Cytotoxicity and potency of mesocellular foam-26 in comparison to layered clays used as hemostatic agents†
Yao
Li
a,
April M.
Sawvel‡
b,
Young-Si
Jun
b,
Sara
Nownes
b,
Ming
Ni
a,
Damien
Kudela
b,
Galen D.
Stucky
*b and
Daniele
Zink
*a
aInstitute of Bioengineering and Nanotechnology, 31 Biopolis Way, The Nanos, Singapore 138669, Singapore. E-mail: dzink@ibn.a-star.edu.sg
bDepartment of Chemistry and Biochemistry and Materials Department, University of California, Santa Barbara, California 93106, USA. E-mail: stucky@chem.ucsb.edu
First published on 16th November 2012
Abstract
Uncontrolled hemorrhage is a leading cause of potentially preventable death. The most effective commercial hemostatic products employ layered clays. Due to safety concerns only a product containing kaolin is currently recommended by the U. S. Department of Defense. A problem related to layered clays, including kaolin, is their cytotoxicity. Also, material left in the wound can lead to thrombosis and other adverse effects. Recently, it has been shown that pure silica mesocellular foams (MCF) with cell window sizes >20 nm are effective in promoting blood clotting. Here, we tested the potency and cytotoxicity of layered clays in comparison to MCF with a cell window size of 26 nm (MCF-26) in vitro. The results showed that the potencies of MCF-26 and layered clays in promoting clotting were comparable. Effects on cell viability were assessed with relevant primary human cell types. The cytotoxic effects of all compounds were cell type-specific and most sensitive were endothelial cells. The IC50 values of MCF-26 were in the mg ml−1 range and its cytotoxicity was ∼1–2 orders of magnitude lower than the cytotoxicity of layered clays. Further, MCF-26 did not adhere strongly to cell surfaces and was not taken up by cells as observed for the layered clays. This suggests that it would be easier to remove MCF-26 from wounds. Altogether, the results suggest that MCF-26 would be effective and safer than currently used hemostatic agents.
Introduction
Uncontrolled hemorrhage is the leading cause of potentially preventable death in combat casualties1,2 and is the second leading cause of death in civilian trauma.3,4 Several enhanced hemostatic dressings have been developed to prevent fatal exsanguinations. The active compounds of these dressings are mainly based on chitosan, zeolite, purified procoagulant factors or layered clays, such as smectite clays or kaolin.5 They act as blood factor concentrators, mucoadhesive agents, procoagulent supplementors, or by initiating the intrinsic clotting cascade.Since 2004, two products, namely HemCon and QuikClot, have been deployed by United States and United Kingdom Armed Forces and clinical data supported their efficacy.5,6 HemCon consists of deacetylated chitosan acetate salt on a sterile foam backing pad and QuikClot is a granular preparation of zeolite. Due to the exothermic reaction between zeolite and water QuikClot can cause severe burns.6,7 Based on these observations modified versions of the product have been developed that reduce wound temperature.8
It was found that HemCon and QuikClot were outperformed by WoundStat (WS, smectite granules), Celox (granular chitosan compounds) and QuikClot CombatGauze (CG, kaolin-coated surgical gauze) in animal models of lethal hemorrhage.5,9,10 As a result, the US Army replaced HemCon dressing with CG and WS in 2008. However, in 2009 the US Army permanently suspended the use of WS due to safety concerns over embolization and tissue inflammation (http://www.stripes.com/news/army-halts-use-of-woundstat-1.90678). CG currently remains the number one recommended agent by the U. S. Department of Defense for treating uncontrolled hemorrhage in the battlefield.
Both, CG and WS obtained clearance by the U. S. Food and Drug Administration (FDA) in 2007. However, one study using a swine model published in 2010 revealed that WS use was associated with substantial local inflammatory response, endothelial and neuronal degeneration and myocyte necrosis.11 Another study published in the same year demonstrated that WS led to formation of occlusive thrombi in the injured blood vessels.12 Furthermore, it was observed that WS caused endothelial injury and damage of the vessels that could interfere with surgical repair. It was also found that the non-biodegradable particles could not be completely removed from the wounds and could enter systemic circulation, causing distal thrombosis in vital organs.12 The study concluded that more relevant in vitro and in vivo tests should be performed for clearance of new hemostatic agents. In this regard it was outlined that according to the manufacturer, the cytotoxicity of WS was tested with fibroblast cell cultures and other standard tests, as recommended by the International Organization for Standardization (ISO) guidelines. It was pointed out that these standard tests might not be sufficient and did not include exposure to endothelial cells, which are a relevant cell type in this setting.
The view that it would be important to perform in vitro tests with endothelial cells and other relevant cell types is supported by studies that demonstrated profound cytotoxic effects of WS and/or layered clays on primary human umbilical vein endothelial cells (HUVEC), primary murine neurons and RAW267.4 murine macrophage-like cells.13–15 However, different human and animal cell lines, such as HeLa cells or murine neuroblastoma cells, remained largely unaffected by these agents,13–15 suggesting that cytotoxic effects were cell type-specific.
In addition to cytotoxicity other major safety-relevant problems associated with WS, such as local and distal thrombosis, were due to the fact that WS was difficult to remove from the wounds. In one case, residual kaolin powder was also detected in a vessel specimen treated with CG.12
These results suggest that safer and less cytotoxic hemostatic agents are needed. Improved materials should be at least as effective as layered clays, but have decreased cytotoxicity. Silica materials provide a negative surface that promotes coagulation via contact activation, or the intrinsic pathway. A promising strategy to develop more effective agents is to increase the surface area accessible to contact activation proteins. Mesocellular foams (MCF) are mesoporous silica materials, with a large surface area and open framework structure. The silica framework structure is made up of cages connected by windows, both of which are monodispersed in size. Both the cages and cage window sizes can be tuned over a relatively large size range. It has been suggested that the nanoporous cage windows play a critical role in providing contact activation proteins access to the inner cell surfaces, and it has been demonstrated that a MCF with a cell window size >20 nm is highly effective in accelerating blood clotting.16 2 mg of MCF-33 (cage window size of 33 nm) accelerated clot formation similarly to 20 mg of QuikClot.16
Here, we examined the potency of MCF-26 (cell window size of 26 nm) in promoting clot formation in vitro. Cytotoxicity, adhesion to the cell surface and cellular uptake were investigated with different relevant primary human cell types and two standard cell lines. The results obtained with MCF-26 are compared to data obtained with layered clays.
Materials and methods
Materials
Kaolin, bentonite, montmorillonite and dimethyl sulfoxide (DMSO) were purchased from Sigma-Aldrich (St. Louis, MO, USA). 10 nm silver nanoparticels (Ag NP) were purchased from Meliorum Technologies (Rochester, NY, USA).Synthesis of MCF-26
MCF-26 was synthesized by dissolving 1.1 g of Pluronic P123 surfactant (poly(ethylene glycol)-block-poly(propylene glycol)-block-poly(ethylene glycol) (BASF, Ludwigshafen, Germany) into a solution of 2.5 ml concentrated HCl (Sigma-Aldrich) and 16.25 ml of 18.2 mΩ water. After stirring at room temperature for two hours or until the surfactant dissolved, 1.447 ml mesitylene (Sigma-Aldrich) was added and the solution was heated at 40 °C for 2 hours under vigorous stirring. Next, 2.3 ml tetraethyl orthosilicate (TEOS, Sigma-Aldrich) was added and the solution was stirred for an additional 5 minutes. The solution was then transferred to a Teflon-lined autoclave device and baked in an oven at 40 °C for 20 hours. After removal from the oven, the device cooled to room temperature overnight. 11.5 mg NH4F (Sigma-Aldrich) was dissolved in 0.1 ml 18.2 mΩ water and added to the solution. The autoclave device was again sealed and placed in an oven at 140 °C for 24 hours. The resulting solution was filtered and the filtrate was washed with water and with ethanol. Calcination at 550 °C for six hours removed any impurities from the resulting white solid.Characterization of MCF-26
The surface area, pore volume, and window size of MCF-26 were determined using a Micromeritics Tristar 3000 Porosimeter (Chemical Instruments AB, Sollentuna, Sverige). Prior to measurement, the samples were dried under 220 °C nitrogen gas overnight. Brunauer–Emmett–Teller (BET) analysis was used to calculate the surface area. Pore size and window size were derived from the adsorption and desorption isotherms using the Broekhoff–de Boer method.Scanning electron microscopy (SEM)
SEM used to identify particle size and morphology was performed either with an XL30 Sirion FEG microscope (FEI, Hillsboro, OR, USA) or with an JSM-7400 field emission scanning electron microscope (Jeol, Tokyo, Japan) after coating with gold.Dynamic light scattering (DLS)
DLS was performed with a ZetaPLAS particle size analyzer (Brookhaven Instruments, Holtsville, NY, USA).Surface area and pore size determination
A Tristar 3000 (Micromeritics Instrument Corporation, Norcross, GA, USA) was used for nitrogen gas sorption analysis of clay and MCF-26 surface areas. Before the surface area measurement these compounds were dried at 220 °C for 12 hours under nitrogen. Data presented for surface areas were calculated using the BET analysis, and pore sizes and external surface area were calculated using the BJH (Barrett–Joyner–Halenda) analysis.Thrombelastography
All samples were pre-weighed into 1 dram glass vials prior to use and MCF-26 was used after calcination at 550 °C. A thrombelastograph (Haemoscope Corporation, Niles, Illinois, USA) was used to determine the clotting properties of all materials investigated. This instrument provides quantitative data regarding time until clot initiation (R), rate of clot formation (Alpha) and strength of the clot formed (MA) by measuring the torsion of a small sample of blood around a wire during coagulation. First, 20 μl of 0.2 M CaCl2 were added to a plastic cup heated to 37.0 °C. Next, 340 μl of pooled human plasma (PHP) was added to the cup, followed immediately by addition of the clotting agent from a 1 dram glass vial. Finally, the sample cup was quickly loaded into position for commencement of the measurement.PHP was purchased in 1 ml aliquots from George King Biomedical (Overland Park, KS, USA) and stored at −80 °C. Samples were warmed to 37 °C for 20 minutes immediately prior to use. All plasma samples were used or discarded within one hour of heating to 37 °C. Experiments involving PHP were approved by and carried out in accordance with the guidelines put forth by the Human Subjects Committee at the University of California, Santa Barbara, USA.
Cell culture
Different batches of primary human umbilical vein endothelial cells (HUVEC) and primary adult human epidermal keratinocytes (HEK) were purchased from ScienCell Research Laboratories (Carlsbad, CA, USA). Different batches of primary adult human dermal fibroblasts (HDF) were purchased from ScienCell Research Laboratories, the American Type Culture Collection (ATCC, Manassas, VA, USA), and Promocell (Heidelberg, Germany). Human primary renal proximal tubular cells (HPTC), HK-2 cells and NIH/3T3 cells were obtained from ATCC. The different cell types were cultivated in the media recommended by the vendors as described.17–20Cell viability assays
The neutral red uptake (NRU) assay was performed as described21 and as recommended by the International Standard for the Biological Evaluation of Medical Devices (Part 5: Tests for In Vitro Cytotoxicity, ISO 10993-5:2009 (E)), with the following modifications: different cell types were used and cells were seeded at a density of 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cells cm−2 into 96-well microplates. Cells were cultivated for 24 h and were then treated overnight with the test materials. Before the NRU assay was performed the cells were washed with phosphate-buffered saline (PBS). Data acquisition and analysis was performed as described.17 The NRU assay was used for the generation of all data on cell viability shown in the main manuscript. Some data that were generated by using assays employing 5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium (MTS) or measuring lactate dehydrogenase (LDH) leakage are provided in the ESI† and details on these assays are described in the ESI.†
000 cells cm−2 into 96-well microplates. Cells were cultivated for 24 h and were then treated overnight with the test materials. Before the NRU assay was performed the cells were washed with phosphate-buffered saline (PBS). Data acquisition and analysis was performed as described.17 The NRU assay was used for the generation of all data on cell viability shown in the main manuscript. Some data that were generated by using assays employing 5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium (MTS) or measuring lactate dehydrogenase (LDH) leakage are provided in the ESI† and details on these assays are described in the ESI.†
Adhesion of test materials to the cell surface
HUVEC were seeded on glass coverslips (Menzel-Gläser, Braunschweig, Germany) and were cultivated for 24 h. 1 mg ml−1 of test material was added for 10 minutes. Subsequently, all samples were rinsed in similar ways with 3 × 10 ml PBS and the samples were fixed with 3.7% formaldehyde/PBS. Imaging was performed with the CytoViva™ high resolution imaging system (CytoViva, Auburn, AL, USA), which is suitable for the imaging of nanoparticles and other types of particles.Cellular uptake of test materials
HUVEC and HDF were seeded on cover slips and were cultivated for 24 h before treatment with the test materials was performed overnight. After treatment cells were washed with PBS, fixed and imaged with the CytoViva™ system.Zeta potential measurements
Measurements of the zeta potentials of layered clays and MCF-26 were performed with 10 μg ml−1 of the materials suspended in PBS or complete endothelial cell culture medium (ScienCell Research Laboratories, Carlsbad, CA, USA; supplemented with 5% fetal bovine serum). Zeta potentials were measured with a Zen 3690 Zetasizer Nano ZS90 (Malvern Instruments, Malvern, Worcestershire, UK).Measurement of reactive oxygen species (ROS)
Intracellular ROS generation was detected using 5-(and-6) chloromethyl-2′,7′-dichlorodihydrofluorescein diacetate, acetyl ester (CM-H2DCFDA) (Invitrogen, Carlsbad, CA, USA). Assays were performed after cells had been treated for 24 h with kaolin or MCF-26 at concentrations of up to 250 μg ml−1. Cells were incubated with 10 μM CM-H2DCFDA for 60 min followed by incubation in PBS for 15 min. Fluorescence at an emission wavelength of 528 nm was measured using a Tecan Safire2™ microplate reader (Männedorf, Switzerland).Statistical analysis
Microsoft Office Excel 2003 was used for all calculations and statistics (unpaired t-test).Results and discussion
Sizes and shapes of the particles in the samples of MCF-26, kaolin, bentonite and montmorillonite were determined by using SEM and DLS. MCF-26 consisted of smooth spheres with a narrow size distribution in the range of ∼2000 nm (Fig. 1). The layered clays consisted of irregularly shaped particles, which showed broader size distributions. In case of bentonite and montmorillonite the sizes of most particles were in the range of ∼1000 nm. Kaolin particles were smaller and the majority displayed sizes in the range of ∼500 nm.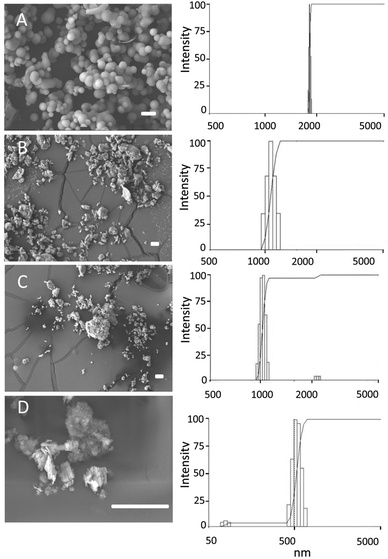 | ||
| Fig. 1 Particle shapes and sizes. MCF-26 (A), bentonite (B), montmorillonite (C) and kaolin (D) were imaged by SEM (left-hand panels). Scale bars: 10 μm. Particle sizes were determined by DLS (right-hand panels). | ||
In addition, surface areas and pore sizes were determined by BET and BJH analysis. MCF-26 had the largest total and external surface areas and the values were 364 m2 g−1 (total surface area) and 278 m2 g−1 (external surface area), respectively. The pore volume was 2.3 cm3 g−1 and the average pore diameter was 26.4 nm. The external surface area of montmorillonite was 51 m2 g−1, and the internal surface area of the layered structure contributed substantially to the total surface area, which was 174 m2 g−1. The spacing of the layered structures was smaller in case of bentonite and kaolin, which resulted in smaller differences between the total surface area (20 m2 g−1 for both compounds) and the external surface area (16 m2 g−1 for both compounds). These results also showed that bentonite and kaolin had the smallest surface areas from the 4 compounds investigated.
The potency of MCF-26, kaolin, bentonite and montmorillonite in promoting clot formation was investigated by measuring the time to clot formation in pooled human plasma (PHP). Bentonite and montmorillonite are smectite clays with hemostatic effects. All of the materials investigated here led to decreased clotting times compared to PHP alone (Fig. 2). This is consistent with previous reports on accelerated clot formation by layered clays and MCFs.16,22 There were no significant differences between the times to clot formation when the effects of layered clays were compared at a given concentration (Fig. 2). At the lowest concentration examined (3 mg ml−1) the time to clot formation was slightly but significantly increased with MCF-26 in comparison to the layered clays. At 7 mg ml−1 no significant differences were observed with respect to all of the materials tested.
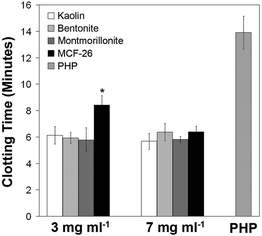 | ||
| Fig. 2 Clotting times in the presence of different hemostatic agents. The clotting times of pure PHP without hemostatic agents (right-hand grey bar) or of PHP exposed to the indicated concentrations of hemostatic agents were determined. The bars show the mean ± standard deviation (s.d., n = 4). Significant differences (p < 0.05) between MCF-26 and layered clays are indicated by an asterisk. | ||
Next, we examined the effects of kaolin on the viability of different cell types in vitro. We examined HUVEC as well as primary human HEK and HDF to investigate the effects on primary human endothelial and skin cells. Three different batches of each cell type were included to address for interdonor variability. For comparison we examined immortalized NIH/3T3 mouse fibroblasts, which represent a widely used standard cell line. We also assessed the effects on cells from a vital internal organ by including human primary (HPTC) and immortalized (HK-2) renal proximal tubular cells. These cell types are well-characterized and widely applied in in vitro nephrotoxicology.
In order to address effects on cell viability and cell damage we compared the results of the NRU, MTS and LDH assays. LDH leakage was not observed when different cell types were treated with increasing concentrations of up to 7 mg ml−1 of bentonite, montmorillonite and MCF-26 (Fig. S1†). This suggested that membrane damage did not play a major role in the cytotoxic effects of these compounds and that the LDH assay was not useful for this study.
Results obtained with the MTS assay (Fig. S2†) and the NRU assay were similar. However, the NRU assay is more reliable as it is not influenced by cell density,17 which compromised the results of the MTS assay (Fig. S3†). Therefore, all experiments shown in the results section below were performed with the NRU assay. This assay is also recommended by the International Standard ISO 10993–5:2009(E).
When cells were treated with kaolin and other layered clays we observed that these compounds strongly adhered to the cell and substrate surface and could not be washed away completely (Fig. 3). Tests with kaolin applied in cell culture medium to cell-free multi-well plates revealed that kaolin interfered with the absorbance measurements of multi-well plate-based assays (data not shown). Therefore, kaolin could not be tested at concentrations >250 μg ml−1. Any potential artifacts due to residual kaolin in the NRU assays would lead to an overestimation of cell viability, as kaolin increased the values measured in the NRU assay.
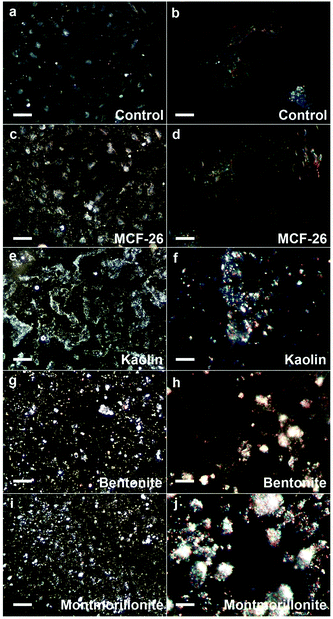 | ||
| Fig. 3 Adhesion of layered clays and MCF-26 to the cell surface. HUVEC were exposed for 10 minutes to 1 mg ml−1 of kaolin, bentonite, montmorillonite or MCF-26 (control: untreated). Subsequently, cells were washed. Images were captured with the CytoViva system after fixation and particles appear white on the images. The left-hand images show fields of cells (scale bars: 100 μm), whereas the right-hand images show individual cells (scale bars: 10 μm). | ||
Kaolin was applied at different concentrations to the cells in order to determine its effects on cell viability and Fig. 4 displays the dose-response curves. Table 1 (left-hand column) provides the IC50 values as well as the percentages of cell viability at the highest concentration tested (250 μg ml−1). The results showed that the effects of kaolin were strongly cell type-specific. The numbers of NIH/3T3 fibroblasts were not significantly decreased by kaolin (P > 0.05) and the numbers of two batches of human skin fibroblasts (HDF1 and HDF2) were even increased (P < 0.05; Fig. 4 and Table 1). In contrast, a dose-dependent decrease of cell viability was observed in all other cases. HUVEC were most sensitive and the IC50 values of the three HUVEC batches ranged between ∼50 μg ml−1 and ∼125 μg ml−1. Substantial cell death was also observed in case of HEK and here the IC50 values ranged between ∼180 μg ml−1 and ∼250 μg ml−1 (Table 1, the cell viability of two HEK batches was slightly above 50% at 250 μg ml−1). Primary and immortalized human renal cells were only moderately affected and their IC50 values were clearly >250 μg ml−1.
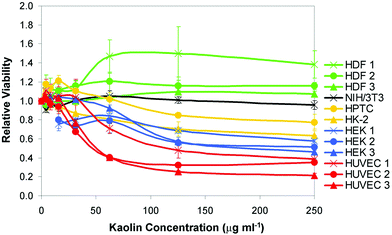 | ||
| Fig. 4 Dose-dependent effects of kaolin on cell viability. Kaolin was applied at concentrations of up to 250 μg ml−1 to the cell types indicated on the right-hand side. Three different batches of HDF, HEK and HUVEC were tested. Cell viability was determined with the NRU assay and all values were normalized to the values obtained with untreated control cells. Error bars show the s.d. (n = 3). | ||
| Cell type | Kaolin | Bentonite | Montmorillonite | MCF-26 | ||||
|---|---|---|---|---|---|---|---|---|
| IC50 (μg ml−1) | % at 250 μg ml−1 | IC50 (μg ml−1) | % at 1000 μg ml−1 | IC50 (μg ml−1) | % at 1000 μg ml−1 | IC50 (μg ml−1) | % at 1000 μg ml−1 | |
| HUVEC 1 | 125 ± 47 | 39 ± 5 | 11 ± 1 | 18 ± 1 | 65 ± 3 | 18 ± 0 | >1000 | 75 ± 2 |
| HUVEC 2 | 48 ± 4 | 35 ± 2 | 17 ± 2 | 37 ± 3 | 33 ± 2 | 25 ± 3 | >1000 | 73 ± 12 |
| HUVEC 3 | 51 ± 2 | 21 ± 2 | 17 ± 4 | 34 ± 2 | 95 ± 13 | 33 ± 4 | >1000 | 65 ± 3 |
| HDF 1 | >250 | 138 ± 14 | 300 ± 85 | 45 ± 3 | 272 ± 99 | 29 ± 1 | >1000 | 66 ± 13 |
| HDF 2 | >250 | 119 ± 8 | >1000 | 60 ± 3 | 335 ± 32 | 38 ± 5 | >1000 | 130 ± 4 |
| HDF 3 | >250 | 107 ± 3 | 468 ± 18 | 52 ± 4 | 454 ± 120 | 57 ± 1 | >1000 | 122 ± 3 |
| HEK 1 | >250 | 58 ± 2 | >1000 | 60 ± 6 | >1000 | 78 ± 6 | >1000 | 100 ± 3 |
| HEK 2 | >250 | 51 ± 12 | 58 ± 14 | 41 ± 6 | >1000 | 62 ± 2 | >1000 | 66 ± 8 |
| HEK 3 | 181 ± 44 | 46 ± 2 | 34 ± 10 | 41 ± 2 | >1000 | 69 ± 2 | >1000 | 102 ± 12 |
| NIH/3T3 | >250 | 96 ± 5 | >1000 | 56 ± 1 | 461 ± 22 | 38 ± 2 | >1000 | 93 ± 9 |
| HK-2 | >250 | 62 ± 6 | >1000 | 82 ± 4 | >1000 | 66 ± 2 | >1000 | 71 ± 13 |
| HPTC | >250 | 77 ± 9 | >1000 | 55 ± 3 | 280 ± 27 | 34 ± 2 | >1000 | 89 ± 11 |
Overall, the results demonstrated cell type-specific cytotoxicity of kaolin and the sensitivity of the cell types used here could be ranked in the following order HUVEC > HEK > HK-2/HPTC > NIH/3T3 > HDF. Of note, the fibroblastic cell types (either immortalized murine or primary human) did not display any significantly reduced cell viability, even at the highest concentration of kaolin. It is also worth mentioning that, although some interdonor variability was observed, cell viability was mainly influenced by cell type-specific effects.
In the following we tested the cytotoxicity of bentonite and montmorillonite. These compounds interfered less strongly with the NRU assay than kaolin and were tested at higher concentrations of up to 1000 μg ml−1. The same cell types were used as before and the results of the NRU assays are displayed in Fig. 5 (black and grey graphs) and Table 1.
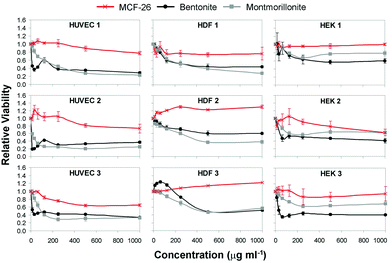 | ||
| Fig. 5 Dose–response curves. HUVEC, HDF and HEK were treated with up to 1000 μg ml−1 of MCF-26 (red graphs), bentonite (black graphs) or montmorillonite (grey graphs). Cell viability was determined and all values were normalized to the values obtained with untreated control cells. Error bars show the s.d. (n = 3). | ||
The results revealed that all layered clays tested had substantial cytotoxic effects. As observed with kaolin, HUVEC were also most sensitive to the effects of bentonite and montmorillonite and the IC50 values ranged from ∼11 μg ml to ∼17 μg ml−1 (bentonite) and from ∼33 μg ml−1 to ∼95 μg ml−1 (montmorillonite). The effects of bentonite and montmorillonite on the different cell types were somewhat variable. However, all cell types and batches displayed IC50 values clearly below 1000 μg ml−1 with at least one of the two compounds, with exception of HK-2 cells and HEK batch 1.
Next, we addressed the cytotoxicity of MCF-26 by performing similar assays. When cells were treated with MCF-26 cell viability was generally higher as compared to bentonite and montmorillonite (Fig. 5), and in all cases the IC50 values were >1000 μg ml−1 (Table 1). This applied also to HUVEC, which were most sensitive to the cytotoxic effects of the layered clays. These results showed that MCF-26 had strongly reduced cytotoxicity in comparison to the layered clays.
As the IC50 values were in all cases >1000 μg ml−1 we assessed the cytotoxicity of MCF-26 also at higher concentrations in the mg ml−1 range with the most relevant cell types (primary human skin cell types and HUVEC). The IC50 values are displayed in Table 2 and the results revealed again cell type-specific effects. HEK were least sensitive with IC50 values of >7.0 mg ml−1 in all cases.
| Cell type | IC50 (mg ml−1) | % at 7 mg ml−1 |
|---|---|---|
| HUVEC 1 | 6.3 ± 0.2 | 50 ± 3 |
| HUVEC 2 | 2.1 ± 0.8 | 27 ± 7 |
| HUVEC 3 | 0.7 ± 0.0 | 21 ± 2 |
| HDF 1 | 2.0 ± 0.2 | 13 ± 3 |
| HDF 2 | 5.6 ± 1.4 | 40 ± 8 |
| HDF 3 | 5.0 ± 0.7 | 41 ± 3 |
| HEK 1 | >7.0 | 92 ± 9 |
| HEK 2 | >7.0 | 60 ± 4 |
| HEK 3 | >7.0 | 78 ± 3 |
In addition, interdonor variability was observed and the IC50 values of the different batches of HDF and HUVEC ranged between ∼2.0 mg ml−1 and ∼6.3 mg ml−1 with the exception of HUVEC batch 3, where an IC50 value of ∼0.7 mg ml−1 was determined. The difference to the previous result, where an IC50 value of >1000 μg ml−1 was obtained with this batch (Table 1), can probably be explained by the fact that this cell batch was used at a higher passage number (P6) when higher concentrations of MCF-26 were tested (P4 was used in the other experiments). Nevertheless, the lowest IC50 value obtained with this cell batch and MCF-26 (0.70 mg ml−1, Table 2) was still ∼7-fold higher than the highest value obtained with this cell batch and a layered clay (95.0 ± 12.5 μg ml−1, Table 1, montmorillonite).
Overall, these results confirmed that MCF-26 was less cytotoxic than layered clays and the IC50 values were in the mg ml−1 range when relevant primary human cell types were tested.
In order to compare the cytotoxicity of MCF-26 and layered clays to the effects of other cytotoxic compounds we performed similar assays with DMSO and Ag NP. DMSO is a mildly cytotoxic agent that is used in concentrations of 10% for the cryopreservation of cells and as solvent for pharmaceuticals in clinical applications. The IC50 values of this compound with low cytotoxicity ranged from ∼25 mg ml−1 to >100 mg ml−1 (Table S1†) and were about 1–2 orders of magnitude higher than the IC50 values obtained with MCF-26 (Table 2 and Table S1†). Again, HUVEC displayed lower IC50 values than most other cell types. The lowest IC50 value was obtained here with HK-2 cells, which were relatively insensitive to layered clays.
Ag NP are applied in consumer products, cosmetics and wound dressings as antibacterial agents.23,24 The cytotoxicity of Ag NP, which is probably due to the leaching of silver ions, is well documented and a point of concern.23,25 Here, the IC50 values of Ag NP were in the same range as those of layered clays (Table 1 and Table S1†). Again, the lowest values were obtained with HUVEC and HK-2 cells. Overall, the IC50 values of Ag NP were at least an order of magnitude lower than those of MCF-26.
Together, the data showed that the cytotoxicity of MCF-26 is relatively mild and ∼1–2 orders of magnitude lower than the cytotoxicity of other materials applied in wound dressings, such as kaolin and AgNP.
It would be important that non-biodegradable wound dressing materials can be effectively removed from the wound and are not taken up by the cells. Material remaining in the body can cause local and systemic effects including thrombosis, inflammation and foreign body reaction. We observed that layered clays strongly adhered to the cell surface and could not be completely washed away (Fig. 3), which would be a problem in this regard. Adhesion of MCF-26 to the cell surface was strongly reduced in comparison to layered clays (Fig. 3). To further address processes that might interfere with efficient removal we also studied cellular uptake of materials currently used in wound dressings in comparison to MCF-26. Fig. 6 shows that kaolin and Ag NP were efficiently taken up by HUVEC and HDF. Although kaolin also strongly adhered to the cell surface (Fig. 3) the fact that much lower concentrations of particles appeared in the nuclear area after overnight exposure (Fig. 6) showed that most particles were taken up into the cytoplasm and were not just distributed over the cell surface. In contrast, no uptake of MCF-26 by HUVEC and HDF could be detected after overnight exposure, even when MCF-26 was applied at 5- to 10-fold higher concentrations than Ag NP or kaolin (Fig. 6). The observed differences in cellular uptake and interactions with the cell surface could account for differences in the cytotoxicity of MCF-26 and layered clays.
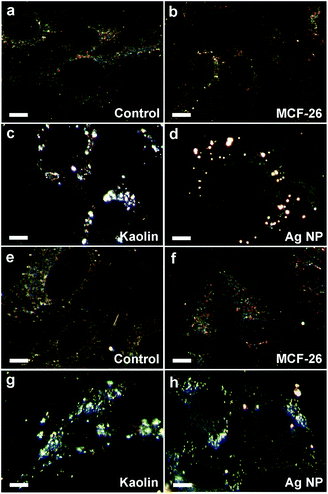 | ||
| Fig. 6 Cellular uptake. HUVEC (a–d) and HDF (e–h) were left untreated (control) or were treated with MCF-26, kaolin or Ag NP as indicated. Ag NP and kaolin were applied at concentrations of 10 μg ml−1 (HUVEC) or 20 μg ml−1 (HDF). MCF-26 was applied to all samples at 100 μg ml−1. Cells were washed after overnight exposure before images were captured with the CytoViva system. Cell nuclei appear as dark ovals within the cells. Scale bars: 10 μm. | ||
As differences in cytotoxicity could also be potentially due to differences in the zeta potential of the particles, which could also account for the observed differences in the interactions with the cell surface, we measured the zeta potential of MCF-26, kaolin and the two smectite compounds. Positively charged compounds are usually cytotoxic. However, all of the compounds investigated here had negative zeta potentials. No significant differences between the zeta potentials of bentonite, kaolin and MCF-26 were observed when the different materials were suspended in cell culture medium (Fig. S4a†).
The generation of ROS is another mechanism that could potentially account for the cytotoxic effects observed. Intracellular ROS generation in the presence of kaolin and MCF-26 was assessed with HUVEC batch 2 (Fig. S4b†). ROS generation in the presence of kaolin peaked when this compound was added at a concentration of 62.5 μg ml−1 and declined at higher concentrations. This could probably be explained by cell death. Only when kaolin and MCF-26 were added at a concentration of 62.5 μg ml−1 kaolin led to higher levels of ROS generation than MCF-26 (∼24%). The concentration of ROS in the presence of MCF-26 increased linearly with the concentration of MCF-26 at higher concentrations of this compound. At 250 μg ml−1 of MCF-26 the concentration of ROS was ∼1.8-fold higher than in untreated controls (Fig. S4b†). However, no negative effects on cell viability were observed at this concentration of MCF-26 (Fig. 5, HUVEC 2). Altogether, the data suggested that generation of ROS was not a primary cause for the negative effects on cell viability observed here.
Here, we addressed the cytotoxicity of MCF-26 and its potency in promoting clot formation in comparison to layered clays with hemostatic activity. Effects on cell viability were investigated in vitro by using different types of primary human cells and two well-characterized standard cell lines. The results revealed that the cytotoxicity of MCF-26 is ∼1–2 orders of magnitude lower than the cytotoxicity of layered clays. The effects of layered clays are strongly cell type-dependent and HUVEC are most sensitive. This result is in agreement with the findings of previous studies demonstrating cell type-specific cytotoxicity of layered clays and high sensitivity of HUVEC.13–15
The results showing a high sensitivity of HUVEC in vitro are in agreement with the finding that WS induced endothelial damage and other degenerative processes in vivo.11,12 WS had been approved by the FDA in 2007 and the adverse effects observed in the more recent in vivo studies11,12 suggested that standard in vitro safety tests with fibroblast cultures are not sufficient (discussed in ref. 12). In agreement with this notion we observed here that fibroblastic cell types are insensitive to the cytotoxic effects of layered clays. Although cell type-specific effects are also observed in case of MCF-26, this compound was only mildly cytotoxic to all cell types tested, and the IC50 values were in the mg ml−1 range.
It is not clear yet why the cytotoxicity of MCF-26 is reduced in comparison to layered clays. The mechanism of cell type-specific toxicity of layered clays does not appear to involve leaching of toxicants or binding of nutrients from the cell culture medium, and direct contact between the cell surface and the layered clay material is required.13 We and others14 observed that layered clays adhered strongly to the cell surface, and it has been discussed that the negative charge of the clay materials is important for associating with and lysing the cells.14 However, we found that MCF-26, which has a similar zeta potential as kaolin and bentonite in cell culture medium, adheres less strongly to the cell surface and is less cytotoxic. Thus, reduced interactions with the cell surface might explain the reduced cytotoxicity of MCF-26, although this does not appear to be charge-dependent. It is also important to note that the results on LDH leakage did not indicate that membrane damage plays a major role in the cytotoxic effects observed.
Cellular uptake might be important for the cytotxic effects, which occurred in case of kaolin, but not in case of MCF-26. If cellular uptake was important for the cytotoxic effects of layered clays, it remains to be explained why the effects are cell type-specific. Substantial cellular uptake of kaolin, for instance, was observed in case of HDF and HUVEC (other cell types not tested), but only HUVEC displayed compromised viability. Our results suggested that the generation of ROS did not play a major role in the negative effects on cell viability observed.
Apart from potential effects on cell viability, adhesion to the cell surface and cellular uptake aggravates removal of non-biodegradable hemostatic agents from the wound. In fact, using porcine vascular injury models it was observed that it was difficult to remove WS from the wound.9,12 Residual material led to local thrombus formation as well as to distal thrombosis in vital organs after entering systemic circulation.12 In another study it was found that WS use was associated with substantial local inflammatory response and neurovascular degeneration up to 5 weeks post injury.11 Severe problems with removal of the agent from the wound and thrombosis were not observed when CG was applied.12 This was attributed to the fact that only a small amount of kaolin powder is incorporated into CG, whereas WS consists of smectite granules that are directly poured into the wound. However, in one case traces of kaolin were found in a specimen and it would be safer to use a compound that does not strongly adhere to the cells and does not show cellular uptake, as has been observed in case of MCF-26.
While we have demonstrated that MCF-26 has decreased cytotoxicity in comparison to layered clays and can be removed more easily, its potency to promote clot formation was in the same range. Previous data suggest that MCFs with larger pore window diameters than those investigated here could be more effective.16 Furthermore, the ability of MCFs to uptake and immobilize proteins can be used to functionalize the surface with thrombin or other factors promoting blood clotting. It has been already demonstrated that the clotting times of plasma exposed to MCFs were dramatically reduced by immobilizing thrombin in the pores.16 Also, the ability to sequester and release proteins26 would allow to use MCFs as delivery vectors for procoagulant clotting factors. Using these strategies it would be possible to generate not only safer, but also more effective hemostatic agents.
Conclusions
Our results showed that MCF-26 displays a strongly reduced cytotoxicity to relevant human cell types in comparison to kaolin and other layered clays. Also, MCF-26 can be more easily removed from cellular material. This suggests that the use of MCF-26 would be safer, while its potency in promoting clot formation is comparable to that of the most effective commercial hemostatic agents. The potency of MCFs in promoting clot formation can be further increased by various strategies, which include increasing the pore window diameter and surface functionalization with thrombin.Acknowledgements
This work was supported by the Institute of Bioengineering and Nanotechnology (Biomedical Research Council, Agency for Science, Technology and Research, Singapore). Part of this work was funded through the Casualty Care and Management Program at the Office of Naval Research, ONR grant numbers N00014-06-1-0145 and N00014-10-1-0191, and by the U. S. Army Medical Research & Materiel Command and the Telemedicine & Advanced Technology Research Center under Contract Number WQ81XWH-11-2-0021. The MRL Central Facilities are supported by the MRSEC Program of the NSF under Award No. DMR 1121053; a member of the NSF-funded Materials Research Facilities Network (http://www.mrfn.org).Notes and references
- R. F. Bellamy, Mil. Med., 1984, 149, 55–62 CAS.
- H. R. Champion, R. F. Bellamy, C. P. Roberts and A. J. Leppaniemi, Trauma, 2003, 54, S13–S19 Search PubMed.
- J. A. Acosta, J. C. Yang, R. J. Winchell, R. K. Simons, D. A. Fortlage, P. Hollingsworth-Fridlund and D. B. Hoyt, J. Am. Coll. Surg., 1998, 186, 528–533 CrossRef CAS.
- A. Sauaia, F. A. Moore, E. E. Moore, K. S. Moser, R. Brennan, R. A. Read and P. T. Pons, J. Trauma, 1995, 38, 185–193 CrossRef CAS.
- J. Granville-Chapman, N. Jacobs and M. J. Midwinter, Injury, 2011, 42, 447–459 CrossRef CAS.
- E. D. Cox, M. A. Schreiber, J. McManus, C. E. Wade and J. B. Holcomb, Transfusion, 2009, 49(Suppl 5), 248S–255S CrossRef.
- P. Rhee, C. Brown, M. Martin, A. Salim, D. Plurad, D. Green, L. Chambers, D. Demetriades, G. Velmahos and H. Alam, J. Trauma, 2008, 64, 1093–1099 CrossRef.
- F. Arnaud, T. Tomori, W. Carr, A. McKeague, K. Teranishi, K. Prusaczyk and R. McCarron, Ann. Biomed. Eng., 2008, 36, 1708–1713 CrossRef.
- B. S. Kheirabadi, J. W. Edens, I. B. Terrazas, J. S. Estep, H. G. Klemcke, M. A. Dubick and J. B. Holcomb, J. Trauma, 2009, 66, 316–328 CrossRef.
- B. S. Kheirabadi, M. R. Scherer, J. S. Estep, M. A. Dubick and J. B. Holcomb, J. Trauma, 2009, 67, 450–460 CrossRef CAS.
- T. Gerlach, J. K. Grayson, K. O. Pichakron, M. J. Sena, S. D. DeMartini, B. Z. Clark, J. S. Estep and D. Zierold, J. Trauma, 2010, 69, 1203–1209 CrossRef CAS.
- B. S. Kheirabadi, J. E. Mace, I. B. Terrazas, C. G. Fedyk, J. S. Estep, M. A. Dubick and L. H. Blackbourne, J. Trauma, 2010, 68, 269–278 CrossRef CAS.
- P. D. Bowman, X. Wang, M. A. Meledeo, M. A. Dubick and B. S. Kheirabadi, J. Trauma, 2011, 71, 727–732 CrossRef CAS.
- E. J. Murphy, E. Roberts, D. K. Anderson and L. A. Horrocks, Neuroscience, 1993, 57, 483–490 CrossRef CAS.
- E. J. Murphy, E. Roberts and L. A. Horrocks, Neuroscience, 1993, 55, 597–605 CrossRef CAS.
- S. E. Baker, A. M. Sawvel, J. Fan, Q. Shi, N. Strandwitz and G. D. Stucky, Langmuir, 2008, 24, 14254–14260 CrossRef CAS.
- Y. Li, Y. Zheng, K. Zhang, J. Y. Ying and D. Zink, Nanotoxicology, 2012, 6, 121–133 CrossRef.
- M. Ni, J. C. M. Teo, M. S. Bin Ibrahim, K. Zhang, F. Tasnim, P. Y. Chow, D. Zink and J. Y. Ying, Biomaterials, 2011, 32, 1465–1476 CrossRef CAS.
- M. Ni, P. K. Zimmermann, K. Kandasamy, W. Lai, Y. Li, M. F. Leong, A. C. A. Wan and D. Zink, Biomaterials, 2012, 33, 353–364 CrossRef CAS.
- Z. Y. Oo, R. Deng, M. Hu, M. Ni, K. Kandasamy, M. S. Bin Ibrahim, J. Y. Ying and D. Zink, Biomaterials, 2011, 32, 8806–8815 CrossRef CAS.
- G. Repetto, A. del Peso and J. L. Zurita, Nat. Protoc., 2008, 3, 1125–1131 CrossRef CAS.
- S. E. Baker, A. M. Sawvel, N. Zheng and G. D. Stucky, Chem. Mater., 2007, 19, 4390–4392 CrossRef CAS.
- H. J. Johnston, G. Hutchison, F. M. Christensen, S. Peters, S. Hankin and V. Stone, Crit. Rev. Toxicol., 2010, 40, 328–346 CrossRef CAS.
- L. J. Wilkinson, R. J. White and J. K. Chipman, J. Wound Care, 2011, 20, 543–549 CAS.
- M. Ahamed, M. S. Alsalhi and M. K. Siddiqui, Clin. Chim. Acta, 2010, 411, 1841–1848 CrossRef CAS.
- Y.-J. Han, G. D. Stucky and A. Butler, J. Am. Chem. Soc., 1999, 121, 9897–9898 CrossRef CAS.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c2tx20065a |
| ‡ Currently at the Molecular Foundry, Lawrence Berkeley National Laboratory, Berkeley, California 94720. |
| This journal is © The Royal Society of Chemistry 2013 |
