Total synthesis of ageliferin via acyl N-amidinyliminium ion rearrangement†
Hui
Ding‡
,
Andrew G.
Roberts‡
and
Patrick G.
Harran
*
Department of Chemistry and Biochemistry, University of California Los Angeles, 607 Charles E. Young Drive, East, Los Angeles, CA 90095-1569, USA. E-mail: harran@chem.ucla.edu
First published on 24th October 2012
Abstract
Ageliferin is a marine natural product having antiviral and antimicrobial activities. These functions remain to be characterized at a molecular level. Ageliferin is also thought a biosynthetic intermediary linking oroidin type alkaloids to more complex polycyclic derivatives. This scenario has the amino tetrahydrobenzimidazole motif in ageliferin serving as a reduced progenitor of oxidized, ring-contracted spirocycles. Here we describe the reverse. Namely, a concise synthesis of ageliferin which features ring expansion of a spirocyclic precursor – itself derived from reduction. The pathway also provides access to unique isosteres of the axinellamine ring system, allowing new synthetic additions to the growing family of pyrrole/imidazole alkaloids.
Ageliferin (1, Fig. 1) is a pyrrole/imidazole alkaloid discovered in extracts of Agelas coniferin. It has since been identified in numerous Agelas sponges; routinely alongside isomers such as sceptrin and nagelamides.1 The molecule is a prototype oroidin dimer and features prominently in discussions as to how, or if, its structure is biosynthetically intermediate en route to more complex relatives.2 Regarding the origin of 1 itself, two pathways have been advocated: (1) a net two-carbon ring expansion of sceptrin3 and (2) formal Diels–Alder dimerization of hymenidin followed by tautomerization.1b Both constructions have been emulated in the laboratory. Baran has synthesized 1 by executing the former, while Ohta has prepared a dimethylated congener (unnatural) using a variant of the latter.4,5 Chen's recent asymmetric synthesis of 1 is not aligned with either pathway.6
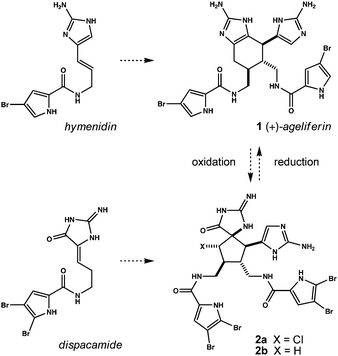 | ||
| Fig. 1 Pyrrole/imidazole alkaloids: monomer oxidation state and the interrelatedness of derived dimers. | ||
The aminotetrahydrobenzimidazole motif in ageliferin is thought a reduced precursor to ring-contracted spirocycles such as those observed in palau'amine, konbu'acidin, axinellamines and massadine.2 Several laboratories have adopted similar logic for converting ageliferin synthons into congeners of dehydro ‘pre-axinellamine’ (i.e.2, X = Cl).7 Our own observations have led us to pursue the reverse outcome.8 Namely, we find that C2-symmetric dimers of the natural product dispacamide readily isomerize to oxidized precursors of 2; wherein X = H (vide infra). We recently discovered methods to partially reduce these materials,8b and herein demonstrate novel ring expansion of the resultant hemiaminals to ageliferin. Depending upon intermediate stereochemistry, we also create previously unknown structural isomers of the axinellamine ring system.
The assembly of C2-symmetric spiroaminals 6 requires twelve steps beginning with γ-butyrolactone, thiourea and carbohydrazide 5 (Fig. 2). As we've shown previously,8b the aminal units in 6 are moderately stable to acid, but not base. In fact, all diastereomers of this structure are susceptible to a base mediated rearrangement cascade; funneling ultimately to alkylidenes 7 following degradation of the oxadiazine rings. In earlier studies, we advanced individual isomers of 7 to axinellamine structures.8b In the current work, separation is not necessary. Geometric isomers of each C14 epimer of 7 can be reduced as a mixture; initially to alkylidene aminoimidazolines 8. These subsequently tautomerize to aminoimidazoles 9.
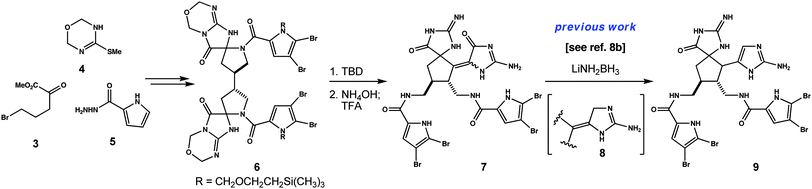 | ||
| Fig. 2 Spirocycles 9 are prepared in 12 steps from γ-butyrolactone, utilizing thiouron 4 and carbohydrazide 5 as key building blocks. | ||
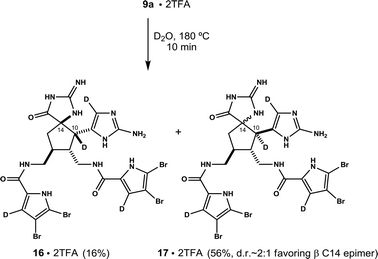
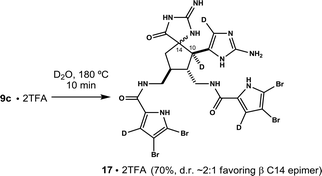
A valuable finding was made while experimenting with 9. The structure is isolated as a mixture of four diastereomers. It turns out the stereochemistry at C10 and C14 can be equilibrated. This is achieved simply by heating salt forms of 9 in water. For example, microwave heating an aqueous solution of pure 9a (bis-TFA salt, 180 °C, 1.0 mM) converts it fully to 9c and its C14 epimer 9d (65% isolated by HPLC, d.r. ∼2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) within 15 minutes (Scheme 1). A similar result is observed when the experiment is repeated on pure 9d, a mixture of 9a and 9b, or a mixture containing 9a, 9b and 9d. In each case, the system converges on C10, C11 trans diastereomers 9c and 9d, wherein 9c predominates in roughly a 2
1) within 15 minutes (Scheme 1). A similar result is observed when the experiment is repeated on pure 9d, a mixture of 9a and 9b, or a mixture containing 9a, 9b and 9d. In each case, the system converges on C10, C11 trans diastereomers 9c and 9d, wherein 9c predominates in roughly a 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio.9 Mechanistic details are not yet known, although data is consistent with reversible cleavage of the C10, C14 bond.10
1 ratio.9 Mechanistic details are not yet known, although data is consistent with reversible cleavage of the C10, C14 bond.10
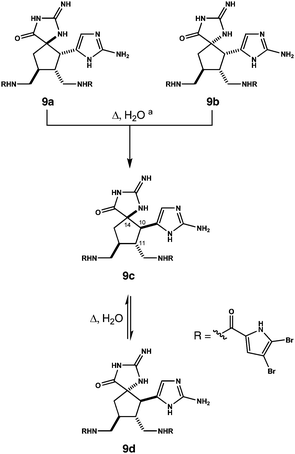 | ||
| Scheme 1 Trifluoroacetate salt forms of diastereomers 9 thermally equilibrate in water. Reagents and conditions: (a) e.g. pure 9a, H2O, 180 °C (microwave), 15 min, 42% of 9c, 23% of 9d. Isolated yields for variations of this experiment are detailed in the ESI† and in ref. 10. | ||
The equilibration process converts four isomers of 9 to two, and the major isomer is used to synthesize ageliferin. 9c is treated with excess SmI2 in aqueous THF. This results in rapid debromination at C6′ and C6′′ followed by gradual reduction of the glycocyamidine carbonyl. The resultant epimeric hemiaminals 10 (d.r. 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) are freed from samarium salts by preparative HPLC and exposed to trifluoroacetic anhydride (TFAA) in TFA.11 This initiates ring-expanding rearrangement to a trifluoroacylated tetrahydrobenzimidazole, from which the racemic natural product (1) is isolated following hydrolytic (1 N HCl) workup (Scheme 2).12
1) are freed from samarium salts by preparative HPLC and exposed to trifluoroacetic anhydride (TFAA) in TFA.11 This initiates ring-expanding rearrangement to a trifluoroacylated tetrahydrobenzimidazole, from which the racemic natural product (1) is isolated following hydrolytic (1 N HCl) workup (Scheme 2).12
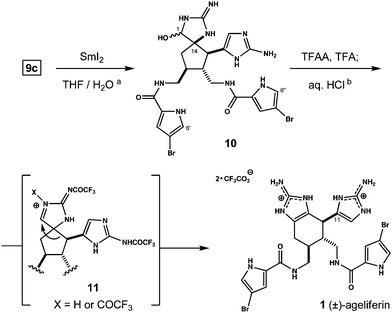 | ||
| Scheme 2 Reagents and conditions: (a) 9c, excess SmI2, THF/H2O, −40 °C to RT, 37%; (b) TFAA/TFA, THF, 70 °C; aq. 1 N HCl, 38% from 10. | ||
Each C1 epimer of 10 converts to 1 using this protocol, which we rationalize in terms of 1,2-alkyl migration occurring within an intermediate acyl N-amidinyliminium ion (11, X = H or COCF3). The result is analogous to acylation-induced ring expansion of partially reduced 1,3-diazaspiro[4.4]nonan-2,4-diones.13,14 However, in the current complex, non-symmetric system, substrate stereochemistry provides for varying outcomes. For example, minor C14 epimer 9d does not lead to ageliferin. Rather, upon SmI2 reduction and TFAA/TFA treatment, this molecule gives polycycle 12, wherein a putative N-amidinyliminium ion intermediate is trapped by the proximal, cis-disposed aminoimidazole (Scheme 3). When the original mixture of 9 is separated by HPLC rather than equilibrated, we observe a similar result beginning with major component 9a. Like 9d, the C14–C10 bond and the aminoimidazole substituent are oriented cis on the cyclopentane ring in 9a. SmI2 reduction and TFAA/TFA treatment of this material affords aminal 13.15
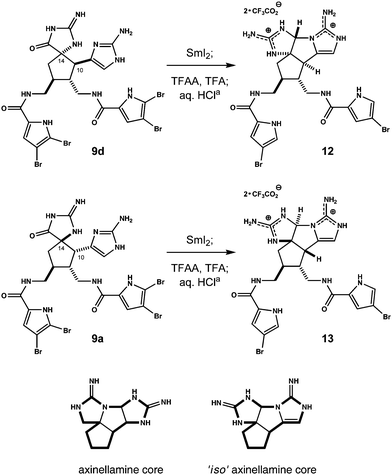 | ||
| Scheme 3 Reagents and conditions: (a) TFAA/TFA, THF, 60 °C; aq. 1 N HCl. Products 12 and 13 isolated as bistrifluoroacetate salts (22 and 13% respectively over two steps, >95% purity) following two rounds of preparative reverse phase HPLC. Lowered yields reflect competing air oxidation of intermediates during isolation. | ||
Structures 12 and 13 have inverted core topologies and represent new synthetic isosteres of the axinellamine ring system (Scheme 3). They exist at the oxidation state of ageliferin, yet this parameter is easily adjusted. For example, exposure of 13 to oxaziridine 14 (55 °C, 2 h) in aqueous THF smoothly oxidizes the aminoimidazole ring (Fig. 3).7b,8b Following preparative HPLC, we isolate two hemiaminal epimers of pentacycle 15 (66%, d.r. ∼1.4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1); a remarkable substance having two imbedded aminal linkages sharing a common nitrogen atom. This unusual connectivity is assigned with the aid of HMBC spectra, wherein C1 and C5 aminal protons correlate to C9. Relative to precursor 13, a new long-range correlation linking C5H to C1′′ and C3′′ is reflective of the installed C9–N2′′ bond.
1); a remarkable substance having two imbedded aminal linkages sharing a common nitrogen atom. This unusual connectivity is assigned with the aid of HMBC spectra, wherein C1 and C5 aminal protons correlate to C9. Relative to precursor 13, a new long-range correlation linking C5H to C1′′ and C3′′ is reflective of the installed C9–N2′′ bond.
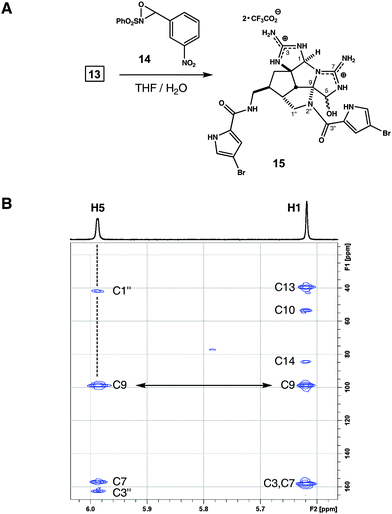 | ||
Fig. 3 (A) Reagents and conditions: 14, THF, 60 °C, 2 h, 66%, d.r. ∼1.4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. (B) Partial HMBC data for 15 (500 MHz, CH3OH-d4); key correlations: H5–C1′′, H5–C3′′, H5–C9 and H1–C9. 1. (B) Partial HMBC data for 15 (500 MHz, CH3OH-d4); key correlations: H5–C1′′, H5–C3′′, H5–C9 and H1–C9. | ||
One can envision numerous oxidative manipulations in this series, providing access to a range of designed congeners. When combined with a short and flexible synthesis of ageliferin, the chemistry provides a wide platform to explore biological functions of complex pyrrole/imidazole natural products; ideally while uncovering synthetic variants having superior and/or more selective activity. Initial screens suggest moderate antibacterial activity for the group. We suspect other venues may be more fruitful. Particularly interactions with gated ion channels, for which there is intriguing precedent.16 Work along these lines is ongoing, as are attempts to exploit these key findings for total syntheses of palau'amine structures.
Acknowledgements
Funding provided by the NIH (RO1-GM60591), the Donald J. & Jane M. Cram Endowment, the Foote Family Endowment (fellowship to A.G.R.) and a major instrumentation grant from the National Science Foundation (CHE-1048804).Notes and references
- (a) K. L. Rinehart, US Pat., 4737510, April 12, 1988; (b) K. L. Rinehart, Pure Appl. Chem., 1989, 61, 525 CrossRef CAS; (c) J. Kobayashi, H. Tsuda, T. Murayama, H. Nakamura, Y. Ohizumi, M. Ishibashi and M. Iwamura, Tetrahedron, 1990, 46, 5579 CrossRef CAS; (d) P. A. Keifer, R. E. Schwartz, M. E. S. Koker, R. G. Hughes, Jr., D. Rittschof and K. L. Rinehart, J. Org. Chem., 1991, 56, 2965 CrossRef CAS; (e) D. H. Williams and D. J. Faulkner, Tetrahedron, 1996, 52, 5381 CrossRef CAS. Ageliferin has drawn attention due to reported antiviral and antimicrobial activities, particularly an intriguing impact on drug resistant biofilms. See: (f) V. Stern, Sci. Am., 2009, 19, 7 Search PubMed; (g) S. A. Rogers, R. W. Huigens III, J. Cavanagh and C. Melander, Antimicrob. Agents Chemother., 2010, 54, 2112 CrossRef CAS and references cited therein.
- D. P. O'Malley, K. Li, M. Maue, A. L. Zografos and P. S. Baran, J. Am. Chem. Soc., 2007, 129, 4762 CrossRef CAS.
- P. S. Baran, D. P. O'Malley and A. L. Zografos, Angew. Chem., Int. Ed., 2004, 43, 2674 CrossRef CAS.
- (a) P. S. Baran, K. Li, D. P. O'Malley and C. Mitsos, Angew. Chem., Int. Ed., 2006, 45, 249 CrossRef CAS; (b) B. H. Northrop, D. P. O'Malley, A. L. Zografos, P. S. Baran and K. N. Houk, Angew. Chem., Int. Ed., 2006, 45, 4126 CrossRef CAS.
- (a) I. Kawasaki, N. Sakaguchi, N. Fukushima, N. Fujioka, F. Nikaido, M. Yamashita and S. Ohta, Tetrahedron Lett., 2002, 43, 4377 CrossRef CAS; (b) I. Kawasaki, N. Sakaguchi, A. Khadeer, M. Yamashita and S. Ohta, Tetrahedron, 2006, 62, 10182 CrossRef CAS.
- (a) X. Wang, Z. Ma, J. Lu, X. Tan and C. Chen, J. Am. Chem. Soc., 2011, 133, 15350 CrossRef CAS; (b) X. Wang, X. Wang, X. Tan, J. Lu, K. W. Cormier, Z. Ma and C. Chen, J. Am. Chem. Soc., 2012 DOI:10.1021/ja309172t.
- (a) R. Sivappa, N. M. Hernandez, Y. He and C. J. Lovely, Org. Lett., 2007, 9, 3861 CrossRef CAS; (b) R. Sivappa, P. Koswatta and C. J. Lovely, Tetrahedron Lett., 2007, 48, 5771 CrossRef CAS; (c) M. A. Zancanella and D. Romo, Org. Lett., 2008, 10, 3685 CrossRef CAS; (d) Y. He, P. Krishnamoorthy, H. Lima, Y. Chen, H. Wu, R. Sivappa, H. V. R. Dias and C. J. Lovely, Org. Biomol. Chem., 2011, 9, 2685 RSC . The term “pre-axinellamine” was coined by P. S. Baran et al. (see: J. Yamaguchi, I. Seiple, I. S. Young, D. P. O'Malley, M. Maue and P. S. Baran, Angew. Chem., Int. Ed., 2008, 47, 3578.).
- (a) Q. Li, P. Hurley, H. Ding, A. G. Roberts, R. Akella and P. G. Harran, J. Org. Chem., 2009, 74, 5909 CrossRef CAS; (b) H. Ding, A. G. Roberts and P. G. Harran, Angew. Chem., Int. Ed., 2012, 51, 4340 CrossRef CAS.
- Equilibration experiments conducted with 9a, 9b and 9d uniformly provide 9c and 9d in a ∼2
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio. In certain instances, competitive hydrolysis
to form hydantoin derivatives of 9c and 9d is observed upon prolonged heating.
1 ratio. In certain instances, competitive hydrolysis
to form hydantoin derivatives of 9c and 9d is observed upon prolonged heating. - Thermolysis of 9a in D2O results in rapid tetradeuteration (as labeled, ≥95% D at each position as determined by 1H NMR and MS) concomitant with epimerization at C10 and C14. Data below reflects product mixture at ∼75% conversion.
The same experiment carried out on 9c results in tetradeuteration and loss of stereochemical integrity at C14. The ratio of C14 epimers is ∼2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, comparable to that produced from 9a, suggesting equilibrium has been established. No C10 epimers (e.g.17) are observed. Yields refer to isolated material following preparative reverse phase HPLC. See ESI† for details.
1, comparable to that produced from 9a, suggesting equilibrium has been established. No C10 epimers (e.g.17) are observed. Yields refer to isolated material following preparative reverse phase HPLC. See ESI† for details.
. - Desalted hemiaminals 10 show diagnostic (see ref. 8b) C1 methine 1H resonances at δ 5.23 and 5.14 (500 MHz, CH3OH-d4). Because the materials degrade readily, seemingly through auto-oxidation, they are reacted with TFAA/TFA immediately after isolation.
- 13C NMR data for synthetic (1) is identical to that reported for natural ageliferin, except for a 3.8 ppm descrepancy in chemical shift for C11 (Scheme 2). The same phenomenon is observed by Baran (ref. 2) and Chen (ref. 6). We attribute the difference to the natural product being purified and characterized as an acetate salt, whereas we isolate 1 by preparative HPLC eluting with 0.1% TFA in CH3CN/H2O. For further discussion see: (a) R. J. Pugmire and D. M. Grant, J. Am. Chem. Soc., 1968, 90, 697 CrossRef CAS; (b) A. Olofson, K. Yakushijin and D. A. Horne, J. Org. Chem., 1998, 63, 5787 CrossRef CAS.
- (a) C. Pedregal, M. Espada, L. Salazar and J. Elguero, J. Heterocycl. Chem., 1986, 23, 487 CrossRef CAS; (b) A. Pesquet, A. Daïch and L. Van Hijfte, J. Org. Chem., 2006, 71, 5303 CrossRef CAS; (c) An interesting base promoted ring expansion of 4-amino-1,3-diaza-2-(methylthio)spiro[4.4]non-1-ene has been observed: B. A. Lanman and L. E. Overman, Heterocycles, 2006, 70, 557 CrossRef CAS.
- The analogous SmI2 mediated reduction on model 2-imino-1,3-diazaspiro[4.4]nonan-4-one provides 2-amino-1,3-diazaspiro[4.4]non-1-en-4-ol which undergoes efficient ring expanding rearrangement to 2-aminotetrahydrobenzimidazole (see ESI†).
- Based on results for 9a, 9c and 9d, we anticipated the remaining series diastereomer, namely 9b, would lead to nagelamide E (i.e. C10 epi-1) upon SmI2 reduction and ring expansion. Interestingly, a mixture (as yet inseparable) of 9b and its C14 epimer (i.e.9a) gives only 13 and ageliferin following these two operations. To the extent 9a leads only to 13 (Scheme 3), the ageliferin produced is derived from 9b. This implies C10 epimerization occurs during and/or prior to ring expansion. Note: thermal equilibration of 1 and nagelamide E has been demonstrated (see ref. 2 and D. P. O'Malley, Ph.D. Dissertation, the Scripps Research Institute, 2008, UMI #3313886).
- U. Bickmeyer, Toxicon, 2005, 45, 627 CrossRef CAS.
Footnotes |
| † Electronic supplementary information (ESI) available: See DOI: 10.1039/c2sc21651e |
| ‡ These authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2013 |