Thiol-containing polymeric embedding materials for nanoskiving†
Robin L.
Mays
a,
Parisa
Pourhossein
b,
Dhanalekshmi
Savithri
a,
Jan
Genzer
a,
Ryan C.
Chiechi
*b and
Michael D.
Dickey
*a
aDepartment of Chemical and Biomolecular Engineering, North Carolina State University, 911 Partners Way, Raleigh, NC 27695, USA. E-mail: mddickey@ncsu.edu; Fax: +1 (919) 513-6243; Tel: +1 (919) 513-0273
bStratingh Institute for Chemistry and Zernike Institute for Advanced Materials, University of Groningen, Nijenborgh 4, Groningen, 9747 AG, The Netherlands
First published on 25th October 2012
Abstract
This paper describes the characterization of new embedding resins for nanoskiving (ultramicrotomy) that contain thiols. Nanoskiving is a technique to produce nanoscale structures using an ultramicrotome to section thin films of materials (e.g., gold) embedded in polymer. Epoxies are used typically as embedding resins for microtomy. Epoxies, however, do not adhere well to gold or other smooth metallic structures that are used commonly for nanoskiving. Thiol–ene and thiol–epoxy polymers provide improved adhesion to gold due to the thiol functional group. In addition, the thiol–ene polymers can be prepared within minutes using photopolymerization, which allows for rapid prototyping. Two commercial thiol-containing adhesives were evaluated as resins in addition to several formulations of commercially available monomers. The important physical and mechanical properties for microtomy of these unconventional embedding resins were characterized and the properties were compared to commercial epoxy resins. Gold nanowires were fabricated using nanoskiving of gold films embedded in these unconventional resins. These studies show that a 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 mixture of thiol (pentaerythritol tetra(3-mercaptopropionate)) and ene (triallyl-1,3,5-triazine-2,4,6-trione) works very well as a resin for nanoskiving and provides improved adhesion and reduced preparation time relative to epoxies.
4 mixture of thiol (pentaerythritol tetra(3-mercaptopropionate)) and ene (triallyl-1,3,5-triazine-2,4,6-trione) works very well as a resin for nanoskiving and provides improved adhesion and reduced preparation time relative to epoxies.
Introduction
This paper evaluates new thiol-containing embedding matrices for nanoskiving and compares these polymeric materials to conventional epoxy-based embedding matrices. Nanoskiving is a form of edge lithography that uses an ultramicrotome equipped with a diamond knife to section thin films (e.g., gold) embedded in a polymer matrix.1 The conventional polymer used for embedding these films is typically a liquid pre-polymer that cures with minimal shrinkage to produce a hard bulk polymer that facilitates handling and is necessary for sectioning. Adhesion between the sample (i.e., often a metal film in the case of nanoskiving) and the polymer matrix is important to assure that the final composite does not disintegrate while sectioning with a microtome.Epoxies are popular embedding resins for microtomy because they are hard, shrink minimally during curing, and adhere conformally to most materials. Epoxies, however, do not adhere well to gold (or other smooth, metallic structures). Gold is often used for nanoskiving because it is easy to deposit and section, is not brittle, and does not oxidize readily.
Araldite and Epofix are the two most commonly used commercial epoxides for nanoskiving. Araldite is advantageous because it is easy to trim (a necessary first step in nanoskiving) and forms mechanically stable sections even at very thin (<100 nm) thicknesses that are common in nanoskiving. However, Araldite is a three-part epoxy with a lengthy curing time (∼48 hours for optimal mechanical properties) and is optimized for soft materials (e.g., biological specimens). Epofix is a two-part, thermally curable epoxy that is designed specifically for sectioning hard materials, but it is considerably harder than Araldite which leads to unstable sections and an increased susceptibility to “chattering”, in which the diamond knife skips across the surface of the epoxy block leading to non-uniform thicknesses in the resulting sections. Neither Araldite nor Epofix adhere particularly well to smooth metal surfaces, especially gold.
We hypothesized that incorporating monomers with thiol functionality into the embedding polymer would improve the adhesion between gold and the polymer during sectioning by introducing chemisorption via the spontaneous formation of gold–thiolate bonds. We evaluated photocurable thiol–ene polymers because they possess properties that are well suited for microtomy, i.e.: (1) their mechanical properties can be tailored by the choice of monomers, (2) they have low viscosity before curing, which facilitates embedding, (3) they photocure quickly on demand to deep thicknesses, which allows for rapid prototyping, and (4) they cure by step-growth polymerization, which leads to low shrinkage compared to most free-radical polymerizations. We also studied a thiol–epoxy formulation, which has many of the same desirable attributes of the epoxy networks, but includes thiol functionality. We compared the most relevant properties of the new embedding resins such as mechanical behavior, polymerization shrinkage, and adhesion to gold to evaluate the advantages and limitations relative to conventional epoxy resins. We also fabricated gold nanowires of various dimensions and examined the resulting sections by optical microscopy, measured the conductance of the nanowires, and compared the etching rates by plasma oxidation to demonstrate that the thiol-containing embedding resins are superior to conventional epoxy resins for nanoskiving.
Background
Fig. 1 outlines the procedure for fabricating nanostructures by nanoskiving. Preparing the sample (or “block”) for sectioning by microtome is a key aspect of nanoskiving. Typically, the fabrication process begins with a substrate consisting of a cured embedding resin that can either be flat or have topography (defined by soft lithography,2–4 for example). Thin films can be deposited onto this substrate with precise thickness by a number of methods (e.g., spin coating, physical vapor deposition, sputtering). The entire substrate is then embedded in additional resin. Prior to sectioning, the face of the block is trimmed manually into a trapezoidal shape (∼1 to 1.5 mm2) using a razor blade. Sectioning the resulting block with an ultramicrotome equipped with a diamond knife attached to a boat filled with water yields thin polymer slabs that float on the surface of the water and that can be transferred to a substrate directly (e.g., by dipping a substrate in the boat) or via a drop of water. Structures formed by nanoskiving are often composed of gold because it is easy to deposit, does not oxidize, and is soft relative to most metals, which facilitates sectioning and results in stable, electrically continuous nanostructures. Exposure to oxygen (or air) plasma removes selectively the embedding resin and generates freestanding nanostructures. The topography of the original substrate, the thickness of the deposited film, and the thickness of the sections cut by the ultramicrotome (as thin as 15 nm) determine the dimensions of these nanostructures. The ability to control the dimensions of the nanostructures without the use of sophisticated lithographic tools or a clean room makes this technique very attractive for rapid prototyping. Nanostructures can also be fabricated from materials that are not compatible with conventional photolithography/etching. Nanoskiving produces hundreds of thousands of identical sections from a single block and the thin polymeric sections can be positioned onto various substrates.5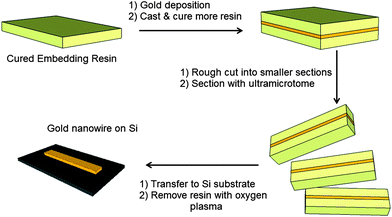 | ||
| Fig. 1 A schematic of the fabrication of a gold nanowire by nanoskiving. | ||
Biologists and material scientists use ultramicrotomy conventionally to produce thin (∼100 nm thick) sections of cells, tissues, and materials for analysis by electron microscopy.6,7 Embedding is done by infiltration of the sample with a liquid embedding medium that polymerizes to produce a solid block that can be handled and sectioned using a microtome. An embedding medium for conventional biological samples should have the following properties: commercial availability, uniformity from one batch to another, solubility in dehydrating agents, low viscosity as a monomer for ease of handling, uniform polymerization, minimal volume change upon polymerization to avoid distortion of the sample, ease of sectioning and stability in an electron beam.6 Embedding resins for nanoskiving do not require solubility in dehydrating agents nor stability in an electron beam, but should be able to be etched by oxygen plasma.
None of the embedding media developed so far possesses all the desired qualities for microtomy, although several commercial epoxies (e.g., Araldite and Epofix) work well for most microtome applications.1,8 Epoxies are generally cross-linked and possess relatively large elastic moduli (Young's modulus, E > 1500 MPa), which is well suited for sectioning at room temperature.9 Hardening of epoxies occurs via an addition reaction that results in very little change of volume. Epoxy resins also adhere well to many materials with polar surfaces (i.e. surface oxides) by hydrogen bonding.10
Most nanoskiving work to date relied on Araldite and Epofix epoxy resins, which offer convenience at the expense of performance.7,11 Unfortunately, epoxy-based embedding resins exhibit poor adhesion to gold, which can lead to catastrophic delamination of the nanostructures from the embedding resin during sectioning or during preparation of the sample blocks (cf., Fig. 6). Sectioning is a mechanical cutting process and relies on the smooth and continuous advance of the block past the edge of the knife. Delamination diminishes significantly the quality of the sectioning process and should be mitigated when possible. For applications in which the epoxy matrix is used to electrically isolate the top face of gold nanostructures, delamination is catastrophic.12 Delamination between the epoxy and gold has been minimized – but not eliminated – previously by careful handling and ensuring that the epoxy completely encapsulates the gold film on all sides.1
We sought to evaluate polymeric embedding materials that possess the important material characteristics required for microtoming, while enhancing the binding to gold and increasing the rate of curing with minimal shrinkage. We chose polymers that contained thiols because they form strong bonds with many metals including gold, silver, palladium, copper, and many others.13 Although in some circumstances it may be possible to improve adhesion between metal and the embedding resin by modifying the gold films with self-assembled monolayers (SAMs), we have had limited success with epoxide and carboxylate-terminated SAMs with both Epofix and Araldite. Thus, we sought to develop a simpler and more universally useful resin that contained thiols so that no additional steps would be required to improve adhesion to gold. In this paper, we evaluate the critical properties of several thiol-containing resins within the context of microtomy and demonstrate the utility of these materials in nanoskiving.
Experimental design
Table 1 summarizes the materials studied as embedding resins. Epofix and Araldite serve as commercial benchmarks to which we compare the thiol-containing resins.Thiol-esters
We evaluated two commercially available, photocurable, mercapto-ester systems: NOA 63 and NOA 81. Norland Products sells many photocurable adhesives, but we selected these polymers because they possess elastic moduli similar to that of conventional epoxy resins. The manufacturer does not provide chemical information other than to call these products ‘mercapto-esters’ and we use the term ‘thiol-ester’ to be consistent with our other notations. These resins are also sold in smaller quantities than Araldite and Epofix, as they are optical adhesives that are optimized for transparency, not embedding.Thiol–enes
The photopolymerization of mixtures of thiols and alkenes is an efficient method for the rapid production of crosslinked polymer networks.14 Thiol–ene photopolymerization proceeds rapidly by a step-growth free-radical chain transfer reaction.14 We formulated thiol–ene resins using commercially available monomers, pentaerythritol tetra(3-mercaptopropionate) and triallyl-1,3,5-triazine-2,4,6-trione (referred to as PETMP and TATATO, respectively). PETMP–TATATO has been studied a potential dental restorative material, which also has the requirements of minimal shrinkage and hardness.15 The properties of this formulation can be tuned by varying the composition and stoichiometry of the reacting monomers. We evaluated 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 and 1
4 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 molar compositions of PETMP–TATATO. The 3
1 molar compositions of PETMP–TATATO. The 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 molar mixtures have stoichiometric amounts of functional groups, whereas 1
4 molar mixtures have stoichiometric amounts of functional groups, whereas 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mixture has an excess of thiol functional groups but comprises stoichiometric amounts of monomer molecules.
1 mixture has an excess of thiol functional groups but comprises stoichiometric amounts of monomer molecules.
Thiol–epoxies
We also evaluated a thiol–epoxy catalyzed by an amine, which involves a multistep reaction resulting in the opening of the epoxide ring followed by the addition of a thiol anion.16 The motivation for using these materials is driven by the low shrinkage and mechanical properties enabled by epoxies and by the ease with which a commercial thiol (PETMP) can be added to a commercial epoxy embedding resin (Epofix).17 These materials are cured thermally at room temperature using the chemistry developed for conventional resins (i.e., using an amine initiator).Benchmark embedding resins
We selected two conventional epoxy-based resins, Araldite 502 and Epofix, as benchmarks to compare the thiol containing resins. Both embedding resins have been used previously for nanoskiving.4,12 Araldite cures at 60 °C over a 24–48 h period, whereas Epofix cures at room temperature over 8–10 h. In Araldite, the curing agent is an anhydride, whereas in Epofix, it is an aliphatic amine.Characterization
We sought to measure the properties and compare the performance of the new resins to the benchmark resins. Previous studies have shown that stress–strain tensile measurements provide the best metric for predicting a priori whether a material will be a satisfactory embedding resin.9 Ideal materials are hard enough to prevent sample deformation (i.e., they possess a large modulus), but are not too brittle so as to prevent cracking. We therefore use stress–strain behavior as a primary metric for predicting ideal embedding resins. Of course, the ultimate metric for evaluating new resins is their performance during nanoskiving.There are also some additional fundamental properties of polymer networks that help predict whether a resin will perform well; these include glass transition temperature (Tg), shrinkage, and adhesion to gold.9 Thermomechanical analysis provides insight into the glass transition temperature of the polymer networks. The Tg is a physical property of polymers that can indicate the best temperature settings needed for microtomy. As a general rule, polymers that are hard (Tg > room temperature) can be sectioned at room temperature while soft materials (Tg < room temperature) need to be sectioned at lower temperatures.9 Shrinkage of the resin during preparation of the block should be minimized to avoid sample distortion and adhesion of the resin to gold during sectioning should be maximized to avoid delamination.
Experimental
Materials and preparation
We purchased Epofix and Araldite 502 as a kit (Electron Microscopy Sciences). The Epofix kit consists of two components, a resin (contains bisphenol-A-diglycidyl ether) and hardener (triethylenetetramine). Following the instructions from the kit, we mixed the resin and hardener in 25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 (w/w) ratios and stirred rigorously for two minutes, keeping the mixture in vacuum for no longer than 20 min removed visible trapped air bubbles. Epofix cures at room temperature for 8–10 h,18 though it performs better when cured at 60 °C presumably because it lowers the viscosity before hardening. Araldite 502 kits consist of three components, Araldite 502 resin, DDSA (dodecenyl succinic anhydride) and DMP-30 (2,4,6-tri-(dimethylaminomethyl)phenol) and those are mixed in 20
3 (w/w) ratios and stirred rigorously for two minutes, keeping the mixture in vacuum for no longer than 20 min removed visible trapped air bubbles. Epofix cures at room temperature for 8–10 h,18 though it performs better when cured at 60 °C presumably because it lowers the viscosity before hardening. Araldite 502 kits consist of three components, Araldite 502 resin, DDSA (dodecenyl succinic anhydride) and DMP-30 (2,4,6-tri-(dimethylaminomethyl)phenol) and those are mixed in 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 22
22![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.65 (v/v) ratios, respectively. Araldite cures at 60 °C in an oven for 24–48 h.18
0.65 (v/v) ratios, respectively. Araldite cures at 60 °C in an oven for 24–48 h.18
We purchased PETMP and TATATO (Sigma Aldrich) monomers and mixed all formulations with 0.1 wt% of 2,2-dimethoxy-2-phenylacetophenone (DMPA, Ciba-Geigy) as the photoinitiator. Fig. 2 depicts the chemical structures of the monomers. All materials were used as received.
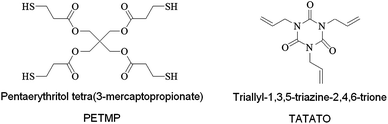 | ||
| Fig. 2 Chemical structures of the thiol and ene monomers. | ||
We purchased NOA 63 and NOA 81 (Norland Optical Adhesive) from Norland Products. We cured the thiol–ene and NOA resins for 60 s (75 mW cm−2) to ensure thorough curing. The NOA curing time varied with sample thickness as outlined in the technical data sheets.18 A mercury UV Floodlight (Intelli-Ray 400 from Uvitron International) cured the formulations.
To formulate the thiol–epoxy, we mixed PETMP (thiol, 0.6 g), Epofix resin (epoxy, 1.4 g) and DMP-30 (0.1 g, 5 wt%) as the catalyst, resulting in stoichiometric amounts of functional groups (epoxy and thiol). The mixture cured at room temperature for 30–60 min.
Polymerization shrinkage
We analyzed the shrinkage by measuring the density of the resin before and after curing. Eqn (1) defines the percentage volumetric shrinkage, ΔV, | (1) |
We measured the density of the cured polymer samples by the flotation method in mixed solvents.20 Cured polymer samples floated on carbon tetrachloride (ρ = 1.594 g cm−3)20 in a graduated cylinder with a magnetic stir bar. A burette added dropwise a light solvent – toluene (ρ = 0.8668 g cm−3)20 – to the carbon tetrachloride. Aluminum foil covered the container except for a small hole for the toluene to drop through to minimize evaporation. Eqn (2) determines the density of the sample at the point when the sample floated halfway in the cylinder:
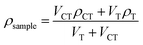 | (2) |
Tensile properties
An extensometer (Instron 5943) measured the stress and elongation at the breaking point as well as the elastic modulus. We followed the ISO 527-2 standard using a specimen type 5A. All samples cured in a stainless steel mold coated with a non-stick layer.Dynamic mechanical analysis
DMA determined the glass transition temperature of the cured polymers. Curing the pre-polymer formulations in a Teflon Petri dish produced the specimens (6–8 mm wide, 0.6–1.0 mm thick and 15–17 mm long) for analysis. We conducted DMA (TA Q800 DMA, TA instruments) studies over a temperature range of 0–120 °C for epoxy and −50 to 120 °C for thiol containing systems, with a ramping rate of 5 °C min−1 using tensile mode (sinusoidal stress of 1 Hz frequency).Peel test
We evaluated the adhesion of polymer to gold using an extensometer in peel test mode.21Fig. 3 describes two approaches used to prepare the samples.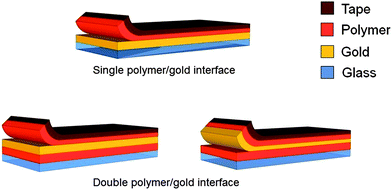 | ||
| Fig. 3 Comparison of peeling using glass–Ti–Au–polymer and glass–polymer–Au–polymer substrates. In the first case (“single polymer–gold interface”) peeling primarily occurs at the Au–polymer interface. In the second case, peeling could occur at two interfaces, as shown. | ||
To study the adhesion of polymer cured on gold, we used electron beam evaporation to deposit titanium (5 nm) followed by gold (30 nm) onto clean glass slides and then spun cast thin films (15–17 μm thick, as verified by profilometry) of monomer onto the gold surface.
An additional set of experiments measured the peel strength of the interface between gold and polymer formed by depositing gold onto cured polymer. We cured a thin film of the polymer on glass and deposited a thin (30 nm) film of gold onto the cured polymer surface by e-beam evaporation. We cast a thin film of the same pre-polymer formulation on the gold surface and cured it against a backing material that can be gripped by the Instron. We pressed a suitable backing material (e.g., Scotch tape) on top of the pre-polymer film and cured the polymer. The backing material had to be thin (so that it could be bent easily) and had to have sufficient adhesion to the polymer to avoid delamination during the peel test. Commercial Scotch tape served as the backing material for most of the measurements, although Kapton film (DuPont) served as the backing material for the Epofix samples because Epofix showed poor adhesion to the Scotch tape. Peeling the backing tape slowly (∼5 mm s−1 by hand) from the substrate provided a qualitative evaluation of the nature of failure. The Instron measured the force required to peel the polymer film (90° peel angle and a 1.0 mm s−1 peel rate) from the gold coated substrate at room temperature.
Nanoskiving
We prepared samples for ultramicrotome sectioning by first depositing a thin film of gold (thickness 50 or 100 nm) on a Si-wafer by e-beam evaporation. We spin coated (or drop cast for Epofix) the pre-polymer on the gold surface and cured it. The gold-film adhered to the polymer as it was peeled from the Si surface and cut into small strips of (5–7 mm long, 3 mm wide). Embedding the gold strips in more pre-polymer formulation in a mold produced the block. A razor blade trimmed the edges of the sample block into a trapezoid shape (about 1 × 1 mm) to expose the metal.A Leica UC-6 Ultramicrotome prepared sections from the blocks containing gold films with thicknesses of 50, 100, 150, and 200 nm using a 2 mm 35° Diatome diamond knife at 1 mm s−1 except for the 50 nm sections which were sectioned at 0.8 mm s−1.
Results and discussion
Tensile testing and Young's modulus
Tensile tests represent the best method for predicting the usefulness of embedding resins.9 The Young's modulus (i.e., the initial slope of the stress–strain plot) is important for sectioning since mechanical damage by compression can occur in softer samples and cracking occurs in hard, brittle samples. Embedding materials with Young's moduli of 1.5–3 GPa generally provide high quality sections in ultramicrotomy.9 Softer resins (with moduli <1.5 GPa) can be cut below room temperature, but so-called “cryo-sectioning” is more challenging than room temperature sectioning. Resins should also undergo minimal plastic flow (i.e., low ductility) to avoid distortions during sectioning. Fig. 4a shows that, as expected, the two conventional epoxy resins have all of these desirable features. In contrast, the commercial NOA resins have highly undesirable deformation and ductility, which reduces the usefulness of the NOA resins for nanoskiving. Only the NOA 81 could be sectioned by a microtome and it resulted in sections with non-uniform thickness.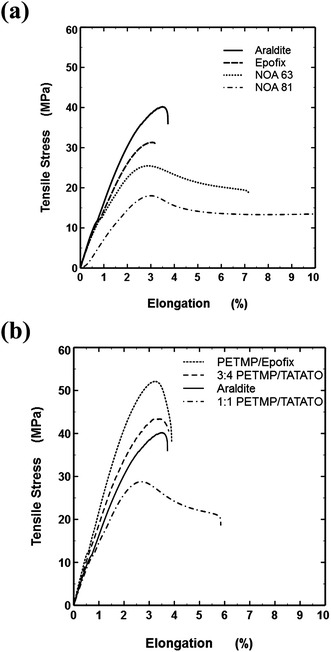 | ||
| Fig. 4 (a) Conventional embedding resins and commercial photocurable ‘thio-esters’ (NOA 63 and 81). Not shown: the break point of NOA 81 occurs at 15.8%. (b) Thiol–ene and thiol–epoxy embedding resins and Araldite (for comparison). | ||
The PETMP-based resins behave favorably (Fig. 4b), with limited yielding and low ductility. Notably, the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 PETMP–TATATO has significant variability in its stress–strain curves (Fig. S2†), likely due to the non-stoichiometric nature of the network. This variability limits the utility of this resin for reliable sectioning. The 3
1 PETMP–TATATO has significant variability in its stress–strain curves (Fig. S2†), likely due to the non-stoichiometric nature of the network. This variability limits the utility of this resin for reliable sectioning. The 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 PETMP–TATATO showed almost identical behavior to the Araldite resin and therefore appeared to be the most promising of all the thiol containing resins studied. The PETMP–Epofix sample had the highest yield stress and showed low ductility, which suggests this thiol–epoxy resin may be useful for nanoskiving too.
4 PETMP–TATATO showed almost identical behavior to the Araldite resin and therefore appeared to be the most promising of all the thiol containing resins studied. The PETMP–Epofix sample had the highest yield stress and showed low ductility, which suggests this thiol–epoxy resin may be useful for nanoskiving too.
The slope of the initial linear portion of the stress–strain traces provided the modulus values listed in Table 2. Using the elastic moduli of Araldite and Epofix as benchmark values (2.01 and 2.15 GPa, respectively), the values for NOA 63 and NOA 81 are low. Although the modulus value of 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 PETMP–TATATO is lower than the benchmark values, it is larger than the 1
4 PETMP–TATATO is lower than the benchmark values, it is larger than the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 PETMP–TATATO and still acceptable for room temperature sectioning. The value also agrees with modulus values found for similar formulations in the literature22,25 and is consistent with the known trend that increasing the amount of ene in the thiol–ene formulation increases hardness.17 The thiol–epoxy formulation also yielded sample blocks with modulus values slightly greater than the benchmark.
1 PETMP–TATATO and still acceptable for room temperature sectioning. The value also agrees with modulus values found for similar formulations in the literature22,25 and is consistent with the known trend that increasing the amount of ene in the thiol–ene formulation increases hardness.17 The thiol–epoxy formulation also yielded sample blocks with modulus values slightly greater than the benchmark.
| Materials | T g (°C) | Young's modulus (GPa) at 25 °C | % Shrinkage |
|---|---|---|---|
| Araldite | 76 | 2.01 ± 0.15 | 4.8 |
| Epofix | 72 | 2.15 ± 0.22 | 6.3 |
| NOA 63 | 44 | 1.54 ± 0.08 | 5.7 |
| NOA 81 | 35 | 0.85 ± 0.05 | 8.8 |
PETMP–TATATO (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
51 | 1.43 ± 0.09 | 7.7 |
PETMP–TATATO (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4) 4) |
73 | 1.70 ± 0.13 | 7.5 |
| PETMP–Epofix | 60 | 2.19 ± 0.08 | 3.0 |
Cure time
The thiol containing polymers evaluated in this study cured rapidly compared to the epoxy based networks (Araldite and Epofix). The benchmark resins require several hours to cure completely. In contrast, the thiol–ene and NOA resins cured within minutes. Previous studies on the curing kinetics of PETMP–TATATO polymerization suggest that 60 s of irradiation (at 5.0 mW cm−2) results in 90% conversion of the TATATO allyl group and 77% conversion of the PETMP thiol functional group.15 The shorter cure time of the thiol–enes and NOAs offers a potential advantage over conventional epoxy-based embedding resins in terms of rapid prototyping.The thiol–epoxy system required ∼30 min to cure at room temperature, as estimated by the time required to get a non-tacky, hard-set polymer. Notably, the polymerization begins almost immediately upon mixing as indicated by the increase in viscosity. This characteristic makes it challenging to process the resin (i.e., mixing it rapidly and uniformly without entraining air bubbles). Of the ∼25 samples we produced for nanoskiving, 3 were too soft to section, presumably due to poor mixing.
Polymerization shrinkage
Table 2 summarizes the shrinkage measurements. Epoxies are known to undergo a low extent of shrinkage during polymerization; a volume reduction around 4–5% is expected for epoxy resins, but can vary based on filler content (embedding resins usually lack filler because the filler can damage diamond knives) and preparation conditions of the resin such as mixing or initial resin temperature.10 Araldite 502 shrunk 4.8% and Epofix shrunk 6.3%. NOA 63 and NOA 81 underwent 5.7% and 8.8% shrinkage, respectively. PETMP–TATATO formulations undergo 7.7% and 7.5% shrinkage for the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and 3
1 and 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 formulations. The shrinkage reported for a 3
4 formulations. The shrinkage reported for a 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 stoichiometric PETMP–TATATO resin composition by the static volume change method is 6.2%.22 We speculate that our shrinkage values are slightly higher than those measured previously because during curing our samples were not confined by the walls of the sample container. We did not observe any adverse effects of shrinkage in the samples prepared by nanoskiving, and the amount of shrinkage is less than some embedding resins that have been used previously (e.g., PMMA which has a 15–20% shrinkage23).
4 stoichiometric PETMP–TATATO resin composition by the static volume change method is 6.2%.22 We speculate that our shrinkage values are slightly higher than those measured previously because during curing our samples were not confined by the walls of the sample container. We did not observe any adverse effects of shrinkage in the samples prepared by nanoskiving, and the amount of shrinkage is less than some embedding resins that have been used previously (e.g., PMMA which has a 15–20% shrinkage23).
Dynamic mechanical analysis
Table 2 summarizes the Tg. As a general rule, the Tg of an embedding resin must be greater than room temperature if sectioning is to be done at room temperature,24 and practically, the Tg should be 47 °C or greater for proper sectioning.9 The benchmark epoxy polymers have Tg values between 70 and 76 °C. The Tg of all the thiol-containing polymers studied are lower than the epoxies, but well above room temperature (and all but the NOAs were above 47 °C), suggesting that the sectioning can be done at room temperature. The PETMP–TATATO based networks exhibited a higher glass transition temperature than both NOAs. As the amount of alkene bonds decreases in the PETMP–TATATO formulation (going from 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 to 1
4 to 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 molar), the Tg decreases. The inclusion of thiol in epoxy networks also reduces the Tg (from 72 °C for Epofix to 60 °C for PETMP–Epofix).
1 molar), the Tg decreases. The inclusion of thiol in epoxy networks also reduces the Tg (from 72 °C for Epofix to 60 °C for PETMP–Epofix).
Peel tests
Peel tests evaluated the adhesion of the polymers to gold (Fig. 3). The peeling mechanism differed for the benchmark resins and thiol containing polymers both qualitatively and quantitatively. Epofix and Araldite delaminated primarily at the gold–polymer interface (i.e., adhesive failure), confirming that the adhesion between Au–epoxy is weak. Based on visual inspection of the substrate, we estimate that the epoxy peeled cleanly from at least 90% of the surface area of the gold substrate. Surprisingly, we observed the same behavior for NOA 63 samples despite the presence of thiols (the technical data sheet from Norland suggests that NOA 81 binds more strongly to metals than NOA 63). In contrast, NOA 81, PETMP–TATATO, and the thiol–epoxy left polymer on the gold substrate after peeling. This qualitative observation suggests that the adhesion of these polymers to gold is better than that of Epofix, Araldite, and NOA 63.A 90° peel test quantified the peel force. PETMP–TATATO, NOA 81, and PETMP–Epofix had larger average peel forces than Epofix and NOA 63 as shown in Fig. 5. It is difficult to assign definitive meanings to the absolute values of the peel force measurements. Regardless, the values obtained provide an insight into relative forces required to induce delamination of polymer from the gold surface.
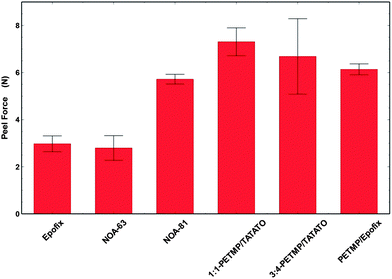 | ||
| Fig. 5 The comparison of average load required to peel the polymers from gold surface of glass–Ti–Au–polymer substrates. | ||
Although the benchmark epoxy resins contain amines, which provide some adhesion to gold,26 thiols are known to bond roughly two times more strongly to gold.27 Thiols are also much more abundant in these resins. It is therefore expected that the force required to peel a thiol-containing resin from a gold layer would be higher than for the epoxy resins; indeed, the peel test results confirm this hypothesis. The addition of a thiol group to Epofix approximately doubles the peel force necessary to remove the polymer from the gold layer relative to pure Epofix.
We also evaluated qualitatively the interface between gold and polymer formed by depositing gold onto pre-cured polymer (polymer–Au–polymer sandwiches). We expected this interface to be weaker than the interface created by curing polymer against gold. During the peeling of Epofix samples, the bottom polymer layer (i.e., the pre-cured polymer layer) and gold failed adhesively suggesting that the adhesion of pre-cured polymer to gold is weaker than that formed by curing polymer against a gold film. In all the thiol-containing samples, patches of gold remained attached to both the top and bottom polymer layers, as might be expected due to the presence of thiol on both sides of the gold. According to theoretical models, Au–Au bonds in bulk gold break with a force of 0.8–0.9 nN.28 It is expected that the S–Au bond, with a strength of 1.4 nN,27 would break after the Au–Au bond which is consistent with our observations.
Nanoskiving
The sample blocks for each polymer system contained an embedded film of gold (50 or 100 nm thick). Prior to sectioning with ultramicrotome, we trimmed these specimens to a rough trapezoid shape (∼1 × 1 mm) as shown in Fig. 6. We only sectioned the Araldite, Epofix, NOA 81, and PETMP–TATATO 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4; the mechanical properties of the blocks formed from the other embedding resins precluded trimming and sectioning, primarily because they were too soft and deformed during the trimming process (Fig. S1†). In some cases, the brittle nature of Epofix, combined with its poor adhesion to gold, cause the polymer to delaminate and break away in chips, requiring the block to be rough-cut and re-trimmed. An example of this type of failure is shown in Fig. 6A, which is contrasted with a block containing thiols (PETMP–TATATO, Fig. 6B).
4; the mechanical properties of the blocks formed from the other embedding resins precluded trimming and sectioning, primarily because they were too soft and deformed during the trimming process (Fig. S1†). In some cases, the brittle nature of Epofix, combined with its poor adhesion to gold, cause the polymer to delaminate and break away in chips, requiring the block to be rough-cut and re-trimmed. An example of this type of failure is shown in Fig. 6A, which is contrasted with a block containing thiols (PETMP–TATATO, Fig. 6B).
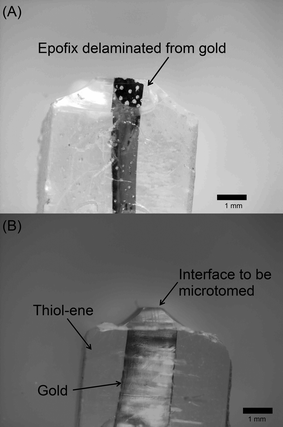 | ||
| Fig. 6 Photographs of sample blocks prepared by cutting polymer with a razor blade. (A) Epofix does not contain thiol and delaminates from gold in some cases. (B) PETMP–TATATO contains thiol and adheres to gold. | ||
The PETMP–Epofix gave the most unexpected result; some of the sample blocks (3 out of 25) were too soft to trim (Fig. S1†) and others were brittle (they could be snapped into two pieces by hand). Although the macroscopic measurements suggest PETMP–Epofix has desirable mechanical properties, we believe this inconsistency arises because the addition of PETMP to Epofix initiates a rapid polymerization that precludes effective mixing on the microscale (mixing is further complicated by the desire to exclude air bubbles from the resin). The relatively small blocks (and extremely small slabs formed during nanoskiving) are sensitive to small, localized variation in mechanical properties arising from the inefficient mixing. This variation manifested itself as block-to-block inconsistencies; some of the blocks could, in fact, be trimmed (Fig. S1†) but we did not continue the nanoskiving test because of the irreproducibility of the blocks.
Fig. 7 shows optical micrographs of the resulting sections of thicknesses of 50, 100, 150, and 200 nm. With the exception of NOA 81, we produced sections down to 40 nm (not shown) before they became too fragile to handle, though the technical limit of the ultramicrotome is 15 nm. Although the resolution of these micrographs (250× magnification) is not sufficient to directly image the gold structures, which are 50–100 nm thick, the mismatch in the indices of refraction between the embedding resins and the gold appears as a dark line. The thickness of the sections can be verified by the color of the sections, which results from thickness-dependent interference; 50 nm appears grey, 100 nm turquoise, 150 nm blue, and 200 nm orange-brown. The NOA 81 sections all appear blue-turquoise, however, indicating that the actual thickness of the sections deviated from the set thickness. This deviation is the result of the mechanical properties of the NOA 81, which is likely too ductile and produces too much friction against the advancing edge of the diamond knife. The result is uneven sections, which is the case in Fig. 7 (lower-left) where only a band of orange-brown (i.e., 200 nm thick resin) is apparent in the 200 nm section. The average thickness of these sections also alternates as the block advances by the set-thickness before each stroke, but differing thicknesses of epoxy are skived (not pictured). The result is that NOA 81 is unsuitable for forming gold nanowires by nanoskiving at any thickness.
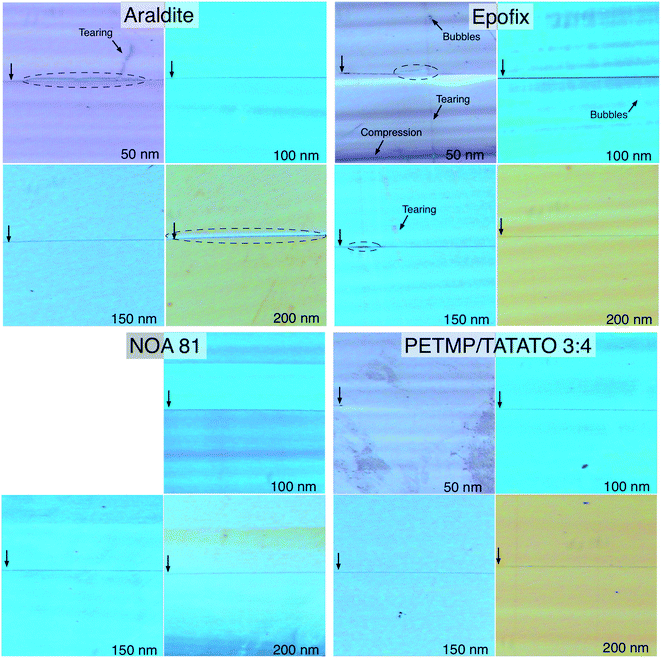 | ||
| Fig. 7 Optical micrographs (250×) of sections of the four embedding resins that could be nanoskived. The vertical arrows at the left of each image indicate the position of the gold nanowire and the dashed ovals indicate delamination of the resin from the gold. The thickness of each section is indicated in the lower right corner, which is confirmed by the color of the section resulting from thickness-dependent interference. The horizontal bands present in some images are the result of chattering, the severity of which depends on the mechanical properties of each resin. Sections of NOA 81 <50 nm disintegrated upon formation and chattering was severe enough to preclude accurate section-thicknesses, which is evident from the predominantly blue color in the 200 nm section. Tearing, bubbles, and compression artifacts were predominant in the Epofix sections, while Araldite exhibited the most severe delamination. | ||
The most obvious feature of the Araldite (Fig. 7; top-left) sections is delamination, which is indicated with dashed ovals and appears as almost complete adhesive failure in the 50 and 200 nm sections. While the degree of delamination varies between sections, the images shown in Fig. 7 are representative. Aside from delamination, Araldite produces – unsurprisingly – very high quality sections with minimal chattering, which appears as horizontal bands of color. These bands of color are the result of the knife skipping (chattering) as it passes through the polymer and are the result of the compressibility of the polymer and the amount of friction generated at the trailing edge of the knife. Chattering should not be confused with transient vibrations, which can be controlled externally (e.g., with a dampening table). From these results we conclude that Araldite, while functional, is not an ideal embedding resin for gold structures.
Epofix, which is designed for ultramicrotomy on hard materials, performs very poorly at thicknesses below 100 nm. The 50 nm thick section pictured in Fig. 7 (top-right) is rife with artifacts, the most severe of which is the compression visible in the lower part of the section. Also present are bubbles, which form when the two parts of the pre-polymer are mixed and which cannot be removed completely before the resin cures. There is also significant tearing, which is usually the result of a knick in the diamond knife, but can also be caused by dust particles. In this case, we ascribe the tearing to a minor defect in the knife (which is very common) that is only apparent in the 50 nm thick sections of Epofix because of the brittle nature of that resin. In addition to being difficult to trim (Fig. 6A), this brittleness leads to sections that craze and are predisposed to separating into pieces in the boat (not shown) as a result of tearing and compression artifacts. The most prominent artifact in the Epofix sections, surprisingly, is not delamination – which is visible to some extent in 50 and 150 nm thick sections – but chattering, which is evident as prominent bands of color in all of the sections. While chattering is not necessarily detrimental to the formation of nanowires of gold, which are cut parallel to the edge of the knife, more complex metallic structures will not have a uniform height/thickness when sectioned using Epofix.
The only embedding resin that produced sections (nearly) free of artifacts and with no delamination at all thicknesses was 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 PETMP–TATATO. The only visible artifacts are smudges, which are the most pronounced in the 50 nm thick sections shown in Fig. 7 (bottom-right). It is not clear what causes these smudges, but they are not tears, bubbles, compression artifacts or the result of chattering nor do they appear to negatively affect the nanoskiving process. The 3
4 PETMP–TATATO. The only visible artifacts are smudges, which are the most pronounced in the 50 nm thick sections shown in Fig. 7 (bottom-right). It is not clear what causes these smudges, but they are not tears, bubbles, compression artifacts or the result of chattering nor do they appear to negatively affect the nanoskiving process. The 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 PETMP–TATATO sections were the easiest to trim and section, show almost no chattering, and form very stable sections even at thicknesses below 50 nm (not shown). Interestingly, the dark line indicating the position of the gold is barely discernible in the 50 nm thick sections, which we ascribe to the small dimension of the gold wire (50 × 50 nm) and the excellent adhesion of the polymer to gold. It is clearly visible and free of any signs of delamination in the 100, 150, and 200 nm thick sections.
4 PETMP–TATATO sections were the easiest to trim and section, show almost no chattering, and form very stable sections even at thicknesses below 50 nm (not shown). Interestingly, the dark line indicating the position of the gold is barely discernible in the 50 nm thick sections, which we ascribe to the small dimension of the gold wire (50 × 50 nm) and the excellent adhesion of the polymer to gold. It is clearly visible and free of any signs of delamination in the 100, 150, and 200 nm thick sections.
To verify that the embedding resins do not interfere with the electrical properties of the gold nanowires, we measured I/V curves for 100 nm thick sections of Araldite, Epofix, NOA 81, and 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 PETMP–TATATO. We measured 50 nm thick gold nanowires (∼1 mm × 50 nm × 100 nm) by painting small (∼1 mm2) pads of silver paste at either end of each nanowire and contacting them in a home-built probe station. The resulting data are plotted in Fig. 8 and show ohmic conduction for all four nanowires, indicating that none of the embedding resins affected the electrical properties of the gold nanowires. The magnitude of the current (∼10−4 A) is consistent with previous measurements on gold nanowires of similar dimensions fabricated using nanoskiving.29 The slightly lower conductivities of NOA 81 and Araldite are the result of differences in the lengths of the nanowires.
4 PETMP–TATATO. We measured 50 nm thick gold nanowires (∼1 mm × 50 nm × 100 nm) by painting small (∼1 mm2) pads of silver paste at either end of each nanowire and contacting them in a home-built probe station. The resulting data are plotted in Fig. 8 and show ohmic conduction for all four nanowires, indicating that none of the embedding resins affected the electrical properties of the gold nanowires. The magnitude of the current (∼10−4 A) is consistent with previous measurements on gold nanowires of similar dimensions fabricated using nanoskiving.29 The slightly lower conductivities of NOA 81 and Araldite are the result of differences in the lengths of the nanowires.
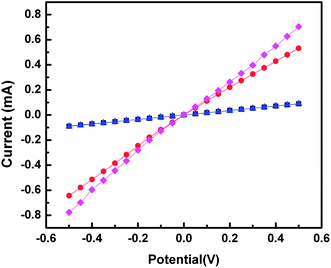 | ||
Fig. 8 Plots of current (mA) versus potential (V) for 50 nm thick gold nanowires fabricated using 100 nm thick sections of Araldite (black squares), NOA 81 (blue triangles), Epofix (red circles), and 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 PETMP–TATATO (pink diamonds). Each trace is an average of four sections. All four wires exhibit ohmic I/V characteristics indicating that the wires are electrically continuous. The slightly lower conductivities of the wires in NOA 81 and Araldite are the result of differences in the lengths of the wires. 4 PETMP–TATATO (pink diamonds). Each trace is an average of four sections. All four wires exhibit ohmic I/V characteristics indicating that the wires are electrically continuous. The slightly lower conductivities of the wires in NOA 81 and Araldite are the result of differences in the lengths of the wires. | ||
From these measurements and the optical micrographs in Fig. 7, we can conclude that all four resins are capable of producing electrically continuous gold nanowires; however, 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 PETMP–TATATO yields the least delamination and, qualitatively, the highest quality sectioning and general ease of handling of the sections. Moreover, 3
4 PETMP–TATATO yields the least delamination and, qualitatively, the highest quality sectioning and general ease of handling of the sections. Moreover, 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 PETMP–TATATO is photo-curable in a fraction of the time of the thermally curable Araldite and Epofix epoxies. While delamination is, in this case, not a critical parameter – it leads to some deformation of the wires, but not catastrophic failure – it is of critical importance for delicate and/or complex metallic features and for applications that leverage the insulation that the polymer slab provides. For instance, nanowires fabricated using 3
4 PETMP–TATATO is photo-curable in a fraction of the time of the thermally curable Araldite and Epofix epoxies. While delamination is, in this case, not a critical parameter – it leads to some deformation of the wires, but not catastrophic failure – it is of critical importance for delicate and/or complex metallic features and for applications that leverage the insulation that the polymer slab provides. For instance, nanowires fabricated using 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 PETMP–TATATO can be contacted using silver paste with no leakage (hence electrical shorts) to the supporting substrate, and the top face of the wire can be selectively functionalized with, for example, a SAM or addressed by a fluid for sensing applications.
4 PETMP–TATATO can be contacted using silver paste with no leakage (hence electrical shorts) to the supporting substrate, and the top face of the wire can be selectively functionalized with, for example, a SAM or addressed by a fluid for sensing applications.
For applications in which it is desirable to remove the polymer slab, leaving only the metallic nanostructure – in our case nanowires – on the substrate, we compared the time required to ash 100 nm thick slabs of each of the four resins completely using oxygen plasma. We exposed each to 1 mbar of pure oxygen plasma until there were no traces of polymer remaining by optical microscopy. These results are summarized in Table 3 and show that the thiol-containing polymers (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 PETMP–TATATO and NOA 81) take considerably longer (30 min) to ash than do the epoxies, and that Araldite ashes in about one third the time (5 min) of Epofix (15 min). We expect these times to scale according to the power output of the plasma oxidizer; we used a relatively low power plasma cleaner.
4 PETMP–TATATO and NOA 81) take considerably longer (30 min) to ash than do the epoxies, and that Araldite ashes in about one third the time (5 min) of Epofix (15 min). We expect these times to scale according to the power output of the plasma oxidizer; we used a relatively low power plasma cleaner.
| Material | Oxygen plasma exposure (min) |
|---|---|
| Epofix | 15 |
| Araldite 502 | 5 |
| NOA 81 | 30 |
PETMP–TATATO (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4) 4) |
30 |
Conclusions
We identified new embedding resins for nanoskiving that have similar properties to conventional epoxy-based embedding resins, but are photocurable and have improved adhesion to gold because of the inclusion of thiol functional groups. We compared these thiol-containing resins to two conventional, benchmark epoxy-based resins (Araldite and Epofix). Of the thiol-containing materials in our study, the 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 PETMP–TATATO appears to have the most promising properties for nanoskiving. It cures rapidly, possesses mechanical properties similar to commercial resins, adheres well to gold, and produces high-quality microtome sections. NOA 81 also produced samples that could be sectioned using an ultramicrotome, but the resulting sections had irregular thicknesses.
4 PETMP–TATATO appears to have the most promising properties for nanoskiving. It cures rapidly, possesses mechanical properties similar to commercial resins, adheres well to gold, and produces high-quality microtome sections. NOA 81 also produced samples that could be sectioned using an ultramicrotome, but the resulting sections had irregular thicknesses.
A draw-back of the 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 PETMP–TATATO system is that it shrinks slightly more than conventional epoxies, which may result in some deformation or stress in delicate samples. We observed no evidence of this being an issue in our samples as determined by measuring the electrical properties of the gold nanowires, but it may be an issue for more sensitive geometries (e.g., fragile gold structures that might deform under stress). For particularly sensitive samples, users may consider exploring techniques from the literature to reduce thiol–ene shrinkage such as allyl sulfide addition–fragmentation chain transfer.30,31 The ability to partially cure thiol–enes to produce oligomers prior to embedding provides an additional route to lower the shrinkage.
4 PETMP–TATATO system is that it shrinks slightly more than conventional epoxies, which may result in some deformation or stress in delicate samples. We observed no evidence of this being an issue in our samples as determined by measuring the electrical properties of the gold nanowires, but it may be an issue for more sensitive geometries (e.g., fragile gold structures that might deform under stress). For particularly sensitive samples, users may consider exploring techniques from the literature to reduce thiol–ene shrinkage such as allyl sulfide addition–fragmentation chain transfer.30,31 The ability to partially cure thiol–enes to produce oligomers prior to embedding provides an additional route to lower the shrinkage.
Physical and mechanical measurements suggest that the thiol–epoxy formulation – which can be formed by simply adding thiol to a commercial epoxy – could be a suitable resin. We found, however, that the rapid polymerization associated with this approach resulted in sample-to-sample variability, presumably due to poor mixing. Future work could focus on optimizing this formulation to control the rate of polymerization, although there appear to be few benefits of this resin relative to the 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 thiol–ene based on this study.
4 thiol–ene based on this study.
The improved adhesion of the thiol-containing embedding polymers to the metal reduces the occurrence of delamination during sample preparation and microtome sectioning. We anticipate that the enhanced properties offered by thiol-containing systems, such as reduced cure time, excellent adhesion to gold, and sufficient hardness, will be useful to those that use nanoskiving. The use of light to initiate the reaction allows for rapid prototyping but may also enable better spatial–temporal control of the resin during sample preparation. Nanoskiving of gold nanowires has already proven useful to numerous applications,11,29 and with this new embedding resin there is increased potential for further use of this unconventional nanofabrication technique in a wider range of areas with more reliable sectioning.
Acknowledgements
This work was supported by the NSF's Research Triangle MRSEC (DMR-1121107) and by the Joint Solar Programme (JSP) of the Stichting voor Fundamenteel Onderzoek der Materie FOM, which is supported financially by Nederlandse Organisatie voor Wetenschappelijk Onderzoek (NWO), and HyET Solar.References
- Q. Xu, R. M. Rioux, M. D. Dickey and G. M. Whitesides, Acc. Chem. Res., 2008, 41, 1566–1577 CrossRef CAS.
- B. D. Gates, Q. Xu, J. C. Love, D. B. Wolfe and G. M. Whitesides, Annu. Rev. Mater. Res., 2004, 34, 339–372 CrossRef CAS.
- B. D. Gates, Q. Xu, M. Stewart, D. Ryan, C. G. Wilson and G. M. Whitesides, Chem. Rev., 2005, 105, 1171–1196 CrossRef CAS.
- Q. Xu, J. Bao, R. M. Rioux, R. P. Castillejos, F. Capasso and G. M. Whitesides, Nano Lett., 2007, 7, 2800–2805 CrossRef CAS.
- D. J. Lipomi, F. Ilievski, B. J. Wiley, P. B. Deotare, M. Lončar and G. M. Whitesides, ACS Nano, 2009, 3, 3315–3325 CrossRef CAS.
- A. M. Glauert, Practical Methods in Electron Microscopy, American Elsevier Publishing Co. Inc., New York, 1974 Search PubMed.
- H. K. Plummer, Microsc. Microanal., 1997, 3, 239–260 CAS.
- D. J. Lipomi, R. V. Martinez, R. M. Rioux, L. Cademartiri, W. F. Reus and G. M. Whitesides, ACS Appl. Mater. Interfaces, 2010, 9, 2503–2514 Search PubMed.
- J. D. Acetarin, E. Carlemalm, E. Kellenberger and W. Villiger, J. Electron Microsc. Tech., 1987, 6, 63–79 CrossRef.
- E. M. Petrie, Epoxy Adhesive Formulations, McGraw-Hill, New York, 2005 Search PubMed.
- D. J. Lipomi, R. V. Martinez and G. M. Whitesides, Angew. Chem., Int. Ed., 2011, 50, 8566–8583 CrossRef CAS.
- P. Pourhossein and R. C. Chiechi, ACS Nano, 2012, 6, 5566–5573 CrossRef CAS.
- J. C. Love, L. A. Estroff, J. K. Kriebel, R. G. Nuzzo and G. M. Whitesides, Chem. Rev., 2005, 105, 1103–1169 CrossRef CAS.
- C. E. Hoyle, T. Y. Lee and T. J. Roper, J. Polym. Sci., Part A: Polym. Chem., 2004, 42, 5301–5338 CrossRef CAS.
- H. Lu, J. A. Carioscia, J. W. Stansbury and C. N. Bowman, Dent. Mater., 2005, 21, 1129–1136 CrossRef CAS.
- C. E. Hoyle, A. B. Lowe and C. N. Bowman, Chem. Soc. Rev., 2010, 39, 1355–1387 RSC.
- J. A. Carioscia, J. W. Stansbury and C. N. Bowman, Polymer, 2007, 48, 1526–1532 CrossRef CAS.
- Technical Data Sheets, Epo-fix: www.emsdiasum.com/microscopy/technical/datasheet/1232.aspx, NOA 63: www.norlandprod.com/adhesives/noa%2063.html, NOA 81: www.norlandproducts.com/adhesives/noa%2081.html, Araldite 502: www.emsdiasum.com/microscopy/technical/datasheet/13900.aspx.
- A. Uhl, R. W. Mills, A. E. Rzanny and K. D. Jandt, Dent. Mater., 2005, 21, 278–286 CrossRef CAS.
- A. Mohan, A. Gurarslan, X. Joyner, R. Child and A. E. Tonelli, Polymer, 2011, 52, 1055–1062 CrossRef CAS.
- R. Song, M. Y. M. Chiang, A. J. Crosby, A. Karim, E. J. Amis and N. Eidelman, Polymer, 2005, 46, 1643–1652 CrossRef CAS.
- J. A. Carioscia, H. Lu, J. W. Stansbury and C. N. Bowman, Dent. Mater., 2005, 21, 1137–1143 CrossRef CAS.
- A. M. Glauert and R. H. Glauert, J. Biophys. Biochem. Cytol., 1958, 4, 191–194 CrossRef CAS.
- H. Gnaegi, Microsc. Microanal., 2000, 6(Suppl. 2: Proceedings), 314–315 Search PubMed.
- E. C. Hagberg, M. Malkoch, Y. Ling, C. J. Hawker and K. R. Carter, Nano Lett., 2007, 7, 233–237 CrossRef CAS.
- M. Frei, S. V. Aradhya, M. Koentopp, M. S. Hybertsen and L. Venkataraman, Nano Lett., 2011, 11, 1518–1523 CrossRef CAS.
- M. Grandbois, M. Beyer, M. Rief, H. Clausen-Schaumann and H. E. Gaub, Science, 1999, 283, 1727–1730 CrossRef CAS.
- G. Rubio-Bollinger, S. R. Bahn, N. Agrait, K. W. Jacobsen and S. Vieira, Phys. Rev. Lett., 2001, 87, 026101 CrossRef.
- K. Dawson, J. Strutwolf, K. P. Rodgers, G. Herzog, D. W. M. Arrigan, A. J. Quinn and A. O'Riordan, Anal. Chem., 2011, 83, 5535–5540 CrossRef CAS.
- T. F. Scott, A. D. Schneider, W. D. Cook and C. D. Bowman, Science, 2005, 308, 1615–1617 CrossRef CAS.
- C. J. Kloxin, T. F. Scott and C. N. Bowman, Macromolecules, 2009, 42, 2551–2556 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c2tc00030j |
| This journal is © The Royal Society of Chemistry 2013 |
