pH responsive MWCNT–star terpolymer nanohybrids†
Zacharoula
Iatridi
ab and
Constantinos
Tsitsilianis
*ab
aDepartment of Chemical Engineering, University of Patras, 26504 Patras, Greece. E-mail: ct@chemeng.upatras.gr; Fax: +30 2610 997266; Tel: +30 2610 969531
bInstitute of Chemical Engineering Sciences ICE/HT-FORTH, P.O. Box 1414, 26504 Patras, Greece
First published on 16th October 2012
Abstract
We present a unique multisegmented, heteroarm star block terpolymer that can be used as an effective dispersing agent for multiwalled carbon nanotubes in aqueous media. The (polystyrene)22(poly(2-vinylpyridine)-b-poly(acrylic acid))22, PS22(P2VP-b-PAA)22, star terpolymer exhibits a pH responsive self-assembly behavior in aqueous solution, due to the ampholytic nature of its diblock copolymer arms. Depending on the pH of the medium, two different self-assemblies were observed. At a low pH (pH 2), core–shell unimolecular micelles, with a hydrophobic PS core and P2VP, PAA segments in the shell, as well as multicore large compound micelles were formed. At pH 8.5, the star was transformed from a bis-hydrophilic into a bis-hydrophobic state, leading to a network-like intermicellar assembly. This star terpolymer was capable of dispersing MWCNTs, through non-covalent interactions, thus leading to stable MWCNT–star nanohybrids in water. More importantly, the resulted MWCNT–star nanohybrids adopted the “smart” pH responsive properties of the physisorbed stars, i.e. the nanohybrids can be positively or negatively charged at low and high pH respectively, while at an intermediate pH window, phase separation occurs due to neutralization of the oppositely charged segments.
Introduction
Carbon nanotubes (CNTs) have attracted great interest, thanks to their mechanical, electrical and thermal properties. They find numerous applications in air and space applications, biomedical applications, or serve as conductors, molecular sensors, electromechanical devices, etc.1,2 One of the major problems that the CNTs encompass is their poor solubility in water as well as in organic solvents. A solution to this issue is the functionalization of the CNTs through covalent or non-covalent bonding between the CNTs and polymers.3–6 In covalent bonding procedures, the polymer chains are chemically grafted onto the CNTs,7–9 which however highly affects the structure and the physicochemical properties of the CNTs. On the other hand, the non-covalent modification of SWCNTs (single walled carbon nanotubes) or MWCNTs (multi-walled carbon nanotubes) by polymers preserves the CNTs' physical and mechanical properties, while at the same time it enhances their solubility in various solvents. This physical procedure (physisorption) can be achieved through π–π stacking and/or van der Waals interactions between the polymeric chains and the CNT surfaces.10,11 These interactions are favored especially with conjugated polymers and polymers that contain aromatic groups.12–15Numerous studies have been published concerning the dispersion of SWCNTs or MWCNTs in organic solvents,16,17 assisted by non-covalent interactions with homopolymers such as polystyrene (PS),18,19 or polymers bearing pyrene groups.20,21 Increasingly high interest is observed as well in the dispersion of CNTs in aqueous media. Several reports have been published including non-covalent functionalization of CNTs with either positively or negatively charged polyelectrolytes,22 polymers such as poly(acrylic acid) (PAA),23 poly(ethylene oxide) (PEO),24 polyethers,25 as well as amphiphilic random26,27 or block copolymers.28–35 Fewer are the studies on the utilization of stimuli responsive polymers to disperse CNTs. Common pH responsive weak polyelectrolytes such as PAA, poly(methacrylic acid) (PMAA) and poly(allylamine) (PAAm) were used to prepare pH tunable SWCNT–polymer dispersions that depend on the degree of ionization of the polymeric chain.36 MWCNT–PAA aqueous dispersions, except for the pH responsive behavior, also show good tolerance to ionic strengths.37,38 Very recently, a double stimuli responsive copolymer, sensitive to both temperature and ionic strength, consisting of N-isopropylacrylamide (NIPAM) and 1-ethyl-3-vinylimidazolium bromide (EVImBr), was used as an effective stabilizer of MWCNTs.39
Star-shaped block terpolymers of various topologies like μ-ABC miktoarm stars,40–45 (ABC)n star block terpolymers,46,47 [A-b-(B-co-C)]n block random star terpolymers,48 A(B-b-C)2 asymmetric heteroarm star block terpolymers49 and An(B-b-C)n symmetric heteroarm star block terpolymers,50,51 comprise a unique type of macromolecules, due to their special architecture and the interesting properties that can be tuned by varying the polymer arms.52,53
An interesting class of water soluble block copolymers are those bearing polyampholyte segments with both negatively and positively charged groups.50,54–63 The self-association of these polymeric species in aqueous media is highly affected by the electrostatic interactions of the oppositely charged segments. If the polyampholyte groups are weak acids and bases, the net charge and the type of the charges can be tuned by varying the pH of the aqueous solution. At a particular pH which is called the isoelectric point (iep), the oppositely charged moieties compensate, leading to a zero net charge.
Recently, the aqueous solution properties of a novel polyampholyte multiarmed star terpolymer PSn(P2VP-b-PAA)n, bearing a number of hydrophobic PS arms and an equal number of ampholytic P2VP-b-PAA diblock arms, was explored.50c It was found that this terpolymer self-organized in aqueous media, forming a wide variety of amphoteric self-assemblies such as core–shell unimolecular micelles, worm-like micelles, multicore large compound micelles (LCMs), network-like large assemblies, and finally, patchy compartmentalized micelles, depending on the pH of the aqueous solution.
In this work we have chosen to use this kind of multi-functional ampholytic star terpolymer as a dispersing agent of MWCNTs in aqueous media. The polymer under investigation is a multiarmed and multisegmented polymer bearing 22 short PS arms and 22 long P2VP-b-PAA diblock copolymer arms, all emanated from a tightly crosslinked poly(divinylbenzene) (PDVB) core. Due to the weak polyampholytic character of the P2VP-b-PAA diblock copolymer arms (positive ionization of P2VP, negative ionization of PAA), this star polymer exhibits pH dependent conformations (Scheme 1).
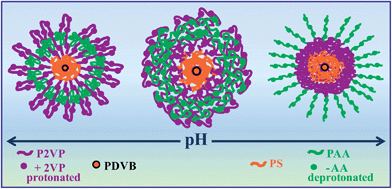 | ||
| Scheme 1 Schematic representation of the pH-dependent arm conformations of the star terpolymer. | ||
To the best of our knowledge, this is the first attempt to use this type of stimuli-responsive stars as dispersing agents of MWCNTs. The results showed efficient dispersion of MWCNTs which is affected by the pH of the medium. More importantly, the resulted MWCNT–star nanohybrids adopted the “smart” pH responsive properties of the physisorbed stars, i.e. the nanohybrids can be positively or negatively charged at low and high pH respectively, while at the isoelectric region they can be removed from the medium by phase separation through neutralization of the oppositely charged segments.
Experimental section
Materials
Multi-walled carbon nanotubes with a purity of 97%, an inner diameter of 3.5–12.5 nm and an outside diameter of 15–35 nm from Nanothinx S.A. (Greece) were used without further purification.Polymer synthesis and characterization
An extended “in–out” method was followed for the synthesis of the (PS34)22(P2VP136-b-PAA119)22 heteroarm star block terpolymer as reported elsewhere.50a In brief, the star terpolymer was prepared by “living” anionic polymerization in a one-pot/four-step reaction and subsequently easy deprotection of the tert-butyl-acrylate moieties of the third block by acidic hydrolysis. First, the PS arms were prepared in THF using s-BuLi as the initiator. After the consumption of styrene, the ‘living’ PS chains were used to polymerize a small quantity of DVB (crosslinking agent), resulting in a ‘living’ star-shaped polystyrene precursor (PSn) bearing active sites in its PDVB core. In the next step, the ‘living’ star was used to polymerize 2VP, leading to the formation of a second generation of P2VP arms. The sites located at the end of P2VP arms were used to polymerize tBA. The final (PS34)22(P2VP136-b-PAA119)22 terpolymer was obtained after acidic hydrolysis of the PtBA blocks in 1,4-dioxane with a 6 fold excess of hydrochloric acid at 80 °C. The terpolymer was characterized by means of size exclusion chromatography (SEC) and static light scattering (SLS). The results are presented in Table 1.| Polymer | Number of arms (average) | M w | Degree of polymerization |
|---|---|---|---|
| a Determined by SEC. b Determined by SLS. c Calculated by subtracting the Mw of the PS22 from that of PS22P2VP22 and dividing by the number of arms (21.7). d Calculated from the Mw of the (PS)22(P2VP-b-PtBA)22 precursor assuming quantitative hydrolysis of the tBA moieties. e Calculated by subtracting the Mw of the PS22P2VP22 from that of (PS)22(P2VP-b-PAA)22 and dividing by the number of arms (21.7). | |||
| PS(arm) | 3500a | 34 | |
| PS22 | 22 | 76![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000b 000b |
|
| PS22P2VP22 | 44 | 386![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000b 000b |
|
| P2VP(block, arm) | 14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 300c 300c |
136 | |
| (PS)22(P2VP-b-PtBA)22 | 44 | 717![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000b 000b |
|
| (PS)22(P2VP-b-PAA)22 | 44 | 573![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000d 000d |
|
| PAA(block,arm) | 8600e | 119 | |
Sample preparation
The experiments were conducted in H2O using Millipore MILLI-Q water. The (PS34)22(P2VP136-b-PAA119)22 star terpolymer was first dissolved in DMF, followed by slow addition of a sufficient quantity of 0.01 M aqueous HCl solution until a 3/7 volume ratio of DMF–H2O was achieved. The prepared solution was then dialyzed against 0.01 M HCl solution for 6 days to remove DMF, using a dialysis membrane (cutoff 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000). The resultant mother solution (pH 2) was again dialyzed against aqueous solutions of different pH values, varying from pH 1.4 to 11.8.
000). The resultant mother solution (pH 2) was again dialyzed against aqueous solutions of different pH values, varying from pH 1.4 to 11.8.
For the preparation of the MWCNT–star dispersions, a specific amount of MWCNTs was added to aqueous star terpolymer solutions of a given pH, keeping the mass ratio of MWCNTs over star terpolymer equal to 1.3. The dispersion was subjected to sonication for 30 min, using a water-bath sonicator (Branson 1200). Afterwards, the samples were centrifuged at 1500 rpm for several minutes and left to stand for several days before the experiments.
Techniques
Results and discussion
Aqueous solution behavior of the star terpolymer dispersing agent
Due to the amphiphilic and ampholytic character of the star terpolymer, initially we investigated its phase behavior and self-assembly in aqueous media and in the absence of MWCNTs. As shown in previous studies,50c,d the PSn(P2VP-b-PAA)n star terpolymers are characterized by high pH sensitivity owed to the protonation/deprotonation equilibrium of the weak P2VP–PAA block polyampholyte arms. The charge status of the (PS34)22(P2VP136-b-PAA119)22 star terpolymer was first explored through zeta potential measurements. For this experiment, a series of aqueous (PS34)22(P2VP136-b-PAA119)22 solutions at a polymer concentration of 0.2 wt% and at different pH values varying from pH 1.4 to pH 11.8 were prepared and the results are presented in Fig. 1.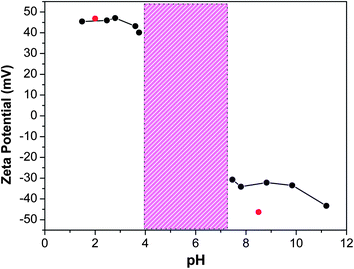 | ||
| Fig. 1 Zeta potential as a function of pH of 0.2 wt% (PS34)22(P2VP136-b-PAA119)22 aqueous solutions. The marked region denotes the precipitation regime. Red symbols represent the zeta potential for MWCNT–star terpolymer dispersions (see text). | ||
Three distinct areas were revealed: (a) the low pH regime for pH < 4, where positive charges predominated, as the P2VP segments are protonated and behave like a cationic polyelectrolyte, while at the same time the PAA blocks are mainly neutral. (b) The intermediate pH regime (4 < pH < 7) (isoelectric point regime), where the terpolymer precipitated due to electrostatic interactions of the oppositely charged PAA and P2VP segments and the hydrophobicity of the latter segment above pH 5. The pH value where the net charge of the terpolymer solution passes through zero was determined at pH 5.5 and it is consistent with the isoelectric point (iep) of the ampholytic arms. (c) The third pH regime (7 < pH < 11.8), where the star terpolymer was again solubilized in the aqueous media and the net charge of the polymer solution switched from zero to negative values, due to the high ionization of the PAA segments.
The self-organization of the star terpolymer in the low and high homogeneous pH regimes was explored by TEM. In Fig. 2, images of nanostructures embedded on carbon substrate from 0.0006% polymer aqueous solutions under acidic and basic conditions are displayed. At pH 2, two main populations were observed: the minor population corresponds to unimolecular micelles with a radius of ca. 35 nm (Fig. 2a) and the major population corresponds to large compound micelles with a radius of ca. 100–140 nm (Fig. 2b and c). These large compound micelles (Fig. 2c) are patchy multicore micelles that are formed due to intermolecular H-bonding interactions between the corona chains of the unimolecular micelles.50c
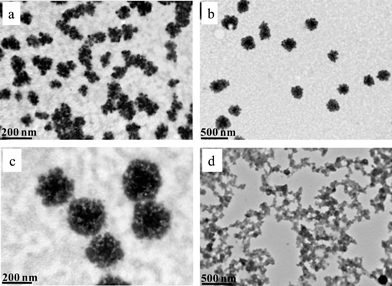 | ||
| Fig. 2 TEM images of 0.0006 wt% (PS34)22(P2VP136-b-PAA119)22 aqueous solutions at (a–c) pH 2 and at (d) pH 8.5. | ||
At pH 8.5, the hydrophilic–hydrophobic balance of the star was significantly altered towards highly hydrophobic due to the entire deprotonation of P2VP. On the other hand, the PAA segments are almost fully ionized, thus leading to different self-assembly of the star terpolymer. A network-like structure was observed, where spherical micelles with negatively charged PAA coronas and PS–P2VP concentric compartmentalized hydrophobic cores50c,d were interacted strongly by hydrophobic interactions (Fig. 2d). These findings are in good agreement with the results obtained by DLS experiments (Fig. S2, ESI†) that showed a tendency of the star terpolymer to form very large aggregates. Note that the TEM images correspond to higher concentrations due to the slow evaporation of the solvent during the preparation of the carbon grids for the TEM observation.
The results on the self-assembly behavior of the (PS34)22(P2VP136-b-PAA119)22 terpolymer are similar to those exhibited by (PS33)9(P2VP126-b-PAA69)9.50c Thus, we can consider that the increase in the number of arms from 18 to 44 did not significantly affect the aqueous self-organization behavior of these star terpolymers under the specific pH conditions investigated.
MWCNT dispersion in aqueous media
In the following, the ability of the star terpolymer to disperse hydrophobic MWCNTs in aqueous media of controlled pH was examined. MWCNT dispersions were prepared in water using 0.06 wt% (PS34)22(P2VP136-b-PAA119)22 aqueous solutions of pH 2 and 8.5. The as obtained mixtures were sonicated for 30 minutes, centrifuged at 1500 rpm for several minutes and left to stand for more than three weeks. As presented in Fig. 3, the MWCNT–star dispersions were stable since macroscopically homogeneous black solutions at both pH (Fig. 3b and c) were obtained, while the pristine MWCNTs in pure water precipitated within the first few minutes (Fig. 3a).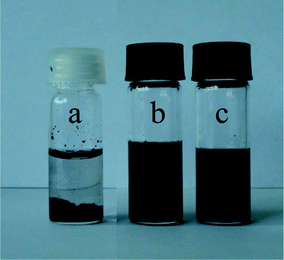 | ||
| Fig. 3 Photographs of MWCNT dispersions in: (a) pure water, (b) 0.06 wt% aqueous (PS34)22(P2VP136-b-PAA119)22 solution (pH 2), and (c) 0.06 wt% aqueous (PS34)22(P2VP136-b-PAA119)22 solution (pH 8.5). Photos were taken 3 weeks after sonication treatment. | ||
The Raman spectra of the drop-cast MWCNT–star dispersions are presented in Fig. 4. In the same figure, the spectrum of the pristine MWCNTs is also included for the sake of comparison. Both the spectra of MWCNT–star and pristine MWCNTs revealed two peaks, characteristic of MWCNTs: the D band at ∼1350 nm which is correlated with the presence of defects in the hexagonal graphitic layers and the G band at ∼1580 nm which is attributed to the vibration of sp2-hybridized carbon atoms. As can be seen, all samples have similar spectra, implying that the MWCNT structure remains intact after the physical adsorption of the star terpolymers on the MWCNT surface.
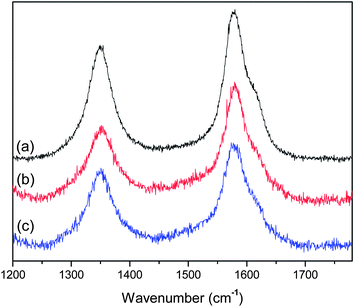 | ||
| Fig. 4 Raman spectra of drop cast samples: pristine MWCNTs (a), MWCNT–star terpolymer nanohybrids at pH 2 (b) and at pH 8.5 (c). | ||
Characterization of MWCNT–star nanohybrids
The morphological characterization of the MWCNTs after the physisorption of the star terpolymers was investigated using electron microscopy. Fig. 5 demonstrates characteristic TEM and SEM images of MWCNT–star nanohybrids at pH 2. It can be clearly seen that the MWCNTs are quite debundled, well dispersed in the aqueous solution and they appear to be straight and long. From the magnified images in Fig. 5c, d and f, a polymer coat wrapped around the MWCNT surface can be observed. This polymer coat is actually the reason that preserves the MWCNTs dispersed in water. On the grid surface, unimolecular micelles that have not interacted with the MWCNTs can be observed. More interestingly, the large compound micelles detected in Fig. 2c do not exist in the presence of MWCNTs. It seems that the star polymer prefers to be adsorbed onto the MWCNT surface rather than to form large compound micelles.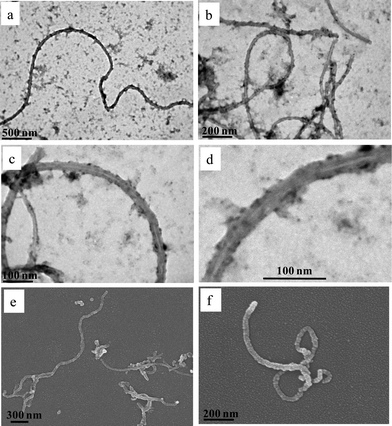 | ||
| Fig. 5 TEM (a–d) and SEM (e and f) images of MWCNT–star nanohybrids embedded on carbon-coated substrate from aqueous solutions of pH 2. | ||
Debundled MWCNTs were also observed at pH 8.5 (Fig. 6). However, the MWCNT–star nanohybrids appear to be different from those at low pH. One can observe that star terpolymer hemi-micelles were formed on the nanotube surface (Fig. 6c and f) similar to those reported for low molecular weight surfactant dispersing agents64 and amphiphilic diblock copolymer polyelectrolytes.30,31
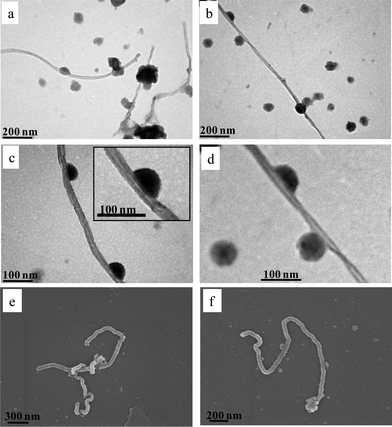 | ||
| Fig. 6 TEM (a–d) and SEM (e and f) images of MWCNT–star nanohybrids embedded on carbon-coated substrate from aqueous solutions of pH 8.5. | ||
Furthermore, free spherical micelles with a radius of approximately 35 nm, some of which were adsorbed along the MWCNT surface, can be seen in Fig. 6a–c. Similar micelles adhered onto the nanotube surface were observed by Shin et al. using a PS-b-P4VP diblock amphiphilic copolymer to disperse MWCNTs in either polar or nonpolar organic solvents.65 Results from SEM displayed the same behavior, verifying the TEM findings.
As has been reported previously, the macromolecular architecture plays a significant role in the micellization phenomena.66 For a heteroarm star copolymer with symmetrical PS and P2VP arms, PS6P2VP6, the star copolymer, exhibited a cmc (critical micelle concentration) three orders of magnitude higher than that of the PS-b-P2VP diblock copolymer counterpart. This seems to be valid for the PSn(P2VP-b-PAA)n star terpolymers at pH 2 where unimolecular micelles are mainly formed.50c Therefore, at pH 2, it is implied that segregated star polymers (unimolecular micelles) interact with the MWCNTs stabilizing the dispersion. On the other hand, at high pH the star terpolymer prefers to self-assemble, forming multimolecular micelles due to the substantial alteration of the hydrophilic–hydrophobic balance arisen from deprotonation of the P2VP segments.
A possible scenario of the way the star terpolymers interact with the MWCNTs is presented in Scheme 2. Under acidic conditions (pH 2), the terpolymer, instead of forming large compound micelles, was physisorbed on the MWCNTs through π–π stacking and/or hydrophobic interactions between the PS arms and the carbon nanotubes. Due to the poor solubility of the outer PAA segments, they should be gathered close to the CNT walls. The approach of the hydrophobic short PS arms on the wall should be attained by retraction of the stretched polyelectrolyte P2VP arms, in a similar manner to interparticle association of spherical polyelectrolyte brushes.67 Due to the high hydrophobicity and glassy character of PS arms the formed nanohybrids should be “frozen”, in analogy to the “frozen” micelles, implying that the hybrid structure depends on the preparation method.68 The positively charged P2VP segments provide electrostatic stabilization in the aqueous media, preventing aggregation. Thus, the entire MWCNT surface was covered by star polymers (Fig. 5d and f), increasing the nanotube roughness as compared to the surface of pristine MWCNTs (Scheme 2a).
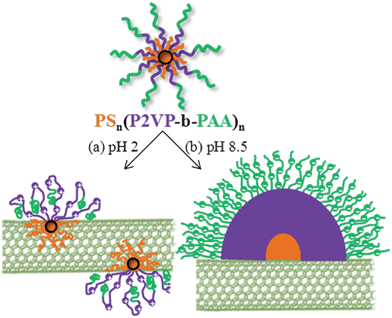 | ||
| Scheme 2 Schematic representation of the (PS34)22(P2VP136-b-PAA119)22 stars physisorbed onto MWCNTs at pH 2 (a) and pH 8.5 (b). | ||
Under basic conditions (pH 8.5) where P2VP segments have been deprotonated, the hydrophobic–hydrophilic balance increased considerably from 0.12 to 0.59. The interaction of the star terpolymer with the MWCNTs at this pH seems to stem from this significant alteration of the hydrophobic content. As seen in Fig. 6, spherical micelles already formed by the star terpolymer are attracted and adsorbed onto the MWCNT surface through hydrophobic interactions, either with the PS arms and/or P2VP segments. On the other hand, the detected hemi-micelles seem to be directly formed onto the MWCNT surface according to the following pathway: the hydrophobic PS arms are strongly anchored onto the CNT surface while hydrophobic attractions between PS and P2VP lead to the formation of compact hemi-micelles (Scheme 2b).
Electrophoresis experiments were conducted in order to characterize the charge status of the MWCNT–(PS34)22(P2VP136-b-PAA119)22 nanohybrids under acidic and basic conditions. At pH 2, the zeta potential of the MWCNT–star dispersion was 46.8 mV, close to that determined in the terpolymer solution in the absence of nanotubes. For the dispersion at pH 8.5, the zeta potential changed dramatically from positive to negative values i.e. −46.3 mV, again close to the value of the star polymer solution (Fig. 1). This behavior suggests that we were able to prepare MWCNT–polymer nanohybrids that can be charged both positively or negatively, depending on the solution pH, and preserving the polyelectrolyte character of the arms.
MWCNT–star nanohybrid responsiveness
As has been shown in Fig. 1, the (PS34)22(P2VP136-b-PAA119)22 star terpolymer remained water soluble at pH < 4 and pH > 7, while it precipitated at the isoelectric point regime (4 < pH < 7), where neutralization of the oppositely charged moieties of P2VP and PAA occurred. Considering this phase separation phenomenon, we were motivated to study the pH responsiveness of the MWCNT–star nanohybrids. Initially, an appropriate volume of aqueous solution of NaOH 1 M was added to the 0.06 wt% MWCNT–star dispersion at pH 2 (Fig. 7a), until the pH value reached 5.5 (iep). After stirring for less than a minute, precipitation of the MWCNT–star nanohybrids occurred at the bottom of the vial, leaving the aqueous solution clear (Fig. 7b).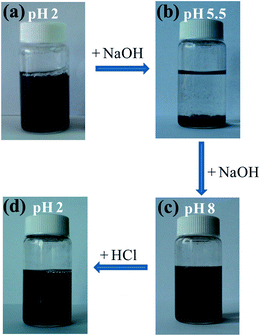 | ||
| Fig. 7 pH responsiveness of MWCNT–star nanohybrids in aqueous media. | ||
In the second step, more NaOH 1 M was added to the dispersion so as to achieve a basic environment, i.e. at pH 8 (Fig. 7c). Interestingly, the MWCNT–star precipitate was easily redispersed (without sonication) in the aqueous solution, presenting the same stability as the initially prepared MWCNT–star dispersion at pH 8.5 (Fig. 7c). Furthermore, after adding an adequate amount of aqueous HCl 1 M solution along with vigorous stirring, in order to prevent precipitation while passing through the isoelectric point regime, a stable MWCNT–star dispersion at pH 2 was formed again (Fig. 7d). It should be noted that if “frozen” nanohybrids were formed, as discussed previously, the structures depend on the preparation method. Thus, the hybrid structure prepared by dialysis at pH 2 may be different from that prepared by dialysis at pH 8 and going to pH 2 by changing pH.
This pH reversibility of the MWCNT–star nanohybrids renders the (PS34)22(P2VP136-b-PAA119)22 star terpolymer a “smart” dispersing agent for “switching-on” or “switching-off” the dispersion of MWCNTs in aqueous solution simply by controlling the pH of the media. This reversible dispersion of CNTs has also been reported for SWCNT/graphene–amphiphile hybrids where pH sensitive cholesterol based carboxylates were used to disperse SWCNTs.69
Comparison with previous results
A number of previous studies concerning the use of block copolymers to disperse CNTs in aqueous media have been reported. Solubilization of CNTs by micelle encapsulation has been accomplished using a PS-b-PAA copolymer.28 The ability of block copolymers to wrap CNTs has been exhibited by triblock copolymers like PNIPAM-b-PS-b-PNIPAM,70 poly(ethylene-co-butylene)-b-poly(methyl methacrylate-co-pyrene methyl 2-methyl-2-propenoate) diblocks71 and amphiphilic pluronic PEO-b-PPO-b-PEO33,34 (where PPO: poly(propylene oxide)) and poly(ethylene glycol)-b-poly(propylene sulfide)35 copolymers. Physical adsorption of a PS-b-PMAA copolymer on CNTs was realized by a non-wrapping mechanism.29 A polystyrene-b-(sodium sulfamate/carboxylate polyisoprene) (PS-b-sPI) block copolymer was also able to solubilize CNTs in water and the polyelectrolyte effect exerted by the hydrophilic part was preserved to a lesser extent in CNT–copolymer dispersions.30a Very recently, the efficiency of amphiphilic block copolymers with PEO as the hydrophilic block and poly(ethylene) (PE), poly(butadiene) (PB), PS, PPO or poly(thiophene) as the hydrophobic block, to disperse MWCNTs in aqueous solution was demonstrated.24Previous reports dealing with the non-covalent interactions of star polymers with CNTs are very limited. For example, the successful dispersion of MWCNTs was accomplished using a well-defined six-armed poly(lactic acid) (PLLA) star with a triphenylene core,32 a pyrene end-capped A6 dendrimer poly(ε-caprolactone) (PCL), star polymer20 or a pyrene bearing amphiphilic miktoarm star polymer (bearing three different arms).21 However, these studies were performed in organic solvents such as THF and not in water.
Dispersion of CNTs in aqueous media by utilizing star-like copolymers is quite rare. Recently, water-soluble C60 derivatives comprised of PEO arms, end-capped by hydrophilic quaternary ammonium groups, were successfully used as dispersing agents.72 These star polymers bear a C60 core which probably adheres to the CNT surface through π–π stacking interactions, while the polymeric hydrophilic arms preserve the stability of the CNTs in the solution. As far as the block copolymers are concerned, they usually consist of a hydrophobic block that adsorbs onto the CNT surface, while a hydrophilic block provides solubility of the system in the aqueous media.
The star terpolymer under investigation differs from the aforesaid star and block copolymers in many respects: first, the PS22(P2VP-b-PAA)22 terpolymer is a multiarmed star consisting of 22 PS and 22 P2VP-b-PAA arms. Its number of arms (44 in total) is much higher than that of the stars reported in the bibliography (ca. 3 to 6 arms). Second, to the best of our knowledge, this is the first attempt to study a heteroarm stimuli responsive star terpolymer as a dispersant of CNTs in aqueous media. The novelty of the star polymer under investigation is its specific architecture and multi-functionality of its arms e.g. hydrophobic PS and ampholytic P2VP-b-PAA arms. Whereas in most CNT–block copolymer dispersions, the hydrophilic block acts just to ensure solubility in the aqueous media; in the present system, the P2VP-b-PAA arms exhibit a multifunctional character, i.e. the P2VP-b-PAA arms are pH sensitive block polyampholytes displaying three states: negatively and positively charged, as well as neutral at iep. The latter feature constitutes the main originality of the present work, as “smart” pH responsive MWCNT–star terpolymer dispersions can be prepared. Although pH responsive reversible CNT dispersion has been reported very recently,69 herein, we demonstrate for the first time the preparation of stable dispersions at both low and high pH regimes, with positively and negatively charged MWCNT–star terpolymer nanohybrids respectively, as well as a precipitation state in an intermediate pH window. Third, while in most of the publications the proportion of polymers in the dispersions is much higher than that of CNTs (e.g. for PS-b-sPI, the polymer–CNT ratio was 3), in our case the dispersion of MWCNTs in the aqueous terpolymer solution was achieved even at a polymer–CNT ratio of 0.77. We speculate that this efficiency is attributable to the star topology as the high number of PS arms makes the interaction with the CNT surface more favorable. To ensure this assumption, further studies have to be addressed. Thus, future studies on dispersion of CNTs by star terpolymers bearing a different number of arms and also by the linear counterpart will allow us to answer the question: what is the role of macromolecular topology on the CNT–polymer dispersion?
Conclusions
The ability of a novel multiarmed and multisegmented PS22(P2VP-b-PAA)22 heteroarm star block terpolymer with hydrophobic (PS) and ampholytic (P2VP-b-PAA) arms to disperse MWCNTs in H2O was investigated.In the first part of this work the phase behavior and self-organization of this star terpolymer in aqueous media was explored by varying the pH of the solution. Three pH windows were revealed by electrophoresis: the low pH, characterized by positively charged nanoparticles; the intermediate pH where a two phase regime existed; and the high pH where negatively charged self-assemblies were found.
DLS and TEM investigations revealed the formation of micellar self-assemblies. At pH 2, positively charged unimolecular micelles and multicore large compound micelles comprised of hydrophobic PS cores and P2VP and PAA segments were observed. At high pH values, ca. pH 8.5, network-like intermicellar large assemblies were formed, with the cores of the neighboring stars interacting through hydrophobic interactions and stabilized by the stretched ionized PAA arms. This responsive behavior was attributed to the pH-dependent properties of the two P2VP and PAA polyelectrolyte segments.
PS22(P2VP-b-PAA)22 proved to be an efficient dispersing agent of MWCNTs in water. Stable, ink-colored MWCNT–star terpolymer aqueous dispersions at pH 2 and pH 8.5 were prepared at a polymer–MWCNT ratio as low as 0.77. Raman experiments revealed that there is no polymer intervention on the MWCNT structure. Depending on the pH of the solution two types of MWCNT–star nanohybrids were formed: (1) in acidic media, MWCNTs coated by positively charged stars; (2) in basic media, MWCNTs with negatively charged multimolecular micelles and/or hemi-micelles adsorbed along the nanotube surface. Around the iep of the polyampholyte arms, the MWCNTs were precipitated. It was shown that the MWCNT–star nanohybrids were pH responsive and that the dispersion of MWCNTs in aqueous solution could be “switched-on” or “switched-off” simply by controlling the pH of the media.
Furthermore, this multifunctional ampholytic star terpolymer can be used to disperse other carbon alloys like e.g. graphene sheets and this is the objective of future work. More interestingly, novel MWCNT–organic/inorganic smart nanohybrids can be fabricated, taking into consideration that P2VP and PAA can be coordinated with various charged molecules and/or inorganic nanoparticles like for instance proteins, m-RNA, magnetic or gold nanoparticles and quantum dots.
Acknowledgements
The authors thank Dr Maria Kollia from the Lab of Electron Microscopy and Microanalysis at the University of Patras for the TEM images, Dr Vasilis Drakopoulos and Dr Amaia Soto Beobide from the Institute of Chemical Engineering Sciences (ICE/HT-FORTH) for the SEM images and Raman spectra, respectively.References
- I. Szleifer and R. Yerushalmi-Rozen, Polymer, 2005, 46, 7803 CrossRef CAS.
- N. G. Sahooa, S. Ranab, J. W. Chob, L. Li and S. H. Chana, Prog. Polym. Sci., 2010, 35, 837 CrossRef.
- D. Tasis, N. Tagmatarchis, A. Bianco and M. Prato, Chem. Rev., 2006, 106, 1105 CrossRef CAS.
- Y.-L. Zhao and J. Fraser Stoddart, Acc. Chem. Res., 2009, 42, 1161 CrossRef CAS.
- Z. Spitalsky, D. Tasis, K. Papagelis and C. Galiotis, Prog. Polym. Sci., 2010, 35, 357 CrossRef CAS.
- J.-T. Sun, C.-Y. Hong and C.-Y. Pan, Polym. Chem., 2011, 2, 998 RSC.
- X. Lou, C. Detrembleur, C. Pagnoulle, R. Jerome, V. Bocharova, A. Kiriy and M. Stamm, Adv. Mater., 2004, 16, 2123 CrossRef CAS.
- Z. Wang, Q. Liu, H. Zhu, H. Liu, Y. Chen and M. Yang, Carbon, 2007, 45, 285 CrossRef CAS.
- C. Li, J.-C. Hsu, K. Sugiyama, A. Hirao, W.-C. Chen and R. Mezzenga, Macromolecules, 2009, 42, 5793 CrossRef CAS.
- P.-C. Maa, N. A. Siddiqui, G. Marom and J.-K. Kim, Composites, Part A, 2010, 41, 1345 CrossRef.
- M. Rahmat and P. Hubert, Compos. Sci. Technol., 2011, 72, 72 CrossRef CAS.
- J. Zhao, J. P. Lu, J. Han and C.-K. Yang, Appl. Phys. Lett., 2003, 82, 3746 CrossRef CAS.
- J. Zou, L. Liu, H. Chen, S. I. Khondaker, R. D. McCullough, Q. Huo and L. Zhai, Adv. Mater., 2008, 20, 2055 CrossRef CAS.
- B. Baykal, V. Ibrahimova, G. Er, E. Bengu and D. Tuncel, Chem. Commun., 2010, 46, 6762 RSC.
- D. Tuncel, Nanoscale, 2011, 3, 3545 RSC.
- R. Shvartzman-Cohen, Y. Levi-Kalisman, E. Nativ-Roth and R. Yerushalmi-Rozen, Langmuir, 2004, 20, 6085 CrossRef CAS.
- J. H. Rouse, Langmuir, 2005, 21, 1055 CrossRef CAS.
- Z. Zhang, J. Zhang, P. Chen, B. Zhang, J. He and G.-H. Hu, Carbon, 2006, 44, 692 CrossRef CAS.
- D. Linton, P. Driva, B. Sumpter, I. Ivanov, D. Geohegan, C. Feigerle and M. D. Dadmun, Soft Matter, 2010, 6, 2801 RSC.
- M. Gorur, F. Yilmaz, A. Kilic, Z. M. Sahin and A. J. Demirci, J. Polym. Sci., Part A: Polym. Chem., 2011, 49, 3193 Search PubMed.
- H. Durmaz, A. Dag, U. Tunca and G. Hizal, J. Polym. Sci., Part A: Polym. Chem., 2012, 50, 2406 Search PubMed.
- (a) S. E. Moya, A. Ilie, J. S. Bendall, J. L. Hernandez-Lopez, J. Ruiz-Garcia and W. T. S. Huck, Macromol. Chem. Phys., 2007, 208, 603 CrossRef CAS; (b) G. Romero and S. E. Moya, Soft Matter, 2012, 8, 9727 RSC.
- A. Liu, I. Honma, M. Ichihara and H. Zhou, Nanotechnology, 2006, 17, 2845 CrossRef CAS.
- C. Semaan, G. Pecastaings, M. Schappacher and A. Soum, Polym. Bull., 2012, 68, 465 Search PubMed.
- H. Gong, G. Xu, T. Liu, J. Pang, W. Dou and X. Xin, Colloid Polym. Sci., 2011, 289, 933 CrossRef CAS.
- V. A. Sinani, M. K. Gheith, A. A. Yaroslavov, A. A. Rakhnyanskaya, K. Sun, A. A. Mamedov, J. P. Wicksted and N. A. Kotov, J. Am. Chem. Soc., 2005, 127, 3463 CrossRef CAS.
- G. Prencipe, S. M. Tabakman, K. Weisher, Z. Liu, A. P. Goodwin, L. Zhang, J. Henry and H. Dai, J. Am. Chem. Soc., 2009, 131, 4793 Search PubMed.
- Y. Kang and T. A. Taton, J. Am. Chem. Soc., 2003, 125, 5650 CrossRef CAS.
- E. Nativ-Roth, R. Shvartzman-Cohen, C. Bounioux, M. Florent, D. Zhang, I. Szleifer and R. Yerushalmi-Rozen, Macromolecules, 2007, 40, 3676 CrossRef CAS.
- (a) G. Mountrichas, N. Tagmatarchis and S. Pispas, J. Phys. Chem. B, 2007, 111, 8369 CrossRef CAS; (b) G. Mountrichas, S. Pispas and N. Tagmatarchis, Small, 2007, 3, 404 CrossRef CAS.
- X. Xin, G. Xu, T. Zhao, Y. Zhu, X. Shi, H. Gong and Z. Zhang, J. Phys. Chem. C, 2008, 112, 16377 CrossRef CAS.
- L.-P. Yang and C.-Y. Pan, Macromol. Chem. Phys., 2008, 209, 783 CrossRef CAS.
- M. Granite, A. Radulescu, W. Pyckhout-Hintzen and Y. Cohen, Langmuir, 2011, 27(2), 751 Search PubMed.
- R. Shvartzman-Cohen, I. Monje, M. Florent, V. Frydman, D. Goldfarb and R. Yerushalmi-Rozen, Macromolecules, 2010, 43, 606 CrossRef CAS.
- E. M. Di Meo, A. Di Crescenzo, D. Velluto, C. P. O'Neil, D. Demurtas, J. A. Hubbell and A. Fontana, Macromolecules, 2010, 43, 3429 CrossRef CAS.
- (a) J. C. Grunlan, L. Liu and Y. S. Kim, Nano Lett., 2006, 6, 911 CrossRef CAS; (b) J. C. Grunlan, L. Liu and O. Regev, J. Colloid Interface Sci., 2008, 317, 346 CrossRef CAS; (c) K. C. Etika, M. A. Cox and J. C. Grunlan, Polymer, 2010, 51, 1761 CrossRef CAS.
- A. Liu, T. Watanabe, I. Honma, J. Wang and H. Zhou, Biosens. Bioelectron., 2006, 22, 694 CrossRef CAS.
- K. Saint-Aubin, P. Poulin, H. Saadaoui, M. Maugey and C. Zakri, Langmuir, 2009, 25, 13206 CrossRef CAS.
- S. Soll, M. Antonietti and J. Yuan, ACS Macro Lett., 2012, 1, 84 Search PubMed.
- (a) E. Kesselman, Y. Talmon, M. A. Hillmyer and T. P. Lodge, Science, 2004, 98(306), 98 Search PubMed; (b) Z. Li, M. A. Hillmyer and T. P. Lodge, Langmuir, 2006, 22, 9409 CrossRef CAS; (c) N. Saito, C. Liu, T. P. Lodge and M. A. Hillmyer, Macromolecules, 2008, 41, 8815 CrossRef CAS; (d) N. Saito, C. Liu, T. P. Lodge and M. A. Hillmyer, ACS Nano, 2010, 4, 1907 CrossRef CAS.
- A. Walther and A. H. E. Muller, Chem. Commun., 2009, 1127 RSC.
- J. Li, W.-D. He, N. He, S.-C. Han, X.-L. Sun, L.-Y. Li and B.-Y. Zhang, J. Polym. Sci., Part A: Polym. Chem., 2009, 47, 1450 CrossRef CAS.
- K. Van Butsele, J. F. Gohy, R. Jerome and C. Jerome, Langmuir, 2009, 25, 107 CrossRef CAS.
- (a) H. Liu, C. Li, H. Liu and S. Liu, Langmuir, 2009, 25, 4724 CrossRef CAS; (b) Y. Zhang, H. Liu, J. Hu, C. Li and S. Liu, Macromol. Rapid Commun., 2009, 30, 941 CrossRef CAS; (c) Y. Zhang, H. Liu, H. Dong, C. Li and S. Liu, J. Polym. Sci., Part A: Polym. Chem., 2009, 47, 1636 CrossRef CAS.
- Y.-Y. Yuan and J. Wang, Colloids Surf., B, 2011, 85, 81 Search PubMed.
- N. Stavrouli, A. I. Triftaridou, C. S. Patrickios and C. Tsitsilianis, Macromol. Rapid Commun., 2007, 28, 560 CrossRef.
- W. Zhu, A. Nese and K. Matyjaszewski, J. Polym. Sci., Part A: Polym. Chem., 2011, 49, 1942 Search PubMed.
- C. Tsitsilianis, G. Gotzamanis and Z. Iatridi, Eur. Polym. J., 2011, 47, 497 CrossRef CAS.
- W. Zhang, W. Zhang, N. Zhou, J. Zhu, Z. Cheng and X. Zhu, J. Polym. Sci., Part A: Polym. Chem., 2009, 47, 6304 CrossRef CAS.
- (a) N. Stavrouli, A. Kyriazis and C. Tsitsilianis, Macromol. Chem. Phys., 2008, 209, 2241 CrossRef CAS; (b) I. Choi, R. Gunawidjaja, R. Suntivich, C. Tsitsilianis and V. V. Tsukruk, Macromolecules, 2010, 43, 6818 Search PubMed; (c) Z. Iatridi and C. Tsitsilianis, Chem. Commun., 2011, 47, 5560 RSC; (d) Z. Iatridi, Y. Roiter, N. Stavrouli, S. Minko and C. Tsitsilianis, Polym. Chem., 2011, 2, 2037 RSC.
- R. C. Hayward and D. J. Pochan, Macromolecules, 2010, 43, 3577 CrossRef CAS.
- K. Khanna, S. Varshney and A. Kakkar, Polym. Chem., 2010, 1, 1171 RSC.
- Z. Iatridi and C. Tsitsilianis, Polymer, 2011, 3, 1911 Search PubMed.
- (a) R. Bieringer, V. Abetz and A. H. E. Muller, Eur. Phys. J. E, 2001, 5, 5 Search PubMed; (b) C. M. Schilli, M. Zhang, E. Rizzardo, S. H. Thang, Y. K. Chong, K. Edwards, G. Karlsson and A. H. E. Muller, Macromolecules, 2004, 37, 7861 CrossRef CAS.
- F. Liu and A. Eisenberg, J. Am. Chem. Soc., 2003, 125, 15059 CrossRef CAS.
- (a) C. S. Patrickios, L. R. Sharma, S. P. Armes and N. C. Billingham, Langmuir, 1999, 15, 1613 CrossRef CAS; (b) J. V. M. Weaver, S. P. Armes and S. Liu, Macromolecules, 2003, 36, 9994 CrossRef CAS; (c) Y. Cai and S. P. Armes, Macromolecules, 2004, 37, 7116 CrossRef CAS.
- (a) F. Bossard, V. Sfika and C. Tsitsilianis, Macromolecules, 2004, 37, 3899 CrossRef CAS; (b) F. Bossard, C. Tsitsilianis, S. N. Yannopoulos, G. Petekidis and V. Sfika, Macromolecules, 2005, 38, 2883 CrossRef CAS; (c) G. Gotzamanis and C. Tsitsilianis, Macromol. Rapid Commun., 2006, 27, 1757 CrossRef CAS; (d) C. Tsitsilianis, N. Stavrouli, V. Bocharova, S. Angelopoulos, A. Kiriy, I. Katsampas and M. Stamm, Polymer, 2008, 49, 2996 CrossRef CAS; (e) N. Stavrouli, I. Katsampas, S. Aggelopoulos and C. Tsitsilianis, Macromol. Rapid Commun., 2008, 29, 130 CrossRef CAS.
- (a) G. Hadjikallis, S. C. Hadjiyannakou, M. Vamvakaki and C. S. Patrickios, Polymer, 2002, 43, 7269 CrossRef CAS; (b) T. K. Georgiou, L. A. Phylactou and C. S. Patrickios, Biomacromolecules, 2006, 7, 3505 CrossRef CAS.
- (a) J. Rodriguez-Hernandez and S. Lecommandoux, J. Am. Chem. Soc., 2005, 127, 2026 CrossRef CAS; (b) W. Agut, A. Brulet, C. Schatz, D. Taton and S. Lecommandoux, Langmuir, 2010, 26, 10546 CrossRef CAS.
- R. G. Ezell and C. L. McCormick, J. Appl. Polym. Sci., 2007, 104, 2812 CrossRef CAS.
- M. Gao, X. Jia, G. Kuang, Y. Li, D. Liang and Y. Wei, Macromolecules, 2009, 42, 4273 CrossRef CAS.
- C. Mantzaridis and S. Pispas, J. Polym. Sci., Part A: Polym. Chem., 2011, 49, 3090 CrossRef CAS.
- C. V. Synatschke, F. H. Schacher, M. Fortsch, M. Drechsler and A. H. E. Muller, Soft Matter, 2011, 7, 1714 RSC.
- M. F. Islam, E. Rojas, D. M. Bergey, A. T. Johnson and A. G. Yodh, Nano Lett., 2003, 3, 269 CrossRef CAS.
- H. I. Shin, B. G. Min, W. Y. Jeong and C. M. Park, Macromol. Rapid Commun., 2005, 26, 1451 CrossRef CAS.
- D. Voulgaris, C. Tsitsilianis, F. J. Esselink and G. Hadziioannou, Polymer, 1998, 39, 6429 CrossRef CAS.
- (a) A. Jusufi, C. N. Likos and M. Ballauff, Colloid Polym. Sci., 2004, 282, 910 CrossRef CAS; (b) A. Wittermann, M. Drechsler, Y. Talmon and M. Ballauff, J. Am. Chem. Soc., 2005, 127, 9688 CrossRef CAS.
- T. Nicolai, O. Colombani and C. Chassenieux, Soft Matter, 2010, 6, 3111 RSC.
- S. Dutta, T. Kar, S. Brahmachari and P. K. Das, J. Mater. Chem., 2012, 22, 6623 RSC.
- H. Kitano, K. Tachimoto, T. Nakaji-Hirabayashi and H. Shinohara, Macromol. Chem. Phys., 2004, 205, 2064 Search PubMed.
- X. Lou, R. Daussin, S. Cuenot, A.-S. Duwez, C. Pagnoulle, C. Detrembleur, C. Bailly and R. Jerome, Chem. Mater., 2004, 16, 4005 CrossRef CAS.
- H. Li, C. Liu, J. Hao, U. Hartnagel and A. Hirsch, J. Nanosci. Nanotechnol., 2009, 9, 2763 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c2sm27211c |
| This journal is © The Royal Society of Chemistry 2013 |
