the interaction of hMSCs with the gels via α5 integrin. As shown in Fig. 2B–D, α5 staining largely localized to dash shaped structures present at the ends of actin fibers. This pattern is expected as integrin binding to ligands results in formation of focal adhesions that act as a scaffold from which actin monomers polymerize to form cell cytoskeleton. Qualitatively, we observe that the majority of actin fibers terminate with α5 staining. This observation agrees with our blocking data and further supports that hMSC bind to c(RRETAWA) presenting gels primarily via α5β1 integrin.
ALP activity and RT-PCR results demonstrate that osteogenic differentiation of hMSCs on c(RRETAWA) hydrogels was achieved without the need for soluble factors (Fig. 6). However, this result was highly dependent on substrate stiffness. hMSCs respond to the changes in the substrate modulus by continuously modulating their contractility that is balanced by the resistive forces from the substrate dictated by their modulus. The mechanical continuum of ECM-integrins-cytoskeleton components at the cell surface is constantly remodelled to maintain this force balance with the underlying substrate. Hence, we hypothesize that engagement of α5β1 through c(RRETAWA) to form stable focal adhesions and upregulation of integrin signaling are achieved only on the stiffer substrates (Fig. 4 and 5) resulting in osteogenic differentiation of hMSCs. This is also supported by previous studies24,31,32 and our results demonstrating the inability to form longer focal adhesions and largely diffuse actin cytoskeleton when hMSCs are cultured on softer gels, which is in contrast to hMSC on stiff gels that form longer focal adhesions and highly organized f-actin. Previous studies by different groups have demonstrated that increased stiffness33 and higher RGD concentration34 resulted in increased osteogenic differentiation. Taken together, our results demonstrate that hMSCs upregulate gene expression in a dose dependent manner with respect to substrate stiffness and ligand concentration. Interestingly, a recent study by Frith et al.35 found that a linear RRETAWA peptide did not induce Runx2 expression in hMSCs cultured on polystyrene-block-poly(ethylene oxide) copolymer substrates in osteogenic media. However, it is possible that the use of osteogenic supplements, which were not used in our study, may have overridden any effects induced by integrin signaling. Furthermore, peptide binding, and consequently integrin signaling, was possibly enhanced in our system due to (1) the high mobility of the PEG chains, which improves peptide accessibility, and (2) the cyclic nature of our peptide, which likely increases integrin binding affinity.36 The results of Friedland et al.37 support our finding that the underlying substrate elasticity plays a major role in conformational stability of α5β1 binding to its ligand. They found that gel stiffness positively correlates with the number of α5β1 integrin bonds formed and FAK phosphorylation at Y397 residue. Y397 phosphorylation of FAK has been previously implicated in osteogenic differentiation of hMSCs.11 Since we observe longer focal adhesions on stiff gels, it is possible that the total FAK phosphorylation at Y397 could be higher, which would result in higher levels of the downstream signal. Future experiments monitoring the osteogenic differentiation in presence of small chemical pFAK inhibitors such as PF-573228 would be interesting to pursue to confirm or negate this hypothesis.
Conclusions
The role of integrin signaling on hMSC osteogenic differentiation was studied on peptide functionalized hydrogel culture substrates. Our data demonstrate that hMSCs interact with c(RRETAWA) functionalized hydrogel substrates primarily via α5 integrins. Monomer formulations were identified to synthesize hydrogels with varying elasticity and ligand concentration independently. hMSC attachment and spreading were significantly higher on stiffer substrates irrespective of the ligand concentration. hMSCs required a higher substrate elasticity to form stable and robust focal adhesions as demonstrated by focal adhesion area data. Collectively, these results demonstrate that higher substrate stiffness upregulated ALP activity and osteogenic related genes in hMSCs in the absence of osteoinductive factors.Acknowledgements
We gratefully acknowledge financial support from National Institute of Health (R01, DE016523) and the Howard Hughes Medical Institute.Notes and references
- S. P. Yun, J. M. Ryu and H. J. Han, J. Cell. Physiol., 2011, 226, 683–692 CrossRef CAS PubMed.
- Z. Hamidouche, et al. , Proc. Natl. Acad. Sci. U. S. A., 2009, 106, 18587–18591 CrossRef CAS PubMed.
- Z. Hamidouche, O. Fromigué, J. Ringe, T. Häupl and P. J. Marie, BMC Cell Biol., 2010, 11, 44 CrossRef PubMed.
- C. Popov, et al. , Cell Death Dis., 2011, 2, e186 CrossRef CAS PubMed.
- M. M. Martino, et al. , Biomaterials, 2009, 30, 1089–1097 CrossRef CAS PubMed.
- J. Veevers-Lowe, S. G. Ball, A. Shuttleworth and C. M. Kielty, J. Cell Sci., 2011, 124, 1288–1300 CrossRef CAS PubMed.
- C. Zou, G. Song, Q. Luo, L. Yuan and L. Yang, In Vitro Cell. Dev. Biol.–Anim., 2011, 47, 241–250 CrossRef CAS PubMed.
- A. J. Engler, S. Sen, H. L. Sweeney and D. E. Discher, Cell, 2006, 126, 677–689 CrossRef CAS PubMed.
- Y.-R. V. Shih, K.-F. Tseng, H.-Y. Lai, C.-H. Lin and O. K. Lee, J. Bone Miner. Res., 2011, 26, 730–738 CrossRef CAS PubMed.
- R. F. Klees, et al. , Mol. Biol. Cell, 2005, 16, 881–890 CrossRef CAS PubMed.
- R. M. Salasznyk, R. F. Klees, W. A. Williams, A. Boskey and G. E. Plopper, Exp. Cell Res., 2007, 313, 22–37 CrossRef CAS PubMed.
- S. L. Cheng, C. F. Lai, S. D. Blystone and L. V. Avioli, J. Bone Miner. Res., 2001, 16, 277–288 CrossRef CAS PubMed.
- E. Koivunen, B. Wang and E. Ruoslahti, J. Cell Biol., 1994, 124, 373–380 CrossRef CAS.
- A. P. Mould, L. Burrows and M. J. Humphries, J. Biol. Chem., 1998, 273, 25664–25672 CrossRef CAS PubMed.
- J. D. Humphries, et al. , J. Biol. Chem., 2000, 275, 20337–20345 CrossRef CAS PubMed.
- A. P. Mould, J. A. Askari and M. J. Humphries, J. Biol. Chem., 2000, 275, 20324–20336 CrossRef CAS PubMed.
- S. B. Anderson, C.-C. Lin, D. V. Kuntzler and K. S. Anseth, Biomaterials, 2011, 32, 3564–3574 CrossRef CAS PubMed.
- S. T. Gould, N. J. Darling and K. S. Anseth, Acta Biomater., 2012, 8, 3201–3209 CrossRef CAS PubMed.
- B. D. Fairbanks, et al. , Adv. Mater., 2009, 21, 5005–5010 CrossRef CAS.
- C. N. Bowman and C. J. Kloxin, AIChE J., 2008, 54, 2775–2795 CrossRef CAS.
- M. A. Wozniak and C. S. Chen, Nat. Rev. Mol. Cell Biol., 2009, 10, 34–43 CrossRef CAS PubMed.
- A. S. Rowlands, P. A. George and J. J. Cooper-White, Am. J. Physiol. Cell Physiol., 2008, 295, C1037–C1044 CrossRef CAS PubMed.
- Y. Sun, C. S. Chen and J. Fu, Annu. Rev. Biophys., 2012, 41, 519–542 CrossRef PubMed.
- B. Geiger, J. P. Spatz and A. D. Bershadsky, Nat. Rev. Mol. Cell Biol., 2009, 10, 21–33 CrossRef CAS PubMed.
- E. H. Danen, et al. , J. Biol. Chem., 1995, 270, 21612–21618 CrossRef CAS PubMed.
- T. A. Petrie, J. R. Capadona, C. D. Reyes and A. J. García, Biomaterials, 2006, 27, 5459–5470 CrossRef CAS PubMed.
- B. G. Keselowsky, D. M. Collard and A. J. García, J. Biomed. Mater. Res. A, 2003, 66, 247–259 Search PubMed.
- B. G. Keselowsky, D. M. Collard and A. J. García, Proc. Natl. Acad. Sci. U. S. A., 2005, 102, 5953–5957 CrossRef CAS PubMed.
- N. B. Guerra, et al. , Soft Matter, 2010, 6, 4748–4755 RSC.
- D. L. Elbert and J. A. Hubbell, Biomacromolecules, 2001, 2, 430–441 CrossRef CAS PubMed.
- T. Shemesh, B. Geiger, A. D. Bershadsky and M. M. F. Kozlov, Proc. Natl. Acad. Sci. U. S. A., 2005, 102, 12383–12388 CrossRef CAS PubMed.
- B. Geiger and A. Bershadsky, Cell, 2002, 110, 139–142 CrossRef CAS.
- K. Chatterjee, et al. , Biomaterials, 2010, 31, 5051–5062 CrossRef CAS PubMed.
- F. Yang, et al. , Biomaterials, 2005, 26, 5991–5998 CrossRef CAS PubMed.
- J. E. Frith, R. J. Mills, J. E. Hudson and J. J. Cooper-White, Stem Cells Dev., 2012, 21, 2442–2456 CrossRef CAS PubMed.
- A. A. Aimetti, M. W. Tibbitt and K. S. Anseth, Biomacromolecules, 2009, 10, 1484–1489 CrossRef CAS PubMed.
- J. C. Friedland, M. H. Lee and D. Boettiger, Science, 2009, 323, 642–644 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c3bm60149h |
| This journal is © The Royal Society of Chemistry 2014 |