Rabbit hair regenerative superhydrophobicity†
Xiangyu Yinab,
Daoai Wanga,
Bo Yu*a and
Feng Zhou*a
aState Key Laboratory of Solid Lubrication, Lanzhou Institute of Chemical Physics, Chinese Academy of Sciences, Lanzhou 730000, China. E-mail: yubo@licp.cas.cn; zhouf@lzb.ac.cn
bUniversity of Chinese Academy of Sciences, Beijing, 100049, China
First published on 2nd December 2013
Abstract
A proof-of-concept example of using fibrous rabbit hair absorption/release of low surface energy materials (perfluorooctyl acid, PFA) to achieve rapid and controllable regenerative superhydrophobicity is reported. The superhydrophobicity can be rapidly restored in different ways including heating, evacuation and rubbing.
In recent years, biomimetic synthesis of superhydrophobic materials has generated considerable interest but has yielded few practical applications.1–6 The susceptibility of their fine and delicate surfaces to damage by mechanical wear, irradiation, and various contaminations is a major barrier for application development.7–11 To overcome this problem, superhydrophobic materials with regenerative property are desired. These materials can regenerate their function by external stimuli upon infliction of damage.12–16 To date, the search for more convenient materials and systems to achieve a more rapid, repeatable, and controllable regenerate property has been in progress. New superhydrophobic materials with long-term stability which can be prepared using a simple, inexpensive, and practical approach are highly desirable. Animal fibrous hairs are known to endow animals with good protection to their bodies, excellent heat preservation and many other properties.17 The hollow fiber structure has the ability to absorb moisture and release perspiration.18,19 Chinese ancestors made writing brushes using animal hairs because they could rapidly absorb and release ink. This application has prompted us to develop a novel regenerate superhydrophobicity system by integrating animal fiber hairs with appropriate low surface energy materials. In this paper, we provide a successful proof-of-concept example of using rabbit fibrous hairs (RFHs) absorption/release of low surface energy materials (perfluorooctyl acid, PFA) to achieve rapid and controllable restore superhydrophobicity by different means including heating, evacuation, and rubbing.
Scheme 1 shows the schematic of producing RFHs regenerative superhydrophobicity (full experimental details are available in the electronic supplementary information). The SEM image reveals that RFHs have a hollow fibrous structure with an average diameter of approximately 12 μm (Fig. S1a, ESI†). This structure provides a potential reservoir for storing low surface energy molecules, which can automatically absorb functional compounds because of the capillary force of the microscale hollow structures, whose process is easy and fast, moreover, the root of RFHs is also filled in such short time. The fibers were observed under SEM to confirm that the RFHs were successfully loaded (Fig. S1). The RFHs were filled with substance in high efficiency and the ends of the fibers were sealed, apart from this, no significant morphological difference was observed between the RFHs and the as-prepared samples (Fig. S1b, ESI†). XPS was used to investigate changes in the surface chemical composition of the RFHs. The unloaded RFHs were mainly composed of C and O, whereas F signals were detected in the XP spectrum of the loaded RFHs. This result confirmed the successful loading with PFA (Fig. S1c and d, ESI†).13,20 Moreover, we also find that the PFA maintained stable in the RFHs under normal condition, and it was unsusceptible to generally rubbing, heating and evacuation.
The surface wettability of the RFHs–PFA was initially evaluated by observing the spreading behaviors of the water droplets on RFHs and RFHs–PFA surfaces. When water droplets were placed on the top of the processed RFHs, which were wetted gradually (Fig. S2a, ESI†). By contrast, the loaded PFA provided the RFHs with a persistent water-resistant property, and the water CA was above 160° (Fig. S2b, ESI†). Meanwhile, to fully further prove their water repellency, we investigated the wettability of a half-side unilateral filled RFHs which were completely immersed in water (Fig. S2c, d and e, ESI†). It turns out that the RFHs-PFA had an excellent and persistent water-resistant property, even underwater. It suggests that the superhydrophobicity can be easily achieved on RFHs substrates due to the multiscale microstructures and low-surface energy materials in the hair fibers.
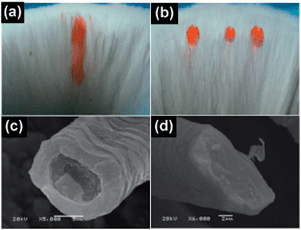
As previously mentioned, superhydrophobic materials are often poor in durability and easily damaged. We expect to heal the surface hydrophobicity using the hair fibers filled with PFA to solve the problem. Oxygen plasma was used to accelerate the superhydrophobicity failure as a result of the damage caused by low surface energy materials on the surface.21 Generally, the surface turned superhydrophilic after treatment as short as 10 s resulting to the water wet and spread quickly on the RFHs surface, and our RFHs had no influence in such a short time.22,23 Then, the water droplets were completely wet and fully absorbed by the RFHs–PFA, leaving a large ink spot on the surface (Fig. 1a). XPS analysis was further carried out to investigate changes in the surface chemical composition of the RFHs surface after O2 plasma treatment (Table, ES1†). The F concentration of the surface decreased sharply from 44.15% to 30.67%. This indicates that O2 plasma can damage the low energy materials on the surface, which increases the superhydrophobicity failure. Fig. 1b shows that the RFHs completely recovered their superhydrophobicity, water droplets could stand on their surface, and no ink stain was observed on the hydrophobic face. The SEM images further confirms this result; the filler obviously decreased on the RFHs–PFA surface after the O2 plasma treatment (Fig. 1c). However, the filler enrichment was clearly observed at the outermost surface after the restore process (Fig. 1d). The outmost surface increased the F concentration to 35.83%, which is consistent with the SEM results. Therefore, we attribute the successful recovery of RFHs–PFA superhydrophobicity to the trapped low surface energy materials. These materials could transfer to the outermost surface automatically, which is also in good agreement with the results reported elsewhere.20,24
The wettability of the RFHs–PFA was further studied by measurement of the water CA on their surface. Fig. 2a and b show the changes in the CAs of the water droplets on the plasma-treated surfaces with time at ambient temperature. The CA of the water droplets on the RFHs–PFA was above 160° and after the O2 plasma treatment, the surface became superhydrophilic. After 3 h, the CA was about 50°, and then hydrophobicity was continually restored for 6 h. After being restored for 12 h, the RFHs–PFA completely recovered its superhydrophobicity (Fig. 2a). The regenerative ability of RFHs–PFA was further confirmed by studying several consecutive damages of the superhydrophobicity treatment and regenerative cycles. Fig. 2b shows the water CAs on the surface with several consecutive failure–healing cycles. This result indicates that the surface was able to maintain its superhydrophobicity for 10 cycles. Therefore, RFHs–PFA is sturdier, which is a very important characteristic of superhydrophobic material under harsh conditions. Meanwhile, the influence of RFHs length on their restored ability was also determined by the water CAs on the different length RFHs in several consecutive failure–healing cycles. Fig. 2c shows that the superhydrophobicity of the 1.2 cm RFHs–PFA was also reduced after seven cycles, and the droplet was rapidly absorbed into RFHs–PFA. Furthermore, no obvious change was observed on the apparent CA of the 0.6 cm RFHs–PFA until three cycles had been completed. However, it gradually decreased (Fig. 2d). A longer RFHs–PFA would result in increased superhydrophobicity. All the advantages previously mentioned suggest that RFHs–PFA has an important function in the utilization of the restored superhydrophobic material.
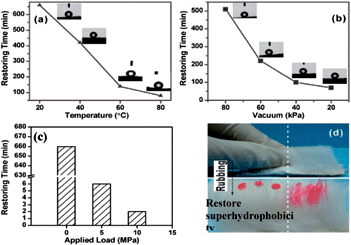
Fig. 3 shows the restore time of RFHs–PFA after O2 plasma treatment under different controlled conditions. As it can be seen in Fig. 3a, the speed of restore superhydrophobicity was greatly accelerated by elevating temperatures. The plasma-treated surface could restore superhydrophobicity within 7 h, 140 min, and 80 min at 40, 60 and 80 °C, respectively, which were much faster than that at ambient temperature (20 °C, about 11 h). The restore time of superhydrophobicity surface under different pressures also presented a similar trend. The restore velocity of the surface was accelerated under low pressure compared with constant pressure. In addition, restore time shortened with the gradual decrease of pressure from 80, 60 to 40 kPa needing about 8, 4 and 2 h, respectively. The restore required about 1 h under 20 kPa, which greatly shortened the time against the ordinary pressure (Fig. 3b). These results imply that the RFHs–PFA surface is more easily restored by either raising the temperature or reducing the pressure. Moreover, the achievement of a controllable and rapid regenerative superhydrophobicity is demonstrated.
RFHs are very flexible and soft, thus, regenerative superhydrophobicity can be easily induced mechanically, such as by squeezing, rubbing, and even rolling about on the ground. Remarkably, RFHs–PFA can also restore its superhydrophobicity in an extremely short time merely through manual squeezing. In the absence of squeezing, the restore process took over 11 h, whereas the process took only 6 min and 2 min under 5 and 10 MPa pressures, respectively. When the applied pressure reached 15 MPa, the superhydrophobicity was immediately recovered (Fig. 3c). The application of pressure can obviously move the infiltrated PFAs out to the surface and considerably increases the speed of recovery of the surface wetting properties. Squeezing the RFHs–PFA to rapidly restore superhydrophobicity is the most facile and fastest healing method compared with the methods used in previous reports.14 Fig. 3d shows that the superhydrophobicity surface recovered by rubbing with hands. One side of the O2 plasma-treated RFHs–PFA was rubbed several times, and then superhydrophobicity restored and water droplets did not spread but form a dried bead with a large CA. By contrast, under the same condition, the side that was not rubbed retaining its superhydrophilicity. The water was wet and spread quickly on the surface. Meanwhile, a larger applied extrusion pressure resulted in faster regeneration. We thus believe that the regenerative superhydrophobic material reported here is vastly superior to many other nanomaterials reported previously.
In conclusion, we demonstrated a facile and feasible method for fabricating a superhydrophobic material by combining the sophisticated and rough array characteristics of RFHs with their hollow interior filled with the low surface energy compound. When the superhydrophobicity of the newly developed material was suppressed through oxygen-plasma treatment, it could be restored. Meanwhile, the superhydrophobicity was healed after 11 h under normal condition. The restore behavior was based on the transfer and enrichment of the PFA loaded in the RFHs onto the top surface. The superhydrophobic regeneration capability of RFHs–PFA was greatly reinforced and controlled through heat or decompression conditions. The most interesting result was that their superhydrophobic surface could be rapidly restored by manual rubbing, and this process only required a few minutes. This study reports a potential breakthrough concept of a unique structural model of animal hair fibers which can be used for many applications. We believe that this cost-effective superhydrophobic material can be simulated in the future and further potential extended to various superhydrophobic field applications.
Acknowledgements
This work was financially supported by NSFC (21125316), 973 project (2013CB632300) and CAS(KJZD-EW-M01).Notes and references
- D. L. Hu, B. Chan and J. W. M. Bush, Nature, 2003, 424, 663 CrossRef CAS PubMed.
- S. A. Kulinich and M. Farzaneh, Cold Reg. Sci. Technol., 2011, 65, 603 CrossRef PubMed.
- I. You, S. M. Kang, S. Lee, Y. O. Cho, J. B. Kim, S. B. Lee, Y. S. Nam and H. Lee, Angew. Chem., Int. Ed., 2012, 124, 6230 CrossRef.
- S. A. Kulinich and M. Farzaneh, Langmuir, 2009, 25, 8854 CrossRef CAS PubMed.
- C. Cottin-Bizonne, J. L. Barrat, L. Bocquet and E. Charlaix, Nat. Mater., 2003, 2, 237 CrossRef CAS PubMed.
- S. M. Kang, I. You, W. K. Cho, H. K. Shon, T. G. Lee, I. S. Choi, J. M. Karp and H. Lee, Angew. Chem., Int. Ed., 2010, 49, 9401 CrossRef CAS PubMed.
- L. Boinovich and A. M. Emelyanenko, Mendeleev Commun., 2013, 23, 3 CrossRef CAS PubMed.
- Y. Y. Wang, J. Xue, Q. J. Wang, Q. M. Chen and J. F. Ding, ACS Appl. Mater. Interfaces, 2013, 5, 3370 CAS.
- S. A. Kulinich, S. Farhadi, K. Nose and X. W. Du, Langmuir, 2011, 27, 25 CrossRef CAS PubMed.
- K. Tadanaga, J. Morinaga, A. Matsuda and T. Minami, Chem. Mater., 2000, 12, 590 CrossRef CAS.
- S. Farhadi, M. Farzaneh and S. A. Kulinich, Appl. Surf. Sci., 2011, 257, 6264 CrossRef CAS PubMed.
- F. Zhang, L. Zhao, H. Chen, S. Xu, D. G. Evans and X. Duan, Angew. Chem., Int. Ed., 2008, 47, 2466 CrossRef CAS PubMed.
- X. L. Wang, X. J. Liu, F. Zhou and W. M. Liu, Chem. Commun., 2011, 47, 2324 RSC.
- B. Bhushan, Y. C. Jung, A. Niemietz and K. Koch, Langmuir, 2009, 25, 1659 CrossRef CAS PubMed.
- T. Verho, C. Bower, P. Andrew, S. Franssila, O. Ikkala and R. H. A. Ras, Adv. Mater., 2011, 23, 673 CrossRef CAS PubMed.
- L. Ionov and A. Synytska, Phys. Chem. Chem. Phys., 2012, 14, 10497 RSC.
- J. W. S. Hearle, Int. J. Biol. Macromol., 2000, 27, 123 CrossRef CAS.
- R. D. B. Fraser and D. A. D. Parry, J. Struct. Biol., 2003, 142, 319 CrossRef.
- F. Wan, Q. Ye, B. Yu, X. W. Pei and F. Zhou, J. Mater. Chem. B, 2013, 1, 3599 RSC.
- J. Tsibouklis, M. Stone, A. A. Thorpe, P. Graham, R. J. Ewen and T. G. Nevell, Langmuir, 1999, 15, 7076 CrossRef CAS.
- Y. Li, L. Li and J. G. Sun, Angew. Chem., Int. Ed., 2010, 49, 6129 CrossRef CAS PubMed.
- J. Tsibouklis, P. Graham, P. J. Eaton, J. R. Smith, T. G. Nevell, J. D. Smart and J. Ewen, Macromolecules, 2000, 33, 8460 CrossRef CAS.
- D. Therriault, S. R. White and J. A. Lewis, Nat. Mater., 2003, 2, 265 CrossRef CAS PubMed.
- X. J. Feng and L. Jiang, Adv. Mater., 2006, 18, 3063 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Full experimental details, photographs, SEM images, XPS spectrum and surface chemical composition data. See DOI: 10.1039/c3ra45188g |
| This journal is © The Royal Society of Chemistry 2014 |