The role of conceptual change texts to improve students' understanding of alkenes
Gulten Sendur* and
Mustafa Toprak
Dokuz Eylul University Buca Faculty of Education, Department of Chemistry Education, Izmir, Turkey. E-mail: gulten.sendur@deu.edu.tr; Fax: +90 232 420 48 95; Tel: +90 232 3012408
First published on 6th June 2013
Abstract
This research aims to investigate the influence of conceptual change texts on students' understanding and misconceptions related to the subject of alkenes. For this, nine conceptual change texts were developed by considering students' misconceptions and learning difficulties regarding alkenes. The participants of this study consist of 63 second year students from the Primary Science Education Department. From the sample group, 33 students were placed in the experimental group and 30 in the control group. Before and after the study, both groups were given an Alkene Concept Test (ACT). Before instruction, a pre-test was applied to all of the students to determine students' prior knowledge and misconceptions. Afterwards, the experimental group was instructed with conceptual change texts while the control group was instructed with traditional teaching methods. Although the pre-test scores showed no statistically meaningful difference between the students of the two groups, the results of the post-test revealed that the mean scores of the students in the experimental group were significantly higher than those in the control group. In addition, students in the experimental group were better in remediating their misconceptions about alkenes. These findings suggest that conceptual change texts are effective in facilitating students' conceptual understanding of alkenes.
Introduction
During the last three decades, thanks to the constructivist learning theory, a learner's prior knowledge has gained importance in various teaching practices around the world. According to the constructivist learning theory, every learner constructs his or her own knowledge by connecting new knowledge with existing knowledge (Hewson and Hewson, 1984). In this process, if new knowledge does not fit with learners' existing knowledge, they may choose to reject it (Sewell, 2002). Many studies have indicated that learners have some prior knowledge that is not scientifically accepted (Driver and Easley, 1978; Posner et al., 1982; Driver and Erickson, 1983; Fleer, 1999; Taber, 2000; Palmer, 2001). Learners' conceptions that are different from those accepted by the scientific community are labelled in science literature as misconceptions (Nussbaum, 1981; Nakhleh, 1992; Gonzalez, 1997; Schmidt, 1997). Although teachers sometimes do not consider the misconceptions of the students, misconceptions highly influence how learners construct new scientific knowledge and interfere with subsequent learning. As a result, determining learners' misconceptions and remedying them are very important to promote conceptual understanding. This process is known as conceptual change. One of the explanations of conceptual change belongs to Posner et al. (1982). Posner et al. suggested four conditions to provide conceptual change. These are:1. There must be dissatisfaction with the existing concepts. If the learner's current understanding or knowledge is satisfactory for understanding a given phenomenon, the learner will be less likely to accept a new concept. For conceptual change to occur, they must become dissatisfied with their existing knowledge.
2. A new concept must be intelligible. Learners must be able to understand what the new concept means.
3. A new concept must appear plausible. It should be consistent with other knowledge and solve a particular problem.
4. A new concept must appear fruitful. It should be useful in a variety of new situations.
Furthermore, many studies have reported that traditional instruction is not sufficient to promote conceptual change (Bodner, 1991; Westbrook and Marek, 1991; Hesse and Anderson, 1992; Kaya, 2007; Hsu, 2008; Tarhan and Acar Sesen, 2012). Therefore, many teaching strategies based on the conceptual change approach, such as cooperative learning methods, concept maps, demonstration, analogies, hands-on activities and conceptual change texts, were developed as an alternative strategy to traditional instruction. In this study, conceptual change texts were used as a teaching strategy to promote conceptual change.
A conceptual change text is a written passage that identifies common misconceptions, explains the reasons for the misconceptions, and then provides the scientifically accepted concepts. The first step in conceptual change text is to test the students' prior knowledge with one or more questions. Through this step, both the instructor and the students will be able to see the existing misconceptions. In addition, some examples and explanations are presented to facilitate learners' understanding. These texts support the conceptual change process by showing the inconsistencies between common misconceptions and scientific knowledge (Kim and Van Dusen, 1998). In the literature, there are many investigations on the effects of conceptual change texts on learners' understanding in chemistry (Yürük and Geban, 2001; Cakır et al., 2002; Palmer, 2003; Uzuntiryaki and Geban, 2005; Canpolat et al., 2006; Özmen, 2007). According to these studies, conceptual change texts are more effective in remedying misconceptions than traditional instruction. These studies emphasised subjects in general chemistry, such as chemical equilibrium, chemical bonding, chemical kinetics, and chemical reactions. There has not been a study that has focused on determining the effect of conceptual change texts instruction in understanding basic concepts and reactions in organic chemistry. Therefore, this study attempts to fill in this gap.
Organic chemistry plays an important role in the curricula of university and secondary school chemistry in many countries. Particularly in Turkey, organic chemistry takes up a large part of the secondary school chemistry curriculum. As a result, the number of questions based on organic chemistry in the university entrance examination is considerable. In this case, the learning of organic chemistry correctly is vital to obtain high scores in the university entrance examination. Also, organic chemistry is one of the most important courses for students majoring in chemistry, chemistry education and science education in Turkey. Moreover, topics of organic chemistry occupy a central place in some countries' high school and university chemistry curricula, for example in Ireland and England. Some studies have revealed that organic chemistry courses were considered difficult for students and their performance in this subject was relatively low. For example, A′ level examiners' reports have highlighted the poor performance in organic chemistry (Chief Examiner's Reports in Chemistry, 2008, 2009; UCLES, 2010). In addition, the results of some studies conducted in these countries indicated that different levels of students (from to secondary school to university) found organic chemistry topics difficult or very difficult (Ratcliffe, 2002; Childs and Sheehan, 2009). In spite of the examiners' reports and other studies, only a few studies concerning the understanding of organic chemistry have been conducted in Turkey and other countries. Mostly, previous studies have been carried out to determine learners' misconceptions about a few concepts in organic chemistry.
Organic chemistry includes many topics such as alkanes, alkenes, alkynes, and functional groups. Within these topics, alkenes are a fundamental topic in organic chemistry since this topic is related to other organic groups such as alkynes, and alcohols. Consequently, students' insufficient knowledge about alkenes affects their understanding of these other topics. For this reason, it was decided to examine the students' understanding of alkenes in this study. Students have some misconceptions concerning alkenes; however, there has been little research conducted. Students' misconceptions and difficulties regarding alkenes identified by previous studies are summarised in Table 1.
| a Students' misconceptions and difficulties about alkenes were identified from the following studies: Lim (2007) and Şendur (2012). |
|---|
| Geometric/cis–trans isomerism |
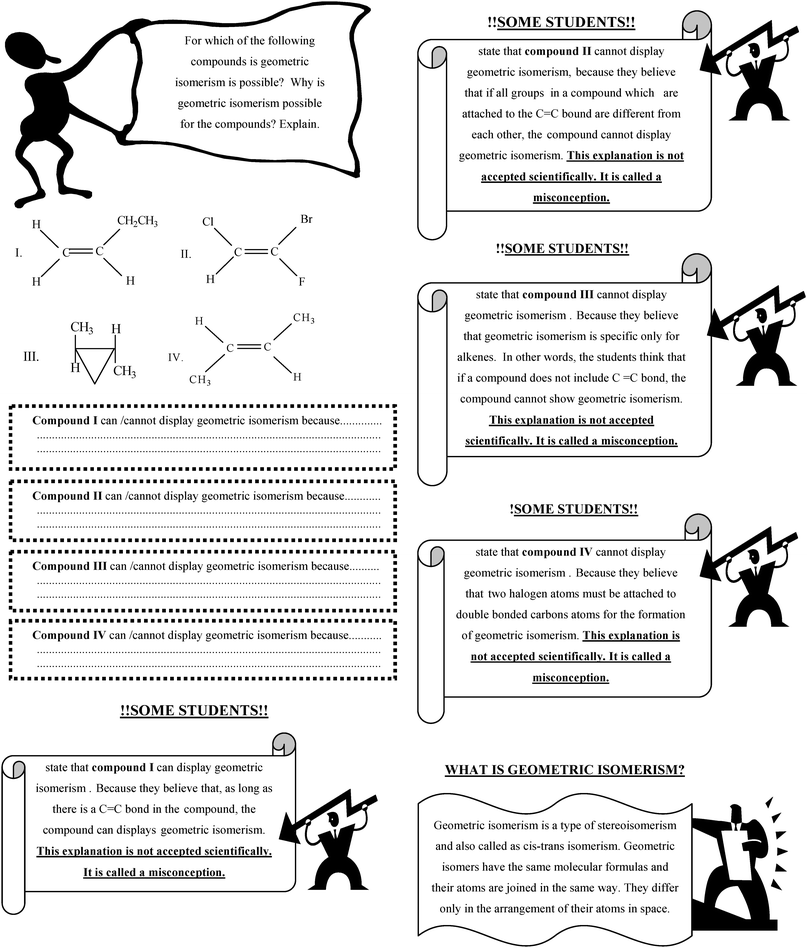
| Two halogen atoms must be attached to double bonded carbon atoms for the formation of geometric isomerism. |
As long as there is a C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bond in the compound, the compound can display geometric isomerism. C bond in the compound, the compound can display geometric isomerism. |
If all groups in a compound which are attached to the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bond are different from each other, the compound cannot display geometric isomerism. C bond are different from each other, the compound cannot display geometric isomerism. |
| Geometric isomerism is specific only for alkenes. |
As long as there is a C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bond with two different groups on each side of the double bond, it can cis–trans geometric isomerism (for example, 1-chloro-2-bromo-cyclohexene can also display cis–trans isomerism). C bond with two different groups on each side of the double bond, it can cis–trans geometric isomerism (for example, 1-chloro-2-bromo-cyclohexene can also display cis–trans isomerism). |
| Physical properties of geometric/cis–trans isomers |
| Boiling points of geometric isomers are the same because geometric isomers have the same chemical formulas. |
| Trans-isomers have higher boiling points than their cis-counterparts. |
| Structural isomerism |
| The cyclic molecule and straight-branched compound are never structural isomers of each other. |
| In alkene chains, the double bond can be located in different positions; these kinds of compounds are not structural isomers of each other. |
| Nomenclature of alkenes |
| When cycloalkenes are named, numbering is always counter-clockwise. |
| When cycloalkenes are named, the highest numbers are always given to alkyl groups attached to the ring. |
| General properties of alkenes |
| The general formula of all alkenes is CnH2n. |
| To call a molecule a cycloalkene, it is enough that its general formula is CnH2n−2. |
| All compounds which have the general formula CnH2n−2 are alkynes. |
| Chemical reactions of alkenes |
| Only the compounds that include π bonds are capable of undergoing addition reactions. |
| In the addition of HX to an unsymmetrical alkene, Markovnikov's rule can always be used to predict the product. |
| The addition of water to an alkene in the presence of acid leads to the formation of ketones. |
| The addition of water to an alkene in the presence of acid leads to the formation of aldehydes. |
| The addition of water to an alkene in the presence of acid leads to the formation of ethers. |
| Only an alkene that has two carbon atoms undergoes polymerisation reactions. |
| Only alkenes that have six or more carbon atoms undergo polymerisation reactions. |
| Synthesis of alkenes |
| During dehydration of 2-butanol as a secondary alcohol in the presence of acid at higher temperature, only 2-butene is formed. |
| During dehydration of 2-butanol as a secondary alcohol in the presence of acid at higher temperature, only 1-butene is formed. |
| When alkyl halides are heated with strong bases such as KOH and NaOH in the presence of alcohols, alcohols are generated as the major product. |
The results of this study will provide information to chemistry teachers, chemistry educators and curriculum developers. The results may be seen as an example study for the effectiveness of conceptual change text instructions on students' understanding of alkenes in chemistry education. Additionally, this study will help chemistry teachers become aware of the misconceptions that students have about alkenes, inform them about the importance of conceptual change texts in the teaching and learning process, and how conceptual change texts can be used in chemistry classes.
Purpose of the study
In the light of the above-mentioned rationale, this research aimed to investigate the effects of conceptual change texts on overcoming students' misconceptions related to alkenes. With this aim, the research questions were addressed as follows:• Is there any significant difference in achievement mean scores between pre-test of the experimental group and the control group?
• Is there any significant difference in achievement mean scores between post-test of the experimental group and the control group?
• How effective is conceptual change text instruction in overcoming students' misconceptions about alkenes when compared to the traditional instruction?
Method
The non-equivalent control group design as a type of quasi-experimental design was preferred in this study. While treatments were randomly assigned to groups, it was not possible to randomly assign the participants to experimental and control groups (Hinkle et al., 1998; Gravetter and Wallnau, 2000). Thus, one class was randomly assigned as the control group (CG) and the other class was randomly assigned as the experimental group (EG) for this study.Participants
The participants of the study consisted of 63 second-year students in the science education department of a public university in Turkey. All of the students volunteered to participate in the study. Also, each participant gave an informed consent form two weeks before the study commenced. The students are admitted to this department only after they have successfully passed a university entrance examination. For this reason, the students have quite similar backgrounds. Also, the socioeconomic status of the students was similar, with the majority of the students coming from low- to middle-class families. The ages of the students ranged from 20 to 22 years. The study was conducted during the 2010–2011 spring semester. Two classes were assigned randomly to the experimental (N = 33) and the control group (N = 30). Students in the control group were instructed with traditionally designed chemistry instruction, whereas students in the experimental group were taught with instruction based on conceptual change texts.Data collection and instrument
• Sound understanding (SU): scientifically complete responses and correct explanations are found in this category.
• Particular understanding with specific misconception (PUSM): this category includes scientifically complete responses and unacceptable explanations.
• Specific misconception (SM): completely scientifically unacceptable responses and explanations that match this category.
All questions included one sound understanding (correct answer), one particular understanding with specific misconception (distracter), and three specific misconception (distracters) choices. Altogether, there are 48 specific misconceptions in ACT.
After the test was prepared, the test was piloted by the participation of 50 students who were separate from the participants of the main study to determine reliability. The reliability coefficient (Cronbach alpha) of the test was found to be 0.75. The content of the ACT is presented in Table 2.
| Content | Question number |
|---|---|
| Geometric isomerism | 1, 2, 3 |
| Structural isomerism | 4 |
| Nomenclature of alkenes | 5, 16 |
| Synthesis of alkenes | 14, 15 |
| Properties of alkenes (general formula of alkenes) | 8 |
| Properties of addition reactions (similarities between cycloalkanes and alkenes) | 6 |
| Reactions of alkenes (addition reactions) | 9, 10, 11, 12 |
| Reactions of alkenes (polymerization reactions) | 7, 13 |
The question below is an example from the ACT (Fig. 1):
 | ||
| Fig. 1 Example of question in ACT. | ||
The ACT was applied to both groups as a pre-test to identify their prior knowledge and misconceptions one week before the treatment. The same test was employed in both groups as a post-test one week after the treatment. There were 6 weeks between the administration of the pre- and post-test. This is sufficient time for the students to forget questions in the ACT (Yürük, 2007).
Treatment
This treatment was conducted over a 6-week period (two 45-minute sessions per week). The subject of alkenes was taught by conceptual change texts in the experimental and by traditional format in the control groups. Both of the groups were taught by the same researcher (first researcher), who was experienced in organic chemistry and conceptual change texts. Both groups were instructed for an equal length of time.Conceptual change texts
In this study, nine conceptual change texts were developed by the researchers based on misconceptions determined in the literature (Lim, 2007; Şendur, 2012). These conceptual change texts and their contents are presented in Table 3. While the conceptual change texts were developed, three steps were followed. In the first step, for content validity, some organic chemistry textbooks were used (Solomons, 1988; McMurry, 1995; Fessenden and Fessenden, 1998 and Atkins and Carey, 2002) and two experts in organic chemistry controlled the content. In the second step, misconceptions about alkenes were determined in the literature. In the third step, the conceptual texts were prepared considering these steps. At the beginning of each text, some questions were asked to identify students' existing knowledge and misconceptions. Then, it was explained that many students have misconceptions and reasons were given as to why these misconceptions are inconsistent with scientific views. Then, correct explanations and several examples related to alkenes were presented to support the scientific explanation. Also, in the last stage, conceptual change texts were examined to determine whether the texts were appropriate to the grade level by two experts in organic chemistry. One example of conceptual change text used in the study is presented in the Appendix.| Conceptual change texts (CCT) | Content |
|---|---|
| CCT 1 | Nomenclature of alkenes |
| CCT 2 | Structural isomerism |
| CCT 3 | Geometric isomerism |
| CCT 4 | Physical properties of geometric isomers |
| CCT 5 | Addition reactions |
| CCT 6 | Anti-Markonikov's rule |
| CCT 7 | Acid catalysed addition of water to alkenes |
| CCT 8 | Polymerisation reactions |
| CCT 9 | Synthesis of alcohols |
Implementation of conceptual change texts
In the experimental group, conceptual change texts instruction was designed to remediate students' misconceptions about alkenes and to replace them with scientific conceptions. During the instruction, four steps suggested by Posner et al. (1982) were followed. At the beginning of each class, conceptual change texts were given to the students. As a first step, the students were asked to read the questions in the texts and the students discussed the questions under the guidance of the teacher. Also, the teacher took some notes about the students' responses whether scientifically accepted and not, and used these responses in the class discussions. For example, many students stated that 1,2-dimethylcyclopropane cannot display geometric isomerism, as they believe that geometric isomerism is specific for alkenes. In addition, some students cannot explain whether 1,2-dimethylcyclopropane can display geometric isomerism or not. On the other hand, some scientific explanations were generated about geometric isomerism. For example, some students stated that 2-butene can show geometric isomerism. Moreover, cis-2-butene and trans-2-butene were explained correctly. By this way, students' prior conceptions had been activated, and their misconceptions were revealed. At this step, many students had difficulties explaining the questions in the texts since their prior conceptions were insufficient. Also, while the teacher emphasised the misconceptions encountered among students, the students read the misconceptions in the texts. As a result, the students became dissatisfied with their existing knowledge (dissatisfaction).For the second and third steps (intelligibility and plausibility), the students read the scientific explanation of alkenes in the text. In order to make the new concepts intelligible and plausible for the students, reasons, situations and examples were provided in the text to explain why the misconceptions were incorrect and why the scientifically accepted concepts were in fact rational. At these stages, the students first read the explanations and examples in the text; then, the teacher repeated the explanations provided in the texts. For instance, the teacher presented explanations in the text supported by examples about what is geometric isomerism, and which compounds can show geometric isomerism after the students read the conceptual change texts. In other words, the cases in the text were used to help the students understand the scientifically accepted concepts. Thus, this process provided intelligibility and plausibility.
As a fourth step (fruitfulness), the lesson continued by presenting students with new situations and examples to enhance the understanding of concepts and reactions about alkenes. Also, to support the fourth step, students were encouraged to use the newly learned information in explaining other examples during the class discussions. This process helped the students to understand why the new explanation is more useful.
Implementation of traditional instruction
Traditional instruction was guided mainly by lecturing methods supported by questions and discussions. At the start of each lesson, the teacher introduced the topic to the class. During the classroom instruction, the teacher (first researcher) wrote the topic on the board without revealing the students' prior conceptions and misconceptions. An explanation was given by writing the main ideas, formulae and reactions of alkenes on the board. After the teacher explanation, the concepts and reactions were discussed with teacher-directed questions. In this method, students were passive as they only listened, took notes, and asked questions about unclear points. The teacher presented answers to the questions posed by the students. In addition, the teacher asked new questions to the students in order to find out whether the concept and reactions were understood well or not. The teacher also wrote some questions on the board to support important concepts and reactions from the lecture. For each question, sufficient time was given to the students to answer them. While the students were responding to the questions, the teacher walked throughout the room to give clues when needed. After that, the teacher or one of the students who solved the question correctly, wrote the solution on the board and gave an explanation for the solution. This process allowed the other students to compare their answers with the correct one. At the end of the lesson, the teacher summarized the lesson for clarification and assigned the same homework for both the experimental and control group. For students in both the control and experimental groups, the topic of alkenes were taught in the same order: general properties of alkenes, nomenclature of alkenes, structural isomerism, geometric isomerism, chemical reactions of alkenes (addition reactions, polymerisation reactions) and synthesis of alcohols (dehydration of alcohols, dehydrohalogenation of alkyl halides). During the instruction, experimental and control groups were assigned the same examples, and questions.Data analysis
In the analysis of the ACT firstly, the total scores of each student in both groups were calculated. For this aim, each correct answer (SU) was given 1 point, making the maximum possible score from the test 16 points. Incorrect answers (PUSM, SM) were given 0 points. The “PUSM” category was given 0 points since, if a student chose a scientifically complete response but chose an unacceptable explanation for the question, it was accepted that this student's response was not scientifically accepted (Cakmakci and Aydogdu, 2011). Similarly, if a student chose a completely scientifically unacceptable response and explanation for the question, this response (SM) was not scientifically accepted. For this reason, the SM category was also given 0 points. In addition, no response was given to 0 points. To analyse statistical data, firstly, the SPSS 15.0 computer program was utilised. In order to understand whether there was a significant difference between the experimental and control groups on pre-test and post-test scores, an independent-sample t-test was used. The significance level of 0.05 was considered when comparing groups. In order to apply parametric tests (independent-sample t-test), the data was initially investigated for normality distribution using the Kolmogorov–Smirnov test. The results of the Kolmogorov–Smirnov test for pre- and post-test, (respectively, experimental group D (33) = 0.133, p = 0.15, control group D (30) = 0.133, p = 0.183, experimental group D (33) = 0.142, p = 0.90, control group D (30) = 0. 157, p = 0.058, p > 0.05) indicated that both distributions were normal in the experimental and control groups. In this study, Cohen's d index was calculated for the post-test as a measure of effect size. Cohen (1992) interpreted the d index according to the following criteria: a d greater than 0.2 and less than 0.5 is considered a “small” effect, a d greater than 0.5 but less than 0.8 is considered a “medium” effect, and a d greater than 0.8 is considered a “large” effect. Also, percentages of students' specific misconceptions in both the pre-test and post-test were determined to compare the effectiveness of conceptual change texts instruction and traditional instruction in remediating students' misconceptions. The percentage difference between pre-test and post-test for each misconception was identified as the percentage of conceptual change. The percentage of conceptual change was classified by effectiveness. A percentage of conceptual change greater than 15% was labelled as “major”, while a percentage of conceptual change between 15% and 10% was labelled as “limited”; the percentage of conceptual change smaller than 10% was labelled “minor”. If conceptual change was not determined relating the misconceptions, it was labelled as “no”. In addition, if a misconception was not determined in the pre- and post-tests, it was labelled as “no specific misconception” (see Table 9).Limitations of the study
In this study, it is not appropriate to say that the improvement in students' conceptual understanding is permanent. This is because ACT was not administered to both groups of students as a delayed test. In addition, as ACT has a multiple-choice item structure, it does not allow the students to write their explanations instead of the explanations presented to them as test item choices. Since the study was conducted with only 63 second-year students in the science education department, further study with a larger population should be conducted to generalise the results. Besides, the conceptual change texts were developed for the organic chemistry curriculum at the science education department. Therefore, the usage of the conceptual change texts only in the context of the organic chemistry curriculum for science education students can be seen as another limitation of the study. The conceptual change text has a low degree of discrimination regarding chemistry departments. In addition, since the study is an experimental design, there are some effects which threaten the external validity of the study. One of them is the novelty effect. The novelty effect refers to the increased interest, motivation, or participation of the students because they are doing something different (Gay and Airasian, 2000). For this reason, the students in the experimental group may have possessed increased interest, or motivation that affected their achievement. The other effect is diffusion of treatment which may occur when new instruction is given to the experimental group to be shared with the control group. This is undesirable for an evaluation because it may decrease the differences between the experimental and control groups in their “before” and “after” changes (Robson et al., 2001). To minimize the effect of treatment diffusion, the lessons of the experimental and control groups were conducted on different days. In addition, the experimental group was not informed that they were receiving a different type of instruction in order for the control group to not be erroneously affected. Moreover, to ensure comparability between the groups the experimental and control groups were exposed to the same content knowledge. Also, lesson plans were examined by experts in organic chemistry each week for both experimental and control groups. All the researchers involved in this study made a continuous effort to behave similarly to both the experimental and control groups. Additionally, experimenter expectancy effect is an external threat to the study. It may occur when the experimenter unconsciously influences the participants, assessment of their performance, or procedure (Gay and Airasian, 2000).Results and discussion
Depending on the first and second research questions of the study, an independent-sample t-test was performed to test the significant difference between the experimental and control groups on pre-test and post-test total mean scores. In this way, it is possible to investigate the effectiveness of teaching with conceptual change texts in enhancing the conceptual understanding of alkenes. The corresponding statistic is presented in Table 4.| Test | Groups | N | Mean | SD | t | p |
|---|---|---|---|---|---|---|
| Pre-test | Experimental | 33 | 5.51 | 1.77 | 1.127 | 0.264 |
| Control | 30 | 5.00 | 1.86 | |||
| Post-test | Experimental | 33 | 12.97 | 2.23 | 3.707 | 0.000 |
| Control | 30 | 10.87 | 2.27 |
In order to identify students' already existing knowledge, the pre-test was administered. As seen in Table 4, the results of the independent-sample t-test showed that there was no significant difference among the experimental and control groups in terms of the pre-test (p > 0.05). These results indicated that the students in the control and experimental groups were similar regarding pre-test scores and prior knowledge.
The results of the post-test reflected that the total mean scores of the experimental group were higher than the control group and there was a significant difference between the total mean scores of the two groups (p < 0.05). These results suggest that teaching with conceptual change texts caused a significantly better acquisition of scientific concepts than teaching with traditional instruction (see Table 4). These findings are consistent with respect to the studies indicating the effect of CCT in students' understanding of chemistry concepts (Özmen, 2007; Yürük, 2007; Taştan et al., 2008; Durmuş and Bayraktar, 2010; Beerenwinkel et al., 2011).
In addition, the size of the mean difference between the groups for the post-test (Cohen's d = 0.94) indicated a large effect. That means, that there was a substantial difference between the post-test scores of the two groups.
In addition, the percentages of the students' correct, incorrect and no responses in the pre- and post-test were calculated to identify whether there was any difference before and after the instruction. With this aim, firstly, the students' responses at the pre-test were classified question by question as SU, PUSM, SM or NR (see Table 5). Secondly, the percentage of correct response (SU), incorrect response (PUSM and SM), and no response were calculated (see Table 6). As seen in Table 6, the experimental and control group students replied correctly in nearly equal proportions before the instruction. Thus, the results of the independent-sample t-test for each question showed that there was no significant difference between the two groups (see Table 6). Also, this result indicated that students in the experimental and control groups could be compared regarding their prior knowledge. From Table 6, it was understood that the percentages of correct responses to some questions were lower than the other questions. Particularly, it was clearly seen in questions 9, 10, 11, 12, 13, 14, and 15 that the percentages of correct responses in both groups were 33.3% and below. When these questions were examined in terms of their content areas, it was determined that these are related to addition reactions (questions 9, 10, 11, 12), polymerisation reactions (question 13), and synthesis of alcohols (question 14 and 15). This result was not surprising because these topics were not emphasised much in high school chemistry in Turkey and mostly, limited numbers of examples were given to the students.
| Question no. | Pre-test | |||||||
|---|---|---|---|---|---|---|---|---|
| Experimental group categories | Control group categories | |||||||
| SU (%) | PUSM (%) | SM (%) | NR (%) | SU (%) | PUSM (%) | SM (%) | NR (%) | |
| SU: sound understanding; PUSM: particular understanding with specific misconception; SM: specific misconception; NR: no response. | ||||||||
| 1 | 45.4 | 9.1 | 36.4 | 9.1 | 43.3 | 16.7 | 33.3 | 6.7 |
| 2 | 42.4 | 15.2 | 33.3 | 9.1 | 40.0 | 13.3 | 33.3 | 13.3 |
| 3 | 45.4 | 6.1 | 36.4 | 12.1 | 36.7 | 13.3 | 40.0 | 10.0 |
| 4 | 39.4 | 9.1 | 45.4 | 6.1 | 33.3 | 20.0 | 43.3 | 3.3 |
| 5 | 33.3 | 21.2 | 27.3 | 18.2 | 36.7 | 26.7 | 26.7 | 10.0 |
| 6 | 39.4 | 12.1 | 30.3 | 18.2 | 26.7 | 13.3 | 26.7 | 33.3 |
| 7 | 36.4 | 12.1 | 9.1 | 42.4 | 33.3 | 6.7 | 6.7 | 53.3 |
| 8 | 39.4 | 15.2 | 33.3 | 12.1 | 33.3 | 16.7 | 36.7 | 13.3 |
| 9 | 30.3 | 45.4 | 9.1 | 15.2 | 33.3 | 33.3 | 6.7 | 26.7 |
| 10 | 33.3 | 18.2 | 15.2 | 33.3 | 30.0 | 13.3 | 20.0 | 36.7 |
| 11 | 30.3 | 15.2 | 33.3 | 21.2 | 33.3 | 6.7 | 33.3 | 26.7 |
| 12 | 30.3 | 30.3 | 6.1 | 33.3 | 26.7 | 33.3 | 10.0 | 30.0 |
| 13 | 33.3 | 24.2 | 21.2 | 21.2 | 20.0 | 20.0 | 26.7 | 33.3 |
| 14 | 27.3 | 15.2 | 27.3 | 30.3 | 16.7 | 26.7 | 23.3 | 33.3 |
| 15 | 21.2 | 12.1 | 21.2 | 45.4 | 20.0 | 13.3 | 23.3 | 43.3 |
| 16 | 42.4 | 12.1 | 42.4 | 3.0 | 36.7 | 16.7 | 40.0 | 6.7 |
| Question no. | Pre-test | t-value | |||||
|---|---|---|---|---|---|---|---|
| Experimental group | Control group | ||||||
| CR (%) | IR (%) | NR (%) | CR (%) | IR (%) | NR (%) | ||
| CR: correct response; IR: incorrect response; NR: no response.a Not significant at 0.05. | |||||||
| 1 | 45.4 | 45.5 | 9.1 | 43.3 | 50.0 | 6.7 | 0.167a |
| 2 | 42.4 | 48.5 | 9.1 | 40.0 | 46.6 | 13.3 | 0.192a |
| 3 | 45.4 | 42.5 | 12.1 | 36.7 | 53.3 | 10.0 | 0.699a |
| 4 | 39.4 | 54.5 | 6.1 | 33.3 | 63.3 | 3.3 | 0.492a |
| 5 | 33.3 | 48.5 | 18.2 | 36.7 | 53.4 | 10.0 | 0.273a |
| 6 | 39.4 | 42.4 | 18.2 | 26.7 | 40.0 | 33.3 | 1.068a |
| 7 | 36.4 | 21.2 | 42.4 | 33.3 | 13.4 | 53.3 | 0.248a |
| 8 | 39.4 | 48.5 | 12.1 | 33.3 | 53.4 | 13.3 | 0.492a |
| 9 | 30.3 | 54.5 | 15.2 | 33.3 | 40.0 | 26.7 | 0.254a |
| 10 | 33.3 | 33.4 | 33.3 | 30.0 | 33.3 | 36.7 | 0.280a |
| 11 | 30.3 | 48.5 | 21.2 | 33.3 | 40.0 | 26.7 | −0.254a |
| 12 | 30.3 | 36.4 | 33.3 | 26.7 | 43.3 | 30.0 | 0.314a |
| 13 | 33.3 | 45.4 | 21.2 | 20.0 | 46.7 | 33.3 | 1.194a |
| 14 | 27.3 | 42.5 | 30.3 | 16.7 | 50.0 | 33.3 | 1.012a |
| 15 | 21.2 | 33.3 | 45.4 | 20.0 | 36.6 | 43.3 | 0.117a |
| 16 | 42.4 | 54.5 | 3.0 | 36.7 | 56.7 | 6.7 | 0.460a |
Similar to the pre-test, at the post-test, the students' responses were first grouped into the levels of understanding and are presented in Table 7. The percentages of correct, incorrect and no responses are shown in Table 8. As can be seen from Table 8, while percentages of correct responses to all the questions in the experimental group were higher than in the control groups at post-test, there was a contrasting situation for percentages of incorrect responses to all questions. Also, when the percentages of specific misconceptions were examined from Table 7, it was determined that these percentages in the experimental group were less than the control group. Even, in the experimental group, the specific misconception category in some questions such as 6, 7, 9, and 11 was not revealed in the post-test. For example, while students in the experimental group did not have any misconceptions in question 6 related to properties of addition reactions, 16.7% of the control group had misconceptions in this question. Similarly, in questions 9 and 11, related to the addition reactions of alkenes, while the misconceptions were not determined in the experimental group, the percentages of misconception determined in these questions were 6.7% and 20%, respectively, in the control group. In the same way, in question 7, related to polymerisation reaction, 6.7% of the control group had misconceptions, but students in the experimental groups had none. In light of these results, it can be said that teaching with conceptual change texts is more effective than traditional teaching regarding overcoming misconceptions about alkenes.
| Question no. | Post-test | |||||||
|---|---|---|---|---|---|---|---|---|
| Experimental group categories | Control group categories | |||||||
| SU (%) | PUSM (%) | SM (%) | NR (%) | SU (%) | PUSM (%) | SM (%) | NR (%) | |
| 1 | 87.9 | 6.1 | 6.1 | — | 83.3 | — | 16.7 | — |
| 2 | 78.8 | 9.1 | 9.1 | 3.0 | 60.0 | 13.3 | 16.7 | 10.0 |
| 3 | 63.7 | 3.0 | 15.2 | 18.2 | 53.3 | 10.0 | 26.7 | 10.0 |
| 4 | 81.8 | — | 18.2 | — | 66.7 | 3.3 | 30.0 | — |
| 5 | 84.8 | 6.1 | 3.0 | 6.1 | 73.3 | 6.7 | 16.7 | 3.3 |
| 6 | 78.8 | 9.1 | — | 12.1 | 46.7 | 20.0 | 16.7 | 16.7 |
| 7 | 84.8 | 6.1 | — | 9.1 | 80.0 | 13.3 | 6.7 | — |
| 8 | 84.8 | — | 15.2 | — | 83.3 | — | 16.7 | — |
| 9 | 87.9 | 12.1 | — | — | 80.0 | 10.0 | 6.7 | 3.3 |
| 10 | 78.8 | 12.1 | 3.0 | 6.1 | 76.7 | 6.7 | 16.7 | — |
| 11 | 87.9 | 12.1 | — | — | 80.0 | — | 20.0 | — |
| 12 | 75.8 | 21.2 | 3.0 | — | 60.0 | 26.7 | 6.7 | 6.7 |
| 13 | 87.9 | 3.0 | 3.0 | 6.1 | 66.7 | 10.0 | 16.7 | 6.7 |
| 14 | 69.7 | 12.1 | 9.1 | 9.1 | 43.3 | 20.0 | 20.0 | 16.7 |
| 15 | 66.7 | 12.1 | 9.1 | 12.1 | 56.7 | 13.3 | 16.7 | 13.3 |
| 16 | 81.8 | 9.1 | 9.1 | — | 76.7 | 3.3 | 20.0 | — |
| Question no. | Post-test | t-value | |||||
|---|---|---|---|---|---|---|---|
| Experimental group | Control group | ||||||
| CR (%) | IR (%) | NR (%) | CR (%) | IR (%) | NR (%) | ||
| a Not significant at 0.05.b Significant at p < 0.05.c Significant at p < 0.01. | |||||||
| 1 | 87.9 | 12.2 | — | 83.3 | 16.7 | — | 0.508a |
| 2 | 78.8 | 18.2 | 3.0 | 60.0 | 30.0 | 10.0 | 1.617a |
| 3 | 63.7 | 18.2 | 18.2 | 53.3 | 36.7 | 10.0 | 0.821a |
| 4 | 81.8 | 18.2 | — | 66.7 | 33.3 | — | 1.366a |
| 5 | 84.8 | 9.1 | 6.1 | 73.3 | 23.4 | 3.3 | 1.110a |
| 6 | 78.8 | 9.1 | 12.1 | 46.7 | 36.7 | 16.7 | 2.734c |
| 7 | 84.8 | 6.1 | 9.1 | 80.0 | 20.0 | — | 0.499a |
| 8 | 84.8 | 15.2 | — | 83.3 | 16.7 | — | 0.162a |
| 9 | 87.9 | 12.1 | — | 80.0 | 16.7 | 3.3 | 0.846a |
| 10 | 78.8 | 15.1 | 6.1 | 76.7 | 23.4 | — | −0.083a |
| 11 | 87.9 | 12.1 | — | 80.0 | 20.0 | — | 0.846a |
| 12 | 75.8 | 24.2 | — | 60.0 | 33.4 | 6.7 | 1.331a |
| 13 | 87.9 | 6.0 | 6.1 | 66.7 | 26.7 | 6.7 | 2.023b |
| 14 | 69.7 | 21.2 | 9.1 | 43.3 | 40.0 | 16.7 | 2.156b |
| 15 | 66.7 | 21.2 | 12.1 | 56.7 | 30.0 | 13.3 | 0.808a |
| 16 | 81.8 | 18.2 | — | 76.7 | 23.3 | — | 0.498a |
At the same time, an independent-sample t-test for each question was used to examine whether there was any statistical difference between the experimental and control groups at post-test. Table 8 indicates that there was a statistical significant difference for questions 6, 13, and 14 between the students of the experimental group and control group. The results indicated that teaching with conceptual change texts had more positive effects on students' understanding about properties of addition reactions (questions 6), polymerisation reactions (questions 13), and the synthesis of alkenes (questions 14). Also, this result is important since it showed that conceptual change texts developed in the study helped the students to further improve their understanding of some topics in alkenes which they learned in detail at first. Depending on the third research question of the study, students' specific misconceptions in the pre- and post-test were determined with ACT, as presented in Table 9. ACT included 48 specific misconceptions; however, 38 specific misconceptions were determined in the pre- and post-test, which are shown in Table 9. At the same time, the percentage of conceptual change (CC) for each misconception is presented in Table 9.
| Question | Specific misconceptions (SM) | Experimental group | Control group | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Pre (%) | Post (%) | CC (%) | Efec.a | Pre (%) | Post (%) | CC (%) | Efec.a | |||
| a Efec: effectiveness. | ||||||||||
| Q-1 | 1. | As long as there is a C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bond in the compound, the compound can display geometric isomerism. C bond in the compound, the compound can display geometric isomerism. |
24 | 6 | +18 | Mj | 23 | 10 | +13 | Lm |
| Q-1 | 2. | Two halogen atoms must be attached to double bonded carbons atoms for the formation of geometric isomerism. | 12 | — | +12 | Lm | 10 | 7 | +3 | Mn |
| Q-2 | 3. | Boiling points of geometric isomers are the same because geometric isomers have the same chemical formulae. | 21 | 6 | +15 | Lm | 20 | 10 | +10 | Lm |
| Q-2 | 4. | trans-Isomers have higher boiling points than their cis-counterparts. | 12 | 3 | +9 | Mn | 13 | 7 | +6 | Mn |
| Q-3 | 5. | If all groups in a compound which are attached to the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bond are different from each other, the compound cannot display geometric isomerism. C bond are different from each other, the compound cannot display geometric isomerism. |
12 | 6 | +6 | Mn | 13 | 10 | +3 | Mn |
| Q-3 | 6. | Geometric isomerism is specific only for alkenes. | 24 | 9 | +15 | Lm | 27 | 17 | +10 | Lm |
| Q-4 | 7. | The cyclic molecule and straight-branched compound are never structural isomers of each other. | 24 | 9 | +15 | Lm | 23 | 20 | +3 | Mn |
| Q-4 | 8. | In alkene chains, the double bond can be located in different positions; these kinds of compounds are not structural isomers of each other. | 21 | 9 | +12 | Lm | 17 | 10 | +7 | Mn |
| Q-4 | 9. | If molecules have the same chemical formulae but their IUPAC names are different, they are not structural isomers of each other. | — | — | — | Nsm | 3 | — | +3 | Mn |
| Q-5 | 10. | When an alkene is named, the substituent groups get first priority for naming. | 27 | 3 | +24 | Mj | 23 | 13 | +10 | Lm |
| Q-5 | 11. | When an alkene is named, the double-bond carbons receive highest possible numbers. | — | — | — | Nsm | 3 | 3 | — | No |
| Q-6 | 12. | Only the compounds which include π bond are capable of undergoing addition reactions. | 18 | — | +18 | Mj | 20 | 13 | +7 | Mn |
| Q-6 | 13. | Only the compounds which include two π bonds are capable of undergoing addition reactions. | 12 | — | +12 | Lm | 7 | 3 | +4 | Mn |
| Q-7 | 14. | When tetrafluoroethylene undergoes substitution reaction, teflon is formed. | 3 | — | +3 | Mn | 3 | 3 | — | No |
| Q-7 | 15. | When tetrafluoroethylene undergoes elimination reaction, teflon is formed. | 3 | — | +3 | Mn | 3 | 3 | — | No |
| Q-7 | 16. | When tetrafluoroethylene undergoes oxidation reaction, teflon is formed. | 3 | — | +3 | Mn | — | — | — | Nsm |
| Q-8 | 17. | The general formula of all alkenes is CnH2n. | 12 | 6 | +6 | Mn | 13 | 7 | +6 | Mn |
| Q-8 | 18. | All compounds which have the general formula CnH2n−2 are alkynes. | 9 | 3 | +6 | Mn | 13 | 7 | +6 | Mn |
| Q-8 | 19. | To call a molecule as cycloalkene, it is enough that its general formula is CnH2n−2. | 12 | 6 | +6 | Mn | 10 | 3 | +7 | Mn |
| Q-9 | 20. | 1-Butene reacts with HCl in accordance with substitution reaction, and 1-choloro-1- butene is formed. | 3 | — | +3 | Mn | 3 | 3 | — | No |
| Q-9 | 21. | 1-Butene reacts with HCl in accordance with substitution reaction, and 2-choloro-1-butene is formed. | 3 | — | +3 | Mn | 3 | 3 | — | No |
| Q-9 | 22. | 1-Butene reacts with HCl in accordance with substitution reaction, and 3-choloro-1-butene is formed. | 3 | — | +3 | Mn | — | — | — | Nsm |
| Q-10 | 23. | In the addition of HX to an unsymmetrical alkene in the presence of peroxides the reaction does not occur. | — | — | — | Nsm | 3 | — | +3 | Mn |
| Q-10 | 24. | In the addition of HX to an unsymmetrical alkene, Markovnikov's rule can always be used to predict the product. | 15 | 3 | +12 | Lm | 17 | 17 | — | No |
| Q-11 | 25. | The addition of water to an alkene in the presence of acid leads to the formation of ketones. | 18 | — | +18 | Mj | 20 | 10 | +10 | Lm |
| Q-11 | 26. | The addition of water to an alkene in the presence of acid leads to the formation of aldehydes. | 9 | — | +9 | Mn | 10 | 7 | +3 | Mn |
| Q-11 | 27. | The addition of water to an alkene in the presence of acid leads to the formation of ethers. | 6 | — | +6 | Mn | 3 | 3 | — | No |
| Q-12 | 28. | 1,3-Butadiene react with one molar equivalent of hydrogen in the presence of catalysts to yield butane. | 3 | 3 | — | No | 3 | 3 | — | No |
| Q-12 | 29. | 1,3-Butadiene react with one hydrogen atom in the presence of catalysts to yield butane. | 3 | — | +3 | Mn | 3 | 3 | — | No |
| Q-12 | 30. | 1,3-Butadiene react with one hydrogen atom in the presence of catalysts to yield butene. | — | — | — | Nsm | 3 | — | +3 | Mn |
| Q-13 | 31. | Only alkene which has two carbon atoms undergoes polymerisation reactions. | 12 | — | +12 | Lm | 13 | 7 | +6 | Mn |
| Q-13 | 32. | Only alkene which has six or more carbon atoms undergoes polymerisation reactions. | 9 | 3 | +6 | Mn | 13 | 10 | +3 | Mn |
| Q-14 | 33. | During dehydration of 2-butanol as a secondary alcohol in the presence of acid at higher temperature, only 2-butene is formed. | 12 | 3 | +9 | Mn | 10 | 10 | — | No |
| Q-14 | 34. | During dehydration of 2-butanol as a secondary alcohol in the presence of acid at higher temperature, only 1-butene is formed. | 15 | 6 | +9 | Mn | 13 | 10 | +3 | Mn |
| Q-15 | 35. | When alkyl halides are heated with concentrated, strong bases such as KOH and NaOH in the presence of alcohols, alcohols are generated as the major product. | 21 | 9 | +12 | Lm | 20 | 17 | +3 | Mn |
| Q-15 | 36. | When alkyl halides are heated with concentrated, strong bases such as KOH and NaOH in the presence of alcohols, alkanes are generated as the major product. | — | — | — | Nsm | 3 | — | +3 | Mn |
| Q-16 | 37. | When cycloalkenes are named, numbering is always counter-clockwise. | 24 | 6 | +18 | Mj | 23 | 13 | +10 | Lm |
| Q-16 | 38. | When cycloalkenes are named, the highest numbers are always given to alkyl groups attached to the ring. | 18 | 3 | +15 | Lm | 17 | 7 | +10 | Lm |
As can be seen in Table 9, there were no remarkable differences between the percentages of misconceptions held by students in the experimental and control groups in the pre-test. On the other hand, students in the experimental group held fewer misconceptions in the post-test. For example, percentages of the experimental group's misconceptions in the post-test ranged from 9% to 3%, while those of the control group ranged from 20% to 3%. As explained in the “data analysis” section, the percentage of conceptual change was classified by effectiveness as “major”, “limited”, “minor” and “no”. Also, if a misconception was not identified in the pre- and post-tests, it was labelled as “no specific misconception (Nsm)”. It can be seen from Table 9 that in the experimental group, five of the conceptual changes were “major (Mj)”, ten were “limited (Lm)”, seventeen were “minor (Mn)”, and one was “no (No)”. Besides, five misconceptions which were determined in the control group were not revealed in the experimental group at pre- and post-test (Nsm). On the other hand, this classification was examined in the control group, and it was seen that none of the percentages of conceptual change was “major”. In addition, it was found that most of the percentages of conceptual change were “minor” (nineteen) and “no” (ten). Only seven of the conceptual changes were “limited” in the control group. Also, two misconceptions which were determined in the experimental group were not revealed in the control group at pre- and post-test (Nsm). With this analysis, it was understood that students in the experimental group were more successful in changing their misconceptions towards scientific ones than the students in the control group. Also, it is understood from Table 9, that some misconceptions are still held by both groups after teaching. The results show that the students resisted changing these misconceptions. Similar results have also been observed in previous studies (Osborne and Freyberg, 1985; Driver, 1989; Hameed et al., 1993).
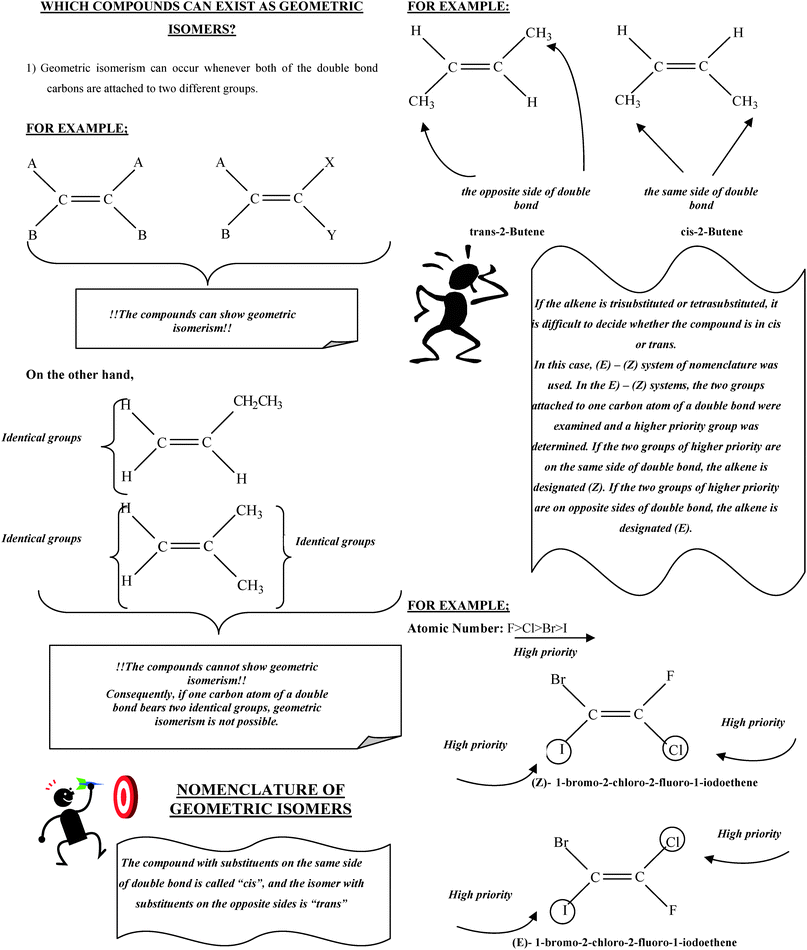
Particularly, these misconceptions were intensified in regards to geometric isomerism. For instance, 24% of students in the experimental group and 23% of students in the control group believed, as in the pre-test, that as long as there is a C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bond in the compound, the compound can display geometric isomerism (SM 1). The reason for this misconception is that students could mostly relate geometric isomerism to alkenes. As can be seen clearly from Table 9, the percentage of this misconception decreased in the post-test in both groups, but the experimental group did better than the control group (6% in the experimental group and 10% in the control group). The conceptual change text related to this misconception (CCT 3) was probably the reason for the better scores of the students in the experimental group. In this text, it was emphasised that all compounds including a C
C bond in the compound, the compound can display geometric isomerism (SM 1). The reason for this misconception is that students could mostly relate geometric isomerism to alkenes. As can be seen clearly from Table 9, the percentage of this misconception decreased in the post-test in both groups, but the experimental group did better than the control group (6% in the experimental group and 10% in the control group). The conceptual change text related to this misconception (CCT 3) was probably the reason for the better scores of the students in the experimental group. In this text, it was emphasised that all compounds including a C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bond cannot display geometric isomerism. Thus, while the percentage of conceptual change about this misconception was classified as major in the experimental group, this percentage was classified by effectiveness as limited in the control group.
C bond cannot display geometric isomerism. Thus, while the percentage of conceptual change about this misconception was classified as major in the experimental group, this percentage was classified by effectiveness as limited in the control group.
Another misconception related to geometric isomerism identified in the study is that two halogen atoms must be attached to double bonded carbons atoms for the formation of geometric isomerism (SM 2). The percentage of the misconception in the control group decreased from pre-test (10%) to post-test (7%). Similarly, in the experimental group, the percentage of this misconception decreased from pre-test to post-test. In fact, this misconception could be remedied in the experimental group after teaching. This result is an indicator of the effectiveness of conceptual change text (CCT 3) on overcoming this misconception. In addition, the difference between the two groups in the context of the effectiveness of conceptual change (limited in the experimental group, minor in the control group) supported the idea that teaching with conceptual change texts had more positive effects on overcoming students' misconceptions than traditional instruction.
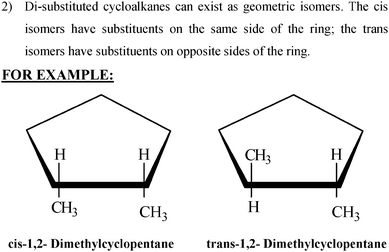
Another important misconception is that geometric isomerism is specific for alkenes only (SM 6). Although the percentage of this misconception decreased from pre-test to post-test, 9% of the students in the experimental group and 17% of the students in the control group held the misconception in the post-test. These students believed that a cycloalkane cannot display geometric isomerism. This is not surprising because students will have learnt that a disubstituted cycloalkane can display geometric isomerism for the first time at university level. Consequently, building new knowledge was easier for some students (especially for experimental group students) since this misconception was emphasised in the CCT 3.
One of the most common misconceptions encountered among students in both groups is that if all groups that are attached to a C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bond in a compound are different from each other, the compound cannot display geometric isomerism (SM 5). It is likely that students interpret this statement according to their prior knowledge. In fact, the Turkish secondary chemistry curriculum and chemistry textbooks did not contain more specific systems or examples. Moreover, chemistry teachers usually use specific examples such as 1,2-dichloroethene when they teach geometric isomerism. In this example, two chlorine atoms may be on opposite or same sides of the double bond; as a result, the compound is called a trans or cis isomer. Thus, students may believe that only compounds where two halogen atoms are attached to double bonded carbons atoms shows geometric isomerism. For this reason, it was explained in detail in CCT 3 that, when four different groups are attached to double bonded carbons; the compounds can show geometric isomerism. When the results of the post-test were investigated, it was seen that students in the experimental group were more successful than the control group students in the context of remedying this misconception (6% of the experimental group, 10% of the control group).
C bond in a compound are different from each other, the compound cannot display geometric isomerism (SM 5). It is likely that students interpret this statement according to their prior knowledge. In fact, the Turkish secondary chemistry curriculum and chemistry textbooks did not contain more specific systems or examples. Moreover, chemistry teachers usually use specific examples such as 1,2-dichloroethene when they teach geometric isomerism. In this example, two chlorine atoms may be on opposite or same sides of the double bond; as a result, the compound is called a trans or cis isomer. Thus, students may believe that only compounds where two halogen atoms are attached to double bonded carbons atoms shows geometric isomerism. For this reason, it was explained in detail in CCT 3 that, when four different groups are attached to double bonded carbons; the compounds can show geometric isomerism. When the results of the post-test were investigated, it was seen that students in the experimental group were more successful than the control group students in the context of remedying this misconception (6% of the experimental group, 10% of the control group).
In addition, the students in both groups had misconceptions about the physical properties of geometric isomers. For example, 21% of the experimental group and 20% of the control group thought that boiling points of geometric isomers are the same because geometric isomers have the same chemical formulae in the pre-test (SM 3). As can be seen from Table 9, the percentage of misconceptions decreased in both groups in the post-test but conceptual change in the experimental group was better than those in the control group. Another misconception is that trans-isomers have higher boiling points than their cis-counterparts (SM 4). In the pre-test, 12% of the experimental group and 13% of the control group held this misconception. These students did not consider the effects of molecular polarity and intermolecular bonds on the physical properties of compounds. Although, it was seen from Table 9 that the percentage of the these misconceptions in the experimental group was less than the control group in the post-test, the experimental and control groups were classified in the same categories (limited and minor) according to the effectiveness of conceptual change. This result showed that CCT 4, the subject of which was physical properties of geometric isomers, was no more effective for remedying students' misconceptions.
Some of the misconceptions encountered among the students were related to structural isomerism. One of the misconceptions was that the cyclic molecules and straight-branched compounds are never structural isomers of each other (SM 7). This misconception was held by 24% of the experimental group and 23% of the control group in the pre-test. These students believed that 1-methylcyclobutene was not a structural isomer with 1,3-pentadiene, 1,4-pentadiene and 2-methyl-1,3-butadiene. After instruction, the percentage of this misconception in the experimental group decreased to 9%, while the percentage in the control group decreased to only 20%. The major reason for this may be that this misconception was discussed in detail in CCT 2. The other misconception related to structural isomerism, as in alkene chains the double bond can be located in different positions; these kinds of compounds are not structural isomers of each other (SM 8). Interestingly, while the percentage of this misconception in the experimental group was higher than the control group in the pre-test, the opposite of this was observed at the post-test (9% of the experimental group, 10% of the control group). Moreover, the effectiveness of conceptual change in the experimental group provided evidence that CCT 2 was effective in changing students' misconceptions into scientific ones.
Another misconception held by the experimental and control groups was related to alkene nomenclature. As seen from Table 9, in the pre-test, 27% of the experimental group and 23% of the control group believed that when an alkene is named, the substituent groups get first priority for naming (SM 10). This may stem from the generalisation of naming alkanes, as it was emphasised that the substituent groups get first priority for naming alkanes. Thus, students named 4-bromo-3-methyl-2-pentane as 2-bromo-3-methyl-3-pentane. After instruction, the percentage of this misconception decreased in both groups, but the effectiveness of conceptual change in the experimental group was better than in the control group (major in the experimental group, limited in the control group). The reason for this achievement in the experimental group may be CCT 1 related to this misconception. Also, any conceptual change was not observed in SM 11 in the control group. This result indicated that traditional instruction was no more effective to promote conceptual change. Similarly, students in both groups had misconceptions related to naming cycloalkenes in the pre-test. For example, 24% of the experimental group and 23% of the control group believed that when cycloalkenes are named, numbering is always counter-clockwise (SM 37). Post-test results revealed that 13% of the students in the control group held this misconception, while only 6% of the students in the experimental group held the misconception. Also, a categorical difference was determined between experimental and control groups in favour of the experimental group regarding the effectiveness of conceptual change. Another misconception was related to naming cycloalkenes: 18% of the experimental group and 17% of the control group believed that when cycloalkenes are named, the highest numbers are always given to alkyl groups attached to ring (SM 38). After instruction, the percentage of this misconception decreased to 3% in the experimental group and 7% in the control group. As above, these results indicate that conceptual change in the experimental group was more meaningful than those in the control group.
The other common misconceptions identified in the study were related to the general formulae of alkenes. For example, 12% of the experimental group and 13% of the control group thought that the general formula of all alkenes is CnH2n in the pre-test (SM 17). These students considered only straight and branched alkenes that contained one carbon–carbon double bond. In other words, the students did not consider cycloalkene and alkadiene as alkenes. After instruction, the percentage of misconception changed in both groups. It was determined that the percentage of conceptual change about this misconception in both groups were equal (6%). As a common misconception, the students in both groups believed that all compounds that have the general formula CnH2n−2 were alkynes in the pre-test (SM 18). This may result from students' prior knowledge since the general formula CnH2n−2 was associated with alkynes in secondary school chemistry textbooks in Turkey. In other words, it was not emphasised in detail that cycloalkenes and alkedienes had the same chemical formulae (CnH2n−2) in these textbooks. In the post-test, the percentage of this misconception decreased in both groups (3% in the experimental group, 7% in the control group). In case of another misconception, 12% of the experimental group and 10% of the control group in the pre-test thought that to call a molecule a cycloalkene, it was enough that its general formula was CnH2n−2 (SM 19). As can be seen from Table 9, although the perception of this misconception in the post-test decreased in both groups, surprisingly the percentage of this in the control group (3%) was less than the experimental group (6%). At this point, it is important to note that the general formulae of alkenes were not the subject of any conceptual change texts. Thus, Table 9 reveals that the percentages of conceptual change related to misconceptions discussed in conceptual change texts such SM 1 and 2 were more meaningful than others. Also, these results showed that conceptual change texts were effective in improving students' understanding and overcoming their misconceptions.
Other important misconceptions were related to alkene reactions, such as addition and polymerisation. As can be seen from Table 9, students' misconceptions mostly intensified on addition reactions among these reactions. The common misconception was that only the compounds that include π bonds are capable of undergoing addition reactions (SM 12). This misconception was held by 18% of the experimental group and 20% of the control group according to the pre-test. The misconception indicated that the students believed that cyclopropane could not undergo addition reaction with bromine because the compound did not include a π bond. The possible reason for this may be related to students' prior knowledge, because it was not emphasised that cyclopropane and cyclobutane could undergo addition reactions in secondary chemistry curriculum and textbooks in Turkey. Due to the importance of this misconception, CCT 5 was prepared and the misconception was discussed in detail in this text. Thus, results of the post-test indicated that CCT was more effective in remedying this misconception than traditional instruction. While 13% of the control group held this misconception after the teaching, the misconception was nonexistent in the experimental group. Thus, the effectiveness of CCT 5 was understood more clearly from differences between two groups in the context of effectiveness of conceptual change (major in the experimental group, minor in the control group). In addition, a similar result was identified for SM 13. In the pre-test, 12% of the experimental group and 7% of the control group believed that only the compounds that include two π bonds are capable of undergoing addition reactions. However, after instruction, the experimental group no longer held this misconception, while 3% of the control group still believed it. Furthermore, students in both groups held misconceptions about the application of Markovnikov's rule. When Table 9 was examined, it was seen that few students in the experimental and control groups interpreted the addition reaction of 1-butene with HCl as a substitution reaction and predicted the product in accordance with this reaction at the pre-test (SM 20, 21, and 22). On the other hand, at the post-test, a different result from the pre-test was revealed. The students in the experimental group did not have these misconceptions at the post-test; nevertheless, the same percentages of students as the pre-test in the control group had these misconceptions after the teaching. When these results were investigated, it was found out that the same students in the control group held these misconceptions at pre- and post-tests. One of these misconceptions was that, in the addition of HX to an unsymmetrical alkene, Markovnikov's rule can always be used to predict the product (SM 24). 15% of the experimental group and 17% of the control group had this misconception in the pre-test. Lim (2007) had reported a similar alternative conception. As seen in Table 9, although the percentage of this misconception in the experimental group decreased on the post-test, any changes were not observed in the control group between pre-test and post-test. This result was not surprising since students learned in high school chemistry that when HX reacts with an unsymmetrical alkene, Markovnikov's rule can always be used to predict the product. Changing prior knowledge was not easy for some students. Thus, mostly the same students in the control group had this misconception before and after the teaching. In light of this result, it can be said that traditionally designed instruction is not sufficient to change the misconceptions into scientific ones. At the same time, this misconception was explained in CCT 6. In this text, it was stated that when HBr was added to alkenes in the presence of peroxides, anti-Markovnikov's addition occurred. In addition, it was emphasised that anti-Markovnikov's addition worked only with HBr, required the presence of peroxides and the reaction followed a free radical mechanism. These explanations could have helped the experimental group to perform better than the control group in the post-test.Table 9 indicates that both the experimental and control group had some misconceptions about acids catalysed with the addition of water to alkenes. In particular, from these misconceptions, it was determined that students had difficulties in the identification of functional groups and their compounds. For example, at the pre-test, as a result of the addition of water to an alkene in the presence of acid, 18% of the experimental group and 20% control group believed that ketones were formed (SM 25). Similarly, while 9% of the experimental group and 10% of the control group believed that aldehydes were formed (SM 26), 6% of the experimental group and 3% of the control group stated that the ethers were formed (SM 27). These students could not apply Markovnikov's rule correctly and identify that alcohol was formed by the reaction. However, when the results of post-test in Table 9 were analysed, it was understood that these misconceptions were remedied in the experimental group and also, a major change was determined regarding the effectiveness of conceptual change (SM 25). The possible reason for this may be that these misconceptions were discussed in the CCT 7. Whereas the percentages of these misconceptions in the control group decreased in the post-test, except for SM 27, most of the students still had these misconceptions.
Table 9 shows that few students in both groups had difficulties in understanding catalytic hydrogenation of alkedienes at the pre-test. One of the reasons for these misconceptions may be students' confusing the concepts of atom and molecule. Thus, some studies reported that different levels of students held misconceptions about atom and molecules (Ben-Zvi et al., 1986; Griffiths and Preston, 1992; Nakhleh and Samarapungavan, 1999; Harrison and Treagust, 2000). Although the addition of hydrogen to alkenes was explained in CCT 5, atomic and molecular structures of hydrogen were not explained in this CCT. After the teaching, no meaningful changes about these misconceptions were seen in either group (SM 28, 29, and 30). In addition, most of the same students in both groups held these misconceptions before and after the teaching. These results indicated that students resisted changing their prior knowledge. Lakatos (1970) stated that if students' misconceptions were well structured, students may reject scientific ideas or new knowledge.
The other major misconceptions encountered by the students of both groups were related to the polymerisation reaction. The misconceptions related to polymerisation reactions were classified into two categories as identification of polymerisation reactions (question 7) and polymerisation of alkenes (question 13). From Table 9, it can be clearly seen that students in both groups held misconceptions about the polymerisation reaction of tetrafluoroethylene before the teaching (SM 14, 15, and 16). Following teaching, these misconceptions were reduced in the experimental group, but the same misconceptions still appeared for the control group. One of the misconceptions about the polymerisation of alkenes was that only an alkene that has two carbon atoms undergoes polymerisation reactions (SM 31). In the pre-test, 12% of the experimental group and 13% of the control group held this misconception. This may be related to students' prior knowledge because the polymerisation reaction of ethene is generally given as an example in secondary school chemistry textbooks. Chemistry teachers largely use this example when teaching the topic of polymerisation reactions. As can be seen from Table 9, 7% of the control group held this misconception in the post-test, while all of the students in the experimental group changed their misconceptions towards scientific ones. Consequently, while the effectiveness of conceptual change was limited in the experimental group, a minor change was observed in the control group. Similar results were observed for SM 32. Table 9 shows that 9% of the experimental group and 13% of the control group believed that only alkenes that have six or more carbon atoms undergo polymerisation reactions in the pre-test. The reason for this may be the meaning of polymer, as polymer means a macromolecule. The percentages of this misconception in both groups were reduced in the post-test. However, the conceptual change in the experimental group was more meaningful than the control group. In light of these results, it can be said that CCT 8, the subject of which was polymerisation reactions, was more effective at promoting conceptual change than traditional teaching methods.
As can be seen from Table 9, many students had misconceptions about the synthesis of alkenes from the alcohols and alkyl halides in the pre-test. Most of the students believed that during the dehydration of 2-butanol as a secondary alcohol in the presence of acid at higher temperature, only 2-butene or 1-butene were formed (SM 33 and 34). In order to overcome these misconceptions, one CCT (CCT 9) was developed in the study. In this CCT, the dehydration of primary, secondary and tertiary alcohols were given as examples and it was explained that when two different alkene products are possible in dehydration of alcohols, the most highly substituted (most stable) alkene was the major product, according to Zaitzev's rule. When the results of the post-test and the percentage of conceptual change between the experimental and control groups were compared, the effectiveness of conceptual change texts can be clearly observed. As a good example, the percentage of SM 33 in the control group did not change from the pre-test to post-test. On the other hand, there was meaningful change related to this misconception in the experimental group. Another important result was determined for SM 34. Unlike SM 33, the percentage of this misconception in the control group was decreased from pre-test to post-test. Nonetheless, this change was not as significant as in the experimental group. At the same time, students in the experimental and control groups held misconceptions about the synthesis of alkenes via elimination reactions of alkyl halides. Many students applied elimination reactions of alkyl halides as substitution reactions at the pre-test. As a result, they believed that alcohols were formed (SM 35). At CCT 9, related to the synthesis of alkenes, differences between elimination and substitution reactions of alkyl halides were explained and this misconception (SM35) was discussed in detail. With the effects of instruction, the percentage of the misconception in both groups decreased; however, there was a greater decrease in the experimental group (a limited change) than the control group (a minor change). In addition, few students in the control group stated that alkanes are generated as a result of reaction of alkyl halides in the pre-test (SM 36). On the other hand, this misconception was not determined in the post-test in either the control or the experimental group.
In addition, one of the analyses which could be used to compare the effectiveness of CCT and traditional teaching methods is presenting how students' responses changed from pre-test to post-test. With this aim, a schema of possible types of changes was constructed, which is presented in Table 10. As can be seen from this table, nine different types of possible changes were identified in the students' responses.
| Possibility of changes | Pre-test | Post-test |
|---|---|---|
| Type 1 | Correct response | Correct response |
| Type 2 | Correct response | Incorrect response |
| Type 3 | Correct response | No response |
| Type 4 | No response | No response |
| Type 5 | No response | Correct response |
| Type 6 | No response | Incorrect response |
| Type 7 | Incorrect response | Incorrect response |
| Type 8 | Incorrect response | Correct response |
| Type 9 | Incorrect response | No response |
Although all types of possibilities were identified, only sample changes related to type 2 and type 3 are presented in Table 11 since these types of changes indicated negative changes from pre-test to post-test. In addition, students' incorrect responses were analysed post- and pre-test, and it was determined that the most frequently identified was type 7; the next most common was type 6. As can be seen from Table 11, in both groups, there were students that changed a correct response into an incorrect one (type 2) or no response (type 3) in the post-test. Particularly, when type 2 changes were examined in both groups, it was revealed that few students acquired specific misconceptions in some questions such as questions 3, 4, 6, 9, 11, 12, 13, 15 and 16. While these specific misconceptions were more common in the control group than the experimental group, unfavourable results were also identified in the experimental group (questions 3, 4, 15 and 16). Also, in some questions, such as questions 9, 11 and 12, few students in the experimental group gave responses that fell into the “PUSM” category in the post-test. This indicates that some students in the experimental group had problems in the application of addition reactions. Similar unfavourable results have been identified in previous conceptual change studies (Hewson and Hewson, 1983; Case and Fraser, 1999; Ebenezer, 2001; Coştu et al., 2007, 2010). This may result from students becoming bored with continued reading of conceptual change texts or interactions with students who strongly held their specific misconceptions in class discussions (Çalık et al., 2009; Coştu et al., 2010).
| Question no. | Experimental group | Control group | ||
|---|---|---|---|---|
| Type 2 | Type 3 | Type 2 | Type 3 | |
| Sample changes | Sample changes | Sample changes | Sample changes | |
| a SE: student in the experimental group. SC: student in the control group. SM: students' specific misconception in Table 9. PUSM: particular understanding with specific misconception. | ||||
| 1 | — | — | — | — |
| 2 | — | — | — | — |
| 3 | SE3 (The 5th SM) | SE23 | SC9 (The 5th SM) | — |
| SE5 (The 6th SM) | SC4 (The 5th SM) | |||
| 4 | SE15 (The 7th SM) | — | SC27 (The 7th SM) | — |
| 5 | — | — | — | SC14 |
| 6 | — | — | SC2 (The 12th SM) | — |
| SC10 (The 12th SM) | ||||
| 7 | — | — | — | — |
| 8 | — | — | — | — |
| 9 | SE9 (PUSM category) | — | SC14 (The 20th SM) | |
| 10 | — | — | — | — |
| 11 | SE23 (PUSM category) | — | SC10 (PUSM category) | — |
| 12 | SE7 (PUSM category) | — | SC21 (The 28th SM) | — |
| 13 | — | — | SC7 (The 35th SM) | — |
| 14 | — | — | — | — |
| 15 | SE11 (The 35th SM) | SE9 | SC5 (The 35th SM) | — |
| 16 | SE23 (The 37th SM) | — | SC2 (The 37th SM) | — |
| SC18 (The 37th SM) | ||||
Conclusions and implications
The main purpose of this study was to compare the effectiveness of conceptual change texts instruction over the traditional instruction on students' conceptual understanding regarding alkenes. The results of data analysis revealed that CCT instruction resulted in a significantly better acquisition of scientific conceptions and remedying misconceptions than the traditionally designed instruction in the context of alkenes. These results also provided evidence that the conceptual change text helped the students in becoming aware of their misconceptions and in distinguishing them from the scientifically accepted concepts. In addition the study showed that students hold a variety of misconceptions about alkenes and these misconceptions may be identified with conceptual change texts and discussion of concepts in the texts. On the other hand, all conceptual change texts did not have the same effect on improving students' understanding and overcoming their misconceptions. For example, only in questions 6, 13 and 14, was there a statistical difference in favour of the experimental group in the context of correct responses in the post-test (see Table 8). At the same time, the effectiveness of conceptual change in misconceptions related to some topics, such as geometric isomerism (question 1), structural isomerism (question 4), nomenclature of alkenes (question 5 and 16), properties of addition reactions (question 6), addition reactions (question 10 and 11), polymerisation reaction (question 13), and the synthesis of alkenes (question 14 and 15), was more meaningful in the experimental group than the control group (see Table 9). However, it was determined that the effectiveness of conceptual change in misconceptions about physical properties of geometric isomers (question 2) was the same in both groups. This situation shows that promoting conceptual change for some concepts is difficult (Campanario, 2002; Tsaparlis and Papaphotis, 2009). Also, several researchers have stated that the conceptual change process may be context dependent (Tao and Gunstone, 1999; Havu-Nuutinen, 2005; Jonassen et al., 2005; Pımthong et al., 2012). One possible reason for this is that these concepts require the application of prior knowledge. For instance, understanding the physical properties of geometric isomers requires the understanding of intermolecular forces and distinguishing between polar and non-polar compounds. If the students lack understanding of those concepts, this could be an obstacle to students' conceptual change. Another reason may be related to language issues. Some terms such as polar and non-polar molecules have similar pronunciation in Turkish. It is possible for the students to confuse the cis isomer as a polar molecule and the trans isomer as non-polar. Thus, confusion in language was found to be an obstacle to students' conceptual change by Wellington and Osborne (2001). In light of these results, it can be said that the conceptual change texts, except CCT 4, were more successful in overcoming the students' misconceptions than the traditionally designed instruction. However, some of the misconceptions were still encountered, and few students acquired the misconceptions after the instruction in the experimental group (see Tables 9 and 11). This shows that CCT instruction also failed to change some students' misconceptions towards scientific ones, totally, and that CCT instruction has a negative effect on a few students' understanding. Also, these results suggest that more than one intervention model should be used to change students' misconceptions towards scientific ones. In this study, it was not possible to identify which features of CCTs were responsible for negative effects on the students' understanding. In future studies, some of the features of the texts such as questions, explanations, and examples may be examined, and their influence on students' understanding may be tested. Future studies may be conducted to investigate the effectiveness of CCTs on students' understanding in different chemistry departments. In addition, this study did not indicate which group of students, when compared to levels of prior knowledge (high, middle, or low), benefited most from CCT instruction. It would be interesting to examine which students benefited more from CCT instruction in a future study. Moreover, in this study, it was not examined whether students' understanding about alkenes were permanent or not. For this reason, future studies may be carried out to investigate the effect of CCT instruction on the retention of students' understanding about alkenes.Appendix
Conceptual change text 3(CCT III): Geometric isomerismReferences
- Abraham M. R., Grzybowski E. B., Renner J. W. and Marek E. A., (1992), Understandings and misunderstandings of eight grades of five chemistry concepts found in textbooks, J. Res. Sci. Teach., 29(2), 105–120.
- Atkins R. C. and Carey F. A., (2002), Organic Chemistry A Brief Course, 3rd edn, New York: McGraw-Hill.
- Beerenwinkel A., Parchmann I. and Gräsel C., (2011), Conceptual change texts in chemistry teaching: a study on the particle model of matter, Int. J. Sci. Math. Educ., 9(5), 1235–1259.
- Ben-Zvi R., Eylon B. and Silberstein J., (1986), Is an atom of copper malleable? J. Chem. Educ., 63(1), 64–66.
- Bodner G., (1991), I have found you an argument: the conceptual knowledge of beginning chemistry graduate students, J. Chem. Educ., 68(5), 385–388.
- Cakmakci G. and Aydogdu C., (2011), Designing and evaluating an evidence-informed instruction in chemical kinetics, Chem. Educ. Res. Pract., 12, 15–28.
- Çakır O., Uzuntiryaki E. and Geban O., (2002), Contribution of conceptual change texts and concept mapping to students' understanding of acids and bases. Paper presented at the annual meeting of the national association for research in science teaching, New Orleans, LA.
- Çalık M., (2005), A cross-age study of different perspectives in solution chemistry from junior to senior high school, Int. J. Sci. Math. Educ., 3, 671–696.
- Çalık M., Ayas A. and Ebenezer J. V., (2009), Analogical reasoning for understanding solution rates: students' conceptual change and chemical explanations, Res. Sci. Technol. Educ., 27(3), 283–308.
- Campanario J. M., (2002), There parallelism between scientist' and students' resistance to new scientific ideas. Int. J. Sci. Educ., 24(10), 1095–1110.
- Canpolat N., Pınarbası T., Bayrakceken S. and Geban O., (2006), The conceptual change approach to teaching chemical equilibrium. Res. Sci. Technol. Educ., 24(2), 217–235.
- Case M. J. and Fraser D. M., (1999), An investigation into chemical engineering students' understanding of the mole and the use of concrete activities to promote conceptual change, Int. J. Sci. Educ., 21, 1237–1249.
- Chief Examiner's Reports in Chemistry, (2008), Retrieved February 15, 2012 from http://examinations.ie.
- Chief Examiner's Reports in Chemistry, (2009), Retrieved February 15, 2012 from http://examinations.ie.
- Childs P. E. and Sheehan M., (2009), What's difficult about chemistry? An Irish perspective, Chem. Educ. Res. Pract., 10, 204–218.
- Cohen J., (1992), A power primer, Psychological Bulletin, 112(1), 155–159.
- Coştu B., Ayas A., Niaz M., Ünal S. and Çalık M., (2007), Facilitating conceptual change in students' understanding of boiling concept, J. Sci. Educ. Technol., 16, 524–536.
- Coştu B., Ayas A. and Niaz M., (2010), Promoting conceptual change in first year students' understanding of evaporation, Chem. Educ. Res. Pract., 11, 5–16.
- Driver R., (1989), Students' conceptions and the learning of science, Int. J. Sci. Educ., 11, 481–490.
- Driver R. and Easley J., (1978), Pupils and paradigms: a review of literature related the concept development in adolescent science students, Stud. Sci. Educ., 5, 61–84.
- Driver R. and Erickson G., (1983), Theories-in-action: some theoretical and empirical issues in the study of students' conceptual frameworks in science, Stud. Sci. Educ., 10, 37–60.
- Durmuş J. and Bayraktar Ş., (2010), Effects of conceptual change texts and laboratory experiments on fourth grade students' understanding of matter and change concepts, J. Sci. Educ. Technol., 19, 498–504.
- Ebenezer J., (2001), A hypermedia environment to explore and negotiate students' conceptions: animation of the solution process of table salt, J. Sci. Educ. Technol., 10, 73–91.
- Fessenden R. J. and Fessenden J. S., (1998), Organic Chemistry, 6th edn, Pacific Grove, CA: Brooks/Cole Publishing.
- Fleer M., (1999), Children's alternative views: alternative to what? Int. J. Sci. Educ., 21, 119–135.
- Gay L. R. and Airasian P., (2000), Educational Research: Competencies for Analysis and Application, New Jersey: Prentice-Hall Inc.
- Gonzalez F.M., (1997), Diagnosis of Spanish primary school students' common alternative science conceptions, School Sci. Math., 97(2), 68–74.
- Gravetter F. J. and Wallnau L. B., (2000), Statistics for the behavioural sciences, 5th edn., Belmont, CA: Wadsworth/Thomson Learning.
- Griffiths A. K. and Preston K. R., (1992), Grade-12 students' misconceptions relating to fundamental characteristics of atoms and molecules, J. Res. Sci. Teach., 29(6), 611–628.
- Hameed H., Hackling M.W. and Garnett P. J., (1993), Facilitating conceptual change in chemical equilibrium using a CAI strategy, Int. J. Sci. Educ., 15, 221–230.
- Harrison A. G. and Treagust D. F., (2000), Learning about atoms, molecules, and chemical bonds: a case study of multiple-model use in grade 11 chemistry, Sci. Educ., 84, 352–381.
- Havu-Nuutinen S., (2005), Examining young children's conceptual change process in floating and sinking from a social constructivist perspective, Int. J. Sci. Educ., 27(3), 259–279.
- Hesse J. and Anderson C., (1992), Students' conception of chemical change, J. Res. Sci. Teach., 29, 277–99.
- Hewson M. G. and Hewson P. W., (1983), Effect of instruction using students' prior knowledge and conceptual change strategies on science learning, J. Res. Sci. Teach., 20, 731–743.
- Hewson P. W. and Hewson M. G., (1984), The role of conceptual conflict in conceptual change and the design of science instruction, Instruct. Sci. 13, 1–13.
- Hinkle D. E., Wiersma W. and Jurs S. G., (1998), Applied statistics for the behavioral sciences, New York: Houghton Mifflin Company.
- Hsu Y. S., (2008), Learning about seasons in a technologically enhanced environment: the impact of teacher-guided and student-centred instructional approaches on the process of students' conceptual change, Sci. Educ., 92, 320–344.
- Jonassen D., Strobel J. and Gottdenker J., (2005), Model building for conceptual change, Interactive Learning Environments, 13(1–2), 15–37.
- Kaya O. N., (2007), A student-centred approach: assessing the changes in prospective science teachers' conceptual understanding by concept mapping in a general chemistry laboratory, Res. Sci. Educ., 38, 91–110.
- Kim S. and Van Dusen L. M., (1998), The role of prior knowledge and elaboration in text comprehension and memory: a comparison of self-generated and text provided elaboration, Am. J. Psychol., 111, 353–378.
- Lakatos I., (1970), Falsification and the methodology of scientific research programmes, in H. Lakatos I., Musgrave A. (ed.), Criticism and the growth of knowledge. Cambridge: Cambridge University Press, pp. 91–196.
- Lim C. H. B., (2007), Identifying students' misconceptions in ‘A-level’ organic chemistry. Paper presented at Redesigning Pedagogy Culture, Knowledge and Understanding Conference, Nanyang.
- McMurry J., (1995), Organic Chemistry, fourth edn, Pacific Grove, CA: Brooks/Cole Publishing.
- Nakhleh M. B., (1992), Why some students don't learn chemistry: chemical misconceptions, J. Chem. Educ., 69(3), 191–196.
- Nakhleh M. B. and Samarapungavan A., (1999), Elementary school children's beliefs about matter, J. Res. Sci. Teach., 36(7), 777–805.
- Nussbaum J., (1981), Towards a diagnosis by science teachers of pupils' misconceptions: an exercise with student teachers, Int. J. Sci. Educ., 3(2), 159–169.
- Osborne R. and Freyberg P., (1985), Learning in science: the implication of children's science, Auckland, New Zealand: Heinemann.
- Özmen H., (2007), The effectiveness of conceptual change texts in remediating high school students' alternative conceptions concerning chemical equilibrium, Asia Pacif. Educ. Rev., 8(3), 413–425.
- Palmer D., (2001), Students' alternative conceptions and scientifically acceptable conceptions about gravity, Int. J. Sci. Educ., 23(7), 691–706.
- Palmer D., (2003), Investigating the relationship between refutational text and conceptual change. Sci. Educ., 87, 663–684.
- Pımthong P., Yutakom N., Roadrangka V., Sanguanruang S., Cowıe B. and Cooper B., (2012), Teaching and learning about matter in grade 6 classrooms: a conceptual change approach, Int. J. Sci. Math. Educ., 10(1), 121–137.
- Posner G. J., Strike K. A., Hewson P. W. and Gertzog W. A., (1982), Accommodation of a scientific conception: toward a theory of conceptual change, Sci. Educ., 66, 211–227.
- Ratcliffe M., (2002), What's difficult about A-level chemistry, Educ. Chem., 39(3), 76–80.
- Robson L. S., Shannon H. S., Goldenhar L. M. and Hale A. R., (2001), Guide to evaluating the effectiveness of strategies for preventing work injuries: how to show whether a safety intervention really works. Columbia: DHHS (NIOSH) Publication.
- Rushton G. T., Hardy R. C., Gwaltney K. P. and Lewis S. E., (2008), Alternative conceptions of organic chemistry topics among fourth year chemistry students, Chem. Educ. Res. Pract., 9, 122–130.
- Schmidt H. J., (1997), Students' misconceptions-looking for a pattern, Sci. Educ., 81(2), 123–135.
- Sewell A., (2002), Constructivism and student misconceptions, Austral. Sci. Teach. J., 48(4), 24–28.
- Sendur G., (2012), Prospective Science Teachers' Misconceptions in Organic Chemistry: The Case of Alkenes (Turkish Title: Fen bilgisi öğretmen adaylarinin organik kimyadaki kavram yanilgilari: alkenler örneği), J. Turkish Sci. Educ., 9(3), 160–185.
- Solomons G. T. W., (1988), Organic Chemistry (fourth edn), USA: John Wiley & Sons.
- Taber K., (2000), Chemistry lessons for universities?: A review of constructivist ideas, Uni. Chem. Educ., 4(2), 63–72.
- Tao P. and Gunstone R., (1999), The process of conceptual change in force and motion during computer-supported physics instruction, J. Res. Sci. Teach., 36, 859–882.
- Tarhan L. and Acar Sesen B., (2012), Jigsaw cooperative learning: acid–base theories. Chem. Educ. Res. Pract., 13, 307–313.
- Taştan Ö., Yalçınkaya E. and Boz Y., (2008), Effectiveness of Conceptual Change Text-oriented Instruction on Students' Understanding of Energy in Chemical Reactions, J. Sci. Educ. Technol., 17, 444–453.
- Tsaparlis G. and Papaphotis G., (2009), High-school students' conceptual difficulties and attempts at conceptual change: the case of basic quantum chemical concepts, Int. J. Sci. Educ., 31(7), 895-930.
- Wellington J. and Osborne J., (2001), Language and literacy in science education, Buckingham, UK: Open University Press.
- Westbrook S. L. and Marek E. A., (1991), A cross-age of student understanding of the concept of diffusion, J. Res. Sci. Teach., 28(8), 649–660.
- UCLES, (2010), Cambridge International Advanced and Advanced Subsidiary Level 9701 Chemistry November 2010 Principal Examiner Report for Teachers. Retrieved February 15, 2012 from. http://www.cie.org.uk/qualifications/academic/uppersec/alevel/subject?assdef_id=736.
- Ünal S., Coştu B. and Ayas A., (2010), Secondary school students' misconceptions of covalent bonding, J. Turkish Sci. Educ., 7(2), 3–29.
- Uzuntiryaki E. and Geban O., (2005), Effect of conceptual change approach accompanied with concept mapping on understanding of solution concepts, Instruct. Sci., 33, 311–339.
- Yürük N., (2007), The effect of supplementing instruction with conceptual change texts on students' conceptions of electrochemical cells, J. Sci. Educ. Technol., 16, 515–523.
- Yürük N. and Geban Ö., (2001), Conceptual change text: a supplementary material to facilitate conceptual change in electrochemical cell concepts. Paper presented at The Annual Meeting of The National Association for Research in Science Teaching, St. Louis, MO.
| This journal is © The Royal Society of Chemistry 2013 |