Exploring conceptual frameworks of models of atomic structures and periodic variations, chemical bonding, and molecular shape and polarity: a comparison of undergraduate general chemistry students with high and low levels of content knowledge
Chia-Yu
Wang
*a and
Lloyd H.
Barrow
b
aInstitute of Education, National Chiao Tung University, 1001 Ta-Hsueh Road, Hsinchu, Taiwan. E-mail: cwg25@mail.nctu.edu.tw
bScience Education Centre, University of Missouri-Columbia, 321 Townsend Hall, Columbia, MO, USA. E-mail: barrowl@missouri.edu
First published on 3rd January 2013
Abstract
The purpose of the study was to explore students’ conceptual frameworks of models of atomic structure and periodic variations, chemical bonding, and molecular shape and polarity, and how these conceptual frameworks influence their quality of explanations and ability to shift among chemical representations. This study employed a purposeful sampling technique and used three diagnostic instruments for conceptual understanding to determine the students’ level of content knowledge of the related concepts. Six student interviews were analyzed to portray students’ conceptual frameworks in high and low content knowledge (HCK and LCK, respectively) groups. The study’s major findings revealed that moving from a high toward a low level of content knowledge, the quality of students’ explanations declined, as did their ability to reconcile new information to their existing knowledge frameworks. Three essential concepts – models of atomic structure, effective core charge and principles of electrostatic force, and quantum mechanics descriptions – were identified that may explain students’ failure to learn the necessary aspects of molecular geometry and polarity. This study provides empirical evidence of how students’ content knowledge influences their understanding about molecular polarity. The findings have implications for college chemistry education with respect to teaching concepts about molecular polarity.
Introduction
Stevens et al. (2010) considered that traditional instruction and assessments often address learning using a piecemeal approach of isolated knowledge and urged future studies to examine how students incorporate and connect ideas in their conceptual frameworks. The present study explored undergraduate general chemistry students’ framework of conceptions and propositions regarding models of atomic structure, periodic trends, chemical bonding, and molecular shape and polarity. These concepts represent a significant portion of the major ideas in the grade 7–12 curricula (Stevens et al., 2010). Also, these concepts involve several unifying concepts (e.g., matter and energy, interactions, models as explanations, evidence, and representations) that the College Board Standards for College Students: Science (a US document published by College Board, 2009) specifies as rigorous knowledge students need to develop for college and their future careers. An understanding of these concepts is also a prerequisite for learning more advanced concepts, including intermolecular forces, properties of solutions, acids and bases, and organic chemistry. Stevens et al. (2010) described learning progression as how students can move towards more sophisticated understanding of the big ideas of science. The move towards expertise requires building a more complex idea upon the understanding of some underpinning knowledge, and incorporating more ideas and connecting to ideas of other related topics. However, previous research has indicated that many students are at a low level in the learning progression and do not possess an adequate understanding required for postsecondary chemistry courses (e.g.Taber, 2003a; Stevens et al., 2010). Although various diagnostic instruments have been developed to investigate students’ alternative conceptions, these alternative ideas or lack of some essential concepts are often addressed as isolated concepts among discrete topics, neglecting the interrelated relationships among the concepts. She (2004) urged that a series of underpinning conceptions lacking in students’ conceptual framework needs to be identified and addressed in order for radical conceptual change to occur. Thus, we felt that research must extend the area of investigation from examining students’ knowledge about a single concept to their conceptual frameworks to reveal relationships between and among related concepts and the impact of those frameworks on student learning. For instructional purposes, analyzing the characteristics of conceptual frameworks possessed by students and how the characteristics of these conceptual frameworks may influence their explanations of the related concepts may help chemistry instructors better predict students’ thinking and reasoning. Also, revealing differences in conceptual frameworks (e.g., content and structure) between students with high and low levels of content knowledge, and pinpointing some key ideas lacking in the conceptual frameworks of the LCK group, may help identify essential concepts that need to be stressed to bridge learners’ understandings to the next level.Investigating characteristics of conceptual frameworks
In the present study, we define conceptual framework as an individual’s background knowledge of a specific topic that consists of a network of interrelated assumptions, knowledge, and beliefs. These conceptual frameworks vary among individuals, and students select ideas or strategies from their conceptual frameworks to support their reasoning and influence about a phenomenon (Posner et al., 1982; Vosniadou, 1994; Jones et al., 2000; Chi, 2008; Talanquer, 2009). Some researchers portray this network of knowledge as having some characteristics of hierarchies, which implies that the learning of some underpinning concepts may influence the understanding of a concept at the higher levels of the hierarchy (Vosniadou, 1994; She, 2004; Chi, 2008). Other researchers consider these conceptual frameworks to be more fragmented, consisting of pieces of knowledge (diSessa, 1993). The assumptions, ideas, and beliefs contained in an individual’s conceptual framework may constrain his or her way of thinking and selection of cues, thus guiding his or her reasoning about a phenomenon (Talanquer, 2009). As a consequence, incorrect, vaguely defined, and missing or fragmented knowledge of this conceptual framework may also influence the quality of the individual’s explanation (Jones et al., 2000; Taber, 2003a). Likewise, learning impediments may result from two circumstances when students do not recognize the relevance of the new information to their existing knowledge and when students do not hold prerequisites that anchor the new information to this network of knowledge (Taber, 2005). For instance, Park and Light (2008) indicated that failing to grasp concepts about probability and energy quantization may underpin difficulties that hinder college general chemistry students to achieve the targeted level of understanding about models of atomic structure. Additionally, existing knowledge may not be applicable to new situations if their ideas are fragmented and lack relational organization (Novak, 2002; Stevens et al., 2010).Within this conceptual framework, both alternative and scientific models of the same concept can coexist, though they are cued by different task features (Vosniadou, 1994; Mortimer, 1995; Chi, 2008). Mortimer (1995) described how students may hold manifold models about a specific concept. Expert students are conscious (a metacognitive awareness) of the distinct features among different models and are able to consider which model has the highest conceptual power within a specific context or problematic situation (Mortimer, 1995). Therefore, differences between experts’ and novices’ reasoning may not be entirely attributed to the differences in the content and structure of their conceptual frameworks (Gupta et al., 2010). Rather, as experts consciously resolve discrepancies within their conceptual frameworks, they possess a well-organized and contextualized knowledge that is readily accessible and provides flexibility when forming explanations (Stevens et al., 2010). In contrast, novices may not reach the level of metacognitive awareness required to structure their conceptual frameworks or to reconcile conflicting ideas within their conceptual frameworks; thus, their conceptual frameworks remain only partially coherent or fragmented (Clark et al., 2011). Park and Light (2008) suggested that researchers should explore the threshold of understanding and the degree of difficulty of a concept by investigating and comparing the different characteristics of the conceptual frameworks held by novices and experts in detail. In this manner, instructors and researchers can become more aware of the threshold of understanding that students need to overcome, and thus, they can prepare appropriate instructions that help students overcome the problems encountered while learning specific conceptions.
Molecular polarity subsumes several underlying concepts, including (1) periodic variation (including models of atomic structure), (2) chemical bonding, (3) electronegativity, and (4) molecular geometry. Because it is a concept at the higher level of the hierarchy and is abstract in nature, it is a difficult concept for students to understand. Furthermore, the conceptualization of the concept requires students to possess a high level of visual-spatial thinking. Research on this topic, however, is limited to identifying chemistry students’ common misconceptions about molecular polarity and its prerequisite concepts (Peterson et al., 1989; Peterson and Treagust, 1989; Nicoll, 2001; Jang, 2003). Only Furió et al. (2000) have attributed one source of learning impediments to students’ common sense reasoning.
To address this gap in the research, the goal of this study was to characterize undergraduate general chemistry students’ conceptual frameworks regarding models of atomic structure, periodic variations, chemical bonding, and molecular shape and polarity. We interviewed students from high and low level of content knowledge groups and investigated their conceptual frameworks when explaining a series of phenomena related to the targeted concepts of the study. The identification of characteristics of conceptual frameworks for each group can help us to understand students’ levels of conceptual understanding and how students at the different levels of expertise explain and reason with the targeted concepts. We also aimed to find distinctions between characteristics of students at the two levels of expertise and to identify essential elements that lower-level students lack in their conceptual frameworks and the underlying implicit assumptions that constrain students’ thinking. The findings of the present study may provide insights into designing a more fruitful instructional approach than directly addressing isolated alternative conceptions individually.
This study utilized a purposeful sampling technique to select three participants each from the high- and low-scoring groups based on their scores on three diagnostic instruments about molecular polarity and its prerequisite concepts. These six participants were interviewed to investigate their utilization of existing conceptual frameworks while solving a series of problems. While investigating students’ conceptual frameworks, we specifically assessed their quality of explanations and their use of chemical representations.
Methods
Context of the study
This study took place in the second course of a three-course general chemistry sequence at a Midwest research-extensive institute in the United States. We adapted three instruments from the science education literature to diagnose the level of understanding and the misconceptions possessed by college students regarding molecular polarity. These three diagnostic instruments included (1) a diagnostic instrument on electronegativity (EN instrument) (adopted from the ionization energy probe in Taber, 2002), (2) a two-tier diagnostic instrument on chemical bonding (CB instrument) by combining selected items from Peterson et al., (1989) and Jang (2003), and (3) a diagnostic instrument on molecular geometry and polarity (GP instrument) (Peterson et al., 1989; Furió et al., 2000). These three diagnostic instruments served three purposes: First, to provide background information of commonalities between learners in terms of their level of understanding of the prerequisite concepts of molecular polarity, and their potential misconceptions. Taber (2000) suggested that data from diagnostic instruments provides information about ideas that students commonly hold in the class. The second purpose is to serve as a sampling technique to select participants for interviews. This increases the generalisability if case studies include other students who are not involved in the interviews (Taber, 2000). The third purpose is to serve for data triangulation. Participants’ responses on the three diagnostic instruments were used to triangulate findings from the interview analysis and interpretation. We removed items from the original instruments if they did not directly address molecular polarity or its prerequisite concepts. Each instrument was administered to the entire class as a concept exercise on a BlackBoard system after corresponding topics were introduced. Participation in the concept exercise was voluntary and did not influence students’ course grades. Students were encouraged to do each concept exercise to clarify their understanding of the related topics, and the BlackBoard system provided feedback as “correct” or “incorrect” when students responded to each item. All students could work from home and complete each instrument more than once, but their final score was used for sampling.We constructed a concept map for molecular polarity (Fig. 1) to illustrate the relationships between and among the concepts based on the primary text for the chemistry course – Chemistry (Chang, 2005). The components and structure of the concept map were validated by a panel of experts, which included a chemistry faculty member and three science education faculty members. Four major concepts are considered essential for understanding molecular polarity: models of atomic structure and periodic variation, chemical bonding, and molecular geometry and polarity.
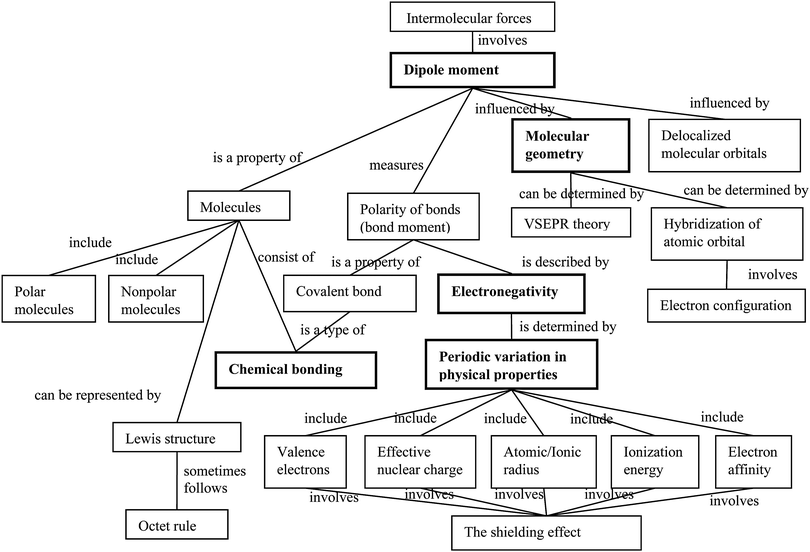 | ||
| Fig. 1 Concept map for molecular polarity (based on Chang, 2005). The bold rectangles are the key concepts addressed in the present study. | ||
Sampling technique
A total of 250 students registered for the course, of whom 159 granted permission to use their data from the diagnostic instruments. The results of the quantitative analysis regarding the 159 students’ responses to the three diagnostic instruments have been reported elsewhere (in Wang, 2007). Among the 159 students, 48 volunteered to participate in the interviews. We divided the volunteer students based on their scores on the three diagnostic instruments. Participants who ranked in the top 30% or lower 50% on two out of the three diagnostic instruments were considered to be in the high and low conceptual knowledge (HCK and LCK, respectively) groups, respectively. Considering the scoring method of the two-tier items (students had to answer both tiers correctly to receive one point for each two-tier item), distributions of students’ scores for the diagnostic instruments were skewed toward lower scores. Also, scores were tightly bunched for students in the lower 50%. Thus, we used the lower 50% rather than the lower 30% as the cut-off point to help us select more representative cases that depict the characteristics of the conceptual frameworks of the lower half of the learners. The HCK students were considered individuals who possess more prerequisite knowledge about molecular polarity as they had more successful performances on all three diagnostic instruments. The LCK students represented the individuals who encounter difficulties in correctly responding to the diagnostic items. Among the 48 volunteers, students who met the criteria for the HCK and LCK groups were invited, and three students from each group were interviewed.The interview
A combination of think-aloud protocol and interview-about-events (White and Gunstone, 1992) was used to collect data of students’ explanations and their constructions of artefacts (including drawings and model constructions). The interview protocol was developed by adopting some questions from the literature regarding investigations of students’ conceptual understandings about atoms and molecules (Taber, 2002) and chemical bonding (Nicoll, 2001). We began the interview by eliciting a participant’s ideas about atomic structure, periodic variations, and procedures to derive a Lewis structure from a chemical formula. Participants were then asked to build a model that represented the shape of a molecule from the Lewis structure, followed by questions prompting him or her to describe features of the model, including chemical bonds, electron distributions, and interactions (e.g. attraction or repulsion) among electron pairs. Students were then asked to determine the polarity of the molecule. Throughout this process, each participant was prompted to describe their thinking processes or strategies, such as memorizing definitions and statements or using routines of problem-solving strategies in his or her mind. We asked participants consistently whether or not they used a mental image while thinking through the tasks, and probed for details about features of their mental images by encouraging them to describe and draw on paper, or build models using play-dough and straws. We also specifically probed for meaning when participants used a specific term and what concepts they associated with each question. All interviews were video-recorded and were transcribed verbatim for analysis.The data in this study included the participants’ responses to the three diagnostic instruments, the student-generated diagrams and models, and the verbal explanations accompanying their diagrams and models. A related article regarding the actions of the students in applying their mental models and their ability to manipulate the mental models to solve tasks was reported in Wang and Barrow (2011).
Within-case analyses of participants’ conceptual frameworks
The present study looked for identifying characteristics (e.g., quality and structure) of an individual’s conceptual frameworks while providing explanations of targeted concepts and what attributed to the variations in the quality of their explanations. Because of our interest in the process of sense-making in one’s mind, we adopted a personal constructivist lens, believing that an individual applies existing knowledge to make sense of a new concept and to incorporate it into their existing knowledge (Ferguson, 2007). We also believe that an individual’s explanations are strongly associated with and need to be interpreted within the individual’s overall structure of existing knowledge.We conducted case studies employing a hermeneutical cycle that allowed us to see a participant’s meaning-making experience by interpreting the dialogues and artefact productions in his or her global meaning of a specific concept (Patton, 2002). The grounded theory approach, employing a constant comparative method, allowed us to examine emergent patterns, themes and categories within and across cases. Semi-structured interview protocols guided each participant as they worked through the same set of thought-revealing tasks, thereby providing a base to compare thinking processes within and across cases. Explanations that students offer provide information about how they organize, relate, and integrate concepts and principles to a specific mode while thinking about interview questions.
To develop each case, we reviewed interview videos and applied an open coding process (Strauss and Corbin, 1998) to students’ explanation and nonverbal data (e.g. drawings and construction of models). Based on the interview questions, the transcripts were divided into three sections about (1) models of atomic structure and periodic variations, (2) chemical bonding, and (3) molecular shape and polarity. We began with the videos of three HCK students (Hugh, Heidi, and Harry), working section by section, and carefully read through each transcript to conceptualize and to extract the conceptual elements (e.g., protons, atomic number, numbers of valence electrons, etc.) used by the HCK students, and then used these elements as nodes of their conceptual frameworks. Their verbal explanations of relationships or propositions connecting the conceptual elements were used as bases to draw links between the conceptual elements. Three conceptual frameworks about (1) models of atomic structure and periodic variation, (2) chemical bonding, and (3) molecular shape and polarity were yielded from the above process for each HCK student. We then revisited their videos and conducted microanalysis (Strauss and Corbin, 1998) to compare the meanings of the conceptual elements and links used by the three HCK students to verify the appropriateness of our labelling of the conceptual elements and links in their conceptual frameworks. Individual participants’ responses to the three diagnostic instruments were included to triangulate the mapping of the conceptual frameworks. Strauss and Corbin (1998) suggested that researchers consider the interplay between qualitative and quantitative methods to inform the emergence of a theory. Most of the conceptual elements and links shared the same labels among the conceptual frameworks of the three HCK students. If a participant possessed a specific explanation that was not shared by other students in the group, the corresponding labels were reassigned or modified to address the meanings unique to the individual during the within-case comparisons.
When analyzing the transcripts of the LCK students, we noticed that their explanatory patterns were different from those of the HCK students. In addition, some conceptual elements and explanatory links identified in the HCK group were missing from the explanations of the LCK students. Thus, we decided to use the conceptual elements and links synthesized from the HCK students’ conceptual frameworks as initial criteria for analyzing the LCK students’ (Lisa, Luke, and Larry) interviews. This may help us depict the differences in the conceptual frameworks between the HCK and LCK groups. We visited individual interviews of the LCK students and analyzed their use of explanatory propositions and patterns of reasoning to examine whether some conceptual elements or links were found or were missing from their explanations. We also identified alternative conceptions or explanatory principles used by the LCK students. Representations of conceptual frameworks for each LCK student were constructed by keeping the conceptual elements and links similar to those of the HCK students, adding the elements and links of alternative explanations, as well as indicating missing elements and links to illustrate features of their quality of understanding. The aforementioned process yielded 18 drawings of conceptual frameworks, three for each interview participant, for later analysis.
We further compared the six participants’ conceptual frameworks within and across the HCK and LCK groups and developed a first draft of categories and dimensions (Strauss and Corbin, 1998) illustrating characteristics of the conceptual frameworks for the HCK and LCK groups. We retested each characteristic of the HCK group against evidence from the interviews of Hugh, Heidi, and Harry. The same validation procedure was performed to examine the characteristics of the LCK students against their interviews. We also contrasted the characteristics of the LCK students with those of the HCK students by revisiting the interview videos of the HCK students, and vice versa. The reviewing and recoding processes were repeated until the generated statements about the characteristics of their conceptual frameworks were saturated. The aforementioned process yielded three sets of characteristics of conceptual frameworks regarding models of atomic structure and periodic variations, chemical bonding, and molecular shape and polarity (please see Tables 1–3).
| HCK (Hugh, Heidi, Harry) | LCK (Lisa, Luke, Larry) | |
|---|---|---|
| Models of atomic structure | • Considered effective core charge and its interaction with electrons (electrostatic force) | • Neglected the influence of effective core charge and interactions of electrostatic force between the nucleus and electrons |
| • Bohr model possessed detailed features of electron shells and the number of valence electrons | • Concepts about electron shells and energy levels were missing from their Bohr model or | |
| ∘ Associated electron shells with energy levels | ∘ Concepts about electron shells were present but not associated with energy levels | |
| • 3D electron-cloud model | • 2D electron-cloud model (as a circle) | |
| ∘ Explained using quantum mechanics descriptions | ∘ Did not use quantum mechanics descriptions | |
| • Used Lewis dot structure, if necessary | • Lewis dot structure was the preferred mental model | |
| Periodic variations | • Descriptions included the numbers of total electrons and valence electrons | • Same as the HCK groups |
| • Descriptions included the numbers of neutrons and protons | • The numbers of neutrons and protons were missing | |
| • Justified electronegativity using electrostatic forces | • Used an algorithm and memorization for electronegativity without justification | |
| • Related trends of atomic radius to the numbers of neutrons and protons | • Used an algorithm to recall the trend of atomic radius without justification | |
| • Justified the trends of reactivity with the octet rule | • Did not include descriptions about reactivity |
| HCK (Hugh, Heidi, Harry) | LCK (Lisa, Luke, Larry) | |
|---|---|---|
| Chemical bonding | • Chemical bonding was viewed as an electrostatic force between two atoms | • Chemical bonding was viewed as some type of (attractive) force between two atoms |
| • Justified the formation of bonding with the octet rule and stability | • Justified the formation of bonding with the octet rule and stability or with teleological explanations | |
| • Comfortable with exceptions to the octet rule | • Not comfortable with exceptions to the octet rule or possessed misconceptions |
| HCK (Hugh, Heidi, Harry) | LCK (Lisa, Luke, Larry) | |
|---|---|---|
| Molecular shape and polarity | • Used electron probability to explain a polar bond | Some students could use an electron-cloud model to conceptualize a polar bond but others explained a polar bond as a pulling of forces by drawing arrows to show its direction |
| ∘ Associated bond polarity with differences in electronegativity | ∘ Same as the HCK students | |
| • Steps used to determine geometry and polarity | • Steps to determine geometry and polarity | |
| 1. Used the VSEPR model to determine the arrangement of electron pairs in 3D and justifies using electrostatic force | 1. Used an algorithm or memorization to determine molecular shape or to consider arrangement of electron pairs on the basis of 2D Lewis structure | |
| 2. Applied propositions of direction of pulls to determine the structure, then cancels out the pulls spatially and determines molecular polarity | 2. Same as the HCK students |
Cross-case analyses of participants’ conceptual frameworks
Next, we performed axial coding (Strauss and Corbin, 1998) to look for answers to three questions regarding (1) how the conceptual frameworks of the HCK and LCK groups were similar to and different from each other, (2) whether the quality of the student explanations varied along with the quality of their conceptual frameworks, and (3) what key concepts were missing from the LCK students’ conceptual frameworks that may impede them from learning molecular shape and polarity successfully. We compared the synthesized characteristic statements and representations of the conceptual frameworks for both groups to depict the differences in the structure and quality of their conceptual frameworks. We also revisited the interviews to relate the differences in structure and quality of the conceptual frameworks for both groups to differences in their explanatory process. A set of characteristic statements was developed to illustrate features of conceptual frameworks for both groups (please see Table 4). We then verified these characteristic statements by constantly revisiting the interview videos of the six participants. The axial coding process resulted in four assertions to provide explanations for the aforementioned three questions in the discussion section.| HCK (Hugh, Heidi, Harry) | LCK (Lisa, Luke, Larry) | |
|---|---|---|
| Features of conceptual framework | • Conceptual frameworks were coherent and consisted of precise, accurate conceptions, with only few misconceptions or missing conceptions | • Some students’ conceptual frameworks were semi-coherent with some conflicting concepts, or they were fragmented and contained many misconceptions and/or missing concepts |
| • Justified their explanations using appropriate concepts | • Many concepts had no justifications or were justified using common-sense reasoning (teleological or anthropomorphic explanations) | |
| • Had the ability to reconcile and switch between models | • Had the ability of reconcile and switch between models | |
| ∘ Reconciled different models in the conceptual framework; sometimes had a hybrid model | ∘ Some students were satisfied with partially accurate propositions, and others adhered to algorithmic strategies and personal theories; therefore, different models and disjointed conceptions were not reconciled. Instead, they used models that “pop-up in my head” and used predominately 2D Lewis structures, but they perceived these structures as collections of letters, lines, and dots | |
| ∘ Models were functional for explanation or problem-solving | ||
| • Associated appropriateness of using models or representations with the context or the problem | • Were not able to select different models according to the context of the problem |
In the following sections, examples from students’ excerpts are provided to portray their understanding at each level and to show the differences among the characteristics of students’ conceptual frameworks.
Findings
The findings of the within-case comparisons are divided into three sections: (1) models of atomic structure and periodic variations, (2) chemical bonding, and (3) molecular shape and polarity. In each section, the content and quality of students’ conceptual frameworks for both the HCK and LCK groups are presented. Students’ excerpts, drawings, and misconceptions identified from the diagnostic instruments are used to portray their understandings at each level. At the end of each section, we present a summary table for the characteristics of the students’ conceptual frameworks to illustrate the similarities and differences between the two student groups.Models of atomic structure and periodic variations
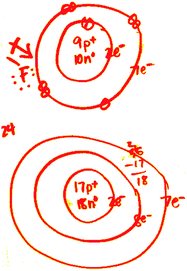 | ||
| Fig. 2 Heidi’s drawings of a fluorine atom and chlorine atom. | ||
The octet rule was an underpinning idea when Heidi and Harry described “highly reactive” as a similar characteristic for a fluorine atom and chlorine atom. Heidi and Harry both considered that a fluorine atom and chlorine atom react very similarly because of their tendency of “getting one valence electron to fill the outer shell” (Harry, interview). Heidi gave a similar reason, attributing the reactivity of a fluorine atom and chlorine atom:
[If] you have a sodium ion, it is going to really easily react with either one of these [fluorine and chlorine] because it has one valence electron and both of these have seven. To get eight in the outer shell, it is going to really easily react. (Heidi, interview)
To Heidi and Harry, removing a valence electron or adding one more, resulting in eight electrons in the outer shell, becomes a driving force to justify the strong reactivity for alkali metals and the halogen group.
In addition, these HCK students preferred using the Bohr model when they drew and explained atomic structures. Furthermore, they visualized an atom in three dimensions (3D) with an electron-cloud surrounding the nucleus (Heidi and Harry, interview) or a ball with layers of concentric orbitals (Hugh, interview). All of the HCK students were able to describe their idea of an electron-cloud model using quantum mechanics descriptions.
The centre is the nucleus, and then the first ring has two electrons because that is all it could hold. The outer ring has seven valance electrons, and I put nine [beside the Bohr model] because it has nine electrons all together. (Lisa, interview)
When describing a chlorine atom, Lisa simply added another shell to the fluorine atom and completed the numbers of total valence electrons, disregarding the change in the numbers of protons and neutrons. Luke preferred the simple Lewis dot structures that elicited only the number of valence electrons on the outer shell (Fig. 3). Larry used a special notion to indicate the numbers of protons, neutrons, and total electrons (Fig. 3). Larry’s drawing also revealed his partial understanding of the atomic structure of chlorine by mistakenly indicating its number of neutrons as 17. The LCK students revealed limited or partial understanding of atomic structure models. Consider Luke’s descriptions about the similarities and differences between a fluorine atom and chlorine atom:
 | ||
| Fig. 3 Drawings of a fluorine atom and chlorine atom by two LCK students. | ||
The first things that come to my mind are the Lewis structures. That is how I differentiate between the differences. I do not really know how to differentiate between elements in the group besides just like a bigger circle basically, just because of the atomic radius… (Luke, interview).
This deficient understanding was also evident in their responses to items on the EN instrument, as all of the LCK students shared several misconceptions about the interactions between electrostatic forces. For example, the LCK students did not associate an inner electron shell with a lower energy level (EN-9) or reduced electron-shielding effect (EN-7). Each of the LCK students’ responses to some items indicated misconceptions about Coulombic principles for the nucleus–electron interactions (EN-6, 8, 13, 14, and 16).
This insufficient understanding about the atomic structure is a disadvantage regarding these students’ comprehension of periodic trends. By using the periodic table, the LCK students could indicate the number of valence electrons and the relative electronegativity for hydrogen, sulfur, boron, fluorine, and chlorine atoms, but the trends of reactivity, atomic radius, and ionization energy were missing from their descriptions. Accordingly, the LCK students conceptualized the periodic trends as general assumed rules and memorized the trends of periodic variations algorithmically because the role of positive core charge was missing from the LCK students’ conceptual frameworks. Larry’s descriptions about his strategy provide a clear example of these shortcomings:
Just with the [imagined] periodic table being in front of me, I am seeing arrows, like less electronegativity from left goes [right], from this [bottom] and this [to the top]; and the size goes from this to this; the radius, atomic radius. I just go through like a checklist. It is just something memorized and applied. (Larry, interview)
The links between the properties of elements in the periodic trends and the characteristics of each element’s atomic structure were missing in these students’ conceptual frameworks.
In addition, the descriptions of the quantum mechanics were absent from the LCK students’ descriptions, and the students had not reconciled the descriptions with the electron-cloud model. Due to a lack of confidence in their understanding about these two models, the LCK students used a Lewis dot model as their preferred mental model. The characteristic statements about the conceptual frameworks of the HCK and LCK groups regarding atomic structure and periodic trends are provided in Table 1.
Chemical bonding
I think of covalent and ionic bonds and just how the electrons are negative and the protons are positive and how those attract and repel. With bonding, we are going to have to talk about how they attract, how the electrons and the protons attract each other. (Hugh, interview)
When the HCK students reconciled the concept of electrostatic force with the ideas of covalent bonding and ionic bonding, they also used the concept that “atoms want to fulfil the octet rule” as an underlying presupposition to justify the formation of ions. The electrostatic force was then attributed to the attractions between cations and anions. Hugh’s explanations implied that ionic bonding is essentially electron-transfer:
[Electrons] can be given up to make a complete orbital that, I guess, is more stable and at the same time creates ions that one would be negative, one would be positive, and that is the reason that they bond because the difference in charges. (Hugh, interview)
The underpinning presupposition about the octet rule was evident in the HCK students’ responses to the EN instrument. They justified the octet rule with an alternative notion of stability. They held a misconception thinking that a sodium atom would be more stable if it “lost an electron” (EN-18) or “gained seven electrons” (EN-20). However, Heidi and Hugh had a correct understanding that “energy is required to remove an electron from the atom” (EN-1), stating that, “the atom will not spontaneously lose an electron to become stable” (EN-3). They also rejected the statement that “if the outermost electron is removed from the [sodium] atom, it will not return because there will be a stable electron configuration” (EN-12). Heidi and Hugh were neither aware of nor did they reconcile these two conflicting ideas by associating them with the octet rule. In Harry’s case, the octet rule was an underlying presupposition that he consistently applied throughout these items.
The HCK students extended this notion of the octet rule to explain formations of covalent bonds. For these students, forming a bond and drawing a Lewis structure of a given chemical formula was a way to share or give up valence electrons from each atom to achieve a stable noble gas electron configuration. For example, when Hugh explained the Lewis structure for hydrogen sulfate (H2S) as follows:
Whenever you are bonding, you want a complete orbital, so you want to achieve, which is this last column [of the periodic table]. So, if you want to get eight [electrons], you have to find a way to share or give up electrons from each of these [atoms] so that each orbital is as complete as it can be. (Hugh, interview)
 | ||
| Fig. 4 Larry’s drawing of chemical bonding. | ||
The charges. Like the cations and anions, opposites attract. In that way, the charges have to be balanced. Like hydrogen is + 1 and fluorine is -1 so those match up. The charges would be the same, and they like to be together. (Larry, interview)
This excerpt indicates that Larry possessed a misconception, thinking that the same amount of opposite charge would match. He also provided an incorrect example by describing the bond between hydrogen and fluorine as an ionic bond. As the concepts that the LCK students described were fragmented and inconsistent, these students neither connected their concepts in a meaningful manner nor supported their explanations using appropriate propositions. As Larry said,
I do not think I have ever thought of this so conceptually before. I just seem to plug it on and try it, I never thought about it in much detail like this. (Larry, interview)
A low level of understanding about chemical bonding was evident in several places in the LCK students’ responses to the CB instrument. The LCK students, for example, related the idea of ionic bonding as electron transfer to perceiving NaCl as an ion-pair molecule (e.g. perceiving NaCl as a molecule, CB-6a). They also shared the same misconception that “electrons are shared equally between two atoms in a polar covalent bond (CB-1ab)” without considering electronegativity differences between the two bonded atoms. The LCK students considered the octet rule as a special rule to be followed. Furthermore, they provided teleological explanations (Talanquer, 2007) to justify the formation of chemical bonds as well as the formation of a chemical compound. For instance, Luke and Lisa thought that “every element wants a full octet. They want eight electrons to be stable. Because the noble gases are the most stable, and they have eight electrons” (Lisa, interview). These students overgeneralized the octet rule and thought that “every element on the periodic table wants to try and be similar to the noble gases, which already have eight valence electrons” (Luke, interview). Although Larry was aware of the nucleus–electron interactions when he talked about atomic models, he did not apply this concept when conceptualizing the octet rule. The LCK students’ responses to the CB instrument revealed a strong commitment to the octet rule (CB-3, 4, 12, 18, and 20), which was consistent with their expressions during the interviews. To these students, the octet rule was the rule to follow, while the exceptions to the octet rule were cases to memorize. As a result, the LCK students would attempt to apply the octet rule to explain the structure of a BF3 molecule and felt uncomfortable accepting the BF3 molecule as an octet rule exception. The characteristic statements about conceptual frameworks of the HCK and LCK groups regarding chemical bonding are provided in Table 2.
Molecular shape and molecular polarity
We have four pairs of electrons around the sulfur and two are bonded to hydrogens; that means the molecular geometry is going to be bent, but the electron configuration will be tetrahedral. It will end up looking like this because all of the electrons are repelling each other so they are getting as far away from each other as possible. These [lone pair] electrons actually have stronger repulsion then these [bonding] pairs so they [lone lairs] are just a little bit more separated than these [bonding pairs] are. (Hugh, interview)
You have the region where there is lots of negative charge. You got a dipole moment from this negative charge going from these lone pairs, and because this area over here [around hydrogen atoms] is like, in a sense, devoid of electrons, there is not that many electrons there. There is not as much probability of electrons being there. So this is going to have an overall more negative charge over here [around the sulfur atom], making it polar. (Harry, interview)
In his mental model, Harry successfully reconciled the quantum mechanics descriptions (probability of electrons) with the electron-cloud model. After identifying which atoms were more and less electronegative, these students determined bond polarities and molecular polarity almost simultaneously. All of the HCK students used their mental model as a thinking tool and were able to effectively apply propositions to the model.
We further used Hugh’s excerpts to illustrate the fluency of the HCK students’ thinking about the geometry and polarity of a molecule. He derived the electron configuration of BF3 by checking the positions of boron and fluorine in the periodic table, and this was followed by immediately forming a mental image of BF3 and applying the VSEPR model to determine its shape and molecular polarity in 3D:
Hugh: I have to look at this [the periodic table]. Boron is where? Right here, okay, and then fluorine. I would say that this one [BF3] is nonpolar because I am pretty sure it has a trigonal planer molecular geometry, which means that all of the fluorines are around the boron equally spaced apart 120°.
Researcher: It looks like you have a picture in your mind. Can you walk me through the thinking process that you just had?
Hugh: Okay. I already knew that fluorine had seven valance electrons, and I found boron had three valance electrons. In order to fill each of these three fluorines which are missing one electron in their orbital, boron would need to give up all three of those electrons, one to each fluorine. It [boron] would be left with zero electron pairs that would not be shared. That means that there would not be that unshared electron pair occupying any of the space around the boron. That would allow for the fluorines to equally repel each other because they do not have that additional force [from the unshared electron pair]. (Hugh, interview)
This fluency in thinking about the geometry and polarity of a molecule is supported by the HCK students’ adequate understanding of propositions within their conceptual framework, for instance, understanding the periodic variation of boron and fluorine and being able to determine the configuration of BF3. Also, Hugh was able to use the principles of electrostatic force and the VSEPR model to derive the molecular shape as well as to determine the direction of the bond and molecular polarity in 3D based on its shape and relative electronegativity for boron and fluorine.
Whenever there is a lone pair like this [a bent, H 2 S molecule], that would mean that there is a dipole moment and those are pushing down. Since there are the electrons [lone pairs] up here, they need the room, that they need the space because they have their outer shells, too. So, that means that they will push down the hydrogens. (Luke, interview)
Luke posited that lone pairs repelled (pushed down) the hydrogen atoms. However, he used anthropomorphic explanations as “they need room, they need space” (Luke, interview) without justifying his explanations with principles of electrostatic force. Missing the principles of electrostatic force in Luke’s explanatory framework was also evident when he was prompted to consider whether the arrangement of electron pairs for H2S should be planar or tetrahedral. He said:
I have a feeling that it would be like this [tetrahedral], but I don’t know why. I cannot think of why it would be like that, but I just have a really strong feeling that it would be [tetrahedral]. (Luke, interview)
Lisa also showed concern “because they do not want to, they just get repelled” (Lisa, interview). Some LCK students, such as Lisa, were aware of the existence of repulsion between electron pairs and indicated that the lone pairs generated greater repulsion than bonding pairs when arranging electron pairs for a given molecule. However, most of the LCK students did not reconcile the principles of electrostatic force with the VSEPR model; therefore, their understanding for predicting molecular geometry appeared fragmented.
In addition, the LCK students, such as Luke and Larry, had difficulties associating the characteristics of lone pairs with the hybridization of the molecular orbital; therefore, they did not illustrate the lone pairs when they constructed a mental or concrete model of an H2S molecule. Luke built a concrete model of an H2S molecule and stated as follows:
Whenever you are doing these models, you do not show the lone [pairs]…it would basically look like that (a ball-and-stick model in a bent shape), and that would be its molecular geometry. Then you have to put in the perspective that there are two lone pairs. (Luke, interview)
To Luke, the concept of lone pairs existed as a propositional statement, such as “lone pairs were what is pushing the hydrogens down instead of keeping them in a line” (Luke, interview). This view is in contrast to the conceptualized and visualized perception of lone pairs as an H2S molecular orbital. Larry had the same problem when considering the lone pairs on an H2S molecule:
Larry: There will be electrons [lone pairs] there, I guess. I mean there are electrons [lone pairs] there, but I would not know how to go into detail why you do not see it there or is it there.
Researcher: Where are the lone pair electrons in this model?
Larry: Electrons would still belong to the sulfur, and it would be like floating around or whatever. I think they are just floating free but still within that gravitational pool or whatever it is called. (Larry, interview)
Although Larry knew, from the Lewis structure, that H2S consists of two bonding pairs and two lone pairs, he neither saw the geometry of the arrangement of electron pairs as a tetrahedral shape nor visualized the two lone pairs as two probability regions of electron distribution. Quantum mechanics is critical for understanding the concept of a probability region of electron distribution and for understanding the hybridization of the atomic orbital and VSEPR model. The LCK students did not have these prerequisite concepts to support their reconciliation between the Lewis structure and 3D geometry of H2S based on the VSEPR model. Thus, the questions, “Why were there four bonding sites on the sulfur atom?” and “Why are the two lone pairs considered as two regions of electron distributions that repelled the two bonding pairs in a tetrahedral geometry?” were mysteries to them.
When the gap of reasoning between the 2D Lewis structure and VSEPR model was too large to fill, the LCK students began to base their thinking on intuitive reasoning or memorization. For example, when Luke was prompted to consider whether the arrangement of electron pairs for H2S should be planar or tetrahedral, he said, “It would be like that [tetrahedral]. I cannot think of why it would be like that. But I just have a, I have a really strong feeling that it would be” (Luke, interview). Larry, who used an algorithmic strategy most of the time, again used an algorithm to recall the corresponding bond angles for an H2S molecule. He described his thinking process as follows: “I just went through linear is 180 [degrees], triangle is 90 [degrees], and that is where it stops. I could not think of what it was” (Larry, interview). When Larry had an opportunity to access the textbook, he found the bond angle of a tetrahedral shape quickly and indicated that the bond angle of an H2S molecule was 109°. However, he failed to consider that the lone pairs in an H2S molecule had repelled to a greater angular separation than the bonding pairs. Larry’s prior experiences with success in using an algorithmic strategy to solve problems had led him to trust the outcomes of this strategy.
Other LCK students, such as Luke, conceptualized a polar molecule as a molecule that had a sum of uneven forces that resulted from its asymmetrical geometry. To explain the concept of the polar molecule, Luke gave a counter example, a CCl4 molecule, and used an analogy about tractors pulling to explain the idea of balancing forces. Using this tractor-pulling analogy, he explained the following:
That would be nonpolar because all of these [chlorine atoms] are equal in their electronegativity, they are all going to pull their own ways. Like, if these are all like tractors or trucks or something pulling, that they all have the same force, so there is nowhere that any of them can go because of the way they are set up. So that would be an example of a nonpolar [molecule]. And that [H 2 S] would be an example of a polar because of the way of their molecular geometry…that they are pulling downwards so it would slightly pull the sulfur downward. (Luke, interview)
Luke’s responses to items in the GP instrument indicated that his thinking processes about molecular geometry and polarity were inconsistent. Sometimes, during the interview, Luke took the geometry of H2S into consideration when determining its polarity, while at other times, he incorrectly attributed the reason that ClF3 and OF2 were polar molecules to the high electronegativity of the fluorine atoms (GP-6b and GP-2b), thus disregarding the influence of geometry. Moreover, Luke mistakenly thought that the strength of the intermolecular forces was greater between CF4 molecules (nonpolar) than between OF2 molecules (polar) because there are four polar bonds in CF4 and only two polar bonds in OF2 (GP-8ab). This misconception suggested that either Luke responded to GP instrument thoughtlessly or that he was operating on a fragmented conceptual framework rather than employing a consistent, logical thinking process, as the fragmented knowledge had no foundation to be assimilated or reconciled upon. The fragmentation of his conceptual frameworks could result from rote learning without meaningfully connecting the newly learned conceptions with the existing knowledge. Also, the student may not be aware of and resolve the conflicts within his conceptual frameworks. Thus, the student lost the segments of knowledge in the memory soon after the exam.
Unlike the HCK students’ fluency in thinking about molecular shape and polarity, missing principles of electrostatic force, inadequate understanding of the VSEPR model, and reliance on memorization or mere use of anthropomorphic explanations to explain molecular shape and polarity hinder the LCK students’ ability to form and adjust a mental model to predict molecular shape and polarity. This may explain why some LCK students determined the molecular shape based on a 2D Lewis structure without adjusting with principles of electrostatic force and the VSEPR model, and therefore, often concluded an incorrect polarity based on the inadequate molecular shape. For instance, both Luke and Larry drew a Lewis structure of BF3 in a T-shape and determined that BF3 was a polar molecule by cancelling out the dipole moments of the three B–F bonds based on the T-shaped, 2D structure (Fig. 5). Larry explained his thinking process:
 | ||
| Fig. 5 Drawings of BF3 by two LCK students. | ||
Boron has three valence electrons. That means it can have three bonds coming from it. There are three fluorines so it will attach. I think that these two [dipole moments of B–F bonds] cancel each other out, so maybe it is polar, since these [two dipole moments] are pulling in the opposite and same directions. I am not sure if there is a double bond or not or a triple bond, but assuming that they are all single bonds, these would cancel out and this only fluorine, would determine which direction [of dipole moment] it would go in. (Larry, interview)
Larry’s fragmented conceptual framework did not support him to realize the need to adjust the 2D Lewis structure based on related principles. Instead, he relied on a rigid, 2D Lewis structure to infer the molecular polarity of BF3. The characteristic statements about the conceptual frameworks of the HCK and LCK groups regarding molecular shape and polarity are summarized in Table 3.
Discussions of cross-case analyses
Cross-case analyses were performed to compare the conceptual frameworks of the HCK and LCK students. We have organized our discussions of cross-case comparisons around four assertions:Assertion 1. The HCK students possessed coherent conceptual frameworks composed of precise and accurate concepts. When moving to the other end, students’ conceptual frameworks became fragmented with many misconceptions and missing concepts. From a high toward a low level of content knowledge, the quality of students’ explanations declined, as did their ability to reconcile new information to their existing conceptual frameworks. LCK students’ lower quality of explanations and fragmented conceptual frameworks were related to their use of algorithmic strategies and common-sense explanations while solving a problem. However, the direction of this relationship remains unclear. The HCK students’ conceptual frameworks were characterized by accurate conception and appropriate paths of reasoning, with only a few misconceptions and missing concepts. The coherent conceptual frameworks allowed the HCK students to justify their explanations using appropriate concepts and to reconcile new information easily, rather than merely following the general assumed rules. When the HCK students became aware of a gap in their understanding, they instantly resolved the inconsistency. For example, Heidi originally represented the two lone pairs of H2S as two sets of two dots when she built a concrete model. After the interviewer gave a hint by asking her, “Do you think the lone pairs will be in a planar shape or a tetrahedral shape with the two S–H bonds?”, she answered as follows:
I guess they could be here as tetrahedral. I never really thought of it that way because mostly what I picture is similar to the Lewis structures. Actually, I never thought of them being there [tetrahedral]. It would make sense that they can be there because that puts everything a little bit further apart, and actually, if I think to where the electrons are, it would be tetrahedral. Then, because you have the two lone pairs, it becomes bent. If it was tetrahedral and these [the lone pairs] were atoms, these would be here. So, I guess that would make sense. (Heidi, interview)
Heidi was able to reconsider her mental model and decided that the arrangement of the electron pairs should be tetrahedral. This reconciled mental model was retained throughout the remainder of the interview.
When the quality of students’ conceptual frameworks decreased, the quality of students’ explanations for a concept or a phenomenon also declined. The conceptual frameworks of the LCK students were fragmented and contained many missing concepts, missing links, and/or misconceptions. For the LCK students, either key concepts that anchored the new information to their conceptual frameworks were missing or the new information was incompatible with their personal theories. Thus, these students experienced greater difficulties with reconciling new information with their conceptual frameworks. Key concepts missing in the LCK students’ conceptual frameworks included the influence of the positive core charges in an atomic model and the principles of electrostatic force when describing chemical bonding. Due to the missing links in their conceptual frameworks, the students frequently followed general assumed rules without justifying their approaches with appropriate propositions or rationalized their approaches using common-sense explanations (e.g. teleological or anthropomorphic explanations, (Talanquer, 2006)). These students were somewhat satisfied with their algorithmic strategies and/or personal theories, and therefore, they did not reconcile their incomplete understandings with textbook explanations. The LCK students’ algorithmic strategies and common-sense explanations are worthy of special attention. Based on our analyses, the HCK students used algorithmic strategies on occasion but closely associated these strategies with the underpinnings of principles of chemistry. Some LCK students used algorithmic strategies more often and justified these strategies with common-sense explanations when their low quality of understanding of the related principles was unable to support their explanations. Other LCK students used algorithmic strategies as a habit of thinking, as indicated by Larry in the interview. “I do not think I have ever thought of this so conceptually before. I just seem to plug it on and try it” (Larry, interview). Larry’s satisfaction with his algorithmic strategies may have hindered him from devoting extra efforts to reconcile his disjointed knowledge. Furió and colleagues (Furió and Calatayud, 1996; Furió et al., 2000) attributed students’ errors in solving molecular geometry and polarity problems to procedural difficulties, which occur when students fail to solve a problem due to the functional reduction of the intrinsic complexity of the problem or due to their use of common-sense explanations without considering scientific knowledge, i.e., functional fixedness. Our findings echo and extend beyond the observations of Furió and colleagues (Furió and Calatayud, 1996; Furió et al., 2000), thus suggesting that the LCK students’ procedural difficulties may have resulted from their fragmented conceptual frameworks.
Assertion 2. The HCK students possessed precise and accurate concepts about atomic and molecular models that supported reconciliation among models. The reconciliation among models permitted these students to shift among models with minimum difficulty during problem-solving processes. In contrast, the LCK students possessed an insufficient understanding, which hindered their reconciliation among models. Therefore, these students preferred a simple model or the one with which they were most familiar when solving a problem. All of the HCK students possessed a better understanding about different representations (e.g. space-filling, ball-and-stick models, and Lewis structure) and models (e.g. Bohr model, electron-cloud model, and descriptions of quantum mechanics). Meanwhile, they tried to connect and reconcile these models and representations. For example, Heidi described an electron cloud model:
You have the molecule, and you have the nucleus that the electrons are just kind of, like, in the cloud around them. You can see where would be more electrons or less electrons, but it is not like where this electron always is because it [electrons] would be moving all around so it would be hard to catch it unless you took a picture, I guess. (Heidi, interview)
Heidi first described an uneven distribution of electrons as a cloud surrounding the nucleus in a molecule. She further clarified that the electron cloud was not composed of static electrons; instead, she used the metaphor “It would be hard to catch it unless you took a picture” to explain quantum mechanics. The reconciliation among chemical models and representations allowed the HCK students to switch between models and representations with minimal difficulties. In addition, the HCK students’ understandings of the meanings, explanatory powers, and limitations of the models and representations permitted the HCK students to identify problems and choose an appropriate model or representation accordingly. For example, students in the HCK group used a simple Lewis structure or a ball-and-stick model when they knew that the simple representation contained enough information to solve the problem. When the simple representation was unable to explain a phenomenon, such as to explain the electron distribution of a polar bond, the students could switch to an electron-cloud model or a quantum mechanics description with no observable difficulties. This observation aligns with the findings of Coll and Treagust (2001) regarding grade 12, undergraduate, and postgraduate Australian students’ mental models of chemical bonding.
When the quality of the students’ understandings about models decreased, the insufficient understanding hindered the LCK students’ reconciliations among these models. The LCK students had a poor understanding of the Bohr model, the electron-cloud model, and quantum mechanics, and they had little understanding of the meanings, explanatory power, and limitations of these models. With the fragmented conceptual frameworks, the LCK students could neither reconcile different models and disjointed conceptions nor supplement appropriate propositions to construct a functional 3D model with accurate spatial features. Instead, these students predominately operated their thinking and solved problems based on 2D Lewis structures. While explaining a concept or phenomenon, they used algorithmic strategies without justification or supported their explanations with personal theories or teleological or anthropomorphic explanations. They perceived the Lewis dot structures as verbal-linguistic representations and viewed these structures as collections of letters, lines, and dots rather than conceptualizing the symbols as representations of atoms and molecules. These students also used the model that “pop-up” in their mind when a given question triggered a specific model. This observation of the LCK students echoed the findings of Shane and Bodner (2006) regarding general students’ understanding of chemical compounds. The characteristic statements regarding features of conceptual frameworks are provided in Table 4.
Bodner and Domin (2000) associated students’ problem-solving abilities with the type of mental models they construct. The authors found that the successful problem solvers constructed more representations per problem than did their counter cohorts. Bodner and Domin also found that students who were unable to spontaneously switch from one representation to the other tend to perform poorly in organic chemistry. In contrast, students who performed well in organic chemistry could switch back and forth between these representation systems as needed. Findings from our study about students’ conceptual frameworks and their reconciliation of different models provide a possible explanation for Bodner and Domin’s observations.
Assertion 3. All participants justified the formation of chemical bonds and a chemical compound using the octet rule. Only two HCK students, Hugh and Harry, associated bond and chemical compound formation with the interactions of electrostatic force among atoms. All of the LCK students merely followed the octet rule as a general assumed rule without understanding the chemical principle behind the octet rule. A misconception of the octet rule was common among the LCK participants. These students responded incorrectly to at least four out of five items regarding questions involving the octet rule on the EN instrument. This use of the octet rule as a general assumed rule prevented students from considering criteria and restrictions when using the octet rule to determine a Lewis structure for a given molecule. Talanquer (2007) considered this line of thinking as teleological reasoning, where students considered “filling the octet” as the driving force for forming a chemical compound. This octet framework was found to be well established by age 16 and tenacious enough to seriously impede the learning of important new ideas presented in the college curriculum (Taber, 2003a; Tan et al., 2005). Talanquer also indicated that many sources of students’ teleological explanations were from textbooks or classroom instruction. For example, teleological statements were found in the textbook used in the course of the present study:
The formation of these molecules illustrates the octet rule: An atom other than hydrogen tends to form bonds until it is surrounded by eight valence electrons (italics in original). In order words, a covalent bond forms when there are not enough electrons for each individual atom to have a complete octet. By sharing electrons in a covalent bond, the individual atoms can complete their octets.… When an atom of one of these elements [in the second period in the periodic table] forms a covalent compound, it can attain the noble gas electron configuration [Ne] by sharing electrons with other atoms in the same compound. (Chang, 2005, p. 355)
According to Talanquer’s (2007) findings, we should not be surprised that the participants espoused these teleological explanations about the octet rule. Talanquer explained that at the general chemistry level, this understanding may be an appropriate pedagogical approach to introducing a complex concept without employing complicated explanations.
However, once the octet rule is perceived by a student as the main explanation for why atoms share or transfer electrons to form chemical bonds or a chemical compound, it is difficult for students to reject the rule and reconcile it with later learned scientific explanations. Accordingly, among the six participants in this study, only two HCK students, Hugh and Heidi, partially reconciled the notion of the octet rule with principles of electrostatic force while considering chemical bonding. For example, when Hugh was asked why a molecule would prefer to meet the octet rule, he replied, “It is about stability. That is what I have always been taught. I guess I have been working off just trusting and not really understanding if an orbital is more stable” (Hugh, interview). This example demonstrates that Hugh simply accepted what he had been taught, that is, a full or a half-filled orbital is more stable, without really understanding why. Teleological explanations, such as those found in the course textbook, leave a void in students’ thinking and reasoning when developing conceptual frameworks. Tan et al. (2005) have expressed similar concerns and provided some pedagogical suggestions.
Assertion 4. Three key concepts must be addressed to learn concepts about molecular shape and polarity successfully: (a) the models of atomic structure, (b) effective core charge and principles of electrostatic force, and (c) descriptions of quantum mechanics . The analyses of students’ conceptual frameworks across the HCK and LCK groups allowed us to study how missing prerequisite concepts or linkages among conceptions, partial or incorrect understandings, or fragmented conceptions may result in a reduced ability to learn the content. It is observed that the LCK students did not demonstrate the level of understanding that they should have acquired regarding the models of atomic structure, the concept of effective core charge and nucleus–electron interactions, and an understanding of quantum mechanics. Lacking these essential prerequisites impeded the ability of these students to develop a sophisticated model of molecular polarity.
Models of atomic structure were the first essential prerequisite missing from the LCK students’ conceptual frameworks. Some LCK students, such as Luke, perceived a fluorine atom as a letter surrounded by seven dots representing seven valence electrons. Their use of symbolic representation may not support a proper understanding of models of atomic structure that contain sufficient features to reconcile the interaction of electrostatic force between the nucleus and electrons. Marais and Jordaan's (2000) study showed that first-year chemistry students had more difficulty with the meanings of symbols than with the meanings of words. As symbols are the most abstract chemical representations, students must understand the entities that the symbols represent before they can master the use of the symbols. Cokelez and Dumon (2005) reported that 34% and 30% of French grade 12 students preferred using symbols with a Lewis dot structure or simple sphere, respectively, to represent an atom. Nonetheless, only 10% of the grade 12 students indicated that the number of protons equals the number of electrons when describing the atomic structure. Cokelez and Dumon’s findings suggest that the concept of atomic structure requires increased attention from college chemistry instructors.
The role of effective core charge and the principles of electrostatic force were the second set of key concepts missing from the conceptual frameworks of the LCK students. Because the influence of effective core charges and nucleus–electron interactions were missing from their conceptual frameworks, these students did not understand the relationships between the numbers of protons and electrons or associate the levels of electron shells with energy levels. Missing the concepts of the nucleus–electron interactions in an atomic model and the magnitude of energy involved may have hindered the LCK students’ comprehension about differences and similarities between elements (e.g. electronegativity and atomic radius), which generalize the periodic variations across groups and periods in the periodic table. Taber (2003b) suggested that the electrostatic force be taught as the basis for chemical bonding to prevent students from basing their explanations solely on atoms trying to fill their electron shells. The missing role of the effective core charge may be the result of pedagogy, as teachers may assume that students have already learned certain basic information and therefore use descriptive statements, such as there is a force between the nucleus and electrons to describe the nucleus–electron interactions without referring to the basic physical principle per se (Taber, 1998). Missing the concept of nucleus–electron interactions and the principles of electrostatic force may have a profound influence on students’ understandings of more advanced concepts. Based on our interview analyses, students who lacked an understanding of the effective core charge could not articulate the principles of electrostatic force and perceived the periodic variations as accepted rules or segments of facts, which they may eventually forget. They described chemical bonding as some type of attractive force between two atoms, but they could not associate bonding with the electrostatic force between the nuclei and electrons of the two atoms. Due to this lack of understanding about effective core charge, these students used word associations to conceptualize covalent bonding, explaining that covalent bond means sharing electrons without considering the attraction from the nucleus of a more electronegative atom. Thus, we propose that an insufficient understanding about the concept of effective core charge and principles of electrostatic force hindered students’ conceptualization about electronegativity and chemical bonding (both covalent bonding and ionic bonding) and possibly enhanced the overgeneralization of the octet rule when developing a Lewis structure of a molecule.
Descriptions of quantum mechanics were the third key concept missing from the conceptual frameworks of the LCK students. The LCK students perceived a molecule with a ball-and-stick model or Lewis structure, and the lone pairs were omitted from the molecular structure. The LCK students had difficulties conceptualizing lone pairs of atomic orbital hybridization as probability regions of electron distribution and misinterpreted an electrostatic potential map as an area of electron distribution. The level of abstraction in the descriptions of quantum mechanics can make the concept difficult to learn (Park and Light, 2008). The analyses of students’ explanations suggested that students were not able to develop precise and accurate understanding of the VSEPR model unless their concepts about quantum mechanics and hybridizations of atomic orbital were resolved.
For general chemistry students to develop an adequate understanding of a concept at the higher level of the hierarchy, such as molecular polarity, instructors need to identify and supplement a series of underpinning mental sets that students lack from their conceptual frameworks (She, 2004). We have represented a concept map in Fig. 1 based on the class text. However, according to our findings, this concept map needs to be modified to incorporate essential concepts including the model of atomic structure, effective core charge and principles of electrostatic force, as well as quantum mechanics descriptions. Also, the map needs to be restructured to illustrate the hieratical relationships among the underpinning concepts and concepts at the higher level of the hierarchy. To supplement the underpinning mental sets for reaching an adequate understanding of molecular polarity, first, we proposed that students are to have proper understanding of atomic structure (consisting of specific numbers of protons, neutrons, and electrons) and to use principles of electrostatic force to explain the nucleus–electron interactions and difference in electronegativity. This may help them conceptualize principles that underpin patterns of the periodic variations and realize that chemical bonding and formation of a molecule involve nucleus–electron interactions. Meanwhile, it is important to help students associate symbolic representations, such as the Lewis structure and the ball-and-stick model, with the aforementioned concepts rather than seeing atoms and molecules as combinations of letters and dots.
Next, when forming the structure of a molecule and determining the spatial arrangement of electron pairs using the VSEPR model, it is essential that students visualize electrons of lone pairs and chemical bonds as a region of probability and incorporate principles of electrostatic force in the VSEPR model to justify the spatial arrangement of the electron pairs. Helping students to associate the Lewis structure and the VSEPR model with quantum mechanics descriptions and hybridization of atomic orbitals may also help them conceptualize why lone pairs occupy space and how lone pairs and bonded atoms influence bond angles.
Finally, to understand that determining molecular polarity involves deriving net dipole by balancing out dipole moment spatially, students need to realize that bond moments are vector quantity to represent their magnitude and direction rather than merely seeing them as pulling forces. Also, it is important to help students reconcile a 3D Lewis structure of a molecule with an electron-cloud model to see a resultant dipole moment of a molecule as a resultant in electron density distribution, rather than merely seeing polarity as trying to cancel out pulling forces from different directions. Supplementing these missing conceptions and restoring missing links between conceptions to the LCK students’ conceptual frameworks may reduce their reliance on memorization or use of anthropomorphic explanations.
Conclusions and implications
Findings of this study reveal the importance of assisting students in developing a precise, coherent conceptual framework of general chemistry, as well as the influence of conceptual frameworks on the quality of student explanations and the ability of students to reconcile and shift between and among models and chemical representations. Additionally, three essential concept areas – (a) models of atomic structure, (b) effective core charge and principles of electrostatic force, and (c) descriptions of quantum mechanics – were identified that must be addressed to develop a higher quality of conceptual frameworks when learning about molecular geometry and polarity. Evidence was provided to illustrate that missing any one of the key concepts may result in misconceptions, deficiencies of understanding, or a lack in the use of common-sense reasoning. These findings provide college chemistry instructors and curriculum developers with information about how these concepts are interrelated in students’ conceptual frameworks and the prerequisite concepts. A chemistry instructor should plan the sequence of instruction to address the prerequisite concepts, thereby facilitating the development of conceptual frameworks. Additionally, it is important to assess whether students have learned the prerequisite concepts before moving on to the next level.Previous research on molecular polarity used a quantitative approach to describe students’ understanding of this topic (Peterson et al., 1989; Furió and Calatayud, 1996; Furió et al., 2000; Jang, 2003). This study investigated students’ use of existing conceptual frameworks while solving molecular polarity problems. The findings of this study went beyond the identification of alternative conceptions in a specific domain to provide potential explanations of the connection between the construction and utilization of conceptual understanding. Additionally, comparisons of the thinking processes between the HCK and LCK groups provided a rich description about students’ use of conceptual frameworks and external representations during problem-solving processes. We suggest that future research on student learning about advanced or more abstract concepts must go beyond examining students’ understanding about a single concept to be studied. Science educators should investigate conceptual frameworks as a whole, including prerequisite concepts and the hierarchical relationship among them. The analyses of conceptual frameworks revealed that students’ level of content knowledge was related to the quality of their explanations. The HCK students could justify their answers and explanations with other correct concepts or propositions. As the quality of conceptual frameworks decreased and knowledge became fragmented, students used algorithmic strategies, developed personal theories, or used teleological or anthropomorphic explanations to explain their answers. Future research should explore the relationship between the quality of students’ conceptual frameworks and quality of explanations from an epistemological perspective, and they should also explore how the quality of conceptual frameworks influences students’ reasoning patterns.
References
- Bodner G. M. and Domin D. S., (2000), Mental models: The role of representations in problem solving in chemistry, University Chemistry Education,4(1), 24–30.
- Chang R., (2005), Chemistry (8th ed.), New York: McGraw-Hill.
- Chi M. T. H., (2008), Three types of conceptual change: Belief revision, mental model transformation, and categorical shift, In S. Vosniadou (Ed.), International handbook of research on conceptual change (pp. 61–82), NY: Routledge.
- Clark D. B., D'Angelo C. M. and Schleigh S. P., (2011), Comparison of students' knowledge structure coherence and understanding of force in the Philippines, Turkey, China, Mexico, and the United States, J. Learn. Sci.,20, 207–261.
- Cokelez A. and Dumon A., (2005), Atom and molecule: Upper secondary school French students' representations in long-term memory, Chem. Educ. Res. Pract.,6, 119–135.
- Coll R. K. and Treagust D. F., (2001), Learners' mental models of chemical bonding, Res. Sci. Educ.,31, 357–382.
- College Board., (2009), College board standards for college success: Science, New York: The College Board.
- diSessa A. A., (1993), Toward an epistemology of physics, Cognition Instruct.,10, 105–225.
- Ferguson R. L., (2007), Constructivism and social constructivism, In M. Orgill and G. M. Bodner (Eds.), Theoretical frameworks for reserch in chemistry/science education (pp. 27–48), Upper Saddle River, NJ: Prentice Hall.
- Furió C. and Calatayud M. L., (1996), Difficulties with the geometry and polarity of molecules: Beyond misconceptions, J. Chem. Educ.,73, 36–41.
- Furió C., Calatayud M. L., Bárcenas S. L. and Padilla O. M., (2000), Functional fixedness and functional reduction as common sense reasonings in chemical equilibrium and in geometry and polarity of molecules, Sci. Educ.,84, 545–565.
- Gupta A., Hammer D. and Redish E. F., (2010), The case for dynamic models of learners' ontologies in physics, J. Learn. Sci.,19, 285–321.
- Jang N. H., (2003), Developing and validating a chemical bonding instrument for Korean high school students, doctoral dissertation, University of Missouri-Columbia.
- Jones M. G., Carter G. and Rua M. J., (2000), Exploring the development of conceptual ecologies: Communities of concepts related to convection and heat, J. Res. Sci. Teach.,37, 139–159.
- Marais P. and Jordaan F., (2000), Are we taking symbolic language for granted? J. Chem. Educ.,77, 1355–1357.
- Mortimer E. F., (1995), Conceptual change or conceptual profile change? Science & Education,4, 267–285.
- Nicoll G., (2001), A report of undergraduates' bonding misconceptions, Int. J. Sci. Educ.,23, 707–730.
- Novak J. D., (2002), Meaningful learning: The essential factor for conceptual change in limited or inappropriate propositonal hierarchies leading to empowerment of learners, Sci. Educ.,86, 548–571.
- Park E. J. and Light G., (2008), Identifying atomic structure as a threshold concept: Student mental models and troublesomeness, Int. J. Sci. Educ.,31, 233–258.
- Patton M. Q., (2002), Qualitative research and evaluation methods (3rd ed.), Thousand Oaks, CA: Sage Publications, Inc.
- Peterson R. F. and Treagust D. F., (1989), Grade-12 students' misconceptions of covalent bonding and structure, J. Chem. Educ.,66, 459–460.
- Peterson R. F., Treagust D. F. and Garnett P., (1989), Development and application of a diagnostic instrument to evaluate grade-11 and -12 students' conceptions of covalent bonding and structure following a course of instruction, J. Res. Sci. Teach.,26, 301–314.
- Posner G. J., Strike K. A., Hewson P. W. and Gertzog W. A., (1982), Accommodation of a scientific conception: Toward a theory of conceptual change, Sci. Educ.,66, 211–227.
- Shane J. W. and Bodner G. M., (2006), General chemistry students' understanding of structure-function relationships, Chemical Educator,11, 130–137.
- She H.-C., (2004), Fostering radical conceptual change through dual-situated learning model, J. Res. Sci. Teach.,41, 142–164.
- Stevens S. Y., Delgado C. and Krajcik J. S., (2010), Developing a hypothetical multi-dimensional learning progression for the nature of matter, J. Res. Sci. Teach.,47, 687–715.
- Strauss A. and Corbin J., (1998), Basics of qualitative research: Techniques and procedures for developing grounded theory, Thousand Oaks, CA: Sage Publications, Inc.
- Taber K. S., (1998), The sharing-out of nuclear attraction: or ‘I can't think about physics in chemistry’, Int. J. Sci. Educ.,20, 1001–1014.
- Taber K. S., (2000), Case studies and generalizability: Grounded theory and research in science education, Int. J. Sci. Educ.,22, 469–487.
- Taber K. S., (2002), Chemical misconception - Prevention, diagnosis and cure: Volume I: Theoretical background, London: Royal Society of Chemistry.
- Taber K. S., (2003a), Mediating mental models of metals: Acknowledging the priority of the learner's prior learning, Sci. Educ.,87, 732–758.
- Taber K. S., (2003b), Understanding ionisation energy: Physical, chemical, and alternative conceptions, Chem. Educ. Res. Pract.,4, 149–169.
- Taber K. S., (2005), Learning quanta: Barriers to stimulating transitions in student understanding of orbital ideas, Sci. Educ.,89, 94–116.
- Talanquer V., (2006), Commonsense chemistry: A model for understanding student's alternative conceptions, J. Chem. Educ.,83, 811–816.
- Talanquer V., (2007), Explanations and teleology in chemistry education, Int. J. Sci. Educ.,29, 853–870.
- Talanquer V., (2009), On cognitive constraints and learning progressions: The case of “structure of matter”, Int. J. Sci. Educ.,31, 2123–2136.
- Tan K.-C. D., Taber K. S., Goh N.-K. and Chia L.-S., (2005), The ionisation energy instrument: A two-tier multiple-choice instrument to determine high school students’ understanding of ionisation energy, Chem. Educ. Res. Pract.,6, 180–197.
- Vosniadou S., (1994), Capturing and modeling the process of conceptual change, Learning and Instruction,4, 45–69.
- Wang C.-Y., (2007), The role of mental-modeling ability, content knowledge, and mental models in general chemistry students' understanding about molecular polarity, doctoral dissertation, University of Missouri-Columbia.
- Wang C.-Y. and Barrow L. H., (2011), Characteristics and levels of sophistication: An analysis of chemistry students' ability to think with mental models, Res. Sci. Educ.,41, 561–586.
- White R. T. and gunstone R. F., (1992), Probing understanding, New York: The Falmer Press.
| This journal is © The Royal Society of Chemistry 2013 |
