The Hofmeister effect on nanodiamonds: how addition of ions provides superior drug loading platforms†
Yong
Guo
,
Song
Li
,
Wengang
Li
,
Basem
Moosa
and
Niveen M.
Khashab
*
Controlled Release and Delivery Lab (CRD), Center of Membrane and Porous Materials, Physical Sciences and Engineering Division, King Abdullah University of Science and Technology (KAUST), Thuwal 23955-6900, Kingdom of Saudi Arabia. E-mail: niveen.khashab@kaust.edu.sa
First published on 4th September 2013
Abstract
Colloidal nanodiamonds (NDs) have emerged as highly versatile platforms for the controlled delivery of therapeutics, proteins, DNA, and other assorted biological agents. The most common mechanism of drug loading onto the ND surface depends mainly on electrostatic interactions. Although a few reports have been published on using NaCl salt to increase the drug loading onto NDs, no comprehensive mechanistic study with a wide range of anions and cations has been reported. In this work, the Hofmeister effect of inorganic salts and amino acids with different isoelectric points was employed to understand the mechanism of doxorubicin (DOXH+) loading onto NDs with different sizes. Inorganic salts including NaCl, NaNO3, Na2SO4, KCl, CaCl2, (NH4)2SO4 and amino acids with an isoelectric point above 7 (positively charged at neutral pH) increase the DOXH+ loading onto small size NDs (SNDs, 5–10 nm). On the other hand, amino acids with an isoelectric point below 7 (negatively charged at neutral pH) increase the DOXH+ loading onto large size NDs (LNDs, 80–100 nm).
Introduction
As a new type of carbon materials, nanodiamonds (ND) are receiving considerable attention since they have been applied in many fields, including lubricants, nanocomposites, magnetic recording media, biomedical imagining and drug delivery.1 NDs are highly attractive owing to their innate biocompatibility, scalability, commercial availability, high surface area-to-volume ratio, and ease of surface functionalization. Most ND applications are related to the interaction between charged molecules and the ND surface. This is especially valid in medical and biological areas where NDs are used to deliver drugs,2 genes3,4 and proteins.5,6 It is well accepted that the electrostatic interaction between cargo molecules and deprotonated carboxylic groups on the ND surface is responsible for their loading onto NDs.2–6 A well-known anticancer drug, doxorubicin hydrochloride (DOX·HCl), is loaded onto the ND surface by the interaction between the protonated DOXH+ and the deprotonated carboxylic groups on the ND.7–9 Previous reports show that the electrostatic potential on the protein surface plays a very important role in binding DNA, which has been used to predict DNA binding sites.10,11 A high resolution transmission electron microscope image of an ND cluster has shown that its surface contains different facets.12 According to first-principles computer simulation results for the reconstruction of the unsaturated carbon atoms on the ND surface,13 the facets of the ND surface have a different electrostatic potential, e.g. the {100} facet is positive, while the significant regions of the {111} facet are negative.14,15 Moreover, NDs with different sizes exhibit different aggregation and dispersion behavior in aqueous media.16,17The Hofmeister or “salting-out” effect was first used as a term to describe the effect of different salts on increasing or decreasing the solubility of proteins in aqueous media. Today, the Hofmeister effect is a very general term and can be used to describe the alteration of the solubility of a wide range of polymers and smaller molecules, which are partially miscible with water.18,19 To this end, the Hofmeister effect on the ND surface and its direct application in drug loading has never been described in the literature. Herein, a mechanistic study of the Hofmeister effect of different inorganic salts and amino acids to promote superior drug loading capabilities of NDs is reported. In addition, commercially available NDs with different sizes are employed in this study. Large size NDs (LNDs, 80–100 nm) are usually produced by chemical vapor deposition (CVD) and mainly used in implant coating and biomarkers. Small size NDs (SNDs, 5–10 nm) are readily produced by a detonation technique and have been extensively used for DNA and drug delivery.
Experimental section
Materials
Small and large size nanodiamond powders were purchased from Advanced Abrasives Corp. and characterized fully before use. Doxorubicin hydrochloride (DOX·HCl), sodium chloride (NaCl), sodium nitrate (NaNO3), sodium sulfate (Na2SO4), potassium chloride (KCl), calcium chloride (CaCl2), ammonium sulfate ((NH4)2SO4), serine, glycine, alanine, arginine and lysine were purchased from Sigma Aldrich and used as received.Instruments
Transmission electron microscopy (TEM) images were obtained using a Tecnai 12 (FEI Company) operating at 120 keV. Fourier-transform infrared (FTIR) spectra results were obtained on a Nicolet iS10 system. DOX·HCl loading was measured using the ultra violet-visible spectrophotometer Cary 5000. Raman spectra were recorded using a confocal micro-Raman spectrometer (Horiba Jobin Yvon/Labram Aramis) with an excitation wavelength of 325 nm. Zeta potential and size measurements were performed using the Zetasizer Nano ZS (Malvern). An inductively-coupled plasmaspectrometer (ICP) was used to detect the sodium elements.Dispersion of SNDs and LNDs in acidic and basic media
SND/LND powder (10 mg) was dispersed in 60 ml deionized water and the as-prepared solution was kept for one week to reach a solvation equilibrium. Then, 8 ml supernatant (pH 7) of the SND solution were distributed over three vials, where 0.1 ml of 1 M HCl was added to obtain pH 3, and 0.1 ml of 1 M NaOH was added to afford pH 11.Dispersion of NDs in inorganic salt solutions
ND powder (10 mg) was dispersed in 60 ml deionized water and the as-synthesized solution was kept for one week to reach a solvation equilibrium. Then, 8 ml supernatant of the as-synthesized ND solution were transferred into each of six glass vials. The different salts NaNO3, NaCl, KCl, Na2SO4, CaCl2 and (NH4)2SO4 (20 mg) were added into these vials.DOX loading
NDs (2.0 mg) were added to 5 ml of a deionized water–DOX·HCl (30 μg ml−1) solution and sonicated for 5 minutes. The DOX·HCl concentration was measured at certain time intervals using a UV-vis spectrophotometer by testing the supernatant after centrifugation (14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm).
000 rpm).
Results and discussion
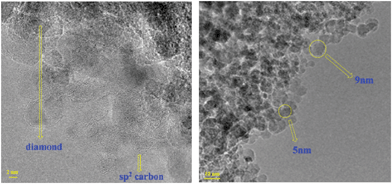
SNDs and LNDs were characterized by IR, TG, ICP, TEM and Raman methods before their dispersibility in water was investigated. According to the FTIR spectrum of the SNDs (Fig. S1†), the carboxylic groups are present on the SND surface, since a peak appears at 1731.8 cm−1.20 The peak at around 3417.3 cm−1 corresponds to the OH stretching vibration, while the one around 1623.8 cm−1 may be caused by the bending mode of the associated OH groups. The ether-like groups in the SNDs may contribute to the absorption peak at 1103.1 cm−1.21 The IR spectra show no evidence of organic impurities. This result was further verified by TG (Fig. S2†), where no significant weight loss was observed from 100 °C to 300 °C.22 ICP analysis was used to detect Zn, Co, Cr, Ni, Ag, Pd, Pt and Cu in the SND samples. No trace metals were found in the samples (the detection limit is 0.03 ppm). Raman spectra of the SNDs (Fig. S3†) show a peak at around 1327 cm−1, which is assigned to the Raman model of the cubic diamond lattice, while the asymmetric peak at around 1640 cm−1 corresponds to the superposition of the sp2 carbon band at 1590 cm−1 and the O–H bending vibration at 1640 cm−1.23 Using high resolution TEM, the sp2 carbon on the SND surface as well as the SND size (5–10 nm) could be easily verified (Fig. 1).22The FTIR spectrum of the LNDs (Fig. S4†) shows a peak at around 1720.3 cm−1, which is attributed to carboxylic groups.20 The peak at around 3417.4 cm−1 corresponds to the OH stretching vibration, while the one at around 1623.8 cm−1 may be caused by the bending mode of the associated OH groups.20,21 The peak at 1083.8 cm−1 is assigned to ether-like groups.24 Similar to the SNDs, TG and ICP tests were performed on the LND samples with no detectable traces of organic or inorganic impurities (Fig. S5†). Raman spectra and TEM images of the LNDs are shown in Fig. S6 and S7,† respectively. To further verify the diamond nature of the LNDs, we compared the XRD spectra of SNDs and LNDs (Fig. S8†) and concluded that both SNDs and LNDs have the same XRD peaks ((111) and (022)), and these peaks are identical to the reported specific XRD peaks for diamond materials.25,26
The NDs were then dispersed in an aqueous solution before the addition of different ions. As the overall charge of the NDs, caused by the protonation and deprotonation of surface functional groups, is the driving force for cargo loading, the dispersion of SNDs and LNDs was tested in both acidic and basic media. SND colloidal solutions at acidic (0.1 M HCl, SNDpH3), basic (0.1 M NaOH, SNDpH11) and neutral (SND) pH values were prepared (Fig. 2). SNDs precipitate out faster from the basic solution (SNDpH11). The order of precipitation is SNDpH11 > SNDpH3 > SND, which was further verified by TEM. Moreover, a time course study of dynamic light scattering (Fig. S9†) confirms this result. The ζ potentials of SNDpH3, SND and SNDpH11 are 24.1 mV, 19.1 mV and −31.8 mV, respectively. The IR spectra of SND, SNDpH3 and SNDpH11 (Fig. S10†) show that the intensity of the OH groups under basic conditions is weaker than under acidic conditions, explaining why the ζ potentials of SNDs under basic conditions are more negative. As SNDs can easily precipitate from basic solutions, it is plausible to deduce that the electrostatic potential on most SND facets is negative.
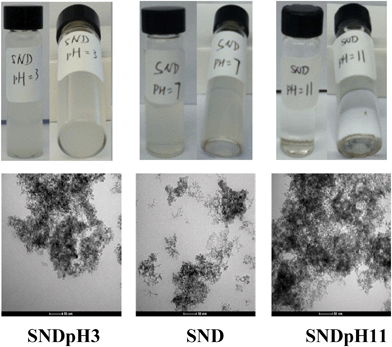 | ||
| Fig. 2 Photographs (top) and TEM images (bottom) of SNDpH3, SND and SNDpH11, in which the aggregation degree of SNDpH11 is larger than that of SNDpH3. | ||
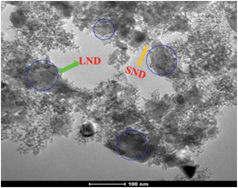
LNDs, on the other hand, precipitate faster from an acidic solution (LNDpH3) than from a basic solution (LNDpH11), which was further verified by the TEM results (Fig. 3). A time course study of dynamic light scattering (Fig. S11†) shows the ease of aggregation of LNDs in acidic media. The size increase of LNDs in basic solution is very slow (Fig. S11†). IR results of LND, LNDpH3 and LNDpH11 are shown in Fig. S12.† As LNDs precipitate faster from acidic solutions, it is logical to deduce that the electrostatic potential on most LND facets is positive. It has been reported15,27 that the electrostatic potential interaction between facets of opposite charge is responsible for ND aggregation. Thus, SND should interact more with LND rather than other SNDs. As a proof of concept, SND and LND colloidal suspensions were mixed and a precipitate was readily formed. A TEM image of the precipitate shows LNDs covered by SNDs (Fig. 4). These deductions were further verified by studying the Hofmeister effect on SNDs and LNDs and its application in drug loading.
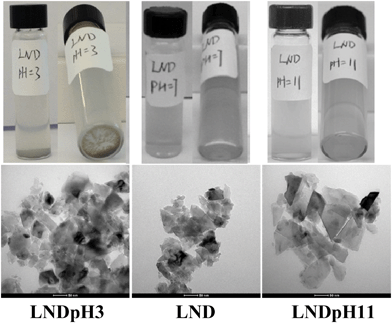 | ||
| Fig. 3 Photographs (top) and TEM images (bottom) of LNDpH3, LND and LNDpH11, in which the aggregation degree of LNDpH3 is larger than that of LNDpH11. | ||
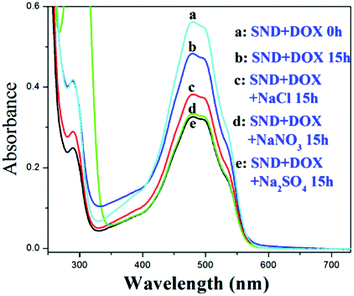
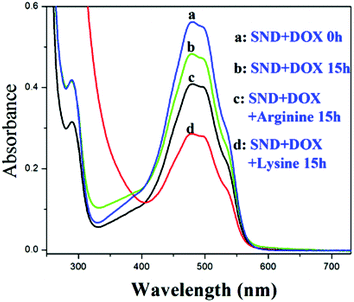
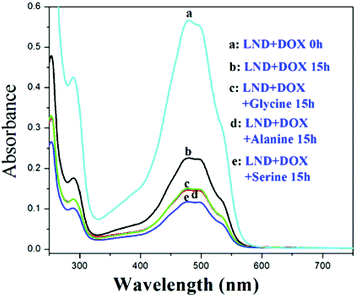
When the biocompatibility of the NDs was established,28,29 reports of the application of these nanomaterials in the delivery of water insoluble drugs such as doxorubicin (DOX), purvalanol A, and 4-hydroxytamoxifen blossomed.30 Adding sodium chloride (NaCl) was reported to increase the loading amount of DOXH+ onto SNDs (ND size ranging from 2 to 8 nm) (Fig. S13†).2 This finding is very significant since many drug carriers have been abandoned because of their low loading efficiency. Generally, DOXH+ is loaded onto the SNDs by the electrostatic interaction between the H+ of DOXH+ and the deprotonated carboxylic groups on the NDs.7,9 Two assumptions were presented to explain why adding NaCl can increase the drug loading without any supporting experimental data. Moreover, no mechanistic study has been reported to better understand the effect of salt addition and its dependence on the surface charge. Following up on our deductions concerning the charged SND facets, we believe that after adding NaCl to this system, cations will first be adsorbed on the negative facets of the SNDs, and then anions are attracted by these cations. These attracted anions can adsorb DOXH+ by the interaction with H+, which leads to the increased loading of DOXH+ onto the SNDs. Also, it has been reported that a cation–anion layer structure can be formed on the metal oxide surface by ion adsorption.31 Moreover, a positive proton and a negative hydride can act as a bridge to connect a Lewis base and a Lewis acid with large substituents.32,33 To verify this observation, we substituted NaCl with NaNO3, Na2SO4, CaCl2, KCl and (NH4)2SO4 and found that they all promote DOXH+ loading onto SNDs (Fig. 5, Fig. S14†). Based on this explanation, other molecules that exist in the ionic form may also be used to promote DOXH+ loading onto SNDs. Amino acids have both carboxylic and amino groups. They are neutral when the pH of the solution is equal to their isoelectric point, negative when the pH of the solution is higher than their isoelectric point and positive when pH of the solution is lower than their isoelectric point.34 Thus, amino acids are also potential accelerators for promoting DOXH+ loading onto SNDs. The advantage of using amino acids as accelerators is that they are more biocompatible than some inorganic salts. Two types of amino acids have been chosen: one with an isoelectric point below 7, including serine (5.68), glycine (5.97) and alanine (6.02), and another with an isoelectric point larger than 7, including lysine (9.74) and arginine (10.76). The loading of DOXH+ onto SND is slightly inhibited by serine, glycine and alanine (all exist in the negative form at pH 7), but is promoted when arginine and lysine (both exist in the positive form at pH 7) are used (Fig. 6 and Fig. S15†). The exact opposite happens when we replace the SNDs with LNDs, as serine, glycine and alanine promote DOXH+ loading onto LNDs (Fig. 7). These experimental results support our original claims of SNDs having negatively charged facets while the charge of LND facets is positive. Future directions include investigating the effect of the manufacturing processes on determining and controlling the surface charge.
In summary, detailed experimental studies have been performed to explore the colloidal stability of SNDs and LNDs in acidic and basic media as well as the Hofmeister effect of inorganic salts (not only NaCl) and amino acids on drug loading. These results suggest that the electrostatic potential on most SND facets is negative, while that of LND facets is mainly positive. These findings are helpful to better understand the surface performance of different sized NDs and promote their application in the biomedical field.
Acknowledgements
We thank King Abdullah University of Science and Technology (KAUST) for the financial support.References
- V. N. Mochalin, O. Shenderova, D. Ho and Y. Gogotsi, Nat. Nanotechnol., 2012, 7, 11–23 CrossRef CAS PubMed.
- H. Huang, E. Pierstorff, E. Osawa and D. Ho, Nano Lett., 2007, 7, 3305–3314 CrossRef CAS PubMed.
- X. Q. Zhang, M. Chen, R. Lam, X. Y. Xu, E. Osawa and D. Ho, ACS Nano, 2009, 3, 2609–2616 CrossRef CAS PubMed.
- M. Chen, X. Q. Zhang, H. B. Man, R. Lam, E. K. Chow and D. Ho, J. Phys. Chem. Lett., 2010, 1, 3167–3171 CrossRef CAS.
- R. A. Shimkunas, E. Robinson, R. Lam, S. Lu, X. Xu, X. Q. Zhang, H. Huang, E. Osawa and D. Ho, Biomaterials, 2009, 30, 5720–5728 CrossRef CAS PubMed.
- K. V. Purtov, A. I. Petunin, A. E. Burov, A. P. Puzyr and V. S. Bondar, Nanoscale Res. Lett., 2010, 5, 631–636 CrossRef CAS PubMed.
- E. K. Chow, X. Q. Zhang, M. Chen, R. Lam, E. Robinson, H. J. Huang, D. Schaffer, E. Osawa, A. Goga and D. Ho, Sci. Transl. Med., 2011, 3, 73ra21 Search PubMed.
- L. C. L. Huang and H. C. Chang, Langmuir, 2004, 20, 5879–5884 CrossRef CAS PubMed.
- S. S. Huang, J. Q. Shao, L. F. Gao, Y. Z. Qi and L. Ye, Appl. Surf. Sci., 2011, 257, 8617–8622 CrossRef CAS.
- S. Jones, H. P. Shanahan, H. M. Berman and J. M. Thornton, Nucleic Acids Res., 2003, 31, 7189–7198 CrossRef CAS PubMed.
- H. P. Shanahan, M. A. Garcia, S. Jones and J. M. Thornton, Nucleic Acids Res., 2004, 32, 4732–4741 CrossRef CAS PubMed.
- T. Tyler, V. V. Zhirnov, A. V. Kvit, D. Kang and J. Hren, Appl. Phys. Lett., 2003, 82, 2904–2906 CrossRef CAS.
- A. S. Barnard and M. Sternberg, J. Mater. Chem., 2007, 17, 4811–4819 RSC.
- E. Osawa, D. Ho, H. Huang, M. V. Korobov and N. N. Rozhkova, Diamond Relat. Mater., 2009, 18, 904–909 CrossRef CAS.
- A. S. Barnard, J. Mater. Chem., 2008, 18, 4038–4041 RSC.
- C. Bradac, T. Gaebel, N. Naidoo, J. R. Rabeau and A. S. Barnard, Nano Lett., 2009, 9, 3555–3564 CrossRef CAS PubMed.
- O. A. Williams, J. Hees, C. Dieker, W. Jager, L. Kirste and C. E. Nebel, ACS Nano, 2010, 4, 4824–4830 CrossRef CAS PubMed.
- E. Thormann, RSC Adv., 2012, 2, 8297–8305 RSC.
- X. Chen, T. Yang, S. Kataoka and P. S. Cremer, J. Am. Chem. Soc., 2007, 129, 12272–12279 CrossRef CAS PubMed.
- J. S. Tu, E. Perevedentseva, P. H. Chung and C. L. Cheng, J. Chem. Phys., 2006, 125, 174713 CrossRef PubMed.
- P. H. Chung, E. Perevedentseva, J. S. Tu, C. C. Chang and C. L. Cheng, Diamond Relat. Mater., 2006, 15, 622–625 CrossRef CAS.
- S. Osswald, G. Yushin, V. Mochalin, S. O. Kucheyev and Y. Gogotsi, J. Am. Chem. Soc., 2006, 128, 11635–11642 CrossRef CAS PubMed.
- V. Mochalin, S. Osswald and Y. Gogotsi, Chem. Mater., 2009, 21, 273–279 CrossRef CAS.
- Y. Liu, Z. N. Gu, J. L. Margrave and V. N. Khabashesku, Chem. Mater., 2004, 16, 3924–3930 CrossRef CAS.
- K. K. Liu, C. C. Wang, C. L. Cheng and J. I. Chao, Biomaterials, 2009, 30, 4249–4259 CrossRef CAS PubMed.
- O. O. Mykhaylyk, Y. M. Solonin, D. N. Batchelder and R. Brydson, J. Appl. Phys., 2005, 97, 074302 CrossRef.
- L. Lai and A. S. Barnard, J. Phys. Chem. Lett., 2012, 3, 896–901 CrossRef CAS PubMed.
- A. M. Schrand, H. Huang, C. Carlaon, J. J. Schlager, E. Osawa, S. M. Hussain and L. Dai, J. Phys. Chem. B, 2007, 111, 2–7 CrossRef CAS PubMed.
- A. M. Schrand, L. Dai, J. J. Schlager, S. M. Hussain and E. Osawa, Diamond Relat. Mater., 2007, 16, 2118–2123 CrossRef CAS.
- M. Chen, E. D. Pierstorff, R. Lam, S. Y. Li, H. Huang, E. Osawa and D. Ho, ACS Nano, 2009, 3, 2016–2022 CrossRef CAS PubMed.
- A. G. Brinkman, Hydrobiologia, 1993, 253, 31–45 CrossRef CAS.
- G. C. Welch and D. W. Stephan, J. Am. Chem. Soc., 2007, 129, 1880–1881 CrossRef CAS PubMed.
- Y. Guo and S. H. Li, Inorg. Chem., 2008, 47, 6212–6219 CrossRef CAS PubMed.
- N. Alende, E. D. J. Nielsen, C. Shields and N. Khaldi, Proteins: Struct., Funct., Bioinf., 2011, 79, 1635–1648 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: TEM, IR, UV and time course dynamic light scattering results are provided. See DOI: 10.1039/c3bm60163c |
| This journal is © The Royal Society of Chemistry 2014 |