The toxicological assessment of two anti-obesity drugs in C. elegans
Layla
Aitlhadj
and
Stephen R.
Stürzenbaum
*
School of Biomedical Sciences, Analytical and Environmental Sciences Division, King's College London, London, UK. E-mail: stephen.sturzenbaum@kcl.ac.uk; Tel: +44 (0) 20078484406
First published on 3rd January 2013
Abstract
Obesity is an ever increasing health concern of global importance. The treatment options for this condition are limited and until recently there was only one FDA approved anti-obesity drug available. The approval of two anti-obesity drugs which have both undergone post-marketing withdrawal undermines consumer confidence and highlights the need for more robust pre-clinical toxicity testing. The nematode Caenorhabditis elegans is an established toxicological model and while it is still in its infancy with regards to the study of obesity, the ease of maintenance and high-throughput assays makes C. elegans an appealing choice. Here, we explore the suitability of C. elegans as an in vivo toxicity model for the mechanistic screening of two anti-obesity drugs. Toxicity profiles identified that a Sibutramine containing drug induced pronounced effects on pharyngeal pumping, defecation, locomotion and reproduction, in concert indicative of a possible neuronal mode of action. Resultant from a drug resistance screen we demonstrate that Sibutramine has non-serotonergic targets, a notion that suggests that serotonin-specific reuptake inhibitors (SSRIs) are less specific than first assumed. These results stress that the interplay between serotonin, dopamine and norepinephrine is not easily dissected. Overall, the data highlight the value of C. elegans as an in vivo toxicity tool in anti-obesity drug research.
Introduction
One of the major challenges in drug development is the accurate assessment of human drug toxicity.1 Overcoming this obstacle would translate into a higher success rate for clinical trials and fewer marketed drugs being withdrawn due to unexpected adverse effects. Although mouse models have proved invaluable, the identification and validation of drug targets as provided by rodent models is relatively low-throughput. As a result, invertebrate models such as the fruit fly Drosophila melanogaster and the nematode Caenorhabditis elegans have gained momentum in the drug discovery process.2Although C. elegans was first proposed as a predictive model for human toxicity in the 1980s,3 the use of the nematode as an in vivo whole animal toxicity screen for pharmaceutical compounds is a more recent venture.4 The increase in disease models available in the worm coupled with the development of high throughput approaches for toxicity screens5,6 places the worm in a desirable position for use by the pharmaceutical industry. Since even mammalian toxicity testing cannot be predictive of drug action in humans, it is unreasonable to expect C. elegans to fully extrapolate into human research. However, the ease of genetic manipulability and maintenance, cost efficacy, and high-throughput drug screening compatibility7 could provide better initial safety profiles and pharmacogenomic testing thus leading to a decrease in drug withdrawals.
The cost of withdrawing a drug from the market is not solely a financial loss due to the inefficient use of time and resources but also one of consumer confidence. Notable cases have arisen from the anti-obesity drug industry, with several high profile drugs being withdrawn within the last decade alone.8 Obesity is a major global health burden which, according to the World Health Organization, affects more than 300 million adults and childhood obesity is increasing at an alarming rate.9 The goal to attain effective long-term weight management has proved challenging due to the multi-causative nature of obesity. Supplementation of diet and behavioural management with pharmacotherapy has nevertheless been shown to provide long-term weight loss.10 However, the success of pharmacotherapy has been inhibited by the lack of drug safety resulting in the poor reputation of anti-obesity drugs and consequent limited therapeutic options.
Until recently, the only existing Food and Drug Administration approved anti-obesity drug was Orlistat, a gastrointestinal lipase inhibitor, which serves as a relatively small aid in weight loss when complemented with lifestyle changes.11,12 The dire need for anti-obesity drugs has led to the approval of two anti-obesity drugs; Lorcaserin (a 5HT2C specific agonist) and Qsymia (a combination of phentermine and topiramate) which have been approved by the FDA despite initial post-marketing withdrawals of both drugs, primarily due to safety-concerns.
A condition of the approval of Lorcaserin and Qsymia is that both will undergo further post-approval clinical trials.13 Although long-term safety studies are invaluable, conducting large-scale toxicity tests at early stages of drug development is expensive and therefore post-marketing outcome studies are proving popular in an attempt to increase the treatment options for obesity and reduce the stringent regulatory mechanisms for FDA approval. However, there is a clear clinical need for the validation of a pre-clinical model for evaluating the efficacy, toxicity and long-term outcomes of anti-obesity drugs. While the nematode is still in its infancy with regards to the study of obesity, it has aided to decipher aspects of insulin signalling, the effect of serotonin on obesity, satiety and feeding and to explore the mechanisms of single gene mutations related to obesity.14 In addition, it is a well established model for ecotoxicological studies and more recently for the toxicity of pharmaceutical compounds.4,15–17 Here, the toxicity profiles of two commercial anti-obesity drugs, namely Xenical® (active ingredient: Orlistat) and Reductil® (active ingredient: Sibutramine) were compared in C. elegans and assessed to determine if the nematode can offer a rational tool for in vivo anti-obesity drug toxicity testing. Gaining an early insight into the toxicology of drug candidates will help to establish safety profiles and reduce preclinical development costs and drug attrition rates by developing safer therapeutic candidates and minimizing off-target effects.
Materials and methods
Growth size assay
Synchronized L1 nematodes (at least 10 per condition) were plated onto standard Nematode Growth Media (NGM) plates seeded with E. coli (OP50). NGM and bacteria were supplemented with appropriate amounts of drugs. Each nematode was photographed every 24 hours over a period of 12 days using a Nikon DS-2Mv digital camera linked to the NIS-Elements F 2.20 software. The nematode images were traced and the average flat surface area calculated using the Image-Pro Express software (Media Cybernetics, Inc.).Brood size/period assay
The brood size refers to the daily and cumulative number of eggs laid per nematode. At least 36 nematodes (L1 stage) were individually placed into 12-well plates supplemented with the appropriated drug concentrations. The plates were incubated at 20 °C for approximately 72 hours (or until reproduction commenced) before transferring the nematodes to fresh NGM plates. They were transferred to new plates every 24 hours thereafter, until reproduction ceased. The number of eggs produced was counted each day thus allowing the calculation of daily and cumulative brood size as well as brood period (defined as the time period between first and last egg laid).Pharyngeal pumping assay
The pharyngeal pumping rate was measured by counting the number of pharyngeal bulb contractions within 30 second intervals. The pharyngeal bulb was followed by videography using a microscope at 12.5× magnification (Leica M205C with DFC420C digital camera and LAS MultiTime Timelapse and Movie Module). The frame rate was slowed down to facilitate consistent counting of pharyngeal contractions. For each condition at least 10 adult worms were assessed at L4 stage (72 hours after hatching).Locomotion
Locomotion was quantified by counting the number of body bends per minute for at least 10 worms per condition. A single body bend was counted each time the tip of the tail moved from the maximal upward deflection to maximal downward deflection, then returning to its original upward deflection (forward movement only).Defecation
Synchronized L4 worms were monitored for 3 cycles of defecation. The defecation rate was counted as the mean length of time between expulsion steps for a total of at least 10 worms per condition. Defecation was assessed by means of high magnification Apochromatic Zoom and FusionOptics™ microscopy (Leica M205C).Results
Anti-obesity drugs cause a concentration-dependent reduction in C. elegans body size
Screening of bioactive compounds in the nematode has been useful in identifying effective pharmacological therapies.16 However, it has recently been demonstrated that the worm is relatively resistant to perturbation by pharmacologically active molecules.17 Consequently, it was deemed necessary to determine whether the drugs are capable of inducing a response in nematodes and at which concentration further studies would be performed. Therefore a range-finder assay was performed using adult body size as the endpoint to define a suitable concentration range that elicits a response and included subsequent two-fold serial dilutions. The same concentration range was applied for both drugs, mainly to allow a direct comparison between the concentrations and effects.In detail, age synchronous L1 worms were maintained on standard NGM plates supplemented with 0.07 mg mL−1, 0.145 mg mL−1 or 0.29 mg mL−1 of Xenical® or Reductil® in both the NGM and the OP50 food source and after 96 hours the mean body size of adults was compared to untreated nematodes. Exposure to Xenical® and Reductil® caused a significant reduction in the body size of C. elegans, namely from 940.9 μM2 ± 27.7 in untreated nematodes to 840.2 μM2 ± 29.1 in Xenical® (0.29 mg mL−1) exposures and from 932.9 μM2 ± 30.4 in untreated nematodes to 73.8 μM2 ± 1.8 in Reductil® (0.29 mg mL−1) treatments, respectively (Fig. 1a and 1b). In response to Reductil®, a statistically robust concentration-dependent reduction in body size was observed, even at the lowest concentration tested. These data suggest that C. elegans is not resistant to exogenously applied Xenical® and Reductil® and effective concentrations are readily obtained using a solid NGM exposure regime. Indeed, it is conceivable that higher throughput assays in liquid media may prove to be even more sensitive.5,6
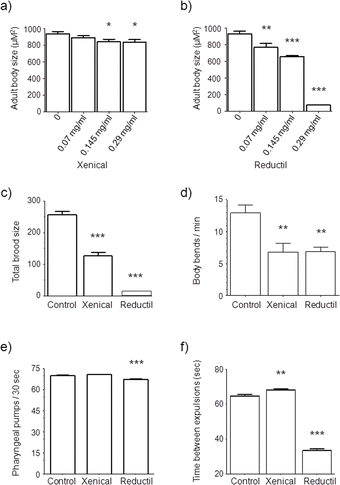 | ||
| Fig. 1 Concentration response analysis of nematodes exposed to (a) Xenical® and (b) Reductil® using mean adult body size as the end point. Each data set represents the mean area of 10 individual worms. (c) The effect of anti-obesity drugs on nematode fecundity. Worms were treated with 0.145 mg mL−1 of either Xenical® or Reductil® and then compared to untreated worms. Each dataset represents 36 replicates. (d) The effect of Xenical® and Reductil® on the locomotion of C. elegans. Locomotion is measured as the number of full sinusoidal movements, with each full movement accounting for 1 body bend. Each data set represents 11 worms each recorded for 1 minute using video capture and is representative of 3 individual experiments. (e) Feeding rate of drug treatments compared to control. Measured by counting the number of pharyngeal pumps per 30 seconds. Each data set represents the mean of 10 individual worms, with each worm counted 3 consecutive times. (f) Defecation rate of nematodes exposed to anti-obesity drugs. Values represent the time between successive expulsions. Each data set represents 10 individual worms each of which were measured three times. *P = <0.05, **P = <0.001 and ***P = <0.0001. | ||
Effects of Xenical® and Reductil® on life history traits of C. elegans
Several life history traits including brood size, locomotion, pharyngeal pumping and defecation were measured in response to Xenical® and Reductil® exposure. Endpoints in nematode reproduction are both ecologically relevant and sensitive for acute toxicity testing with the distinct advantage of escaping the caveats of impregnation.18 Reproduction provides an indication of the effects of a potential toxicant on development and indirectly, on the neuromuscular activity of egg-laying.5 The total reproductive output of 36 worms was measured in response to Xenical® and Reductil® and was compared to untreated nematodes (Fig. 1c). Whilst the mean total brood size of control worms was 256.4 ± 9.5, both Xenical® and Reductil® treatment resulted in a reduction in reproductive performance. Xenical® exposure resulted in a 49% reduction in brood size (125.8 ± 11.3), whereas Reductil® treatment lead to the complete cessation of egg-laying. Close inspection of the Reductil® exposed worms revealed that adults did not produce eggs indicating a severe disruption in egg development/embryonic lethality (data not shown).Egg-laying is a complex process which is co-ordinated with several other motor programs and shown to be mediated by feedback from the HSN motorneurons to interneurons in the head that promote forward movement. The contraction and relaxation of body muscles is co-ordinated by acetylcholine and simultaneously affects GABA transmission. Since egg-laying was disrupted in Xenical® and Reductil® treated worms, the consequent effect on locomotion was investigated. Fig. 1d reveals that the mean body bend count per minute is 12.9 ± 1.2 in control worms, whereas both Xenical® and Reductil® exposure led to a reduction in locomotion by approximately 50% (6.8 ± 1.4 and 6.9 ± 0.7 for Xenical® and Reductil®, respectively). Since both drugs disrupt the locomotory behaviour, a common mechanism of toxicity may exert this effect, a possible candidate is the cholinergic system, as Orlistat and Sibutramine have been shown to affect acetylcholine in vivo.19,20
The effect of Reductil® was also assessed by measuring the pharyngeal pumping rate of worms chronically exposed to 0.145 mg mL−1 Reductil®. Indeed, pharyngeal pumping is a valid endpoint in toxicology as it is susceptible to a broad spectrum of chemicals and can directly measure neurotoxicity. Fig. 1e shows that the mean number of pharyngeal pumps was significantly reduced in the Reductil® treated worms (67.3 ± 0.3) compared to control (70.0 ± 0.2), whereas exposure to Xenical® did not cause a notable difference in pharyngeal pumping rate (70.6 ± 0.3). Chemotaxis assays confirmed that these effects were specific to the drug action and not a consequence of chemo-repulsion (data not shown). These data suggest that the Reductil® induced reduction in pharyngeal pumping may cause a reduced food intake. Although, reduced feeding could be indicative of a similar mode of action for Reductil in C. elegans as in mammals, it may more likely be due to a neurotoxicological effect of the drug.
The defecation rate of C. elegans was analysed by measuring the time between expulsion events (Fig. 1f). In untreated worms, the mean time between each expulsion step was 64.6 ± 0.8 s. The time between expulsions was extended in worms treated with Xenical® (68.0 ± 0.8 s), but reduced in worms treated with Reductil® (33.5 ± 1.0 s). These data align well with previous studies which show an increased transit time in patients treated with Xenical® and enhanced gastric emptying upon treatment with Reductil®.21,22 Taken together these data suggest that Reductil® drug action affects serotonergic systems in the nematode.
Identifying targets of Reductil® in C. elegans
Many genes and proteins are conserved between mammals and C. elegans including those that have been identified as playing a role in the serotonergic system including the selective serotonin re-uptake inhibitors (SSRIs). Since Reductil® treatment led to the modulation of serotonin controlled behaviour and is a known SSRI in mammals, the targets for Reductil® were investigated further using a genetic drug-resistance screen of body size involving C. elegans mutants of the serotonergic system. Genetic screens for C. elegans mutants with altered responses including drug resistance can not only identify direct drug targets but also the pathways associated with off-targets, thus helping to unravel mechanisms of toxicity. It was hypothesized that mutant strains for the target proteins of Reductil® would be smaller in body size in control (drug-devoid) conditions. Furthermore, it was predicted that these mutants would not display a significant reduction in body size upon exposure to Reductil®. Several mutants, dat-1 (tm903), ser-1 (ok345) and ser-4 (ok512) as well as the double mutant ser-1;ser-7 (ok345;tm1325) (Fig. 2 and Table 1) were indeed smaller than the wild-type worms and their body size was proportionally less significantly reduced in response to Reductil® treatment (approximately 20% in the 4 mutants compared to 34.5% in wild-type). Since ser-7 did not exhibit this effect it is likely that the response from the double mutant ser-1;ser-7 is due to the effect of ser-1 alone. Conversely, the mod-5 mutant which is the only reported serotonin reuptake transporter in C. elegans, did not display the dat-1 and ser-1 phenotype, suggesting that a hitherto unidentified receptor may be responsible for reuptake of serotonin which in turn increases the availability of serotonin to post-synaptic receptors.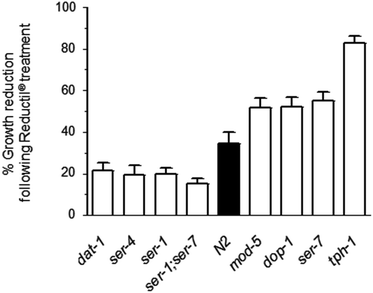 | ||
| Fig. 2 The mean adult body size of mutant strains was measured every 24 hours and compared to wild-type worms (N2) in the presence or absence of Reductil®. Results represent the percentage reduction of body size in each mutant as opposed to the untreated counterpart. Compared to the effects measured in wild-type worms, the difference between Reductil® treated and untreated animals was significantly smaller in dat-1 (tm903), ser-1 (ok345) and ser-4 (ok512) and ser-1;ser-7 (ok345;tm1325) mutants. In contrast, mod-5 (mt9772), tph-1 (mg280), dop-1 (ok398), and ser-7 (ok1944) exhibited a Reductil® induced reduction in body size which was greater than wild-type worms. | ||
| Mutant strain | Orthologous to human gene | Description of gene action |
|---|---|---|
| ser-1 (ok345) | 5HT2 receptor | Required in both vulval muscle and neurons for the stimulation of egg-laying by serotonin (5-HT), but is completely dispensable for stimulation by the uptake inhibitor fluoxetine, and mostly dispensable for stimulation by the tricyclic antidepressant imipramine |
| ser-4 (ok512) | 5-HT7 metabotropic serotonin/dopamine receptor | SER-4 is required for normal inhibition of movement by 5-HT. Dispensable for the stimulation of egg-laying by 5-HT and by the uptake inhibitor fluoxetine |
| ser-7 (ok1944) | 5-HT7 metabotropic serotonin receptor | Required for stimulation of egg-laying or pharyngeal pumping by 5-HT, for regular pumping in response to bacteria. Expressed in head and tail neurons, pharyngeal neurons, vulval muscles, and intestine; stimulates intracellular adenylate cyclase activity; has high affinity for 5-HT and tryptamine |
| ser-1;ser-7 (ok345;tm1325) | Double mutant serotonin receptor | SER-7 and SER-1 are redundantly required for normal egg-laying |
| dat-1 (tm903) | Dopamine transporter | Predicted to regulate dopaminergic neuro transmission via reuptake of dopamine into pre-synaptic neurons |
Discussion
Our ever advancing lifestyles will continue to induce toxic side-effects and emerging pharmacological research will likely focus on damage-control, such as the development of anti-obesity drugs. Post-marketing withdrawal of anti-obesity drugs due to serious adverse effects results will undoubtedly result in the loss of consumer confidence as well as the reputation of the company. Consequently there is a growing need for sensitive and specific pre-screening tools to predict toxicity in humans.C. elegans has already been established as an excellent model for toxicological testing which, coupled with the growing interest in obesity models, allows C. elegans to compete with mammalian models. Here we show that two anti-obesity drugs cause a measurable response in several life history parameters in worms. Of the two toxicity profiles generated, Reductil® appears to be more potent in disrupting pharyngeal pumping, defecation, locomotion and reproduction, all indicative of a neuronal mode of toxic action. These changes were observed whilst exposures were carried out on solid agar with worms exposed via NGM and food source. It is possible that an increase in sensitivity and throughput capacity may be achieved by means of liquid media. Since the decreased feeding rate in Reductil® treated worms is reminiscent of the satiety induced in patients taking Reductil®, we predict that the mechanism of action is similar in mammals and C. elegans but we cannot neglect the possibility that the effects may have a neurotoxicological origin. Targets of Reductil® in C. elegans were screened and the results suggested that the presence of other, so far unidentified, non-serotonergic targets are likely. This is in accordance with previous findings that have highlighted that SSRIs may cause significant changes in the overlapping functions associated with dopamine and/or norepinephrine signalling.23 Considering the overlapping and interconnected functions of dopamine, serotonin and norepinephrine, it is possible that the alternate targets are also neurotransmitters.
Conclusions
The worm nervous system, specifically the serotonergic system, can and has been successfully exploited to study therapeutic approaches of drugs such as the anti-depressant, fluoxetine.24,25 Here we argue that research using C. elegans is not merely an academic exercise but rather has the potential to develop in vivo toxicity assays for anti-obesity drugs and ultimately push forward the frontiers of 3R research, namely the Replacement, Refinement and Reduction of scientific experimentation with higher animals. Although the use of C. elegans to replace higher organisms for toxicity testing is not unique, this is the first time that anti-obesity drugs have been tested in the worm, more specifically holding promise for the serotonergic anti-obesity drugs in deciphering their mechanisms of action as well as the development of high throughput screens to be exploited by the pharmaceutical industries.5,6Acknowledgements
This work was supported by a Medical Research Council (MRC) studentship. Furthermore we wish to acknowledge the Caenorhabditis Genetics Center (CGC) which is funded by the National Institutes of Health – National Centre for Research Resources for the supply of nematodes and the bacterial feeding strain.References
- A. P. Li, Chem.–Biol. Interact., 2004, 150, 3–7 CrossRef CAS.
- P. M. Carroll, B. Dougherty, P. Ross-Macdonald, K. Browman and K. FitzGerald, Pharmacol. Ther., 2003, 99, 183–220 CrossRef CAS.
- P. L. Williams and D. B. Dusenbery, Toxicol. Ind. Health, 1988, 4, 469–478 CAS.
- M. Dengg and J. C. van Meel, J. Pharmacol. Toxicol. Methods, 2004, 50, 209–214 CrossRef CAS.
- W. A. Boyd, S. J. McBride, J. R. Rice, D. W. Snyder and J. H. Freedman, Toxicol. Appl. Pharmacol., 2010, 245, 153–159 CrossRef CAS.
- M. C. Leung, P. L. Williams, A. Benedetto, C. Au, K. J. Helmcke, M. Aschner and J. N. Meyer, Toxicol. Sci., 2008, 106, 5–28 CrossRef CAS.
- K. J. Helmcke, D. S. Avila and M. Aschner, Neurotoxicol. Teratol., 2010, 32, 62–67 CrossRef CAS.
- R. J. Rodgers, M. H. Tschöp and J. P. Wilding, Dis. Model. Mech., 2012, 5, 621–626 CrossRef CAS.
- WHO (World Health Organization) Obesity and overweight. 2010. [accessed 10/12/2012]. Available: http://www.who.int/dietphysicalactivity/media/en/gsfs_obesity.pdf.
- S. Phelan and T. A. Wadden, Obes. Res., 2002, 10, 50–57 CrossRef.
- P. Anagnostis, D. Selalmatzidou, M. Sapranidis, A. Panagiotou, S. A. Polyzos, A. Slavakis and M. Kita, Indian J. Endocrinol. Metab., 2012, 16, 146–147 CrossRef CAS.
- J. G. Kang and C. Y. Park, Diabetes Metab. J., 2012, 36, 13–25 CrossRef.
- E. Colman, J. Golden, M. Roberts, A. Egan, J. Weaver and C. Rosebraugh, N. Engl. J. Med., 2012, 367, 1577–1579 CrossRef CAS.
- L. Aitlhadj, D. S. Avila, A. Benedetto, M. Aschner and S. R. Stürzenbaum, Environ. Health Perspect., 2011, 119, 20–28 CrossRef CAS.
- T. Kaletta and M. O. Hengartner, Nat. Rev. Drug Discovery, 2006, 5, 387–398 CrossRef CAS.
- S. J. Gosai, J. H. Kwak, C. J. Luke, O. S. Long, D. E. King, K. J. Kovatch, P. A. Johnston, T. Y. Shun, J. S. Lazo, D. H. Perlmutter, G. A. Silverman and S. C. Pak, PLoS ONE, 2010, 5(11), e15460 Search PubMed.
- A. R. Burns, I. M. Wallace, J. Wildenhain, M. Tyers, G. Giaever, G. D. Bader, C. Nislow, S. R. Cutler and P. J. Roy, Nat. Chem. Biol., 2010, 6, 549–557 CrossRef CAS.
- Y. Guo, Y. Yang and D. Wang, Reprod. Toxicol., 2009, 28, 90–95 CrossRef CAS.
- R. Bergholm, M. Tiikkainen, S. Vehkavaara, M. Tamminen, K. Teramo, A. Rissanen and H. Yki-Järvinen, Diabetes Care, 2003, 26, 1667–1672 CrossRef CAS.
- R. J. Davis and G. G. Nomikos, Mol. Psychiatry, 2008, 13, 240–241 CrossRef CAS.
- P. Kocełak, B. Zahorska-Markiewicz, K. Jonderko, M. Olszanecka-Glinianowicz, A. Zak-Gołab, M. Holecki, M. Kamińska and M. Szymszal, J. Gastroenterol., 2008, 43, 609–617 CrossRef.
- J. Xu and J. D. Chen, Neurogastroenterol. Motil., 2008, 3, 236–242 CrossRef.
- E. Morelli, H. Moore, T. J. Rebello, N. Gray, K. Steele, E. Esposito, J. A. Gingrich and M. S. Ansorge, J. Neurosci., 2011, 31, 15742–15750 CrossRef CAS.
- A. Kullyev, C. M. Dempsey, S. Miller, C. J. Kuan, V. M. Hapiak, R. W. Komuniecki, C. T. Griffin and J. Y. Sze, Genetics, 2010, 186, 929–941 CrossRef CAS.
- C. M. Dempsey, S. M. Mackenzie, A. Gargus, G. Blanco and J. Y. Sze, Genetics, 2005, 169, 1425–1436 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2013 |
