Semantic mistakes and didactic difficulties in teaching the “amount of substance” concept: a useful model
Bülent
Pekdağ
* and
Nursen
Azizoğlu
Balıkesir University, Necatibey Education Faculty, Department of Secondary Science and Mathematics Education, Chemistry Education, 10100 Balıkesir, Turkey. E-mail: pekdag@balikesir.edu.tr
First published on 2nd January 2013
Abstract
Textbooks still have the distinction of being the most dominant teaching tool in science teaching. The manner in which a scientific concept is expressed in a textbook is of importance in the in-depth learning process of that concept. With this in mind, problems with expressing the “amount of substance” concept were reviewed in 15 chemistry textbooks published in three different countries (United States, France and Turkey). The problems were analyzed in terms of semantic and didactic perspectives. It was found that the semantic mistakes in the presentation of the amount of substance concept in chemistry textbooks stemmed from: (i) missing concepts, (ii) the use of knowledge at the incorrect level, (iii) the use of the term “number of moles”, (iv) limitations in meaning resulting from a lower concept being used in place of a higher concept, and (v) the use of the amount of substance concept as equivalent to mass, Avogadro’s constant, molar volume and molar mass. Additionally, difficulties were observed that arose from the inappropriate didactic transposition of textbook subjects. These didactic difficulties stemmed from associating the amount of substance with more than one physical quantity. Teaching models for meaningfully teaching the amount of substance at the macroscopic, microscopic and symbolic levels of chemistry have been suggested and discussed.
Introduction
The “amount of substance” is a concept that has been a frequent subject of discussion among science education researchers (Novick and Menis, 1976; Khang and Sai, 1987; Staver and Lumpe, 1993; Furió et al., 2000; Akçay et al., 2003; Gorin, 2003; Ünlü, 2006). The very fact that so much discussion has been devoted to this topic is evidence of how teaching and learning the concept is of such essential importance (Cervellati et al., 1982; De Berg, 1986; Ainley, 1991; Strömdahl et al., 1994; Staver and Lumpe, 1995). There are few subjects involving chemical reasoning that are harder to comprehend than the concept of amount of substance. As this wide interest shows, teaching and learning the amount of substance concept is a difficult task (Graham, 1983; Abraham et al., 1992; Case and Fraser, 1999; Claesgens and Stacy, 2003; Akbal, 2009; Yiğit, 2010). To understand this difficulty and to better assess the results of the present study, it would be useful to first provide a brief review of the historical development of the amount of substance and mole concepts.Amount of substance and mole
In Latin, the word moles means “big mass”; adding the suffix –cula converts the word to the term molecula, meaning “small and tiny”. The term molar, which was derived from the word moles, was first introduced by the German chemist Hofmann, who used it to mean a large or macroscopic mass, the opposite of the term “molecular mass”. In other words, the words “molar” and “molecular” were preferred in place of the terms “macroscopic” and “microscopic”. A limited use of the term molar as the mass of a substance, expressed in grams of the molecules of which it is comprised, as well as of the word mole was introduced by the German physical chemist Ostwald (Jensen, 2004). Ostwald introduced the concept of mole because of his skepticism of Dalton’s atomic and Avogadro’s molecular hypotheses (Furió et al., 2000). In fact, when Ostwald brought out the concept of mole in 1900, he was in the process of trying to determine the chemical formula for “oxygenated water” and the normal weight of this substance by making use of the proportionality between the decrease in the freezing point and the concentration of a solution of the compound. Ostwald as an equivalentist rejected the atomic and molecular hypotheses, defining the mole as the normal or molecular weight of a substance expressed in grams. Thus, the term mole began to be widely used without any discussion about the nature of the quantity it referred to (Milton and Mills, 2009). Ostwald aimed to treat the term mole as a macroscopic term which could be used to discuss the laws of stoichiometry. In those days, however, the atomic-molecular theory had begun to be widely accepted. The solution this theory provided for quantitative relations in chemical reactions was based on the meaning of a reaction. Some proportions that exist between the particles of the reagents and the products of a reaction are shown by coefficients indicated before the chemical formulas. Weight and volumetric relations may be deduced by using these proportions, given the masses of the particles that take part in a reaction. In other words, to move from the microscopic relations of particles to weight and volumetric relations on a macroscopic level, however, the number of particles had to be known. Since these could not be counted one by one, there had to be some indirect way of counting. The solution that was found to this problem was the concept of amount of substance. In 1961, the amount of substance concept was introduced as part of the atomistic paradigm (Padilla and Furio-Mas, 2008). The amount of substance is proportional to the number of constituent particles. The proportional constant is a universal constant that is the same for all substances (BIPM, 2006). The symbol for the amount of substance is n. In the 14th meeting of the General Conference on Weights and Measures (CGPM) in 1971, the International Union of Pure and Applied Chemistry (IUPAC), the International Union of Pure and Applied Physics (IUPAP) and the International Organization for Standardization (ISO) made the recommendation that in the International System of Units (SI), “mole” would be added as a basic unit of the amount of substance. The mole was defined as the following:1. The mole is the amount of substance of a system which contains as many elementary entities as there are atoms in 0.012 kilogram of carbon 12; its symbol is “mol”.
2. When the mole is used, the elementary entities must be specified and may be atoms, molecules, ions, electrons, other particles, or specified groups of such particles.
3. The mole is a base unit of the International System of Units (BIPM, 2006, p. 115).
This definition relates the mass of one mole of carbon-12 atoms to the atomic mass of one carbon-12 atom, the numerical values being the same although the units are different. Thus, one mole of carbon atoms has a mass of 12 grams while the carbon atom has 12 amu. It has been emphasized that the basic structures should be explicitly expressed when quantitative expressions of amount of substance are used. The importance of this has been stressed in the IUPAC book, Quantities, Units and Symbols in Physical Chemistry:
The amount of substance is proportional to the number of specified elementary entities of that substance; the proportionality factor is the same for all substances and is the reciprocal of the Avogadro constant. The elementary entities may be chosen as convenient, not necessarily as physically real individual particles. Since the amount of substance and all physical quantities derived from it depend on this choice it is essential to specify the entities to avoid ambiguities (Mills et al., 1993, p. 46).
This reference states that one mole of all substances contain the same number of particles. This number is the constant that has been set down as the result of experimentation and is called the Avogadro constant, NA. The ratio between a mole and the constituent particles in a sample is defined as the “vehicle to indirectly count atomic/molecular particles of substance by weighing macroscopic amounts” (Staver and Lumpe, 1995).
The amount of substance facilitates the counting of elementary entities. This calculation is performed indirectly since it is impossible to count particles one by one. However, the amount of substance (n), the mass (m), the volume (V) and the number of elementary entities (N) must be clearly distinguished from one another. Although the quantity of the amount of substance is related to quantities of mass, volume and number of particles, expressed by the formulas n = m/M, n = V/Vm and n = N/NA, all of which are taught by teachers and textbooks and known well by students, it is incorrect to define this quantity with any one of the terms in these formulas (Gorin, 1994).
SI’s definition of mole, then, indicates that a mole has both a quantitative and a conceptual nature. Research in science education has shown that students understand the quantitative nature of the mole (Graham, 1983; Gabel et al., 1984; Schmidt, 1990). For students to understand its conceptual nature, however, they must be able to perceive the macroscopic world they see in reality as numbers and associate this with the world of particles (Claesgens and Stacy, 2003).
Literature review
This section will provide some theoretical knowledge about the concepts of semantics, didactic transformation and the three levels of chemistry (macroscopic, microscopic and symbolic levels) that will be useful in subsequently reviewing the semantic mistakes and didactic difficulties that arise when the amount of substance concept is introduced in chemistry textbooks. The review will form the basis for the recommendation of a teaching model that is believed will facilitate the teaching of the amount of substance.Learning is defined as “the process of making meaning” (Halliday, 1993a) and language is the basic tool with which an individual makes meaning out of expressions (Seah et al., 2011). Language provides scientists with the opportunity to form associations between claims and proofs in order to develop scientific theories, evaluate scientific assertions, create new knowledge, and use writing to communicate and disseminate scientific research (Yore et al., 2004). The importance of language lies not only in its being a tool for social and cultural interaction, but also in the fact that it is a mental tool for verbalizing thought (Vygotski, 1934/1997).
Modern science relies on the written text (Norris and Phillips, 2003) and to learn science, one first has to learn the scientific knowledge that is in writing. For the meaning of a concept to be accurately and scientifically created, the manner in which that concept is expressed, and therefore the organization of the scientific information, is of the greatest importance (Halliday, 1993b; Duran et al., 1998). The expression that is used in description is what brings the conceptual structure into light (Talmy, 2000).
Many students are unable to acquire and understand scientific knowledge (Fang, 2005). One of the biggest obstacles standing in the way of science education is the language that is unique to science itself (Wellington and Osborne, 2001; Pozo and Lorenzo, 2009). Many students reading a scientific text often misunderstand it (Craig and Yore, 1995). Although textbooks studied in schools are essential in teaching science (Chiu, 2007; dos Santos et al., 2012; Uhden et al., 2012), it is also true that the scientific knowledge introduced in these books often leads to comprehension difficulties (Adbo and Taber, 2009; van Eijck et al., 2011). Some technical terms, sentences containing a great deal of concentrated information, and complex and long sentences create problems when students are reading a text. This makes learning science difficult and even causes students to shy away from science as a whole (Fang, 2006). Under these circumstances, it can only be expected that students gain only a superficial understanding of scientific knowledge (Reif and Larkin, 1991).
The didactic transposition process can be summarized in three stages (Chevallard, 1985):
1. The stage at which scientific knowledge is transposed into knowledge that can be taught—this stage defines the transposition of knowledge from the form constructed by the scientist to knowledge in a form that can be taught in school. Academic institutions create knowledge true to the purposes of science and meeting the needs of society, formulating this knowledge in line with certain conditions within the framework of scientific rules. Knowledge constructed by academic institutions can only be transposed into knowledge that can be taught in school by first delineating its borders and then carrying out a process of reorganization.
2. The stage at which knowledge is transposed from knowledge to be taught into knowledge to be learned—this stage is performed by the teacher. This step involves the teacher’s use of different classroom activities to transpose the knowledge in the curriculum and the textbook into knowledge that can be presented to students.
3. The stage at which the knowledge taught is transposed into assimilated knowledge—this stage is performed by the student. The student must interpret the knowledge taught in school in order to integrate it with prior knowledge. The information must then be reorganized and, through mental processes, transformed into a unique and comprehensible form.
The unsuitable didactic transposition of scientific knowledge into knowledge to be taught causes learning difficulties. These difficulties stem from didactic obstacles (Brousseau, 1998). Didactic obstacles that hinder a student’s learning derive from the manner in which authors have arranged the knowledge and transposed it into knowledge to be taught or from the way teachers have transferred that knowledge to the class (the expression of the knowledge to be taught, the comprehensibility level of the knowledge, the suitability of the knowledge to the student’s age, the suitability of the method and techniques of teaching, etc.). In the presence of obstacles, it cannot be said that knowledge will be learned meaningfully and in depth. Lack of student achievement, however, is often attributed to the system of education, the curriculum, the textbook, the teacher or the student (Brousseau, 1998).
Research in chemical education has revealed that students have difficulty explaining chemical phenomena at the microscopic level or in learning symbolic level representations (Ben-Zvi et al., 1987; Abraham et al., 1992; Kozma and Russell, 1997; Ardac and Akaygun, 2004; Pekdağ and Le Maréchal, 2010). In addition, students have a general problem with forming meaningful relationships between microscopic and macroscopic levels (Taber, 2001). The theoretical structure of chemistry is based to a large extent on entities that are at the microscopic level (atoms, molecules, ions, orbitals, etc.). The problem at this point is that all of these entities and their properties are abstract concepts to the student and impossible to observe. Taber (2000) has said that although students discuss chemistry at a molecular level, they see the molecular world as a small version of their own macroscopic experiences. Students can therefore attribute properties belonging to the macroscopic level to microscopic structures. Attributing to atoms the ability of melting, being malleable and expanding is an example of this. Explaining the phenomenon of the freezing of a solid on the basis of the interaction between atoms is a sign of the perception of the relationship between macro- and micro-levels, but to say that an atom has frozen would be to attribute a macro-level property to a micro-level entity, which would render the association meaningless. Students can only attain in-depth learning of concepts and phenomena in chemistry if they can form meaningful relationships between levels of chemistry (macroscopic, microscopic and symbolic) (Russell et al., 1997; Johnstone, 2000; Treagust et al., 2003; Pekdağ and Le Maréchal, 2010).
The amount of substance, which is the subject of the present study, is a concept that connects the macro-world with the micro-world. Learning the amount of substance concept meaningfully and in-depth is closely dependent on how well teachers and textbook authors are able to present the concept in a manner that creates meaningful relationships between the three levels of chemistry. Examples of such meaningful relationships have been offered under the section “Useful models for teaching the amount of substance concept” (see Tables 6–8).
Purpose of the study
Meaningful and in-depth understanding of the amount of substance concept forms the foundation for learning many topics in chemistry, among which are chemical reaction equations, concentration calculations, stoichiometric problems, electrochemistry, gases, and the like. There is therefore a need for a teaching approach that will ensure the conceptual and in-depth learning of the amount of substance concept. The present study aims to review the semantic mistakes made in the presentation of the concept of amount of substance in chemistry textbooks and to pinpoint the didactic difficulties involved. In the light of their findings, the authors attempt to recommend a teaching model in which the scientific definition of the concept is correctly expressed semantically. Previous studies have suggested different methods for teaching the amount of substance. For example, Dori and Hameiri (1998) developed “mole environment”-oriented problem-solving studyware. Geban (1995) and Akçay et al. (2003) proposed computer-aided instruction for teaching the amount of substance. The explicit method of problem-solving accompanied with analogies or conceptual change texts have also been used in teaching the amount of substance (Ünlü, 2006; Akbal, 2009). No study has been encountered in chemical education, however, where a model for teaching the amount of substance concept has been suggested from the perspective of semantics. Adopting a semantic outlook in chemical education is important in providing researchers with new and workable ideas. It is believed that this teaching model will be of immeasurable use to teachers introducing the topic of the amount of substance in the classroom. It will also be of help to authors presenting the concept in textbooks, and to students in helping them to learn the topic well.Research questions
The present study aims to answer the following questions:1. What are the semantic mistakes and didactic difficulties in the presentation of the amount of substance concept in chemistry textbooks?
2. What are the useful models that can be used in teaching the amount of substance concept?
Method
Sample
The sample for the research consisted of 15 chemistry textbooks in which the topic of the amount of substance was treated (see Appendix). These books are teaching aids used in the United States, France and Turkey; the distribution of the books by country and grade level has been given in Table 1. Five of the books targeted the 10th or 11th grades in the secondary education program, depending upon the structure of the educational system in the respective countries. Ten of the books reviewed were university-level and were being used in the first-year General Chemistry course.| Country | Secondary | University | Total |
|---|---|---|---|
| USA | 1 | 2 | 3 |
| France | 3 | 2 | 5 |
| Turkey | 1 | 6 | 7 |
| Total | 5 | 10 | 15 |
The objective of working with a study sample chosen from three different countries and two different educational levels was not to make a comparison between countries and curriculum levels, nor to determine which chemistry textbook at which educational level or in which country presented the concept of amount of substance in a more scientifically accurate and effective manner. Rather, the sample was chosen for the purpose of finding different examples of semantic mistakes and didactic difficulties. Almost all of the chemistry textbooks in Turkey in which the amount of substance concept was described were reviewed for the study. The only book treating the concept in the secondary schools, i.e., the 10th-grade chemistry textbook distributed to students in the public schools, and almost all of the first-year university level chemistry textbooks were included in the research. Also reviewed in the study were the secondary school and university-level textbooks treating the topic of the amount of substance published in the US and France by recognized publishers. These textbooks were being used in the 11th grade and in the first year of the university. Although the number of chemistry textbooks from different countries and educational levels varied, this was not in contradiction to the purpose of the research and in fact contributed to the diversity of the study findings.
Data analysis
In this study the researchers used an iterative process of describing and interpreting the data obtained from chemistry textbooks. The fifteen chemistry textbooks were qualitatively analyzed (content analysis) to discover the semantic mistakes and didactic difficulties (Fraenkel and Wallen, 2006). The semantic mistakes made with regard to the concept of amount of substance were classified under three different sub-groups: missing concepts, mismatching and inappropriate expression. The “inappropriate expression” sub-group was adopted from the literature (e.g.Staver and Lumpe, 1993; Furió et al., 2000) while the other sub-groups were developed by the researchers in order to represent the data more meaningfully.In this study, missing concept was defined as “a concept that is absent at the macroscopic (gas, element, compound, etc.), microscopic (atoms, ions, molecules) or symbolic level (symbols, formulas)”. The term mismatching was defined as “using the amount of substance concept as equivalent to the concepts of mass, molar mass, number of particles or molar volume”.
Inappropriate expression was defined as “the observed incorrect usage of a microscopic level concept in place of a macroscopic level concept or vice versa”. In addition, “including the term the number of moles in the expression” or “limiting the meaning by using a lower concept (e.g., atoms, molecules, ions, electrons or protons) for a higher concept (particles)” was also classified as inappropriate expression. In a concept hierarchy, the meaning of a concept is built on a small number of simpler concepts; each in turn is defined at a lower level using other concepts. Each concept is a union of lower-level categories (Tan and Soon, 1996; Foster et al., 2012). In this study, higher concepts mean more general terms while lower concepts correspond to more specific terms. For example, in the concept hierarchy of the topic of chemical bonding, the higher concept is bonding while the lower concepts would include ionic bonding and covalent bonding. Lower concepts are classified under higher concepts and such concepts must be compatible with the higher concepts to which they are subordinate (Daley, 2010).
In order to transform “incorrect level of knowledge”, “number of moles” and “limitation of meaning” into something meaningful, the researchers gathered these under the sub-group “inappropriate expression”.
Besides the semantic mistakes, the didactic difficulties that made it hard to meaningfully learn the amount of substance concept were also determined.
Results
Semantic mistakes
The semantic mistakes found in fifteen chemistry textbooks in the presentation of the amount of substance concept stemmed from scientifically inappropriate expression, missing concepts, or mismatching. The semantic mistakes related to these three circumstances are shown in Tables 2–4.| Expression with missing concept | Correct expression | Level of missing concept |
|---|---|---|
| How many moles are in 45.6 g NH3? | What is the amount of substance in 45.6 g of compound NH3(g)? | Macro |
| How many moles is 14.47 g K? | What is the amount of substance in 14.47 g of element K(s)? | |
| How many moles is 51.7 g C9H8O4? | What is the amount of substance in a sample of 51.7 g compound C9H8O4(s)? | |
| 0.1 mol OH− | 0.1 mol OH− ions | Micro |
| How many grams is 0.25 mol Au? | What is the mass (in grams) of 0.25 mol Au atoms? | |
| How many grams is 0.5 mol H2O? | What is the mass (in grams) of 0.5 mol H2O molecules? | |
| There are 2 mol Na in 1 mol Na2CO3. | There are 2 mol Na atoms in 1 mol Na2CO3 formula units. | |
| How many moles Si are in 30.5 g Si? | What is the amount of silicon atoms in a sample of 30.5 g element Si(s)? | Micro and macro |
| How many moles of molecules are in 3.36 L Cl2. | What is the amount of chlorine, Cl2 in 3.36 L of element Cl2(g)? | Symbolic and macro |
| Inappropriate expression | Correct expression | Mistake |
|---|---|---|
| 1 mol hydrogen gas | 1 mol hydrogen molecules | Incorrect level of knowledge |
| How can you express 1 mol element Fe in different way? | How can you express 1 mol Fe atoms in different way? | (Macro in place of micro) |
| 1.008 grams hydrogen contain the same number of atoms as 1 mol of any other element contains. | 1 mol of hydrogen atoms is the amount of substance that contains the same number of atoms as 1.008 grams element hydrogen. | |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||
| How many moles are 8 g oxygen molecules? | What is the amount of oxygen, O2 in a sample of 8 g element O2(g)? | Incorrect level of knowledge (Micro in place of macro) |
| The molar mass of the Na atom is 22.99 g. | The molar mass of the element Na(s) is 22.99 g mol−1. | |
| The molar mass of a compound is the sum of molar masses of atoms in 1 mol compound. | The molar mass of a compound is the sum of the molar masses of its constituent elements. | |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||
| The relation between the number of moles and the mass of a substance is given with equation n = m/MA. | The amount of substance (n) and mass (m) are related via molar mass (MA), n = m/MA. | Number of moles |
| The coefficients of a balanced equation represent numbers of moles of reactants and products. | The coefficients of a balanced equation represent the amount of reactants and products in moles. | |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||
| There are as many molecules as Avogadro’s number in 1 mol substance. | There are as many particles (atoms, molecules, ions, electrons, protons, etc.) as Avogadro’s constant in one mole. | Limitation of meaning |
| The mass of one mole atoms is named as molar mass, MA. | The mass of one mole of the elementary entities of a particular substance is named as molar mass, MA. | |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||
| What is the relation between the number of moles and the number of atoms? | What is the relation between the amount of substance and the number of elementary entities (atoms, molecules, etc.)? | Number of moles and Limitation of meaning |
| Expression with mismatching | Formulation of mismatching | Correct expression |
|---|---|---|
| Mass, m, corresponds to n mol. | n = m | The mass of a substance is related to the amount of substance via m = n × MA. |
| Molar mass, MA, corresponds to one mole. | n = MA | The mass of one mole atoms or molecules of a substance is called molar mass, MA. |
| 1 mol Au is equal to 197 g Au. | 1 mol Au atoms has a mass of 197 g. | |
| The quantity represented by Avogadro’s number is mole. | n = NA | The amount of substance is proportional to the number of elementary entities of a substance and the proportionality factor is Avogadro constant, n = N/NA. |
| 1 mol represents 6.022 × 1023 particles. | One mole is the amount of substance that contains 6.022 × 1023 elementary entities. | |
| At STP one mole gas is equal to 22.4 L. | n = Vm | At STP, one mole atoms or molecules of a gas occupy 22.4 liters. |
When the SI definition of the mole is considered, there must be an association formed between the mole and the microscopic form of a substance (ion, atom, molecule, etc.). For example, instead of saying “How many grams is 0.25 mol Au?” the expression “What is the mass (in grams) of 0.25 mol Au atoms?” is the semantically correct form of expression. If the amount of substance is to be expressed by associating it with physical quantities such as mass and volume, then the macroscopic form of the substance (element, compound, etc.) should also be expressed. For example, in the expression associating the amount of substance concept with the mass concept, the macroscopic form of potassium or ammonia has not been expressed. In other words, instead of asking “How many moles is 14.47 g K?” it is semantically correct to express this as, “What is the amount of substance in 14.47 g of the element K(s)?”. In Table 2, there are other examples of missing concepts at the macroscopic, microscopic and symbolic levels.
The expressions “amount of substance in …”, “amount of silicon atoms in…” or “amount of chlorine, Cl2 in…” are all compatible with IUPAC definitions (Mills et al., 1993; BIPM, 2006).
The second sentence of the SI definition of the mole states that elementary entities should be specified. Contrary to this definition, however, the chemistry textbooks contain expressions in which the mole is associated with the macroscopic form (element, gas, etc.) of a substance. For example (see Table 3), while the expression should be “1 mol hydrogen molecules”, it is frequently referred to in the textbooks as “1 mol hydrogen gas”. While the microscopic form needs to be used in the expression, it can be seen that it is the macroscopic form that is used. Since this form of expression is not consistent with the scientific definition, it is semantically incorrect.
The reverse of this was also observed in the textbooks that were reviewed. For example, rather than saying “The molar mass of the Na atom is 22.99 g”, expressing this as, “The molar mass of the element Na(s) is 22.99 g mol−1” is the semantically correct form. This is because we can speak of the molar mass of an element but not of an atom. In this case, it can be seen that the microscopic form of the entity (Na atom) has been used instead of its macroscopic form (the element Na(s)). Another example is what is often expressed when it is asked how many grams an oxygen molecule is. Students could actually answer this question because, if they can form associations between the number of particles, amount of substance, molar mass, the mass of one particle, then they would be able to calculate how many grams an oxygen molecule is. When we speak of molar masses of elements or compounds, these are expressed as grams. However, it is more correct to express small particles such as atoms or molecules in terms of atomic mass units (amu).
Another inappropriate form of expression frequently seen in chemistry textbooks is the use of the term “number of moles”. Since a mole expresses a unit in itself, it is not logically correct to speak of a “number of moles”. Instead of the “number of moles”, the semantically correct expression is the “amount of substance”. Furthermore, some expressions that associate the mole with only one kind of microscopic form (e.g., atom or molecule or ion, etc.) limit the meaning. For example, the expression “There are as many molecules as Avogadro’s number in 1 mol substance” limits the elementary entities to the concept of molecule and is therefore restrictive, both in terms of the definition of the mole and in terms of the building blocks of the substance. Instead of this limiting expression, it is more correct to express this as, “There are as many particles (atoms, molecules, ions, electrons, protons, etc.) as Avogadro’s constant in one mole”.
As can be seen in the examples given in Table 3, semantic mistakes are created when expressions used in teaching the amount of substance concept in chemistry textbooks contain knowledge at an incorrect level, speak of a “number of moles” and otherwise cause limitations in meaning.
As an example of correctly expressing mismatched expressions (see Table 4), instead of saying “Mass, m, corresponds to n mol”, it is meaningful and correct to express this as, “The mass of a substance is related to the amount of substance via m = n × MA”. Similarly, chemistry textbooks mismatch amount of substance and molar mass. Again, instead of saying “Molar mass, MA, corresponds to one mole”, it is more scientific and correct to express this as, “The mass of one mole atoms or molecules of a substance is called molar mass, MA” since molar mass and amount of substance are not equivalent. A student reading a definition of this kind may use these concepts interchangeably; at the same time, the concepts of amount of substance, number of particles and molar volume are used equivalently in chemistry textbooks. Although the magnitude amount of substance with mole as its unit was accepted by the atomic-molecular theory, the equivalentist paradigm still exists in the textbooks. The amount of substance is not equivalent to mass (m), molar mass (MA), the Avogadro’s constant (NA) or molar volume (Vm) whereas the amount of substance (whose unit is the mole) is directly related to mass, volume and the number of particles.
The semantic mistakes are not only capable of obstructing a student’s scientific understanding and learning of the quantity of amount of substance and its unit the mole, but also have the potential of creating misconceptions as well.
Didactic difficulties
In chemistry textbooks some expressions associate the amount of substance with more than one physical quantity. The didactic difficulties that stem from such associations are shown in Table 5.| Expression with multiple associations of physical quantities | Association |
|---|---|
| In general, the molar mass, MA, in grams per mole, is numerically equal to the formula mass. | n ↔ MA ↔ amu |
| A mole represents not only a specific number of particles but also a definite mass of a substance. | N A ↔ n ↔ m |
| A mole contains as many particles as Avogadro’s number and has a mass in grams numerically equal to formula mass of the substance. | n ↔ NA × m ↔ MA |
As seen in Table 5, a definition in the form of “In general, the molar mass, MA, in grams per mole, is numerically equal to the formula mass” expresses the relationship between molar mass, amount of substance and formula mass in the same sentence. Since such a relationship stems from the manner in which authors have organized this scientific knowledge, a didactic difficulty confronts us when this knowledge is to be taught and students will thus find it difficult to comprehend the definition of molar mass. This learning difficulty originates from didactic transposition. In another example, an explanation in the form of “A mole represents not only a specific number of particles but also a definite mass of a substance” expresses the relationship between amount of substance, Avogadro’s constant and molar mass in the same sentence. The difficulty arises because the author of the textbook has associated the three concepts in the same sentence and this inevitably hinders meaningful associations of the scientific concepts in students’ minds. Associating a scientific concept in the same sentence simultaneously with many other scientific concepts makes comprehension of the knowledge difficult and should thus be avoided. If it is at all necessary to associate a scientific concept with more than one other concept, each relationship should be stated in a separate sentence. This will eliminate difficulties that stem from the manner in which the knowledge has been organized.
Useful models for teaching the amount of substance concept
Research in chemical education has shown that meaningful and in-depth learning of the concept of amount of substance is no easy task (Case and Fraser, 1999; Claesgens and Stacy, 2003; Yiğit, 2010). The difficulty lies in the fact that the concept of amount of substance has been associated with knowledge at the macroscopic, microscopic and symbolic levels. Correctly associating the amount of substance with knowledge at these three levels is necessary from a semantic perspective if this concept is to be learned meaningfully and thoroughly. The new teaching models constructed within the scope of this study are based on the SI definition of the mole. Tables 6–8 display the suggested models together with the different examples of semantically correct expressions.| Correct expression | Incorrect expression | |
|---|---|---|

|
1 mol He atoms | 1 mol gaseous He |
| 1 mol Mg atoms | 1 mol element Mg | |
| 1 mol Cl− ions | 1 mol Cl− | |
| 1 mol CO2 molecules | 1 mol gaseous CO2 | |
| 1 mol H2O molecules | 1 mol compound H2O | |
| 1 mol NaCl formula units | 1 mol compound NaCl | |
| 1 mol electrons | 1 mol solid NaCl | |
| 1 mol protons | ||
| 1 mol neutrons |
| Correct expression | |
|---|---|
|
1 mol Be atoms = 6.022 × 1023 Be atoms |
| 4 mol O2 molecules = 4.818 × 1024 O atoms | |
| 4 mol O2 molecules = 2.409 × 1024 O2 molecules | |
| 3 mol electrons = 1.807 × 1024 electrons |
As shown in Table 6, Model 1 in a formula of “mole + microscopic level” is the correct scientific representation of the mole and is also correct in terms of semantics. It is not semantically correct to have knowledge at the macroscopic level (element, compound, etc.) immediately follow the term mole. Because it is a unit of the amount of substance that defines the number of particles, the term mole should be immediately followed by microscopic-level knowledge (atom, ion, molecule, formula unit, electron, etc.).
Model 2, suggested in Table 7, can be applied to expressions where physical quantities such as mass and volume are being used.
Formulated in the form of “mole + microscopic level = symbolic level (m, V) + macroscopic level”, Model 2 shows how the concept of amount of substance can be correctly associated with knowledge pertaining to the symbolic and macroscopic levels in terms of semantics.
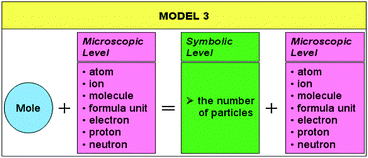
Model 3, shown in Table 8, can be used in representations in which the amount of substance concept is associated with the number of particles.
This model, formulated as “mole + microscopic level = symbolic level (N)+microscopic level”, shows how the amount of substance can be correctly and semantically associated with knowledge that is at the symbolic and microscopic levels.
It is believed that bringing an economic teaching model into the service of chemical education will ensure that meaningful associations are made regarding the amount of substance concept at the three levels of chemistry. Such a model will also be useful in overcoming the difficulties experienced in teaching and learning this concept and additionally serve the function of preventing the formation of student misconceptions.
Discussion and implications for teaching
In the definition accepted at the 14th meeting of the General Conference on Weights and Measures, it is clearly and openly stated that elementary entities must be specified when using the unit mole. None of the textbooks reviewed in this study made any reference to this requirement. The statement may have been omitted by textbook authors in the didactic transposition process, perhaps as a shortcut to explaining the amount of substance. This omission, however, leads to an incorrect expression of the relationship between the amount of substance and the unit of this, which is the mole.Before 1961, the term ‘mole’ was used to refer to a quantity of something that contained units such as Avogadro’s number (Padilla and Furio-Mas, 2008). Nowadays, the quantity of the amount of substance measures the number of particles contained in it (Milton and Mills, 2009). Although the symbol n is the symbol of the amount of substance, the textbooks generally treat it, in expressions and representations, as if it were the symbol of the mole. For example, in the books reviewed, the equation n = m/MA is presented as an equation to be used to calculate “the number of moles”. This approach in the textbooks clearly ignores the elementary quantity of the amount of substance. Almost a quarter of a century ago, Dierks (1981) called attention to the fact that the mole and the amount of substance were being treated as equivalents. It was also found that the definitions in the books (for example, “the relationship between the number of moles and the mass of the substance is expressed with the equation n = m/MA”) and the questions in the exercises in the topics (for example, “What is the relationship between the number of moles and the total number of atoms?”) included the use of the term “number of moles”. Sometimes, even if the expression “the number of moles” was not used, there were other expressions used to suggest this (for example, “how many moles are there in…?”). The quantity of mass may be expressed in different units (e.g., kilogram, gram or ton). However, to express mass in terms of the number of kilograms, the number of grams or the number of tons would not cross anyone’s mind. It is similarly meaningless to define the amount of substance in terms of “the number of moles” (Strömdahl et al., 1994; Gorin, 2003).
Unless one has a very clear understanding of what the chemical definition of “amount of substance” is, the term is likely to be confusing to students who are apt to think of it as referring to mass, volume, or number of individual pieces. On the contrary, the phrase “number of moles” may appear very specific. One could claim that, given that the mole is the par excellence unit of amount of substance, it is pedagogically sound to use “number of moles” when describing or asking questions about amount of substance. One of the reasons this usage appears in the books is that when introducing the mole concept, the fact that the mole is the unit of the amount of substance has not been adequately emphasized. Similarly, the usage of mass instead of amount of substance regrettably shows that the chemistry textbooks still use the ontological meaning of mole given by Ostwald (Padilla and Furio-Mas, 2008).
What is needed here is a recommendation for a concrete solution. It would undoubtedly be correct for chemistry teachers and textbook authors to replace expressions such as “how many moles are …?” or “calculate the number of moles…” with the expression “calculate the amount of substance…”. The BIPM’s brochure published in 2006, The International System of Units (SI), has stated that the name of the specific substance meant by “substance” in the term “amount of substance” should be used together with the empirical chemical formula for this substance, as in the “amount of hydrogen chloride, HCl” or “amount of hexane, C6H14”. The precise specification of the structure is rendered in this way and is consistent with the second sentence in the definition of the mole.
Another result of the analysis of the chemistry textbooks was the discovery of many expressions that emphasized the equivalence of the amount of substance to other quantities (mass, molar volume, etc.). This is the equivalentists’ view that has remained intact until now, and within which the meaning of “mole” is wrongly associated to meanings such as mass or particles numbers. The use of such kinds of expressions leads to semantic mistakes. Although the mole is a unit of the amount of substance that is used to count the number of particles, no counting is performed when the substance is being measured. The mole should be considered a base unit when it appears in calculations and should not be converted into any other base unit (Gorin, 1994). The term “mole” should never remind the student of “MA”, “22.4 L” or “6.022 × 1023”. Attributing meanings such as “chemical mass” and/or “the number of particles” to the mole is not just something that teachers do or that is encountered in chemistry textbooks. To find that this is also done in reputable academic journals is certainly thought-provoking (Furió et al., 2000).
Still another significant result of the study was the observation that some definitions or explanations in the chemistry textbooks expressed the concept of amount of substance by associating it with more than one physical quantity. Including numerous concepts or units of knowledge or complex expressions in the same sentence adversely affects conceptual and in-depth learning since human beings have a limited visual and audial memory and can only retain a limited amount of comprehensible information (Sweller, 1988; Baddeley, 1992). Students learn a topic in-depth only if they have not been overloaded with visual and/or audial information. The knowledge imparted to students in teaching should be designed so as to avoid an overload on students’ cognitive systems (Mayer, 2003) because learning/retaining is adversely affected by extreme cognitive load (Pekdağ and Le Maréchal, 2007; Winberg and Berg, 2007). Knowledge transposition that is based on intense and complex relationships leads to didactic difficulties.
Using semantically correct examples, definitions and explanations in chemistry textbooks and chemistry courses are of the greatest importance for meaningful teaching and learning of the scientific concepts. It is without doubt that chemistry teachers, students and chemistry textbook authors need to make use of models in which the concept of amount of substance is semantically associated with knowledge at the macroscopic, microscopic and symbolic levels. The models, which have been suggested in this study, may be quite useful in teaching the correct meaning attributed to the amount of substance. Since Model 1 introduces a simple formula with which to use the mole correctly as a unit, the researchers’ belief is that students will be able to readily understand and use this model. When the quantity of the amount of substance is associated with other quantities, a correct expression or representation is certain to be utilized when either Model 2 or Model 3 is implemented.
It has been understood that the importance of amount of substance in providing a bridge between macroscopic, microscopic and symbolic levels has not been sufficiently recognized by either chemistry teachers or textbook authors. The use of these models will ensure that students see meaningful relationships and can easily go back and forth between macroscopic, microscopic and symbolic levels. It can be only in this way that conceptual and meaningful learning can take place (Johnstone, 1991; Gabel, 1999; Treagust et al., 2003; Taber, 2009). Research has shown that students have difficulty in interpreting conceptual explanations but yet are able to keep formulas in their minds and more successful in solving “algorithmic-type” questions rather than “conceptual-type” questions (Azizoğlu et al., 2006; Salta and Tzougraki, 2011). In order to support students’ conceptual learning, teachers should make explicit the differences and link between the different sets of symbols (Taber, 2009).
As a first step in overcoming the obstacles, teachers in particular must first have themselves comprehended the ontological meaning of the amount of substance concept and the didactic transposition the concept has gone through before being ready to be used in a classroom. In a study by Strömdahl et al. (1994), only 3 out of 28 teachers had conceptualized the amount of substance in a manner consistent with SI. The authors believe that this fact sheds light on why the amount of substance concept is difficult to understand. When teachers have a problem with a concept, it is readily understandable that students will be unable to learn that concept meaningfully. Teachers must remember that the amount of substance is the chemical magnitude and “mole” is only its unit. Unfortunately, teachers always talk to their students about “number of mole”, but never talk about “amount of substance”. The quantity of amount of substance must be considered as equally important as other fundamental quantities and this expression should be used instead of “number of moles” (Padilla and Furio-Mas, 2008).
A study by Tullberg et al. (1994) has stated that educators’ own comprehension of the amount of substance plays an important role in making decisions about teaching strategies. It is therefore not surprising to find that students actually “echo” their educators. It can be said, in fact, that it is not sufficient to have teachers change their teaching approaches. To help students increase their scientific knowledge, teachers should design and organize learning environments that stimulate epistemological discussions. The semantic models formulated in this study can be used as a theoretical construct for developing teaching chemistry activities related to the amount of substance concept.
Textbooks remain the most dominant teaching tool in today’s science education (Lin et al., 2010; Bucholtz, 2011; Eisenmann and Even, 2011; Kalman, 2011). There are therefore many unanswered questions to probe into about the role of books and the teaching approaches that those books should adopt. The semantic errors related to the presentation of amount of substance in chemistry textbooks and the difficulties of teaching of this concept have existed for more than a quarter of a century. The semantic models in the present study have been suggested to overcome these difficulties. It is believed that these teaching models will be of immeasurable use to teachers in presenting the subject of the amount of substance to the classroom, to authors introducing the concept in textbooks, and to students in helping them to learn the topic well.
Chemistry textbooks in USA
Masterton, W. L. and Hurley, C. N. (2009). Chemistry: Principles and reactions. 6th Edition. CA: Brooks/Cole, Cengage Learning.Mazza, M., Clancy, C., Heimbecker, B., Mustoe, F., Jansen, M., Finkle, T., Doram, T. and McNulty, P. (2010). Chemistry 11. New York: McGraw-Hill Ryerson.
Silberberg, M. (2009). Chemistry: The molecular nature of matter and change. 5th Edition. New York: McGraw-Hill Higher Education.
Chemistry textbooks in France
Depovere, P. (2006). Chimie générale. 3e Edition. Paris: De Boeck Université.Durand, P., Fort, L., Langrand, C., Pierens, E., Pierens, P. and Prévost, V. (2005). Chimie 1re S. Paris: Nathan.
Le Maréchal, J.-F., Mathevet, S., Vasseur, K., Thoral, J. and Garcia, G. (2005). Chimie 1re S. Paris: Hatier.
Parisi, J.-M., Chapelain, D., Fanjeaux, J., Guêtré, M. and Lambert, D. (2005). Chimie 1re S. Paris: Belin.
Zumdahl, S. S. (2000). Chimie générale. 2e Edition. Paris: De Boeck Université.
Chemistry textbooks in Turkey
Bağ, H. (Ed.) (2010). Genel kimya 1 [General chemistry 1]. 4. Baskı [4th Edition]. Ankara: Pegem A Yayıncılık.Dursun, M. F., Gülbay, İ., Çetin, S., Tek, Ü., Özkoç, F. F. and Güntut, M. (2010). Ortaöğretim 10. sınıf kimya ders kitabı [Chemistry 10]. İstanbul: MEB Devlet Kitapları Dergah Ofset.
Erdik, E. and Sarıkaya, Y. (2007). Temel üniversite kimyası [Fundamental university chemistry]. 19. Baskı [19th Edition]. Ankara: Gazi Kitabevi.
Ergül, S. (2009). Genel kimya [General chemistry]. 2. Baskı [2nd Edition]. Ankara: Anı Yayıncılık.
Mortimer, C. E. (2004). Modern üniversite kimyası 1 [Modern university chemistry 1]. 5. Baskı [5th Edition]. İstanbul: Çağlayan Kitabevi.
Özcan, M. (2000). Modern temel kimya 1 [Modern fundamental chemistry 1]. 2. Baskı [2nd Edition]. Ankara: Nobel Yayın Dağıtım.
Petrucci, R. H., Harwood, W. S. and Herring, F. G. (2010). Genel kimya 1: lkeler ve modern uygulamalar [General chemistry 1: Principles and Modern Applications]. 8. Baskı [8th Edition]. Ankara: Palme Yayıncılık.
References
- Adbo K. and Taber K. S., (2009), Learners’ mental models of the particle nature of matter: A study of 16-year-old Swedish science students. Int. J. Sci. Educ., 31(6), 757–786.
- Abraham M. R., Grzybowski E. B., Renner J. W. and Marek E. A., (1992), Understandings and misunderstandings of eighth graders of five chemistry concepts found in textbooks. J. Res. Sci. Teach., 29(2), 105–120.
- Ainley D., (1991), Mole catchers? Educ. Chem., 28(1), 18–19.
- Akbal E., (2009), Ortaöğretim kimya eğitiminde mol konusunun öğretiminde kavramsal değişim metinlerinin başarıya etkisi [The effects of conceptual change texts in teaching the subject of mol in secondary school success]. Unpublished Master’s Thesis, Marmara Üniversitesi, İstanbul.
- Akçay H., Tüysüz C. and Feyzioğlu B., (2003), Bilgisayar destekli fen bilgisi öğretiminin öğrenci başarısına ve tutumuna etkisine bir örnek: Mol kavramı ve Avogadro sayısı [The effect of computer-aided teaching of science on students’ achievement and attitude: The case of the mole concept and Avogadro’s number]. Turk. Online J. Educ. Technol., 2(2), 57–66.
- Ardac D. and Akaygun S., (2004), Effectiveness of multimedia-based instruction that emphasizes molecular representations on students’ understanding of chemical change. J. Res. Sci. Teach., 41(4), 317–337.
- Azizoğlu N., Alkan M. and Geban Ö., (2006), Undergraduate pre-service teachers’ understandings and misconceptions of phase equilibrium. J. Chem. Educ., 83(6), 947–953.
- Baddeley A., (1992), Working memory. Science, 255(5044), 556–559.
- Baylon C. and Mignot X., (1995), Sémantique du langage [Semantics of the language]. Paris: Nathan.
- Ben-Zvi R., Eylon B. and Silberstein J., (1987), Students’ visualization of a chemical reaction. Educ. Chem., 24(4), 117–120.
- BIPM (Bureau International des Poids et Mesures), (2006), The International System of Units (8th ed). France, Sevres: http://www.bipm.org/utils/common/pdf/si_brochure_8_en.pdf. Accessed 06 March 2012.
- Brousseau G., (1998), Théorie des situations didactiques [Theory of didactical situations]. Grenoble: La Pensée Sauvage.
- Bucholtz K. M., (2011), Spicing things up by adding color and relieving pain: The use of “Napoleon’s buttons” in organic chemistry. J. Chem. Educ., 88(2), 158–161.
- Case J. M. and Fraser D. M., (1999), An investigation into chemical engineering students’ understanding of the mole and the use of concrete activities to promote conceptual change. Int. J. Sci. Educ., 21(12), 1237–1249.
- Cervellati R., Montuschi A., Perugini D., Grimellini-Tomasini N. and Pecori Balandi B., (1982), Investigation of secondary school students’ understanding of the mole concept in Italy. J. Chem. Educ., 59(10), 852–856.
- Chandrasegaran A. L., Treagust D. F. and Mocerino M., (2008), An evaluation of a teaching intervention to promote students’ ability to use multiple levels of representation when describing and explaining chemical reactions. Res. Sci. Educ., 38(2), 237–248.
- Chevallard Y., (1985), La transposition didactique [The didactical transposition]. Grenoble: La Pensée Sauvage.
- Chiu M.-H., (2007), A national survey of students’ conceptions of chemistry in Taiwan. Int. J. Sci. Educ., 29(4), 421–452.
- Claesgens J. and Stacy A., (2003), What are students’ initial ideas about amount of substance? “Is there a specific weight for a mole?”. Paper presented at the Annual Meeting of the American Educational Research Association (Chicago, IL, April, 2003). ED477844.
- Cohen L., Manion L. and Morrison K., (2000), Research methods in education (5th ed.). London: Routledge Falmer.
- Craig M. T. and Yore L. D., (1995), Middle school students’ metacognitive knowledge about science reading and science text: An interview study. Read. Psychol., 16(2), 169–213.
- Daley B., (2010), Concept maps: Practice applications in adult education and human resource development. New Horiz. Adult Educ. Hum. Resour. Dev., 24(2–4), 31–37.
- De Berg K. C., (1986), Text book analysis of the mole and its underlying concepts. A teaching-learning perspective. Aust. Sci. Teach. J., 32(4) 33–43.
- Dierks W., (1981), Teaching the mole. Eur. J. Sci. Educ., 3(2), 145–158.
- dos Santos V. C., Joaquim L. M. and El-Hani C. N., (2012), Hybrid deterministic views about genes in biology textbooks: A key problem in genetics teaching. Sci. Educ., 21(4), 543–578.
- Dori Y. J. and Hameiri M., (1998), The ‘mole environment’ studyware: Applying multidimensional analysis to quantitative chemistry problems. Int. J. Sci. Educ., 20(3), 317–333.
- Duran B. J., Dugan T. and Weffer R., (1998), Language minority students in high school: The role of language in learning biology concepts. Sci. Educ., 82(3), 311–341.
- Eisenmann T. and Even R., (2011), Enacted types of algebraic activity in different classes taught by the same teacher. Int. J. Sci. Math. Educ., 9(4), 867–891.
- Fang Z., (2005), Scientific literacy: A systemic functional linguistics perspective. Sci. Educ., 89(2), 335–347.
- Fang Z., (2006), The language demands of science reading in middle school. Int. J. Sci. Educ., 28(5), 491–520.
- Foster J. M, Cañas F. and Jones M., (2012), Learning conceptual hierarchies by iterated relational consolidation. In N. Miyake, D. Peebles & R. P. Cooper (Eds.), Proceedings of the 34th Annual Conference of the Cognitive Science Society (pp. 324–329). Austin, TX: Cognitive Science Society.
- Fraenkel J. R. and Wallen N. E., (2006), How to design and evaluate research in education (6th ed.). New York: McGraw-Hill International Edition.
- Furió C., Azcona R., Guisasola J. and Ratcliffe M., (2000), Difficulties in teaching the concepts of ‘amount of substance’ and ‘mole’. Int. J. Sci. Educ., 22(12), 1285–1304.
- Gabel D., (1999), Improving teaching and learning through chemistry education research: A look to the future. J. Chem. Educ., 76(4), 548–554.
- Gabel D. L., Sherwood R. D. and Enochs L., (1984), Problem-solving skills of high school chemistry students. J. Res. Sci. Teach., 21(2), 221–233.
- Geban Ö., (1995), The effect of microcomputer use in a chemistry course. Hacettepe Univ. J. Educ., 11, 25–28.
- Gorin G., (1994), Mole and chemical amount. J. Chem. Educ., 71(2), 114–116.
- Gorin G., (2003), Mole, mole per liter, and molar. J. Chem. Educ., 80(1), 103–104.
- Graham I., (1983), Difficulties encountered by biology students in understanding and applying the mole concept. J. Biol. Educ., 17(4), 339–342.
- Halliday M. A. K., (1993a), Towards a language-based theory of learning. Linguist. Educ., 5(2), 93–116.
- Halliday M. A. K., (1993b), The construction of knowledge and value in the grammar of scientific discourse: Charles Darwin’s the origin of the species. In M. A. K. Halliday and J. R. Martin (Eds.), Writing Science: Literacy and Discursive Power (pp. 86–105). Pittsburgh, PA: University of Pittsburgh Press.
- Jensen W. B., (2004), The origin of the mole concept. J. Chem. Educ., 81(10), 1409.
- Johnstone A. H., (1982), Macro- and micro-chemistry. Sch. Sci. Rev., 64(227), 377–379.
- Johnstone A. H., (1991), Why is science difficult to learn? Things are seldom what they seem. J. Comput. Assist. Learn., 7(2), 75–83.
- Johnstone A. H., (2000), Teaching of chemistry – logical or psychological? Chem. Educ. Res. Pract., 1(1), 9–15.
- Joly M., (1994), L’image et les signes. Approche sémiologique de l’image fixe [The image and signs: Semiological approach to static image]. Paris: Nathan.
- Jonnaert P., (1988), Conflits de savoirs et didactique [Conflicts of knowledge and learning]. Paris-Bruxelles: De Boeck Université.
- Kalman C. S., (2011), Enhancing students’ conceptual understanding by engaging science text with reflective writing as a hermeneutical circle. Sci. Educ., 20(2), 159–172.
- Khang G. N. and Sai C. L., (1987). Secondary school students’ difficulties in learning the ‘mole concept’ – A preliminary study in Singapore. Asia Pacific J. Educ., 8(1), 80–88.
- Kozma R. B. and Russell J., (1997), Multimedia and understanding: Expert and novice responses to different representations of chemical phenomena. J. Res. Sci. Teach., 34(9), 949–968.
- Lin C.-Y., Cheng J.-H. and Chang W.-H., (2010), Making science vivid: Using a historical episodes map. Int. J. Sci. Educ., 32(18), 2521–2531.
- Mayer R. E., (2003), The promise of multimedia learning: Using the same instructional design methods across different media. Learn. Instruct., 13(2), 125–139.
- Mills I. M., Cvitas T., Homann K., Kallay N. and Kuchitsu K., (1993), IUPAC, quantities, units and symbols in physical chemistry. Oxford: Blackwell.
- Milton M. J. T. and Mills I. M., (2009), Amount of substance and the proposed redefinition of the mole. Metrologia, 46(3), 332–338.
- Norris S. and Phillips L., (2003), How literacy in its fundamental sense is central to scientific literacy. Sci. Educ., 87(2), 224–240.
- Novick S. and Menis J., (1976), A study of student perceptions of the mole concept. J. Chem. Educ., 53(11), 720–722.
- Pekdağ B. and Le Maréchal J.-F., (2007), Memorisation of information from scientific movies. In R. Pintó and D. Couso (Eds.), Contributions from Science Education Research (pp. 199–210). Dordrecht: Springer.
- Pekdağ B. and Le Maréchal J.-F., (2010), An explanatory framework for chemistry education: The two-world model. Educ. Sci., 35(157), 84–99.
- Padilla K. and Furio-Mas C., (2008), The importance of history and philosophy of science in correcting distorted views of ‘amount of substance’ and ‘mole’ concepts in chemistry teaching. Sci. Educ., 17(4), 403–424.
- Pozo J. I. and Lorenzo M. G., (2009), Representing organic molecules: The use of chemical languages by university students. In C. Andersen, N. Scheuer, M. P. Pérez Echeverría and E. V. Teubal (Eds.), Representational Systems and Practices as Learning Tools (pp. 243–266). Rotterdam: Sense Publishers.
- Raisky C. and Caillot M. (Eds.), (1996), Au-delà des didactiques, le didactique [Beyond teaching, the didactics]. Paris-Bruxelles: De Boeck Université.
- Rastier F., (1991), Sémantique et recherches cognitives [Semantics and cognitive research]. Paris: PUF.
- Reif F. and Larkin J., (1991), Cognition in scientific and everyday domains: Comparison and learning implications. J. Res. Sci. Teach., 28(9), 733–760.
- Russell J. W., Kozma R. B., Jones T., Wykoff J., Marx N. and Davis J., (1997), Use of simultaneous-synchronized macroscopic, microscopic, and symbolic representations to enhance the teaching and learning of chemical concepts. J. Chem. Educ., 74(3), 330–334.
- Salta K. and Tzougraki C., (2011), Conceptual versus algorithmic problem-solving: Focusing on problems dealing with conservation of matter in chemistry. Res. Sci. Educ., 41(4), 587–609.
- Schmidt H. J., (1990), Secondary school students’ strategies in stoichiometry. Int. J. Sci. Educ., 12(4), 457–471.
- Seah L. H., Clarke D. J. and Hart C. E., (2011), Understanding students’ language use about expansion through analyzing their lexicogrammatical resources. Sci. Educ., 95(5), 852–876.
- Staver J. R. and Lumpe A. T., (1993), A content analysis of the presentation of the mol concept in chemistry textbooks. J. Res. Sci. Teach., 30(4), 321–337.
- Staver J. R. and Lumpe A. T., (1995), Two investigations of students’ understanding of the mole concept and its use in problem solving. J. Res. Sci. Teach., 32(2), 177–193.
- Strömdahl H., Tullberg A. and Lybeck L., (1994), The qualitatively different conceptions of 1 mol. Int. J. Sci. Educ., 16(1), 17–26.
- Sweller J., (1988), Cognitive load during problem solving: Effects on learning. Cog. Sci., 12(2), 257–285.
- Taber K. S., (2000), Molar and molecular conceptions of research into learning chemistry: Towards a synthesis. Variety in Chemistry Teaching Meeting organised by the Royal Society of Chemistry Tertiary Education Group with the Chemical Education Research Group. University of Lancaster, 5th September, 2000. Full text available at http://www.rsc.org/images/2000-ktaber_tcm18-49179.pdf. Accessed 27 February 2012.
- Taber K. S., (2001), Building the structural concepts of chemistry: Some considerations from educational research. Chem. Educ. Res. Pract., 2(2), 123–158.
- Taber K. S., (2009), Learning at the symbolic level. In J. K. Gilbert & D. Treagust (Eds.), Multiple Representations in Chemical Education (pp. 75–105). Dordrecht: Springer.
- Talmy L., (2000), Fictive motion in language and “ception.” In L. Talmy (Ed.), Toward a Cognitive Semantics (Volume I, pp. 99–175). Cambridge, MA: MIT Press.
- Tan A. H. and Soon H. S. V., (1996), Concept hierarchy memory model: A neural architecture for conceptual knowledge representation, learning, and commonsense reasoning. Int. J. Neural Syst., 7(3), 305–319.
- Treagust D. F., Chittleborough G. and Mamiala T. L., (2003), The role of submicroscopic and symbolic representations in chemical explanations. Int. J. Sci. Educ., 25(11),1353–1368.
- Tullberg A., Strömdahl H. and Lybeck L., (1994), Students’ conceptions of 1 mol and educators’ conceptions of how they teach ‘the mole’. Int. J. Sci. Educ., 16(2), 145–156.
- Uhden O., Karam R., Pietrocola M. and Pospiech G., (2012), Modelling mathematical reasoning in physics education. Sci. Educ., 21(4), 485–506.
- Ünlü Y., (2006), The effect of explicit method of problem solving accompanied with analogies on understanding of mole concept. Unpublished Master’s Thesis, Middle East Technical University, Ankara.
- van Eijck M., Goedhart M. J. and Ellermeijer T., (2011), Polysemy in the domain-specific pedagogical use of graphs in science textbooks: the case of an electrocardiogram. Res. Sci. Educ., 41(1), 1–18.
- Vygotski L. S., (1934/1997), Pensée et langage [Thought and language] (3rd ed.). Paris: La Dispute.
- Wellington J. and Osborne J., (2001), Language and literacy in science education. Philadelphia, PA: Open University Press.
- Winberg T. M. and Berg C. A. R., (2007), Students’ cognitive focus during a chemistry laboratory exercise: Effects of a computer-simulated prelab. J. Res. Sci. Teach., 44(8), 1108–1133.
- Yiğit D., (2010), Lise öğrencilerinin “tanecik sayısı” ve “mol” kavramlarını öğrenmede karşılaştıkları zorluklar [Difficulties in learning the concepts of “number of particle” and “mole” of high school students]. Unpublished Master’s Thesis, Selçuk Üniversitesi, Konya.
- Yore L., Hand B., Goldman S., Hildebrand G., Osborne J., Treagust D. and Wallace C., (2004), New directions in language and science education research. Read. Res. Quart., 39(3), 347–352.
| This journal is © The Royal Society of Chemistry 2013 |

