High-performance inverted solar cells with a controlled ZnO buffer layer
Abstract
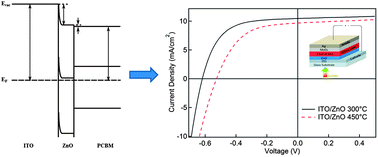
ZnO is a versatile cathode buffer layer for organic photovoltaics (OPV) due to its appealing optical and electronic properties. Using the sol–gel method, we find that the processing temperature of ZnO cathode buffer layers significantly influences the device performance of inverted polymer OPVs composed of blended films of poly(3-hexylthiophene) (P3HT) and [6,6]-phenyl-C61-butyric acid methyl ester (PCBM). In particular, ZnO processed at relatively low temperatures results in better device performance than those processed at higher temperatures despite the improved crystallinity and electron mobility of the latter. We attribute this finding to the tuning of the ZnO work function with the annealing temperature, which determines the interface energetics at the cathode and thus influences the open circuit voltage, series resistance and fill factor.

 Please wait while we load your content...
Please wait while we load your content...