Students' understanding of the nature of matter and chemical reactions – a longitudinal study of conceptual restructuring
Anne Bergliot Øyehaug* and
Anne Holt
Faculty of Education and Natural Sciences, Hedmark University College, Norway. E-mail: anne.oyehaug@hihm.no; Fax: +47 6251 7601; Tel: +47 5271 7873
First published on 6th June 2013
Abstract
This longitudinal study aims to provide greater insight into how students' understanding of matter and chemical reactions develops over time and how their knowledge structures are restructured. Four case-study students in a Norwegian primary school were followed for two years from age 10–11 to age 12–13. Researchers were responsible for implementation of science teaching promoting systematic development of students' understanding of the nature of matter and chemical reactions in many contexts across science disciplines. The four case-study students' expressed understanding was recorded and analyzed throughout the period. Results indicate that students develop fragmented and incomplete understanding, and drawing wrong conclusions may be necessary steps in the learning process. Moreover, students seem to develop a somewhat more integrated and cohesive understanding of the nature of matter and chemical reactions, indicating that the students restructure and reorganize their knowledge structures (i.e. differentiation, coalescence and promoting).
Introduction
Several groups have conducted longitudinal research investigating how students conceive the particulate nature of matter and how their conceptual learning of these concepts progresses in a school environment (Johnson, 1998; Smith et al., 2006; Stevens et al., 2007; Adbo and Taber, 2009). Research shows that students who learn about atomic and molecular theory do it gradually (Arzi, 1988; Johnson, 2000, 2002). It takes a long time and great intellectual effort to build such abstract understanding. Meheut and Chomat (1990) found that if the curriculum and instruction focus on the essentials of atomic and molecular theory, students can use this to explain macroscopic properties. They suggest that students often change a whole set of concepts as they learn about the atomic and molecular theory. In addition, it seems to take a long time and great intellectual effort to build an understanding of matter at macroscopic and sub-microscopic levels. Some studies have taken a special interest in the longitudinal perspectives of building an understanding of matter. Based on a study on the properties of substances, Eskilsson and Helldén (2003) recommend introducing basic models in early science instruction, using students' own ideas when talking about gases and everyday phenomena that deal with changes of substances. Johnson (2002) found that atomic and molecular theory is central for students to gain an understanding of chemical changes. Thus it seems as if having sub-microscopic ideas is essential for developing a deep understanding of the nature of matter and chemical changes, and that conceptual change is gradual. diSessa et al. (2004) and Hunt and Minstrell (1994) have also suggested that conceptual change is gradual and that students in addition hold intuitive ideas alongside instructed ideas.The present study aims to provide greater insight into how students' understanding of matter and chemical reactions develops over time and to what extent their knowledge structures can be characterized as knowledge-as-elements. Research questions in this study are:
How do students' conceptions of matter and chemical reactions develop when students are introduced to these concepts in different contexts and in increasingly complex ways? What characterizes students' understanding in different phases of their actual learning trajectory?
A theoretical framework for conceptual restructuring
The analysis and discussion in this paper are conducted primarily from a so-called conceptual restructuring perspective, but considers ideas from additional perspectives as well. The term conceptual restructuring relates most directly to knowledge-as-elements perspectives. Knowledge-as-elements perspectives characterize students' understanding in terms of collections of multiple quasi-independent elements (diSessa, 1993, 2006; Linn et al., 2004; Clark, 2006). However, more distantly, conceptual restructuring has roots in early work about conceptual ecologies (Posner et al., 1982; Strike and Posner, 1992). Strike and Posner (1992) suggested that students maintain conceptual ecologies, including “anomalies, analogies, metaphors, epistemological beliefs, metaphysical beliefs, knowledge from other areas of inquiry, and knowledge of competing conceptions”. This conceptual perspective is especially relevant to the perspectives that view a student's knowledge structures as a collection of independent elements (diSessa, 1993, 2006; Hunt and Minstrell, 1994; Linn et al., 2004). diSessa's (1993) perspective is the most renowned of knowledge-as-elements perspectives. He suggests that the knowledge structures of novices consist primarily of unstructured collections of many simple elements that he calls p-prims (phenomenological primitives). Linn et al. (2004) focus on the process through which students reorganize, revise, and connect these elements.In the current study we use the term conceptual restructuring for students' conceptual change. This implies that students potentially hold multiple conceptual elements at various levels of connection, contradiction, and organization. These elements and ideas are considered to include but not be limited to nominal facts, experiences, intuitive conceptions such as p-prims (diSessa, 1993), some mental models and concepts (Carey, 2000) at various stages of development and sophistication (diSessa, 1993, 2006; Linn et al., 2004).
Learning about sub-microscopic ideas
From the knowledge-as-elements perspectives it is assumed that understanding is built up over a long period of time, by the collection and coordination of knowledge and experience in many different contexts (diSessa, 2006). According to diSessa, there seems to be a need for studies evaluating processes where a conceptual change takes place over an extended period of time. The current study examines students' longitudinal conceptual change concerning the nature of matter and chemical reactions on both macroscopic and sub-microscopic levels. Students' ideas of sub-microscopic entities are of particular interest. There are several examples of other studies that have examined students' ideas about macroscopic entities. For example, it is well documented that students' ideas about macroscopic entities, especially in classical mechanics, are often context-sensitive (Viennot, 1985; diSessa, 1988, 1993) and that students seldom perform consistently in different contexts (diSessa et al., 2004). These studies focus on fragmentation and context dependence of students' knowledge of physics, in situations using their own experiences on the macroscopic level.However, students who learn about phenomena which can only be deeply understood in terms of sub-microscopic ideas will not bring along their own experiences from daily life to the same extent when they explain for example chemical reactions by means of atomic and molecular theory. Teichert and colleagues (2008) found that although students were able to represent NaCl dissolved in water as separate ions in a conductivity context, they were less able to do the same just a few minutes later in a boiling point context. Thus it appears that the students' ability to connect sub-microscopic ideas (separate Na+ and Cl− ions dissolved in water) to macroscopic phenomena (conductivity and boiling point) is context dependent. A related issue would then be how students learn about these phenomena in a longitudinal perspective.
Clark (2006) conducted a longitudinal study of conceptual learning in a related theme, thermodynamics. He suggests that students' understanding can be explained through a shifting nature of conceptions, and focuses on the longitudinal processes of change. The results clarify the conceptual change processes through which students' understanding of thermal equilibrium evolves from incoherent sets of context dependent ideas toward integrated cohesive perspectives. A key question is if learning about matter and chemical reactions on both macroscopic and sub-microscopic levels in a longitudinal perspective can be characterized in a similar way.
Understanding matter on a sub-microscopic level (atomic and molecular theory) is central for the perception of many phenomena across science disciplines; it explains a wide range of phenomena and serves as a building block in the development of scientific understanding (Duschl and Grandy, 2007). In addition, atomic and molecular theory can be understood in more sophisticated ways as students obtain increased cognitive understanding and experience phenomena in different contexts and representations (Smith et al., 2006).
In order to investigate how knowledge acquisition in matter and chemical reactions happens in a longitudinal perspective, results from research on how children learn about the nature of matter and chemical reactions (Johnson, 2002; Clark, 2006; Adbo and Taber, 2009) were applied in the instruction in the current study. Thus the lesson plans were guided by research on how children learn and recommended learning progressions for matter and molecular theory (Smith et al., 2006). In addition, the plans were adapted to the Norwegian National Curriculum in Science.
Nature of matter and chemical reactions in the Norwegian Science Curriculum
Table 1 shows how the Norwegian National Curriculum (Utdanningsdirektoratet., s.a.) includes ideas of matter and atomic and molecular theory in the first seven years of schooling. Smith et al. (2006) recommend that students experience matter in various contexts before they are introduced to atomic and molecular theory. For example, students should observe, measure and discuss the idea that matter does not change even though a substance changes shape or breaks down into smaller parts. On the basis of the Norwegian curriculum this seems to be feasible.| Year and age | Learning outcome |
|---|---|
| Year 1–2 (age 6–8) | Students should be able to sort the various substances by easily observable characteristics and talk about the characteristics (M) |
| Year 3–4 (age 8–10) | Students should be able to carry out experiments that show that matter can change character under different conditions (M and C) |
| Year 5–7 (age 10–13) | Students should be able to |
| • describe the key properties of gases, liquids, solids and phase transitions by using the particle model of matter (M) • explain how matter is built and how matter can change by using the concepts of atoms and molecules (M and C) • carry out experiments with chemical reactions and explain the characteristics of these reactions (C) |
|
| Learning outcomes in which ideas about matter at the sub-microscopic level will be helpful | |
| • carry out experiments with sound, hearing and noise, describe and explain the results and how to protect against unwanted sound • elaborate on how one has used transfer of motion to utilize energy in wind and water in different times • carry out experiments with magnetism and electricity, describe and explain the results • describe the most important organs in the human body and their functions |
At the age of 10–13, students should be introduced to matter at the sub-microscopic level according to the curriculum. Students are supposed to ‘describe the key properties of gases, liquids, solids and phase transitions by using the particle model of matter’. No distinction between atoms and molecules is made and the generic term particle is used. Later, students are supposed to expand their sub-microscopic ideas and be able to ‘explain how matter is built and how matter can change by using the concepts of atoms and molecules’. In addition, students should be able to ‘carry out experiments with chemical reactions and explain the characteristics of these reactions’. This description of the expected learning outcome does not explicitly say whether the explanations should be on a macroscopic or a sub-microscopic level. In order to give students a basic understanding of chemical reactions, it can be argued that students should understand that new substances with new properties are formed from the original substances. Moreover, they should be able to understand that bonds in the molecules of the original substance(s) are broken and that new bonds and new molecules are formed.
Method
A longitudinal study
Four students' conceptual learning about matter and chemical reactions was examined over time. The study was performed in a small, rural primary school in Norway, following students for two years (from the age 10–11 to age 12–13). During this two-year period, the students learned about matter and/or chemical reactions in four instructional periods. Each period consisted of five to six full-days of science. Systematic development of students' understanding was emphasized. In other words, the teaching was influenced by characteristics of the so-called learning progressions. Table 2 outlines how important aspects of matter and chemical change were emphasized in successively more complex ways during the four instruction periods. In addition, the table shows how data were collected to record the students' expressed understanding over time.| Period (student age) | Theme | Matter (M) and chemical reactions (C) content (and instructional methods) | Data collection |
|---|---|---|---|
| Period 1 Spring year 5, Autumn year 6 (age 10–11) |
Properties of matter | Air is something, weigh something and occupy space (experiments, dialogues) (M) Gases, liquids and solids consist of particles. Bonds between particles are changed during phase transitions. The higher the temperature the faster the particles move. Air pressure is due to the collective impact of the air particles (role play, experiments, dialogues and scientific inquiry) (M). |
Interview with focus students Videotapes from instruction Student work/tests |
| Period 2 Spring year 6 (age 11–12) |
Photosynthesis | Particles are atoms and molecules (dialogues, molecular kit) (M) In photosynthesis molecules react with each other and form new ones (experiments, dialogues, animations, student presentations and scientific inquiry) (C) |
Interview with focus students Videotapes from instruction Student work/tests |
| Period 3 Autumn year 7 (age 12) |
Sound Energy Electricity |
There are particles in gases and solids and these are important for sound transmission and energy transfer. Matter conducts electric current (electrons) to varying degrees (experiments, dialogues, role plays and student presentations) (M) | Interview with focus students Videotapes from instruction Student work/tests |
| Period 4 (Spring year 7) (age 12–13) |
Chemical reactions in general and in combustion reactions in particular | In chemical reactions new substances with new properties are made. Bonds between atoms in molecules are broken and new ones are formed (demonstrations, animations and concept maps) (C) Oxygen molecules are always included in combustion reactions, both with metals and in cell metabolism (experiments, dialogues, animations, poster and scientific inquiry and role-play) (C) |
Interview with focus students Videotapes from instruction Poster Student work/tests |
| Final interview Spring year 7 (age 12–13) |
Nature of matter Chemical reactions |
Case 1: the water cycle (M) Case 2: the chemical reaction involved when wood is burning (M and C) Case 3: the effect of blood doping on a person's circulatory system (M and C) Case 4: formation and decomposition of blueberries (M and C) |
Interview with focus students |
Central concepts related to matter and chemical change (for instance the concept of particles) were revisited and refined during the two years study. In Period 1 the students participated in different experiments with the substances air and water. They were introduced to the idea that air, water and ice consist of particles. In addition, they conducted a study on the difference between intake and output of fluid in their own body. The students measured volume, and were set to interpret the difference in volume using the concept of evaporation. In the end of this period, the students were expected to be able to describe the characteristics of gases, solids and liquids by using the particle model. In addition, they should be able to explain phase transitions in terms of the particle model.
Period 2 was also characterized by experimental activities. First of all, students were introduced to particles as atoms and molecules. Moreover, students interpreted the features of flowering plants by applying key concepts related to photosynthesis. They were expected to be able to describe the difference between atoms and molecules, and that molecules react with each other and form new ones – in photosynthesis. Details about the breaking and making of bonds between atoms were not emphasized. The students were introduced to one particular chemical reaction (photosynthesis), without focusing on the characteristics of chemical reactions in general.
In period 3 the students participated in smaller experiments related to sound, energy and electricity. They were expected to explain sound transmission, energy transfer and why matter conducts electric current to varying degrees in terms of how the particles in different substances and states of matter are organized.
In period 4 students were expected to characterize chemical reactions as a process in which new substances with new properties are made and bonds between atoms in molecules are broken and new ones are formed. In addition, they learned about the circulatory and respiratory system and conducted an inquiry of the circulatory system in humans or animals. In the research report, they explained the chemical reaction of cell metabolism. The students participated in small experiments related to burning of metals, and were expected to make the inference that oxygen molecules are always included in combustion reactions (both in cell metabolism and burning of metals). Moreover, the chemist Antoine Lavoisier appeared in the classroom (travelling by time machine), telling students about his own experiments and important findings a long time ago.
Thus, students should be able to apply central concepts related to matter and chemical reactions in various subjects and in different situations. Instruction aimed to engage students in various ways, through experiments, scientific inquiry, dialogues in plenary, role play, use of models and animations as well as in writing and drawing activities. Important concepts were taught in different contexts in each instructional period. Moreover, these four periods were the only teaching periods in the two years during which students were exposed to content related to matter and chemical reactions.
Researchers and the teacher cooperated in planning for instruction, researchers being responsible for progression and application of central concepts in different contexts in accordance with the Norwegian curriculum and the teacher for the implementation of these principles. Lesson plans were developed in detail to ensure these characteristics. Researchers had a withdrawn role in the classroom, mainly as observers.
Data collection
The main data in this study consist of video tape recordings from group interviews (two groups with three students in each). All interviews were transcribed. Excerpts from two girls and two boys (two from each group) relating to matter and chemical reactions were then coded and analysed. The four were chosen because they represented four different student types. The two boys (Thomas and Martin) were both participating actively in classroom discussions, preferring dialogues and experiments rather than written work. However, one of them (Thomas) performed higher than the other. One of the girls (Anna) was a high performing student; moreover, she was willingly participating in discussions, experiments and written work. The other girl (Elisabeth) was seldom participating in classroom discussions, and seemed to like reading better than the others. Her performance was somewhat below average for the student group. According to information from students' performance on science tests, the four performed at different levels. In general, Thomas and Anna scored on a high level, Elisabeth on a medium level and Martin on a low level.The four students were interviewed by the end of each instruction period. These interviews (post-interviews) aimed to reveal the development of understanding of matter and chemical reactions of the four students. After the first period the students were interviewed about gases, solids and liquids and after the second period about atoms, molecules and photosynthesis. At the end of the third period students were interviewed about sound, energy and electricity and after the fourth period about chemical reactions in general and about burning of metals and cell metabolism in particular. Students were challenged to apply their knowledge by discussing the above-mentioned scientific issues in various contexts.
In addition, the data consist of about 40 hours video tape recordings from instruction and a variety of written student work (see Table 2). All classroom dialogues and a selection of group discussions were videotaped. Video recordings, student work and tests were transcribed, and excerpts from the four case-study students regarding matter and chemical reactions were coded and analysed for all periods. The excerpts were then translated into English, implying that quotes will not be verbatim. In addition, translation is an opportunity for varying interpretations of data. To counter this, we tried to ensure the original meaning of student's utterances.
By the end of the longitudinal study 15 students including the four case-study students were interviewed individually. The final interview was conducted late in the spring term in year 7 (age 13), three weeks after the fourth instruction period about chemical reactions in general and in living organisms was completed. A semi-structured interview guide outlined in Appendix A was used. This interview guide was tested in a pilot study on a group of students who had not been a part of this intervention study. In the interview students were asked questions in four different cases (phase transitions, burning of a match, blood doping and formation and decomposition of blueberries). In each case, students were introduced to various artifacts (photos, texts, and a burning match).
The intention was that students would apply their understanding of matter and chemical reactions in the four cases. Thus students were challenged to apply their knowledge in contexts they had not met in previous school settings. If the student gave no answer, the interviewer should continue with more specific follow-up questions described in detail in the interview guide. The intention was that the dialogue should be a supportive scaffolding for the student during the interview (Vygotsky and Cole, 1978). The interviewer contributed to bring forth students' knowledge. In addition, the students could be asked follow-up questions in order to clarify the meaning of their statements, for instance to get the student to express his/her meaning of the word ‘oxygen’. The interviewer also consciously avoided referring to situations during instruction, in which the students had participated. In addition, student statements were not evaluated and the correct answers were not given. Longer sequences of student utterances were coded to extract their conceptual understanding.
Categorization of students' expressed understanding
Table 3 shows the categories that were developed to determine the level of expressed understanding of matter and chemical reactions (Level 1–4).| Level | Understanding of matter and chemical reactions | |
|---|---|---|
| 1 | No understanding | Macro and sub-micro |
| 2 | Some understanding | Macro – no ability to link sub-microscopic and macroscopic ideas |
| Sub-micro – limited ability to link sub-microscopic and macroscopic ideas | ||
| 3 | Good understanding | Macro – limited ability to link sub-microscopic and macroscopic ideas |
| Sub-micro – limited ability to link sub-microscopic and macroscopic ideas | ||
| 4 | Good understanding | Macro and sub-micro – ability to link sub-microscopic and macroscopic ideas (needs scaffolding) |
| Macro and sub-micro – ability to link sub-microscopic and macroscopic ideas (needs no scaffolding) | ||
Level 1–4 implies an increasing level of sophistication in students' understanding of matter and chemical reactions, from vague, fragmented and incorrect ideas towards a more coherent, theory-like perspective. A key step in the development of students' understanding of particle theory is the move from viewing the particles as small pieces carrying the macroscopic properties to understanding that macroscopic properties emerge from the collective behaviour of the particles. This perspective is taken into consideration in the level descriptions.
The levels of understanding are defined as follows: no understanding at either the macroscopic or the sub-microscopic level is labelled Level 1. Some macroscopic understanding with no ability to link this to sub-microscopic ideas is labelled 1,5. Furthermore, some sub-microscopic understanding with limited ability to link macroscopic and sub-microscopic ideas is labelled Level 2. The student may for instance have the incorrect view of particles as small pieces carrying macroscopic properties (i.e., one single air particle gets higher/lower temperature or changes its volume). Further, good macroscopic understanding, but with limited ability to link macroscopic and sub-microscopic ideas, is labelled 2,5. The student shows through his/her use of concepts such as temperature, pressure, mass, volume and change of substances that (s)he has a clear macroscopic understanding. Moreover, good sub-microscopic understanding, but with limited ability to link macroscopic and sub-microscopic ideas, is labelled Level 3. In this case good sub-microscopic understanding means that the student expresses a clear understanding of matter consisting of smaller units and how these smaller units move and are inter-connected in different states of matter and how bonds between atoms are broken and formed in chemical reactions. In addition, the next two levels require student understanding of macroscopic properties emerging from the collective behaviour of the particles. The levels are graded by how much scaffolding the student needs to complete the reasoning. Good macroscopic and sub-microscopic understanding and the ability (under guidance) to link sub-microscopic and macroscopic ideas is labelled 3,5. Ultimately, good macroscopic and sub-microscopic understanding with the ability to link relevant sub-microscopic ideas and macroscopic ideas on an independent basis without any support is labelled Level 4. A more detailed level-description with examples is given in Appendix B.
Level descriptions shown in Table 3 were developed after the interview guide had been tested in a pilot study. For instance, utterances from the pilot study were helpful in describing the low levels, because these students' ideas about matter often indicated little or fragmented understanding for sub-microscopic entities, i.e. ideas which were less common among the case-study students. Moreover, level descriptions were guided by the proposed learning progression by Smith et al. (2006) and by the prescribed learning outcomes in the Norwegian National Curriculum (Utdanningsdirektoratet., s.a.).
Student expressions including imprecise explanations and descriptions could be coded at high levels. This would be the case when students explain cell metabolism using imprecise energy considerations, since the more correct details about energy transfer in this chemical process were not emphasized during instruction. Students' expressed understanding of matter and chemical reactions was analyzed in Transana, a computer program for analyzing digitalized video and audio files. Early in the analysing process, certain portions of the data (10%) were coded independently by two persons. The coding largely coincided, but variations in coding were discussed and adjusted.
The analysis was carried out in several steps. First, the sequences where the students' expressed understanding of matter and chemical reactions were identified. These sequences were defined and coded as one of the two main categories matter or chemical reaction. Furthermore, the sequences were coded by the level of expressed understanding. Levels of understanding were set on basis of the student’s overall utterances in each interview or case.
Analysis of results
Fig. 1–4 show the four students' level of understanding of matter (●) and chemical reactions (▲). Each data point shows the student's highest expressed level of understanding in interviews (by the end of each instruction period and in each of the four cases in the final interview). | ||
| Fig. 1 Thomas' expressed understanding of matter (●) and chemical reactions (▲) in interviews through the two-year period. | ||
 | ||
| Fig. 2 Anna's expressed understanding of matter (●) and chemical reactions (▲) in interviews through the two-year period. | ||
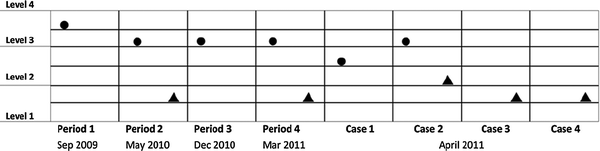 | ||
| Fig. 3 Martin's expressed understanding of matter (●) and chemical reactions (▲) in interviews through the two-year period. | ||
 | ||
| Fig. 4 Elisabeth's expressed understanding of matter (●) and chemical reactions (▲) in interviews through the two-year period. | ||
Learning trajectories for the four students
This section will present excerpts from the four students subsequently, providing the basis for data shown in Fig. 1–4. Excerpts and description of critical episodes from instruction are included to illuminate students' reasoning in the interviews.Thomas' conceptions of matter
In period 1 the students were introduced to gases, liquids and solids (see Table 1). In contrast to the other three students, Thomas reasoned exclusively on a macroscopic level in the post interview. He answered in the following way when he was asked to choose between different claims about the ability of compressing air and water:Uh.. I have a theory here. I believe the girl who thinks it is the air (that can be compressed). For the air – when we took the syringe – we thought it was easier to put together. Water it was so hard and solid – and if you put a rock in a syringe it will be destroyed.
He offered no explanation on the sub-microscopic level, although he expressed understanding of matter as particles in the classroom setting before the interview took place. For instance, Thomas reasoned about empty spaces between particles in liquids. The students had just filled a syringe with water, and discovered that it was not possible to get the pressure piston further down in contrast to an air-filled syringe. The following excerpt shows Thomas in dialogue with another student and the teacher about whether there is empty space between particles in liquids:
Other student: For you cannot press water together, and then there is no, when we press, nowhere the water can go and then there can't be space between the water particles.
Teacher: Do we have any other suggestions here?
Thomas: Yes. We cannot press them (water particles) together… There are gaps and inside there is oxygen and there are very small gaps (between the water particles)
This discussion started because a student claimed that there had to be empty space between the water particles, wherein oxygen particles could hide. Later, in a dialogue with the teacher, Thomas reconfirmed his sub-microscopic understanding, by describing particles in fluids as moving spheres.
In period 2, the students were introduced to concepts of molecules and atoms in the context of photosynthesis. In the post-interview students were not directly asked about properties of matter. Thomas did not express understanding of matter on his own initiative. Therefore data are missing for this period (Fig. 1). However, during instruction, Thomas used the terms atom and molecule in a dialogue with the teacher about water molecules.
In period 3 the students applied the atomic and molecular theory in contexts related to the topics sound, energy and electricity. In the post-interview, Thomas participated in a discussion about why curtains above an oven are moving. He explained this by particles moving faster because of the heat from the oven, indicating an ability to distinguish between individual particle movement and the bulk movement of batches of particles (convection currents).
Chemical reactions in general and combustion reactions in particular were introduced in period 4. All four students expressed understanding of matter at high levels of understanding in the post-interview (Fig. 1–4). The term particle is now replaced by concepts like molecule, atom and the symbol H2O. In the post-interview, Thomas used the term molecule when he explained what happens when sugar is mixed with water. He claimed that ‘…sugar is hiding between the molecules of water’ and that ‘…the volume is approximately the same, just a little bit more.’ Thus, Thomas is able to make links between the macroscopic property volume and sub-microscopic ideas.
Thomas expressed overall good understanding of matter in the final interview. For instance, he was asked about the water cycle and answered in this way:
Thomas: The bonds break up […] or the bonds will be more apart […] in the ocean there are […] many H2O molecules bond together to form water. And as they fly up their bonds get looser. When they evaporate their bonds get looser. […] The molecules do not change; there will be no chemical reaction, right. They are exactly the same.
Interviewer: And what is changing then?
Thomas: Water level, consistency, clouds
Here, Thomas expressed a deep understanding of matter since he responded by linking macroscopic properties such as evaporation and sub-microscopic ideas such as bonds become looser but molecules remain the same.
Furthermore, in an explanation of what happens when a match burns, Thomas expressed understanding of matter in the following way:
Thomas: Glucose is changed; […] air will be changed for now it's CO2 instead of O2 […]
Interviewer: What was the formula for glucose?
Thomas: C6H12O6 […]
Interviewer: Is there anything that is the same here? […] Do the atoms change?
Thomas: Yes, they change … no atoms do not change they stay the same.
In comparison, he claimed that ‘Molecules change from oxygen and glucose to carbon dioxide and water’ when he was asked about cell metabolism in the same case. Furthermore, he said this about photosynthesis:
To make blueberry a berry […] carbon dioxide, water and sunshine are needed. Inside the plant in leafs, glucose is formed. This glucose is transformed to berries … or formed glucose that enters the berries so that it becomes large… and out comes oxygen
In this example, which is representative of Thomas' level of understanding when he talked about photosynthesis, he expressed himself mainly on a macroscopic level and thus showed limited ability to link macroscopic properties to sub-microscopic ideas.
Thomas' conceptions of chemical reactions
The topic chemical reaction was introduced in period 2. To get some understanding of chemical reactions, students learned about substances needed and formed during photosynthesis. In the interview after this instructional period, Thomas succeeded to relate the weight gain of the plant to the contribution of CO2 molecules from the air, though with scaffolding questions from the interviewer. In other words, he was able to link the macroscopic property, weight, to the sub-microscopic idea of the atoms having a definite mass that adds up.The topic chemical reactions was repeated and elaborated in period 4. The emphasis was on the characteristics of chemical reactions, both from a macroscopic (change of matter) and sub-microscopic perspective (bonds between molecules are broken and new ones are formed). As mentioned earlier, students learned about combustion reactions (of metals and organic materials) and cellular respiration. For instance, the students were introduced to the combustion of magnesium and steel wool (iron) as examples of chemical reactions. In the post-interview, Thomas discussed the reaction between magnesium and oxygen in the following way:
Interviewer: Do you remember what happened when the magnesium was burning?
Thomas: It flashed and then the oxygen was added to magnesium so that it became magnesium oxide.
In the above excerpt, Thomas expressed his understanding only at the macroscopic level. It does not mean that he is not able to explain at sub-microscopic level, but it may reflect that he considered the response to be satisfactory in the present context. In the same interview, Thomas explained cell metabolism in this way:
Interviewer: What happens when this gets to the cell: oxygen and glucose?
Thomas: (…) yes oxygen… uh… Then the bonds will be broken and bind to new ones as CO2 and H2O
In a classroom dialogue prior to the interview, Thomas expressed his understanding of the chemical reaction involved when steel wool burns in this way:
Thomas: When you set fire to steel wool more atoms will be added to the molecules of steel wool, several atoms come in there and it becomes heavier.
Teacher: And what kind of other atoms do you think that came in there?
Thomas: Oxygen […]
In a post-test Thomas answered that rust is heavier than iron, which is the opposite of what he said in the pretest before period 4. It appears that he expanded and refined his understanding of chemical reactions during this period.
In the final interview, the students expressed their understanding of chemical reactions in case 2 (the chemical reaction involved when burning wood), case 3 (the effect of blood doping on a person's circulatory system) and case 4 (formation and decomposition of blueberries). In case 2, Thomas showed clear understanding of how matter changes in the reaction on a macroscopic level as well as on a sub-microscopic level. He moved without difficulty between these two representations (for instance he talked about the air we breathe in, and that the air around changes because of increasing amount of CO2 molecules). He needed scaffolding questions from the interviewer to realize that atoms are unchanged in the certain chemical reaction. In the same case, Thomas was asked what he thinks happens to the weight of a burning match:
Thomas: I think it becomes heavier
Interviewer: Because…?
Thomas: It takes in oxygen
Interviewer: But what did you say happened to the oxygen?
Thomas: It goes into the match and CO2 goes out of it …
In this situation it seems like Thomas generalized about combustion reactions resulting in a wrong conclusion. He argued that the match will be heavier because the oxygen atoms add to the wood in the match. Profound understanding of combustion of wood is complex. It involves keeping hold of phase transitions as well as a multiple step chemical process, details of which are beyond what can be expected from students in this age-group. It may seem as if Thomas transferred his experience with combustion of metals (Mg and Fe) to the combustion of organic materials.
In case 3, Thomas had a clear understanding on the macroscopic as well as on the sub-microscopic level of how matter changes in cell metabolism. He showed the ability to link sub-microscopic ideas to macroscopic ideas of the actual chemical reactions. Thomas claimed this about why blood doping has a positive effect on a person's performance:
[…] It (the body) is doped so that it will increase the number of red blood cells do so you can transport more glucose and oxygen to the cells… so that they can work at a higher level […] Without the heart having to pump too much
In case 4, when Thomas was asked about photosynthesis, he only answered on a macroscopic level. He knew what was needed and produced, but gave no explanations about breaking and making of bonds and did not use the terms atoms and molecules.
Anna's conceptions of matter
Anna showed the ability to link sub-microscopic and macroscopic ideas about matter in the post-interview after period 1. During a classroom experiment in this period, Anna explained that ‘particles move faster and hit harder, making pressure when they are warmed up’. However, when talking about perfume in the air, she stated that ‘there are particles, and when you squirt, the perfume will attach to the particles, and they collide all the time… so then it will spread’. In the first situation Anna showed a clear understanding of matter consisting of smaller units. At the same time she used the concept of pressure and showed the ability to link sub-microscopic ideas to this macroscopic property. However, in the latter situation she revealed an unclear idea about what can be considered as particles.In the post-interview of period 2, Anna claimed that plants take up air from the water. In this context, which considered how water plants perform photosynthesis, she was not able to identify different components of air which could be elements in photosynthesis. During instruction in the same period, she described particles in the stem as wet molecules. When Anna was asked a follow-up question about the structure of wet molecules, she replied that it consists of hydrogen and oxygen molecules. In this situation she mixed up concepts of atoms and molecules.
However, in the post-interview after period 3, Anna expressed understanding of matter on a high level. For example, in a discussion about why curtains above an oven are moving, she suggested that the heat from the oven made the particles move faster.
In the final interview, Anna expressed overall good understanding of matter. In case 1 and 2, she linked macroscopic properties to sub-microscopic ideas. When talking about evaporation, Anna expressed a deep understanding of matter since she (similar to Thomas) responded by linking macroscopic properties such as evaporation and sub-microscopic ideas like bonds become looser and molecules remain the same. She responded this (with less elaboration than Thomas):
Uh. In water, then the bonds between molecules are a bit loose, and so when it evaporates the bindings go away and they pop around.
Anna did not talk about matter in case 3 and 4, and therefore these data are missing in Fig. 2.
Anna's conceptions of chemical reactions
Even though Anna often expressed good understanding of chemical reactions during instruction in period 2, she only mentioned some of the substances involved in photosynthesis, and showed little understanding of how matter changes in the reaction in the post-interview. An example of when she was asked to describe a model of photosynthesis is as follows:Anna: Do you want me to describe how photosynthesis happens?
Interviewer: Yes, and what are the different arrows […]?
Anna: This arrow is sun light. Uh… and so it is CO2 and water
Interviewer: What about this purple arrow?
Anna: It is… is it oxygen that leaves the plant? […] and sugar
In the post-interview by the end of period 4, Anna's level of expressed understanding of chemical reactions was still fairly low. For instance, when she was asked about what happened to the oxygen molecules during the burning of magnesium, she answered that ‘They (the oxygen molecules) moved between magnesium’. Furthermore, she guessed that the piece of magnesium would weigh less after it has burned. However, in prepared presentations (on posters and post-test) Anna managed to link sub-microscopic ideas about chemical reactions to macroscopic properties. In the post-test in period 4, one of the questions considered the weight of a 600 g heavy horse shoe after several years in the soil. Anna's answer was:
It weighs more that 600 g, because […] some of the air has penetrated into it and mixed with the horse shoe particles.
In the final interview, Anna expressed understanding of chemical reactions at a high level. In case 2, she gave this answer when she was asked what changes in the actual reaction (burning of a match):
Anna: Molecules and the outside of the match… […] It became carbon dioxide and water.
Interviewer: And what has happened to the glucose?
Anna: It has changed so… maybe it will become those molecules
However, with scaffolding questions referring to glucose (suggested by the student earlier in the dialogue), she realized that atoms are unchanged in this chemical reaction.
In case 3 Anna had, similar to Thomas, a clear understanding of how matter changes in cell metabolism on a macroscopic level as well as on a sub-microscopic level. The student showed the ability to link sub-microscopic ideas to macroscopic ideas of the actual chemical reactions. In case 3, Anna expressed an even more comprehensive understanding. She suggests this about why blood doping has a positive effect on a person's performance:
I think that if you get more access to… no get new blood and then you get more red blood cells and then they can carry eh… oxygen and glucose to the cells… and then we see more combustion and then you work better
She accounted for cell metabolism in a thorough way and she expressed the content independently.
In case 4, Anna continued to express understanding of chemical reactions on a high level. Anna for instance said:
Bonds are broken and then new bonds are formed and then the plant uses glucose itself and so it emits oxygen which can help us to inhale.
Again, she accounted for photosynthesis in a thorough way. In addition, she expresses the content independently. In case 3 and 4, the idea that molecules change and that atoms do not in chemical reactions was clear to Anna. Seemingly, she did not need the same degree of guidance as in case 2.
Martin's conceptions of matter
Similar to Anna, Martin showed the ability to link sub-microscopic and macroscopic ideas about matter in the post-interview after period 1. During a teaching sequence about particles, which took place in the gym, Martin expressed this understanding of the particle's movement:Teacher: Can you show how air particles move? You can show it with your body.
Martin (stands up, beckoning another boy and goes towards the big mattress): if we are here, then we walk like this all the time. If I crash into here (crashes with the big mattress, describing while performing), and walk like this (walking backwards)
This demonstration/dialogue took place after the students had experimented with air in syringes, dramatized how particles move inside a syringe, drawn models of the particles in air, observed a demonstration of how heating the air inside a balloon caused it to expand, and finally talked about all this in a plenary classroom session.
In the beginning of period 2, Martin responded to questions about the content of air and about the behaviour of particles in air. He expressed a clear understanding of matter consisting of smaller units and explained how these smaller units move in air in a similar way as he did in period 1.
In the post-interview after period 3, Martin responded in a similar way to Thomas and Anna in the discussion about why curtains above an oven are moving. In an oral presentation about energy transfer during instruction, where a paper spiral started moving because of heat from a lit candle, Martin explained that ‘energy hits the air…or air particles in the air, before it also goes up to the paper spiral that makes the particles to bounce around and collide so it will go around all the time’. The teacher did not use the term particle during instruction in period 3, but Martin chose to explain the phenomena (convection current) in terms of the bulk movement of batches of particles on own initiative in a new context. Martin used the term air particle, although he had been introduced to the concept of molecule and the symbolic names of the molecules in the air in the previous instruction period.
Similar to Thomas and Anna, Martin expressed good understanding of matter in the final interview. In case 1, Martin used concepts such as H2O, O2 and expressed understanding of matter consisting of smaller units. To some extent he could tell how the molecules move. As shown in the following excerpt Martin had no clear sub-microscopic idea of the details in the transition process of water from liquid to gas:
Interviewer: What does clouds consist of?
Martin: H2O
Interviewer: And what is H2O … and it is?
Martin: It is water […] Water molecule
Interviewer asked scaffolding questions, trying to help the student to reason about how water molecules in the lake ends up in the cloud:
Interviewer: And what happens when something evaporates? What happens to the water molecules?
Martin: Then they will move much more and replacing water molecules… or mix them a little more with O2
In addition, Martin showed limited ability to link sub-microscopic ideas to macroscopic properties of matter. However in case 2, Martin compared the size of different molecules, expressing sub-microscopic understanding. He did not talk about matter in case 3 and 4.
Martin's conceptions of chemical reactions
In the post-interview (period 2), Martin expressed understanding about photosynthesis in this way:Interviewer: What comes from the air into the plant?
Martin: Uh… oxygen into the plant…
Interviewer: Is it oxygen?
Martin: No, carbon dioxide goes into the plant, and it takes out oxygen.
This somewhat leading question (Is it oxygen?) made Martin change his mind, adding more information about reactants and products in photosynthesis. Moreover, he participated to a limited extent in oral presentations, and he expressed very limited understanding of what happens in photosynthesis in a post-test.
Martin expressed a vague understanding of what happens in chemical reactions in the post-interview after period 4:
Interviewer: What does the drawing show?
Martin: There are the lungs with pipelines up to the mouth and nose, and here oxygen and carbon dioxide and stuff go down here to the heart and so it strikes, then it comes to the cells, the cells gives away carbon dioxide to the heart… […]
Moreover, he expressed a low level of understanding of chemical reactions both during instruction and in the post-test. It seems like Martin did not understand the central features of chemical reactions.
In the final interview Martin mentioned some of the elements included in the chemical reaction (burning match, case 1), but showed only a vague understanding of how molecules change (he thought that the glucose molecule would be “broken”):
Interviewer: You said that something happened to the glucose molecules?
Martin: Yes, that it maybe splits
Interviewer: Yes, and what could you get from that?
Martin: Oxygen, hydrogen, water and…
In case 3, Martin mentioned some of the substances involved in cell metabolism, but showed little understanding of what is really going on in the chemical reaction at the sub-microscopic level. He answered questions about cell metabolism in this way:
Interviewer: Yes. And how did you get those energy packages (..)?
Martin: Glucose
Interviewer: And?
Martin: Oxygen… That stays in the cell, a little combustion of those things… and then it goes out again… as carbon dioxide
Martin also expressed a vague understanding of photosynthesis (case 4):
Interviewer: What does a plant need for living? (..)
Martin: Uh… Like this glucose… and water
Interviewer: Yes. Or?
Martin: … and oxygen
Interviewer: What does a plant need to survive?
Martin: Hydrogen…no not hydrogen but carbon dioxide
With scaffolding questions he was able to describe the elements needed and formed during photosynthesis, and in the end he said:
Martin: Then this will be… a chemical reaction or something, wouldn't it? A chemical reaction that one gets… makes a change in shape in a way… and gets bigger
In spite of fairly good conceptual understanding of matter, Martin showed limited understanding of chemical reactions. His explanations were imprecise, and it was often unclear whether he talked about macroscopic or sub-microscopic entities.
Elisabeth's conceptions of chemical matter
Similar to Anna and Martin, Elisabeth showed the ability to link sub-microscopic and macroscopic ideas about matter in the post-interview (period 1). She replied in this way when she was asked to evaluate different statements about compression of materials like air and water:If you have it in a syringe it is possible. So that if you seal the hole … there is pretty much in the syringe … particles … and then the particles cannot leave when you push them, they are pushed together and ultimately it is not possible to push anymore
During the interview, Elisabeth expressed contradictory ideas in two different, but comparable situations. First she talked about particles moving faster when air is warmed up, later she expressed what happens to particles in air when it gets colder in this way:
I think maybe the particles in air gets smaller when it gets colder
In contrast to the other students, Elisabeth only gave short answers on the macroscopic level to the question about air and the behaviour of the particles (post-interview, period 2).
In the post-interview (period 4), Elisabeth reasons about the solution of sugar molecules and water molecules in the following way:
Elisabeth: The sugar grains […]. They float, or go into the void of water molecules and put themselves between the gaps.
Interviewer: The volume becomes larger, but how much larger does it get?
Elisabeth: It gets a little less than twice as large […] Sugar molecules lie between gaps between water molecules […] It will not be as much as twice as large
Thus, Elisabeth is able to make links between the macroscopic property volume and sub-microscopic ideas.
In the final interview Elisabeth expressed understanding of concepts such as H2O, O2, particle and molecule, but showed limited ability to link sub-microscopic ideas to macroscopic properties of matter. Elisabeth had problems with answering questions about the water cycle (case 1). With thorough guidance she manages to link water particles' behavior in liquids to gases. After several scaffolding questions she concludes that water molecules in the gas phase move faster and are further apart than in the liquid phase. Elisabeth neither linked macroscopic properties to sub-microscopic ideas, nor described the molecules in air or in a match (case 2), even though she was asked several scaffolding questions about this. The contexts, water cycle and burning match, were new for the students, and it seemed like Elisabeth had problems with new contexts in general. In case 3 and 4 Elisabeth showed better understanding of matter (Fig. 4) than in the two first cases. In case 3, for instance, she described cell metabolism in this way:
Glucose enters while eating, and oxygen we breathe in. Firstly, this goes into the right and left atrium and then it goes to the heart chambers, then it is pumped out to the body to every cell. And then inside the cells the bonds are broken (…) and then new bonds will be made, and energy packages are made
Elisabeth's conceptions of chemical reactions
Elisabeth expressed limited understanding of chemical reactions in the post-interview (period 2). It is not clear whether she talked about macroscopic or sub-microscopic entities and she was not sure about what is needed and formed during photosynthesis. She answered this when she was asked what she knows about photosynthesis:Elisabeth: That it makes simple sugar
Interviewer: From what?
Elisabeth: From oxygen
In the post-interview (period 4), she answered in this way when she was asked about what happened to the oxygen molecules during burning of magnesium:
Elisabeth: It will turn to ashes […]
Interviewer: This piece of magnesium. What joined this piece… or?
Elisabeth: Warmth and oxygen
In spite of such a leading question (What joined this piece), she shows only a vague understanding of the principles of chemical reactions.
In the final interview (case 2), Elisabeth was not sure of the substances involved in the combustion reaction, but she knew it was a chemical reaction. However, she was aware that bonds within the molecules are broken and new molecules are formed, and therefore the atoms stay the same. She had a relatively good general understanding of the sub-microscopic level, but struggled to apply this in the example of the match and showed little ability to link macroscopic properties to sub-microscopic ideas.
Similar to Thomas and Anna, Elisabeth had a clear understanding of how matter changes in cell metabolism on a macroscopic level as well as on a sub-microscopic level in case 3. The student showed the ability to link sub-microscopic ideas to macroscopic ideas of the actual chemical reactions. Elisabeth answered this when she was asked about what will happen to glucose when it enters the cell:
The bonds will be broken and there are six oxygen molecules in addition. And so then they will make new bonds together
Furthermore, she replied in this way when she was asked about what happens with the atoms and molecules involved in photosynthesis (case 4):
They have changed shape, their bonds are broken and so they have made new molecules.
Both in case 3 and 4 it seems like Elisabeth transferred the ideas from case 2 that molecules change and that atoms do not in chemical reactions.
To sum up, the four case-study students followed different learning trajectories during the two-year period. Anna's and Elisabeth's level of understanding of chemical reactions increased whereas Thomas' and Martin's level of understanding was more steady (steady but high for Thomas and steady but low for Martin).
Discussion
The four students expressed a varying, but often deep and broad understanding of matter and chemical reactions. It seems like students' understanding of matter and chemical reactions has evolved towards being somewhat more integrated and cohesive, indicating that the students restructured and reorganized their knowledge structures during the two year period. However, the individual student's conceptual knowledge and actual learning trajectory was expressed in slightly different ways. In the following some important aspects of their conceptual restructuring will be discussed.Students' understanding of matter
Already during the first period and also throughout the study, the four students got an idea of matter consisting of moving particles. Similarly, they were in most situations able to assert this in the contexts of low and high temperatures. The students did not use these ideas consistently across all contexts (i.e. Elisabeth in case 1 and 2 in the final interview), but the quite rapid appearance of these ideas in students' explanations suggests that they readily added ideas about particles and temperature to their understanding. This quick appearance of new ideas is similar to what happened to some older students learning about metals as conductors (Clark, 2006). Moreover, when students learn about sub-microscopic ideas, they will not necessarily bring their own experiences from daily life. It is possible that this makes the learning process easier, because there are fewer underlying knowledge elements influencing these processes (Teichert et al., 2008).However, there are some examples of students expressing incomplete knowledge elements that can be characterized as p-primes, for example, Anna's imprecise statements about smell. Her ideas about the difference between the smell particles and air particles are not clear, and the descriptions of how smell spreads are incomplete. This is most likely example of a student using underlying, unarticulated elements (imprecise ideas about perfume) on which she based her reasoning (diSessa, 1993). Anna seems to have an underlying idea that the perfume fluid is something different from the particles in the air. Even though she expresses incomplete understanding about spreading of perfume particles in this case, her understanding of matter at the sub-microscopic level is still good.
In addition, it seems like the students have a stepwise advancement in their understanding of matter. Exemplified with Thomas who early in the study describes particles in fluids as moving spheres, then later replacing this concept with concepts like molecule, atom and symbol (e.g. H2O), this can be classified as a differentiation process (Carey, 2000), whereby the idea of particle is decomposed to the distinct components molecules, atoms and chemical symbols.
According to Clark (2006), as students learn and develop an integrated understanding of a topic, they reorganize these ideas and connections in productive ways. As part of this process, some ideas become central as students use them as focal points around which to integrate other ideas. It seems like particles, later atom and molecules, are such a central idea. diSessa (1993) defines promoting as what happens when students increase the activation strength and centrality of an idea to other ideas and cuing contexts. The use of the concept particle, molecule and atom in new contexts can be classified as the student promoting a central idea.
Students' understanding of chemical reactions
An understanding of chemical reactions represents an extension and elaboration of understanding of the nature of matter at the macroscopic and sub-microscopic level, meaning that further reorganizing and restructuring of knowledge structures is necessary. Even though their learning trajectories differed, all four students increased their understanding of chemical reactions to a more sophisticated level during the two year period. As with the nature of matter, it seems like the students are making a stepwise advancement in their understanding of chemical reactions. For instance, Elisabeth is confused about what is needed and formed during photosynthesis in the interview after period 2. In addition, her answers about photosynthesis are mainly on a macroscopic level. She was able to mention some of the molecules involved, but showed no ability to link macroscopic properties to sub-microscopic ideas. In the interview after period 4, Elisabeth expressed some understanding of combustion of metals on a macroscopic level. However, in case 3 in the final interview, Elisabeth was able to represent cellular respiration on a sub-microscopic level, now describing breaking and making of new bonds between atoms. The process can be described as coalescence (Carey, 2000), a process where two ideas have merged (the idea of atoms and molecules have merged with the idea of what a cell needs and gets rid of into the idea of chemical reactions). However, Elisabeth claimed somewhat imprecisely that the bonds of the molecules will make new bonds together. This does not imply a full and correct understanding of the processes going on, but can be regarded as an important step towards a deeper understanding.Each student's expressed understanding varied and evolved throughout the two year period. There can be several reasons for this. Firstly, the data were collected in different situations of performance (interviews, teaching situations and student work). For instance, Martin performed better in interviews than on written post-tests. In addition, the data reflect students' expressed understanding in different subject contexts (air pressure under varying temperatures, photosynthesis, propagation of sound, etc.). The final interview included different subject contexts and assumed that students' expressed comprehension level could vary from one case to the other. For instance, Elisabeth expressed a lower understanding of matter in case 1 and 2 than in case 3 and 4. In case 1 (a picture of the water cycle) and 2 (the burning of a match) Elisabeth had to express matter in a context that was more unfamiliar to her and further away in time than in case 3 (blood doping's effect on the circulatory system) and case 4 (photosynthesis and decomposition). It seemed like Elisabeth performed at her best when she had studied thoroughly and was mentally prepared to present the subject matter. Moreover, Thomas expressed lower understanding of chemical reactions in case 4 than in case 2 and 3. This may be due to the fact that he considered the response to be satisfactory in the present context.
In the final interview students generalized about combustion reactions resulting in a wrong conclusion about the weight of a burnt match. It may be due to the strong emphasis on combustion of metals during instruction in period 4, which seems to have dominated instruction about burning candles. The students' statements about the match's weight are examples of unstable and contextual reasoning. However, this fallacy can be considered as an important step in building bridges between knowledge elements. The students' idea that oxygen binds to the reactant in combustion reactions is so strong that they use it as a focal point around which to integrate other ideas. According to diSessa (1993), this can be classified as promoting.
Knowledge-as-elements perspectives
The current study examines the complexity of the reorganization process when students learn about matter and chemical reactions in a longitudinal perspective. The learning processes seem to involve addition and integration of instructed ideas as well as differentiation, coalescence and promoting early ideas. These processes were not typically quick and clean. Rather, it seems like there were periods during which student's interpretation of ideas remained fluid and unstable. The different situation and subject contexts heavily influenced their interpretation of ideas and how they organised the relationships between ideas. To understand a new concept involves collecting and organizing a set of loosely bound elements so that they make sense. To recognize that the same concept works in the same way in another context, or does not work, will take time. The findings of the current study align with findings of other research (Wiser and Amin, 2001; Linn et al., 2004; Clark, 2006), suggesting an evolutionary component within the conceptual restructuring process.Clearly, the results from the current study support the knowledge-as-elements perspectives, because the students' knowledge did not appear coherent and theory-like at any stage of the two year study. However, the results clarify that conceptual change seems to evolve from disjointed sets of context-dependent ideas toward a more integrated cohesive perspective. Moreover, learning about sub-microscopic entities for the first time is not that much dependent on previous, underlying ideas. This could have led to a quicker step towards a more integrated cohesive understanding of matter consisting of particles. There is probably not one simple truth about the processes of conceptual restructuring and knowledge structures. Wiser and Amin (2001) suggest, for example, that conceptual change involves both revolutionary and evolutionary components.
Implications for curriculum design
Özdemir and Clark (2007) claim that it will be of major significance for instruction whether you see knowledge as a network of theories, such as the knowledge-as-theory perspectives, or as many semi-independent elements as in the knowledge-as-elements perspectives. This will also apply to instruction following certain learning progressions. If a student's understanding is like a theory, he will be dissatisfied with existing terms if examples that come into conflict with existing ideas are introduced. Özdemir and Clark (2007) believe that if we only aim to replace the students' alternative conceptions in a replacement procedure proposed by the revolutionary change model, there will only be a replacement in a limited number of contexts, regardless of the student's interpretations of experiences outside the classroom.The four students expressed quite a deep understanding of matter and chemical reactions, and the reason for this could be their many opportunities for applying, extending and refining their understanding in different contexts within the two-year period. Özdemir and Clark (2007) argue that if knowledge is made up of fragmented elements, teaching should focus on how these elements are activated in appropriate contexts. In this perspective, productive lesson plans confront students with the same phenomenon in different contexts. The case students in this study received such opportunities. Their learning about matter and chemical reactions were related to biological phenomena such as photosynthesis, cell metabolism and phenomena in physics such as sound, electricity and energy. In addition, the students had opportunities to apply and reapply ideas about matter and chemical reactions in different contexts and therefore developed appropriate ideas. Moreover, atomic and molecular theory and chemical reactions are abstract concepts. Taber (2004) argues that it will be fruitful to develop such abstract concepts over a long period of time. According to Özdemir and Clark (2007) science curricula should focus more on refining processes, including adding, modifying, eliminating and organizing knowledge as student knowledge structures over time.
Therefore, suggested by prior researchers (Taber, 2004; Özdemir and Clark, 2007) and supported by our findings, the curriculum should clarify the importance of introducing the central idea of matter on a macroscopic level for students at an early age, for instance at age 8–10. The prescribed learning outcomes in the Norwegian Science Curriculum enable this. How macroscopic properties of matter (like temperature, pressure, density) can be understood in terms of sub-microscopic ideas should be introduced not later than the following year (age 10–11), enabling opportunities for applying, extending and refining their understanding in different contexts. The curriculum should then confront students with this phenomenon in different situation contexts and in different domains of science during the following years. The Norwegian Science Curriculum does not recommend any particular order in which the different topics should be introduced. If sub-microscopic ideas are introduced late in the students' learning process they may lose optimal opportunities to refine their understanding, including adding, modifying, eliminating and organizing knowledge as knowledge structures develop over time.
Appendix A
Interview guide
The semi-structured interview guide used in the final interviews is presented in Table 4 (Case 1: the water cycle), Table 5 (Case 2: burning match), Table 6 (Case 3: blood doping) and Table 7 (Case 4: blueberry).| Case 1: The water cycle (show a picture of water and clouds) | Phase transitions |
|---|---|
| 1.1.a. The rain drops that fall when it rains, where does this water come from? 1.1.b. If we consider the ocean as the starting point, can you tell how rain drops are formed? 1.1.c. (Mention that rain drops are formed inside the clouds) How does the water in the ocean end up in the clouds? |
• The water cycle |
| 1.2.a. What substance or substances are included here? 1.2.b. What sort of substance or substances do we start with in the ocean? What sort of substance or substances are present in clouds? Which substance or substances are present in rain drops? |
• The substance water, eventually water molecules If water or oxygen is mentioned, check sub-microscopic understanding |
| 1.3.a. In this process – is there anything about the substance or substances that change? If yes – what? 1.3.b. What happens to the substance or substances present from when they are in the ocean until they are in the clouds? What happens to the substance or substances present from when they are in a cloud until they fall down like rain drops? |
• The state of matter changes |
| 1.4.a. Does anything happen with the atoms and molecules (i.e. particles) of the substance or the substances you mentioned? If yes – what? (this may already have been answered) 1.4.b. What happens with the atoms or molecules of the substances included from when they are in the ocean to they are in the cloud? What happens to the atoms or molecules of the substances included from when they are in the clouds until they fall down like rain drops? 1.4.c. Which atoms/molecules (i.e. particles) are in the ocean? Can you tell me what happens to them when they go from sea to cloud and from cloud to rain drop? |
• The substance remains the same (physical change) |
| 1.5.a. Can you now summarize the changes (all you've talked about so far) that have happened with the substance or these substances? 1.5.b. What changes and what remains unchanged in these processes? |
• Cycles and phase transitions |
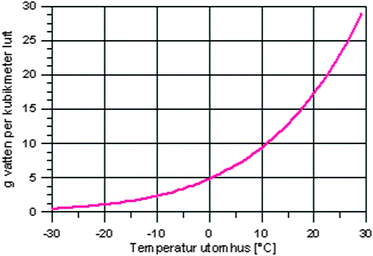 |
|
| 1.6.a. What do you think this graph shows? What has this graph to do with what we have been talking about? 1.6.b. What do the axes tell? Try to read a point on the graph (i.e. 0 °C, 5 grams of water in the air). The graph shows conditions in a cloud. Can you now use the graph to explain the relationship between water and air in a cloud? |
• Interpreting graphs, sub-microscopic understanding |
| Case 2: burning match (light a match, put it in a bowl, wait until it has burned out) | Chemical reactions |
|---|---|
| 2.1.a. What happens with the wood in the match when it burns? 2.1.b. Describe what you see and try to explain what has happened |
• Macroscopic understanding of combustion |
| 2.2.a. Which substances take part when the match burns? (stop the students if they start talking about the phosphorus part of the match) 2.2.b. What kind of substance or substances are present in the wood in the match? Think about how the wood is created. What kind of substance or substances are present in the air around the match before, during and after it has burned? 2.2.c. What kind of substances are present in trees and plants? Think of what is formed during photosynthesis. What kind of substance or substances are needed for something to burn? What is formed when for example candles burn? |
• Sub-microscopic understanding of combustion (oxygen, carbon dioxide and water) If water, oxygen, carbon dioxide or cellulose is mentioned, check sub-microscopic understanding • Sub-microscopic understanding of combustion, chemical reactions |
| 2.3.a. In this process – is there anything with the substances which changes when a match burns. If yes – what? (this may already have been answered) 2.3.b. What happens to substances in the air from before the match burns to after it has burned? What happens to substances in the match from before it burns to after it has burned? |
|
| 2.4.a. Does anything happen with the atoms and molecules of the substances you mentioned? If yes – what? (this may already have been answered) 2.4.b. What happens to the atoms or molecules of substances involved when a match burns? (What kind of substance or substances are present in the wood in the match? Think about how the wood is created. What kind of substance or substances are present in the air around the match before, during and after it has burned?) 2.4.c. Which atoms/molecules (i.e. particles) are present in matches/wood? Which atoms/molecules (i.e. particles) are present in the air? (What kind of substances are present in trees and plants? Think of what is formed during photosynthesis. What kind of substance or substances are needed for something to burn? What is formed when for example candles burn?) |
• Sub-microscopic understanding of combustion (O2, cellulose-C6H12O6, CO2, H2O) |
| 2.5.a. Can you now summarize the changes (all you've talked about so far) that have happened with the substance or these substances? 2.5.b. What changes and what remains unchanged in these processes? |
• Chemical reaction which is dependent on oxygen |
| 2.6.a. What happens to the weight of the match when it burns? Why do you think this? How could you find it out? | |
| 2.7.a. Now we have talked a lot about what happens when wood burns. How do you know all this? 2.7.b. What do you think people believed about this, five hundred years ago? And what did scientists do to find out about combustion? |
• Formation of gases, description of test design • i.e. Antoine Lavoiser's experiments |
| 2.8.a. How did you learn about this? Which instructional methods have helped you to understand what happens when something burns? | • Meta perspective on own learning processes |
| Case 3: Blood doping (see attachment about blood doping) | Chemical reactions |
|---|---|
| 3.1.a. What benefits do athletes achieve with blood doping? Explain what happens in a body that is blood doped. 3.1.b. What are the consequences of increased number of red blood cells in the body? Think about the function of red blood cells. |
• Red blood cells transport oxygen |
| The red blood cells transport substances to the cells of the body. | |
| 3.2.a. Which substances take part in the combustion in the cells? 3.2.b. Which substances do the cells need for combustion? Which waste products do the cells give off in the combustion? 3.2.c. What kind of substance or substances are present in food that the cells need? What kind of substance or substances are present in the air we breathe that the cells need? What kind of substance or substances do the red blood cells take with them back to the lungs? |
• Sub-microscopic understanding of combustion in the cells (oxygen, glucose, carbon dioxide, water) If water, oxygen, carbon dioxide or glucose is mentioned, check sub-microscopic understanding |
| 3.3.a. In this process (combustion in the cells) is there anything with the substances that changes? If yes – what? (this may already have been answered) 3.3.b. What happens to the substance/substances that originally came from the air and the substances that come from the food we eat during the combustion process in the cell? (Which substances do the cells need for combustion? Which waste products do the cells give off in the combustion?) |
• Sub-microscopic understanding of circulation, combustion in the cells, chemical reactions |
| 3.4.a. Does anything happen with the atoms and molecules of the substances you mentioned? If yes – what? (this may already have been answered) 3.4.b. What happens to the atoms or molecules of the substance/substances taking part in combustion in the cells? (Which substances do the cells need for combustion? Which waste products do the cells give off in the combustion?) 3.4.c. Which atoms/molecules (i.e. particles) are present in food, such as sugar? Which atoms/molecules (i.e. particles) are present in air? (What kind of substance or substances are in food that the cells need? What kind of substance or substances are in the air we breathe that the cells need? What kind of substance or substances do the red blood cells take with them back to the lungs?) |
• Sub-microscopic understanding of combustion in the cells (O2, C6H12O6, CO2, H2O) |
| 3.5.a. Can you now summarize the changes that have happened with the substance or substances? 3.5.b. What changes and what remains unchanged in these processes? |
• Chemical reaction which is dependent on oxygen |
| See attachment on research assignment from WADA (let the student read) | |
| 3.6.a. What is the research question? 3.6.b. What are you assumed to find out here? What is your task? |
|
| 3.7.a. What would your hypothesis be? Why? 3.7.b. What do you think the outcome will be and why you think that? |
• Research question, hypothesis, research design |
| 3.8.a. What method will you use to answer this question? 3.8.b. What kind of data will you collect? What kind of simple inquiries or measurements on the circulatory system can you do? 3.8.c. Imagine that you measure pulse using a stethoscope. How could you have performed such a study? |
| Case 4: Blueberry | Chemical reactions |
|---|---|
| 4.1.a. Imagine a blueberry flower on a blueberry plant. What will happen with this flower from spring towards winter? | • The process from berries are formed to decomposition |
During the summer the flower turns into a berry. |
|
| 4.2.a. Which substances take part in the formation of blueberries? 4.2.b. What do plant cells need to live? Which substance or substances do plant cells make? 4.2.c. What kind of substance or substances are coming from the air? What kind of substance or substances are coming from the soil? What substance or substances go into the air? And what substance or substances remain in the blueberries? (mention photosynthesis) |
• Sub-microscopic understanding of photosynthesis (oxygen, glucose, carbon dioxide and water) If water, oxygen, carbon dioxide or glucose is mentioned, check sub-microscopic understanding |
| 4.3.a. In this process called photosynthesis, is there anything about the substances that changes. If yes – what? (this may already have been answered) 4.3.b. What happens to the substance/substances that originally came from the air and the substance or substances that came from the soil? (Which substances do plant cells need to live? Which substance or substances do plant cells make?) |
• Sub-microscopic understanding of photosynthesis, chemical reactions |
| 4.4.a. Does anything happen with the atoms and molecules of the substances you mentioned? If yes – what? (this may already have been answered) 4.4.b. What happens to the atoms or molecules of the substance/substances taking part in photosynthesis (What do plant cells need to live? Which substance or substances do plant cells make?) 4.4.c. Which atoms/molecules (i.e. particles) are present in the air? Which atoms/molecules (i.e. particles) are present in water? (What kind of substance or substances are coming from the air? What kind of substance or substances are coming from the soil? What substance or substances go into the air? And what substance or substances remain in the blueberries?) |
• Sub-microscopic understanding of photosynthesis in the plant cells (O2, C6H12O6, CO2, H2O) |
| 4.5.a. Can you now summarize the changes that have happened with the substance or these substances? 4.5.b. What changes and what remains unchanged in these processes? |
• Chemical reactions which are dependent on light |
| 4.6.a. What happens to substances that blueberries are made of when it falls to the ground/when someone eats it? 4.6.b. What happens with blueberries when they rot/in the digestion process? 4.6.c. Which substances are in blueberries? Which substances are in soil? Which substances are in poop? Is there any substances going into the air? |
• Decomposition |
| 4.7.a. How did you learn about this? Which instruction methods have helped you to understand what is happening in the plants when they are alive and when they die? | • Meta perspective on own learning processes |
| 4.8.a. Do you see any connections between the four cases we have been talking about (water cycle, match that burns, blood doping and blueberry)? | • Ability to connect |
Appendix B
Code descriptions
The detailed description of the categories developed to determine the students' level of understanding of matter and chemical reactions is presented in Table 8.| Level | Matter | Chemical reactions |
|---|---|---|
| 1 | No understanding (macro and sub-micro) | |
| The student shows none or very vague macroscopic and sub-microscopic understanding of matter. One or no concepts of matter (such as air, gas, liquid, solid) or chemical symbols (such as H2O) are mentioned. | The student shows none or very vague macroscopic and sub-microscopic understanding of chemical reactions. One or no substances or chemical symbols (such as H2O) involved in the reaction are mentioned. | |
| 1, 5 | Some understanding (macro) – no ability to link sub-microscopic and macroscopic ideas | |
| The student mentions some macroscopic properties of matter, with no ability to link this to sub-microscopic ideas. Uses phrases such as hot air will rise, without linking it to the particles' behaviour in gases. Concepts such as H2O and O2 are mentioned, but it is unclear whether they refer to matter as a macroscopic entity or to the individual molecules. | The student mentions some of the substances involved in the actual chemical reaction, but shows no understanding of how matter changes in the reaction (i.e. no ability to link this to sub-microscopic ideas). Concepts such as H2O and O2 are mentioned, but it is unclear whether they refer to matter as a macroscopic entity or to the individual molecules. | |
| 2 | Some understanding (sub-micro) – limited ability to link sub-microscopic and macroscopic ideas | |
| The student uses concepts such as H2O, O2, particle, molecule or atom, but expresses only a vague understanding of matter consisting of smaller units. Shows limited ability to link sub-microscopic ideas to macroscopic properties of matter. The student may have the incorrect view of particles as small pieces carrying macroscopic properties (i.e., one single air particle gets higher/lower temperature or changes its volume). | The student mentions some of the molecules involved in the actual chemical reaction, but expresses only a vague understanding of how these molecules change in the reaction. Does not link the sub-microscopic ideas about chemical reactions to macroscopic ideas, like conservation of mass or visual indication that a chemical reaction has taken place. | |
| 2, 5 | Good understanding (macro) – limited ability to link sub-microscopic and macroscopic ideas | |
| Concepts such as H2O and O2 are mentioned, referring to matter as a macroscopic entity. The student shows through his/her use of concepts such as temperature, pressure, mass, volume and substance that (s)he has a clear understanding of matter, but shows limited ability to link these macroscopic properties to sub-microscopic ideas. | The student mentions most of the relevant substances in the actual chemical reaction and expresses understanding of how matter changes in the reaction. The student shows limited ability to link macroscopic properties to sub-microscopic ideas. | |
| 3 | Good understanding (sub-micro) – limited ability to link sub-microscopic and macroscopic ideas | |
| The student uses concepts such as H2O, O2, particle, molecule or atom, and expresses a clear understanding of matter consisting of smaller units and how these smaller units move and are inter-connected in different states of matter. The student shows limited ability to link these sub-microscopic ideas to macroscopic properties of matter, for instance pressure and temperature. | The student mentions most of the relevant molecules and symbols in the actual chemical reaction, and expresses sub-microscopic understanding of how bonds between atoms are broken and formed in this reaction. The student shows limited ability to link these sub-microscopic ideas to macroscopic ideas of chemical reactions. | |
| 3, 5 | Good understanding (macro and sub-micro) – ability to link sub-microscopic and macroscopic ideas (needs scaffolding) | |
| The student has a clear understanding of matter consisting of smaller units. At the same time (s)he uses concepts such as mass, volume, temperature and pressure and shows the ability to link sub-microscopic ideas to these macroscopic properties, for example that the pressure exerted by a gas is due to the collective impact of the particles on the walls of the container. However, the content is not expressed independently. | The student mentions most of the relevant substances as well as molecules involved in the actual chemical reaction. The student has a clear understanding of how matter changes in the reaction on a macroscopic level as well as on a sub-microscopic level. The student shows the ability to link sub-microscopic ideas to macroscopic ideas of chemical reactions (for example that conservation of mass implies that matter is neither created nor destroyed, i.e. the atoms remain the same). However, the content is not expressed independently. | |
| 4 | Good understanding (macro and sub-micro) – ability to link sub-microscopic and macroscopic ideas (needs no scaffolding) | |
| The student has a clear understanding of matter consisting of smaller units. At the same time (s)he uses concepts such as mass, volume, temperature and pressure and shows the ability to link sub-microscopic ideas to these macroscopic properties (for example that atoms are the same even if the substance, which the atom is part of, may change in various ways). Moreover, the content is expressed independently. | The student mentions most of the relevant substances as well as molecules involved the actual chemical reaction. The student expresses understanding of how matter changes in the reaction on a macroscopic level as well as on a sub-microscopic level. The student shows the ability to link sub-microscopic ideas to macroscopic ideas of chemical reactions, for example that conservation of mass implies that matter is neither created nor destroyed, i.e. the atoms remain the same. Moreover, the content is expressed independently. | |
References
- Adbo K. and Taber K. S., (2009), Learners' mental models of the particle nature of matter, A study of 16-year-old Swedish science students, Int. J. Sci. Educ., 31(6), 757–786.
- Arzi H. J., (1988), From short to long-term: studying science education longitudinally, Stud. Sci. Educ., 15, 17–53.
- Carey S., (2000), Science education as conceptual change, J. Appl. Dev. Psychol., 21(1), 13–19.
- Clark D. B., (2006), Longitudinal conceptual change in students' understanding of thermal equilibrium: an examination of the process of conceptual restructuring, Cognition Instruct., 24(4), 467–563.
- diSessa A. A., (1988), Knowledge in pieces, in Forman G. and Pufall P. (ed.), Constructivism in the computer age, Hillsdale, NJ: Lawrence Erlbaum Associates, pp. 49–70.
- diSessa A. A., (1993), Toward an epistemology of physics, Cognition Instruct., 10(2–3), 105–225.
- diSessa A. A., (2006), A history of conceptual change research: threads and fault lines, in Sawyer K. (ed.), Cambridge handbook of the learning sciences, Cambridge, UK: Cambridge University Press, pp. 265–281.
- diSessa A. A., Gillespie N. and Esterly J., (2004), Coherence versus fragmentation in the development of the concepts of force, Cognitive Sci., 28, 843–900.
- Duschl R. A. and Grandy R. E., (2007), Teaching scientific inquiry: recommendations for research and implementation, Rotterdam: Sense Publishers.
- Eskilsson O. and Helldén G., (2003), A longitudinal study on 10–12-year-olds' conceptions of the transformations of matter, Chem. Educ., 4(3), 291–304.
- Hunt E. and Minstrell J., (1994), A cognitive approach to the teaching of physics, in McGilly K. (ed.), Classroom lessons: integrating cognitive theory and classroom practice, Cambridge, MA: MIT Press, pp. 51–54.
- Johnson P., (1998), Children's understanding of changes of state involving the gas state, Part 2: evaporation and condensation below the boiling point, Int. J. Sci. Educ., 20, 695–709.
- Johnson P., (2000), Childrens' understanding of substances, part 1: recognising chemical change, Int. J. Sci. Educ., 20, 697–709.
- Johnson P., (2002), Children's understanding of substances, part 2: explaining chemical change, Int. J. Sci. Educ., 24, 748–769.
- Linn M. C., Eylon B. and Davis E. A., (2004), The Knowledge Integration Perspective on Learning Associates, in Linn M. C., Eylon B. and Davis E. A. (ed.), Internet environments for science education, Mahwah, NJ: Lawrence Erlbaum.
- Meheut M. and Chomat A., (1990), Les limites de l'atomisme enfantin: experimentation d'une demarche d'elaboration d'un modele particulaire par des eleves de college (The limits of childhood atomism: experimenting with involving eight grader students in elaborating on a particulate model), Eur. J. Psychol. Educ., 4, 417–437.
- Özdemir G. and Clark D. B., (2007), An Overview of Conceptual Change Theories, Sci. Technol. Educ., 3(4), 351–361.
- Posner G., Strike K., Hewson P. and Gertzog W., (1982), Accommodation of a scientific conception: toward a theory of conceptual change, Sci. Educ., 66(2), 211–227.
- Smith C., Wiser M., Andersen C. W. and Krajick J., (2006), Implications of research on children's learning for assessment: matter and atomic molecular theory, Meas.: Interdiscip. Res. Perspect., 14(1–2), 1–98.
- Stevens S. Y., Shin N., Delgado C. and Krajcik J., (2007), Developing a learning progression for the nature of matter as it relates to Nanoscience, Paper presented at the American Educational Research Association, Chicago, IL.
- Strike K. A. and Posner G., (1992), A revisionist theory of conceptual change, in Duschl R. A. and Hamiltin R. J. (ed.), Philosophy of Science, Cognitive Psychology, and Educational Theory and Practice, New York: Academic, pp. 211–231.
- Taber K. S., (2004), Learning quanta: barriers to stimulating transitions in students understanding of orbital ideas, Sci. Educ., 89, 94–116.
- Teichert M. A., Tien L. T., Anthony S. and Rickey D., (2008), Effects of Context on Students' Molecular-Level Ideas, Int. J. Sci. Educ., 30(8), 1095–1114.
- Utdanningsdirektoratet., (s.a.), Læreplan i naturfag, Retrieved 14.02, 2013, from http://www.udir.no/kl06/NAT1-02/.
- Viennot L., (1985), Analyzing students' reasoning: tendencies in representation, Am. J. Phys., 53, 432–436.
- Vygotsky L. S. and Cole M. L., (1978), Mind in society: the development of higher psychological processes, Cambridge, Mass: Harvard University Press.
- Wiser M. and Amin T., (2001), Is that hot? Inducing conceptual change by integrating everyday and scientific perspectives on the thermal phenomena, Learn. Instr., 11, 331–355.
| This journal is © The Royal Society of Chemistry 2013 |
