Adenosine capped QDs based fluorescent sensor for detection of dopamine with high selectivity and sensitivity†
Qin
Mu
,
Hu
Xu
,
Yan
Li
*,
Shijian
Ma
and
Xinhua
Zhong
*
Institute of Applied Chemistry, Department of Chemistry, East China University of Science and Technology, Shanghai 200237, PR China. E-mail: yli@ecust.edu.cn; zhongxh@ecust.edu.cn; Fax: +86 21 6425 0281
First published on 30th September 2013
Abstract
Facile detection of dopamine (DA) in biological samples for diagnostics remains a challenge. This paper reported an effective fluorescent sensor based on adenosine capped CdSe/ZnS quantum dots (A-QDs) for highly sensitive detection of DA in human urine samples. In this assay, adenosine serves as a capping ligand or stabilizer for QDs to render high-quality QDs dispersed in water, and as a receptor for DA to attach DA onto the surface of A-QDs. DA molecules can bind to A-QDs via non-covalent bonding, leading to the fluorescence quenching of A-QDs due to electron transfer. The A-QDs based fluorescence probe showed a limit of detection (LOD) of ca. 29.3 nM for DA detection. This facile method exhibited high selectivity and anti-interference in the presence of amino acid, ascorbic acid (AA), uric acid (UA) and glucide with 100-fold higher concentration in PBS solution. Furthermore, it was also successfully used in the detection of DA in the human urine samples with quantitative recoveries (94.80–103.40%).
1. Introduction
Dopamine (DA) is one of the most important catecholamine neurotransmitters of the human central nervous system in the brain and it is involved in many brain functions and behavioral responses.1,2 Excess amounts of DA in the brain often cause pleasurable, rewarding feelings, and sometimes even euphoria,3 while the deficiency of DA in the brain may lead to neurological disorders, such as schizophrenia and Parkinson's disease.4 Therefore, a sensitive and accurate determination of DA is extremely important in the diagnostics of various mental diseases. Many strategies have been documented in the literature for the quantification of DA, including electrochemistry,5–10 enzymatic methods,11,12 spectrophotometry,13–17 capillary electrophoresis,18,19 and high performance liquid chromatography (HPLC).20,21 However, most of these methods are complicated and laborious, or require complicated and expensive instruments. Therefore, the further development of simple, cost-effective methods with high selectivity and sensitivity for detecting DA is highly desirable.Colloidal quantum dots (QDs) have triggered extensive attention because of their unique size and composition dependent electrical and optical properties. They exhibit broad absorption profiles and narrow emission with high quantum yields (QY).22–25 Furthermore, QDs allow the chemical modification of functional groups on their surfaces. These properties make QDs naturally suitable for multiplexing applications. One of the most promising applications of luminescent QDs is to serve as fluorescent platforms for sensing and imaging in biology,26–30 which has exhibited remarkable advantages over traditional detection systems for sensitivity and selectivity. In the presence of a large fraction of atoms on the surface, the photoluminescence (PL) of QDs originating from the recombination of the electrons and holes within particles can be strongly affected by surroundings,31,32 which is the pre-requisite for the development of sensing assemblies. However, a great challenge to detection reliability and selectivity arises from the possible instability in QD emission with environment and time. Accordingly, besides hydrophilicity, good chemical and photostability are all essential for the development of QD probes for sensing biomolecules.
Phase transfer has been proven to be an important route to obtain high-quality water-soluble QDs because high-quality luminescent QDs are usually synthesized in organic media.33 In a previous work, we have designed and synthesized a series of bifunctional multidentate thiol ligands to transfer oil-soluble QDs into the water phase with high luminescent brightness.34,35 Recently, we found that commercially available nucleotides and their corresponding nucleosides possess the capability of rendering the initial oil-soluble QDs water-soluble.36 The obtained nucleotides capped water-soluble QDs not only preserved high PL QY but also exhibited excellent stability even under severe conditions. Also, nucleotides contain amino and hydroxyl groups, offering the possibility to interact with DA via hydrogen-bonding and electrostatic interaction. It is well known that DA can quench the fluorescence of QDs because of the non-radiative electron–hole recombination annihilation through effective electron transfer.37,38 A novel fluorescence probe based on the adenosine capped QDs (A-QDs) following the approach illustrated in Scheme 1 has been designed to satisfy the quantitative detection of DA. Adenosine was used as a capping ligand to render the initially oil-soluble QDs water-soluble, serving as a receptor to link DA with QDs via non-covalent hydrogen-bonding and electrostatic attraction at the same time. As an electron acceptor, the oxidized DA on the surface of QDs led to luminescence quenching of QDs via electron transfer, through which the DA was detected quantitatively based on the dependence of amount of DA on the luminescent intensity.
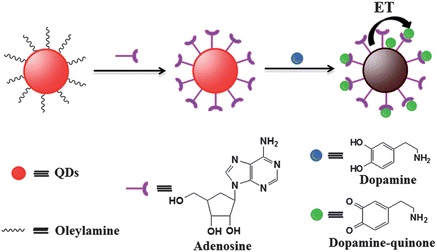 | ||
| Scheme 1 Schematic illustration of fabrication of A-QDs probe and the working principle for the detection of DA. | ||
2. Experimental section
2.1 Chemicals
Cadmium oxide (CdO, 99.99%), zinc acetate dihydrate (Zn(OAc)2·2H2O, 99.8%), selenium powder (Se, 99.99%), sulfur powder (S, 99.98%), trioctylphosphine (TOP, 90%), trioctylphosphine oxide (TOPO, 90%), oleic acid (OA, 90%), oleylamine (OAm, 70%), 1-octadecene (ODE, 90%), glutathione (GSH, reduced form, 99%), adenosine (A, ≥99.0%) and DA (98%) were purchased from Aldrich. Sodium dihydrogen phosphate dehydrate (NaH2PO4·2H2O, ≥99.0%), disodium hydrogen phosphate dodehydrate (Na2HPO4·12H2O, ≥99.0%), hexane, methanol, ethanol and acetone were purchased from Sinopharm Chemcial Reagent Co., Ltd. Tetramethylammonium hydroxide pentahydrate (TMAH, 98%) was purchased from Alfa Aesar. Ultrapure water was used throughout. All reagents purchased from the indicated suppliers were of analytical grade and used without further purification.2.2 Characterization
UV-vis and fluorescence spectra were recorded on a Shimadzu UV-2450 spectrophotometer and a Cary Eclipse (Varian) fluorescence spectrophotometer, respectively. Transmission electron microscopy (TEM) images of the QDs were obtained using a JEOL JEM-1400 transmission electron microscope at an acceleration voltage of 100 kV. FT-IR spectra were recorded on a Magna-IR 550 spectrometer with KBr pellet. The fluorescence lifetime study was performed on an Edinburgh Instruments LifeSpec-ps spectrometer equipped with a Hamamatsu C8898 picosecond light pulser. The excitation light (440 nm) was obtained from a 441 nm laser with frequency of 1.0 MHz. The photographs were taken by a Canon IXUS 110 IS digital camera.2.3 Preparation of water-soluble adenosine capped QDs via ligand exchange
The initial oil-soluble CdSe/CdS/ZnS core/shell/shell QDs were prepared by a minor modified reported method (see ESI†). The water-soluble A-QDs were obtained by ligands exchange from native hydrophobic QDs according to our reported method.36 Typically, 0.1 g (0.37 mmol) of adenosine was mixed with 0.5 mL of ethanol solution, into which TMAH ethanol solution (1.0 M) was added dropwise. The final pH value of the above solution is between 9 and 10. The obtained adenosine solution was added dropwise into the purified oil-soluble QDs dispersed in hexane (5.0 mL) with ultrasonication for 10 minutes. With continual stirring for 10 minutes, the precipitate appeared. The supernatant was discarded, and the precipitate was collected and then dispersed in 5 mL of ultrapure water. To remove the excess amount of ligands, the sample was then precipitated out by the addition of acetone followed by centrifuging. The supernatant was discarded, and the precipitate was redispersed in ultrapure water.2.4 Detection of DA in aqueous solution and biological fluids
2 mL of QDs dispersion (ca. 40 nM) in a clean spectrophotometer quartz cuvette was prepared from QDs stock solution by dilution with PBS solution (10 mM, pH = 7.4). Then different amounts of DA solution of different concentrations were added into the above QDs solution, respectively. After stirring for 20 minutes, the fluorescence spectra were recorded with an excitation wavelength of 350 nm. The obtained PL intensity was noted as I, while the PL intensity of A-QDs suspension without the presence of DA was noted as I0.In order to realize the detection of DA in a real sample, human urine collected from two healthy adult volunteers was used as biological fluid. The urine was first subjected a 100-fold dilution with PBS solution (10 mM, pH = 7.4). The diluted urine samples for DA detection were first spiked with known amounts of DA at different known concentrations. Then A-QDs suspension was introduced into 2 mL of the above simulated biological samples under stirring, the final concentration of A-QDs in the urine samples was 40 nM. After stirring for 20 minutes, the A-QDs suspension was scanned with a fluorescence spectrophotometer. The concentration of DA in the urine samples can be obtained by comparing the PL response I0/I with the linear working plot obtained in ultrapure water. The recovery was calculated as the ratio of the amount of determinated DA to that of the spiked sample. The relative standard deviation was calculated from three consistent determinations.
3. Results and discussion
3.1 Preparation of adenosine capped QDs via ligand exchange
The initial oil-soluble QDs (OAm-QDs) were synthesized according to the reported procedure using oleylamine as capping ligands. A-QDs were obtained from native hydrophobic QDs via ligand exchange following our reported method. The FT-IR spectra of the original oleylamine-capped QDs and the as-received A-QDs clearly showed the successful displacement of the oleylamine shell by the adenosine after phase transfer (Fig. S1†). Compared to the oil-soluble QDs, the characteristic C–H stretching vibrations of OAm-QDs at 2800–3000 cm−1 almost disappeared after ligand exchange, indicating that the original oleylamine molecules attached to the QD surface were almost completely removed and replaced by adenosine ligands. From the TEM images (Fig. S2†), the obtained A-QDs have a nearly identical size (6.0 nm in average) and shape to their initial hydrophobic counterparts.As expected, the A-QDs continued to show the excellent optical properties of the native hydrophobic QDs. As shown in Fig. S3,† the absorption and PL emission profiles of A-QDs exhibited a negligible change in comparison with the original hydrophobic QDs, indicating that the surface adenosine ligands had no effects on the electronic properties of the inorganic QDs cores and no aggregation occurred upon phase transfer. The obtained A-QDs maintained high fluorescence intensity, which is similar to that of initial oil-soluble QDs. We also investigated the pH stability of the obtained A-QDs. It is expected that no obvious aggregation was observed over extended periods of time (months) over a broad pH range (5–12), and A-QDs exhibited a high PL QY in the pH range 6 to 11, as can be seen in Fig. S4.† Importantly, the fluorescence intensity of A-QDs showed no significant change in 24 hours in PBS solution with pH = 7.4 (Fig. S5†), which is crucial for developing the fluorescence probe because the use of QDs in sensing systems requires them to exhibit long-term chemical and photostability in solution. For the detection of DA, the physiological pH value of 7.4 (10 mM PBS solution) was used in the following experimentsl.
3.2 Adenosine capped QDs based optosensing strategy
Scheme 1 shows the schematic illustration of the A-QD sensor for the fluorescence detection of DA. The adenosine ligands at the surface of QDs contain endocyclic and exocyclic amines, heterocyclic nitrogen atoms and hydroxyl groups, which not only can chelate metal cations on the surface of QDs to make the QDs have good water solubility and stability, but they also enable hydrogen-bonding interactions with amine and diols functional groups in DA. Since DA is cationic (pKa 8.86)39 and adenosine is anionic (pKa 3.63) at 7.4, there also exists the possibility of electrostatic interaction between adenosine and DA. Therefore, adenosine plays an important role as a receptor to bind DA species through non-covalent interaction to recognize DA with high specificity. On the other hand, DA could be oxidized by ambient O2 to give dopamine–quinone. The resultant dopamine–quinone bound onto the surface of QDs can quench the fluorescence emission of QDs based on an electron-transfer process from QDs to dopamine–quinone species, allowing specific detection of DA in biological samples.3.3 Possible quenching mechanism of the proposed sensor
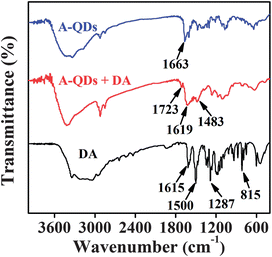
The PL of QDs originates from the recombination of the electrons and holes within the nanocrystal core. Therefore, the changes of the surface or environment of QDs would affect the efficiency of electron–hole recombination and consequently alter the optical properties of these particles. Previous works have shown that DA molecules or conjugation may quench the fluorescence of the QDs, which is a property that has been exploited for sensing applications.37,40–44 It is well known that DA as a type of hydroquinone can be easily oxidized by molecular ambient O2 in basic solution, generating oxidized dopamine–quinone.37 In some studies, the oxidized benzoquinone was suggested as an electron acceptor for QDs through the provision of a favorable non-radiative channel, leading to the fluorescence quenching of QDs.37,38,40,41In this study, as stated above, adenosine on the surface of QDs not only plays a role as a stabilizer for QD stability but also plays an important role as a receptor for DA through non-covalent interactions such as hydrogen-bonding interaction and electrostatic interaction, which could be verified by FT-IR spectra (Fig. 1). Compared to A-QDs, a new peak at 1619 cm−1 for A-QDs–DA system could be observed, which can be attributed to the stretching vibration of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C of benzene ring of DA. It was also observed that there is a new peak at 1723 cm−1 for A-QDs–DA in comparison with free DA. This new peak at 1723 cm−1 for A-QDs–DA is characteristic of the oxidized aromatic conjugated ketones,40 indicating a possible conversion of DA to dopamine–quinone. Moreover, the characteristic peak at 1500 cm−1 for DA, ascribed to –OH vibration stretching of diols, disappeared in the FT-IR spectra of A-QDs–DA. The above results suggest the attachment of DA on the surface of QDs as well as the probable oxidation of DA into dopamine–quinone.
C of benzene ring of DA. It was also observed that there is a new peak at 1723 cm−1 for A-QDs–DA in comparison with free DA. This new peak at 1723 cm−1 for A-QDs–DA is characteristic of the oxidized aromatic conjugated ketones,40 indicating a possible conversion of DA to dopamine–quinone. Moreover, the characteristic peak at 1500 cm−1 for DA, ascribed to –OH vibration stretching of diols, disappeared in the FT-IR spectra of A-QDs–DA. The above results suggest the attachment of DA on the surface of QDs as well as the probable oxidation of DA into dopamine–quinone.
For the PL quenching of A-QDs, fluorescence resonance energy transfer (FRET) was excluded because of no overlap between the absorption spectrum of DA or dopamine–quinone and the emission spectrum of A-QDs (Fig. S6†). This result was also verified by the simultaneous disappearance of the emission spectrum of DA and A-QDs, as shown in Fig. S7.† Therefore, the PL quenching of A-QDs was presumably attributed to electron transfer. To better understand the quenching effect, optical response of A-QDs towards DA was investigated by time-resolved fluorescence spectroscopy (Fig. 2). In general, the PL decay curve for II–VI group QDs can be well fitted by a bi-exponential function:
| I(t) = a1exp(−t/τ1) + a2exp(−t/τ2) |
 | ||
| Fig. 2 Normalized PL decay curves of A-QDs in the absence (closed squares) and presence of DA (5 μM, open squares). The red lines represent the corresponding fitting curves. | ||
| Sample | Spiked (μM) | Measured (μM) | Recovery (%) | RSD (%) |
|---|---|---|---|---|
| Human urine-1 | 0.0 | Not detected | ||
| 5.00 | 4.86 | 97.20 | 2.1 | |
| 10.00 | 9.48 | 94.80 | 4.0 | |
| Human urine-2 | 0.0 | Not detected | ||
| 5.00 | 5.13 | 102.60 | 2.8 | |
| 10.00 | 10.34 | 103.40 | 3.6 |
In order to further confirm the quenching effect of DA on A-QDs, glutathione (GSH) was introduced into the A-QDs–DA system because the GSH is a well-known efficient reducing agent for dopamine–quinone,40,42 generating DA species. As shown in Fig. 3, when GSH was added into the A-QDs–DA system, the fluorescence of quenched A-QDs was recovered. This result demonstrated that dopamine–quinone was a real quencher for A-QDs, providing an efficient electron transfer process from QDs to oxidized DA.
 | ||
| Fig. 3 The PL spectra of A-QDs (black curve), A-QDs–DA system (blue curve) and the A-QDs–DA system after the addition of GSH (25 μM) (red curve) in PBS solution (10 mM, pH 7.4). | ||
3.4 Adenosine capped QDs sensor for dopamine detection
Fig. 4(a) shows the fluorescence response of A-QDs to DA analyte in aqueous solution containing 40 nM QDs. In the absence of DA, the QDs displayed strong fluorescence emission centered at 609 nm. With the addition of DA species, the QDs emission exhibited obvious fluorescence quenching, while the fluorescence emission peak has no obvious shift. Meanwhile, the solution changes from bright yellow-red to colorless under a UV lamp which can be seen with the naked eye (inset of Fig. 4(a)). The fluorescence intensities decreased with increasing successive aliquots of DA concentrations. When the DA concentration went up to 20 μM, the QDs emission was almost completely quenched.Fig. 4(b) shows the plot of fluorescence quenching of A-QDs vs. the concentration of DA. Unambiguously, the fluorescence quenching is closely related to the amount of DA added to the A-QD probe solution, which can be used for the quantification of DA analyte. The Stern–Volmer equation was applied to investigate the relationship between quenching efficiency of QDs and concentration of DA: (I0/I) = 1 + Ksv[A], where I0 and I are the fluorescence intensities in the absence and presence of analyte, respectively. [A] is the concentration of the analyte, and Ksv is the quenching constant of the analyte. As shown in Fig. 4(b) (inset), the linear relationship between I0/I and the concentration of DA could be observed when the concentration of DA was below 20 μM with standard deviation R = 0.994. The limit of detection (LOD) for DA was calculated to be as low as 29.3 nM. These results indicated that the evolution of fluorescence intensity is suitable for the determination of DA within a wide range of 100 nM to 20 μM.
3.5 Selectivity of adenosine capped QDs sensor for DA detection
Together with the sensitivity requirement, it is crucial for chemosensors to specifically detect an analyte.49 To verify the performance of the designed sensor for DA, the influence of possible foreign substances which have either common functional groups or zwitterion structures was tested in the present assay. It was found that the fluorescence of A-QDs showed a selective quenching effect by DA over other substances including ascorbic acid (AA), uric acid (UA), glucide, and amino acids in the present assay, as can be seen in Fig. 5. Other interfering biological molecules in concentrations up to 300 μM have no obvious effect on the fluorescence of A-QDs. However, the introduction of DA with a concentration as low as 3 μM could effectively quench the florescence of A-QDs. This means that these interfering molecules could not produce the wrong signals even if present at 100-fold higher concentrations. Therefore, the developed A-QDs based fluorescence probe offers high selectivity for DA because of the strong noncovalent interactions between DA and adenosine on the surface of the QDs.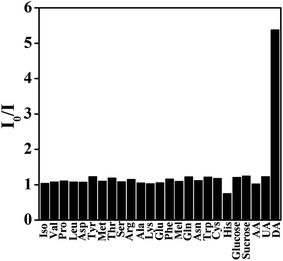 | ||
| Fig. 5 Relative PL response (I0/I) of A-QDs in the presence of DA (3 μM) and other interfering molecules (300 μM) in PBS solution (10 mM, pH 7.4). | ||
3.6 A-QDs probe for the detection of DA in biological fluids
Since it had been reported that the DA concentration in urine reflected the activity of the sympathoadrenal system, the detection of DA in urine is very important for the diagnosis of pheochromocytoma and related diseases.50 In this report, the human urine was used as real sample to estimate the performance of the present assay for DA detection. The A-QDs were added to human urine samples which were prepared by mixing diluted urine with known amounts of DA, and the corresponding PL intensities of A-QDs were recorded. The determined concentration of DA can be obtained from above working plot (Fig. 4(b), inset). The results were summarized in Table 1. It was found that the obtained results were in good agreement with those we added. It is well known that many inorganic cations and anions, uric acid, glucose, amino acids and other organic components are present in human urine. The recovery results here indicated that the presence of these co-existing inorganic and organic components did not significantly interfere with the detection of DA in diluted urine samples. These results demonstrated the practicability of our developed A-QDs probe for the detection of DA in biological samples.4. Conclusions
In summary, a facile and simple A-QDs based chemosensor was investigated for sensitive and selective detection of DA. The QDs obtained from oil-soluble QDs via ligands exchange by using adenosine exhibited a good photo and chemical stability, and it has been demonstrated that the utilization of adenosine as a stabilizer of QDs could also effectively bind DA to the surface of QDs through non-covalent bonding, resulting in fluorescence quenching of the A-QDs to achieve fast detection of DA. The analytical performance of this assay for DA was assessed and it was found that the limit of detection was 29.3 nM. This A-QDs based sensor also exhibited high selectivity and anti-interference among other analogues such as amino acids, UA and AA with 100-fold higher concentrations due to the strong interaction between adenosine and DA, and the electron transfer quenching mechanism.Acknowledgements
This work was supported by Chenguang Program of the Shanghai Education Commission (11CG31), the National Natural Science Foundation of China (no. 21175043), the Fujian Provincial Key Laboratory of Nanomaterials (NM10-06), the Science and Technology Commission of Shanghai Municipality (11JC1403100, 12NM0504101), and the Fundamental Research Funds for the Central Universities.References
- R. N. Adams, Anal. Chem., 1976, 48, 1128A–1138A CrossRef.
- R. D. O'Neill, Analyst, 1994, 119, 767–779 RSC.
- P. N. Tobler, C. D. Fiorillo and W. Schultz, Science, 2005, 307, 1642–1645 CrossRef CAS PubMed.
- S. E. Hyman and R. C. Malenka, Nat. Rev. Neurosci., 2001, 2, 695–703 CrossRef CAS PubMed.
- D. L. Robinson, A. Hermans, A. T. Seipel and R. M. Wightman, Chem. Rev., 2008, 108, 2554–2584 CrossRef CAS PubMed.
- E. Baldrich, R. Gómez, G. Gabriel and F. X. Muñoz, Biosens. Bioelectron., 2011, 26, 1876–1882 CrossRef CAS PubMed.
- P. Biji and A. Patnaik, Analyst, 2012, 137, 4795–4801 RSC.
- D. J. Yu, Y. B. Zeng, Y. X. Qi, T. S. Zhou and G. Y. Shi, Biosens. Bioelectron., 2012, 38, 270–277 CrossRef CAS PubMed.
- M. P. dos Santos, A. Rahim, N. Fattori, L. T. Kubota and Y. Gushikem, Sens. Actuators, B, 2012, 171–172, 712–718 CrossRef CAS PubMed.
- L. Sasso, A. Heiskanen, F. Diazzi, M. Dimaki, J. Castillo-León, M. Vergani, E. Landini, R. Raiteri, G. Ferrari, M. Carminati, M. Sampietro, W. E. Svendsen and J. Emnéus, Analyst, 2013, 138, 3651–3659 RSC.
- M. B. Fritzen-Garcia, F. F. Monteiroa, T. Cristofolini, J. J. S. Acuña, B. G. Zanetti-Ramos, I. R. W. Z. Oliveira, V. Soldi, A. A. Pasa and T. B. Creczynski-Pasa, Sens. Actuators, B, 2013, 182, 264–272 CrossRef CAS PubMed.
- H. Y. Wang, X. G. Feng, M. Zhang and H. Zhao, Anal. Sci., 2007, 23, 1297–1300 CrossRef CAS.
- Q. M. Li, J. Li and Z. J. Yang, Anal. Chim. Acta, 2007, 583, 147–152 CrossRef CAS PubMed.
- Q. L. Xu and J. Y. Yoon, Chem. Commun., 2011, 47, 12497–12499 RSC.
- Y. H. Lin, C. Chen, C. Y. Wang, F. Pu, J. S. Ren and X. G. Qu, Chem. Commun., 2011, 47, 1181–1183 RSC.
- H. C. Lee, T. H. Chen, W. L. Tseng and C. H. Lin, Analyst, 2012, 137, 5352–5357 RSC.
- J. J. Feng, H. Guo, Y. F. Li, Y. H. Wang, W. Y. Chen and A. J. Wang, ACS Appl. Mater. Interfaces, 2013, 5, 1226–1231 CAS.
- Y. S. Zhao, S. L. Zhao, J. M. Huang and F. G. Ye, Talanta, 2011, 85, 2650–2654 CrossRef CAS PubMed.
- M. Bouri, M. J. Lerma-García, R. Salghi, M. Zougagh and A. Ríos, Talanta, 2012, 99, 897–903 CrossRef CAS PubMed.
- K. Syslová, L. Rambousek, M. Kuzma, V. Najmanová, V. B. Valěsová, R. Slamberová and P. Kăcer, J. Chromatogr., A, 2011, 1218, 3382–3391 CrossRef PubMed.
- V. Carrera, E. Sabater, E. Vilanova and M. A. Sogorb, J. Chromatogr., B: Biomed. Appl., 2007, 847, 88–94 CrossRef CAS PubMed.
- X. Peng, L. Manna, W. Yang, J. Wickham, E. Scher, A. Kadavanich and A. P. Alivisatos, Nature, 2000, 404, 59–61 CrossRef CAS PubMed.
- C. Burda, X. Chen, R. Narayanan and M. A. El-Sayed, Chem. Rev., 2005, 105, 1025–1102 CrossRef CAS PubMed.
- N. Tessler, V. Medvedev, M. Kazes, S. H. Kan and U. Banin, Science, 2002, 295, 1506–1508 CrossRef PubMed.
- X. Michalet, F. F. Pinaud, L. A. Bentolila, J. M. Tsay, S. Doose, J. J. Li, G. Sundaresan and A. M. Wu, Science, 2005, 307, 538–544 CrossRef CAS PubMed.
- P. Alivisatos, Nat. Biotechnol., 2004, 22, 47–52 CrossRef CAS PubMed.
- P. Zrazhevskiy, M. Sena and X. H. Gao, Chem. Soc. Rev., 2010, 39, 4326–4354 RSC.
- R. C. Somers, M. G. Bawendi and D. G. Nocera, Chem. Soc. Rev., 2007, 36, 579–591 RSC.
- R. Freeman and I. Willner, Chem. Soc. Rev., 2012, 41, 4067–4085 RSC.
- K. Zhang, H. Zhou, Q. Mei, S. Wang, G. Guan, R. Liu, J. Zhang and Z. Zhang, J. Am. Chem. Soc., 2011, 133, 8424–8427 CrossRef CAS PubMed.
- J. M. Dubach, D. I. Harjes and H. A. Clark, J. Am. Chem. Soc., 2007, 129, 8418–8419 CrossRef CAS PubMed.
- A. Wolcott, D. Gerion, M. Visconte, J. Sun, A. Schwartzberg, S. W. Chen and J. Z. Zhang, J. Phys. Chem. B, 2006, 110, 5779–5789 CrossRef CAS PubMed.
- I. L. Medintz, H. T. Uyeda, E. R. Goldman and H. Mattoussi, Nat. Mater., 2005, 4, 435–446 CrossRef CAS PubMed.
- L. Liu, X. H. Guo, Y. Li and X. H. Zhong, Inorg. Chem., 2010, 49, 3768–3775 CrossRef CAS PubMed.
- Y. Li, B. Shen, L. Liu, H. Xu and X. H. Zhong, Colloids Surf., A, 2012, 410, 144–152 CrossRef CAS PubMed.
- L. Liu and X. H. Zhong, Chem. Commun., 2012, 48, 5718–5720 RSC.
- I. L. Medintz, M. H. Stewart, S. A. Trammell, K. Susumu, J. B. Delehanty, B. C. Mei, J. S. Melinger, J. B. Blanco-Canosa, P. E. Dawson and H. Mattoussi, Nat. Mater., 2010, 9, 676–684 CrossRef CAS PubMed.
- X. Ji, G. Palui, T. Avellini, H. B. Na, C. Y. Yi, K. L. Knappenberger Jr and H. Mattoussi, J. Am. Chem. Soc., 2012, 134, 6006–6017 CrossRef CAS PubMed.
- J. L. Berfield, L. J. C. Wang and M. E. A. Reith, J. Biol. Chem., 1999, 274, 4876–4882 CrossRef CAS PubMed.
- S. Banerjee, S. Kar, J. M. Perez and S. Santra, J. Phys. Chem. C, 2009, 113, 9659–9663 CAS.
- D. Zhao, H. J. Song, L. Y. Hao, X. Liu, L. C. Zhang and Y. Lv, Talanta, 2013, 107, 133–139 CrossRef CAS PubMed.
- X. Z. Ai, Q. Ma and X. G. Su, Microchim. Acta, 2013, 180, 269–277 CrossRef CAS.
- M. Shamsipur, M. Shanehasz, K. Khajeh, N. Mollaniad and S. H. Kazemi, Analyst, 2012, 137, 5553–5559 RSC.
- Q. S. Wen, L. B. Liu, Q. Yang, F. T. Lv and S. Wang, Adv. Funct. Mater., 2013, 23, 764–769 CrossRef CAS.
- J. J. Li, Y. A. Wang, W. Z. Guo, J. C. Keay, T. D. Mishima, M. B. Hohnson and X. G. Peng, J. Am. Chem. Soc., 2003, 125, 12567–12575 CrossRef CAS PubMed.
- S. L. Cumberland, K. M. Hanif, A. Javier, G. A. Khitrov, S. M. Wowssner and C. S. Yun, Chem. Mater., 2002, 14, 1576–1584 CrossRef CAS.
- W. Zhang and X. Zhong, Inorg. Chem., 2011, 50, 4065–4072 CrossRef CAS PubMed.
- P. K. Santra, P. V. Nair, K. G. Thomas and P. V. Kamat, J. Phys. Chem. Lett., 2013, 4, 722–729 CrossRef CAS.
- Q. Mei and Z. Zhang, Angew. Chem., Int. Ed., 2012, 51, 5602–5606 CrossRef CAS PubMed.
- T. J. Panholzer, J. Beyer and K. Lichtwald, Clin. Chem., 1999, 45, 262–268 CAS.
Footnote |
| † Electronic supplementary information (ESI) available: See DOI: 10.1039/c3an01592k |
| This journal is © The Royal Society of Chemistry 2014 |