High adsorption performance polymers modified by small molecules containing functional groups for CO2 separation†
Zhihua Qiao, Zhi Wang*, Song Zhao, Shuangjie Yuan, Jixiao Wang and Shichang Wang
School of Chemical Engineering and Technology, State Key Laboratory of Chemical Engineering, Tianjin Key Laboratory of Membrane Science and Desalination Technology, Tianjin University, Tianjin, PR China. E-mail: wangzhi@tju.edu.cn
First published on 30th October 2012
Abstract
We proposed the novel idea of using small molecules with functional groups to modify the functional groups and molecular aggregation of a polymer simultaneously. Small molecular amine-modified PVAm samples were prepared as novel adsorbents that have high CO2 adsorption performance and good stability.
Global energy and environmental problems create an urgent need for new functional materials to be used in areas such as energy storage and conversion, catalysis and gas separation.1,2 Much attention has been paid to CO2 separation processes, such as CO2 separation from flue gas (mainly N2) for environmental remediation2–5 and CO2 separation from natural gas (mainly CH4) and synthesis gas (mainly H2) for clean energy supply.6 In processes such as pressure swing adsorption (PSA) for CO2 separation, adsorbents such as metal–organic frameworks and molecular sieves have been greatly developed.7–10 These adsorbents are adequate but difficult to regenerate and be desorbed without significant heating, entailing low productivity and high costs.8 And thus the CO2 adsorption capacity of the adsorbents decreases with time, which will result in a large decrease of the CO2 selectivity. For CO2 separation, polymers are one of the most attractive materials, exhibiting fast CO2 adsorption and desorption and low energy requirements for recycling.7,11–16 During the past ten years, polyvinylamine (PVAm) has been developed greatly as a potential polymer for CO2 separation.17–20 CO2 separation is estimated to consume more than 30% of the power of a plant using the presently available polymer adsorption technologies, far above the theoretical minimum work of separation because the CO2 selectivity and adsorption capacity of the polymer is not high.14,15 The CO2 adsorption performance of the polymer depends on two aspects of the polymer, namely functional groups and molecular aggregation. Functional groups such as amine groups19 and carboxylate groups21 can make a chemical interaction with CO2 and metal ions can make a physical interaction with CO2,22 which are beneficial for CO2 adsorption. However, a large increase in the amount of functional groups will result in a great increase in the interactions between the functional groups, which will result in a decrease of the CO2 adsorption capacity and selectivity. The densified molecular aggregation of the polymer will increase the CO2 selectivity due to CO2 having a low kinetic diameter and high condensability, which also results in a decrease of the CO2 adsorption capacity of the polymer. In recent studies, to increase the CO2 selectivity of the polymer, only the molecular aggregation of the polymer was densified by heat treatment,23 chemical cross-linking24 and hydrogen bond cross-linking.25 However, densified molecular aggregation of the polymer will decrease CO2 adsorption capacity.23–25 Furthermore, for some polymers, the methods used to densify the polymer will decrease the concentration of original functional groups such as amine groups used to adsorb the CO2, which will make the CO2 adsorption capacity of the polymer decrease significantly.23,25 To greatly increase the CO2 adsorption capacity and selectivity of the polymer simultaneously, we proposed the novel idea of using small molecules with functional groups to modify the functional groups and molecular aggregation of the polymer simultaneously. To confirm this idea, a typical polymer material PVAm was modified by small molecules with amine groups (ethanediamine (EDA), piperazine (PIP), monoethanolamine (MEA) and methylcarbamate (MC)).
The specific chemical materials of the experiments are described in the Supplementary Information S1, ESI.† Small molecular amine (SMA)-modified PVAm was prepared by mixing 3 wt% PVAm aqueous solutions and SMA, wherein the molar ratio of amine groups contained in SMA to that contained in PVAm is 3. Then the mixtures were stirred for 12 h and stood for 24 h at room temperature (22 °C).
The pure PVAm or SMA-modified PVAm samples were obtained by dropping the aqueous solutions of the pure PVAm or SMA-modified PVAm on silicone rubber substrate, then drying for at least 24 h at 30 °C and 40% relative humidity in an artificial climate chamber (climacell 222R, Germany), and finally peeling from the silicone rubber substrate.
The pure PVAm or SMA-modified PVAm samples were characterized by an attenuated total reflectance Fourier transform infrared (ATR-FTIR) spectrometer (FTS-6000, Bio-Rad of America), volume measurement, mass stability measurement, an elemental analyzer (Carlo Erba EA1110, Italy), X-ray diffractometer (XRD) (D/MAX—2500) and Brunauer–Emmett–Teller measurement.
The chemical characterization of the SMA-modified PVAm samples was accomplished by ATR-FTIR. According to analysis of the ATR-FTIR spectra (see the Supplementary Information S2, ESI†), it can be concluded that intermolecular hydrogen bonds formed between SMA and PVAm in the SMA-modified PVAm samples. The intermolecular hydrogen bonds can be summarized as in the schematic diagram Fig. 1.
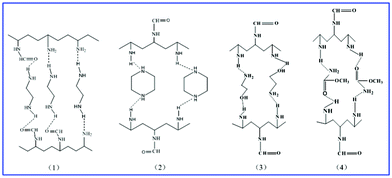 | ||
| Fig. 1 Schematic diagram of the intermolecular hydrogen bonds in the SMA-modified PVAm samples: (1) EDA-modified PVAm; (2) PIP-modified PVAm; (3) MEA-modified PVAm; (4) MC-modified PVAm. | ||
With the same amount of PVAm, the volume of the pure PVAm and SMA-modified PVAm samples tested is almost the same due to SMA having low molecular weights and intermolecular hydrogen bonds between SMA and PVAm. Therefore, compared with the pure PVAm sample, the molecular aggregation of the SMA-modified PVAm sample was densified.
In the PSA process, the adsorbents may be lost under pressure alteration during the adsorption and desorption processes.26 For investigating the mass stability of the SMA-modified PVAm, the mass of the SMA-modified PVAm samples treated under different pressure alterations was tested at 50 °C (the specific treatment of the sample is described in the supplementary information S3, ESI†).
The mass of the SMA-modified PVAm samples under various pressure alterations can be considered as the same in the error range of the analytical balance measurement. After treatment under pressure, the SMA-modified PVAm samples were investigated by ATR-FTIR again. According to a comparison of the ATR-FTIR spectra before and after treatment, it was found that the intermolecular hydrogen bonds formed between SMA and PVAm had not changed. Therefore, it can be deduced that under pressure almost all the molecules of the SMAs were fixed stably in the SMA-modified PVAm by intermolecular hydrogen bonds, which will result in a great increase of CO2 selectivity.
The mass ratio of C![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) N in the pure PVAm and SMA-modified PVAm samples was measured by an elemental analyzer. Table 1 shows the amine group concentration of the pure PVAm sample, which was calculated according to the molar mass of PVAm and the mass ratio of C
N in the pure PVAm and SMA-modified PVAm samples was measured by an elemental analyzer. Table 1 shows the amine group concentration of the pure PVAm sample, which was calculated according to the molar mass of PVAm and the mass ratio of C![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) N in the pure PVAm sample. The molar ratio of SMA
N in the pure PVAm sample. The molar ratio of SMA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PVAm (mSMA
PVAm (mSMA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) mPVAm) was calculated according to the mass ratio of C
mPVAm) was calculated according to the mass ratio of C![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) N. Table 1 also shows the amine group concentration of the SMA-modified PVAm, which was calculated according to the mSMA
N. Table 1 also shows the amine group concentration of the SMA-modified PVAm, which was calculated according to the mSMA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) mPVAm in the SMA-modified PVAm samples, the molar mass of SMA and the amine group concentration of the pure PVAm sample. The primary or secondary amine groups contained in the SMA-modified PVAm samples can react with CO2 as shown in the following formulas:27,28
mPVAm in the SMA-modified PVAm samples, the molar mass of SMA and the amine group concentration of the pure PVAm sample. The primary or secondary amine groups contained in the SMA-modified PVAm samples can react with CO2 as shown in the following formulas:27,28
| CO2 + RR′NH ⇌ RR′NH+COO− | (1) |
| RR′NH+COO− + RR′NH ⇌ RR′NCOO− + RR′NH2+ | (2) |
| RR′NH+COO− + H2O ⇌ RR′NCOO− + H3O+ | (3) |
| RR′NCOO− + H2O ⇌ RR′NH + HCO3− | (4) |
| The amine group concentration (10−2 mol carriers/g PVAm) | Crystallinity | |
|---|---|---|
| Pure PVAm | 1.110 | 0.380 |
| EDA-modified PVAm | 1.721 | 0.451 |
| PIP-modified PVAm | 4.200 | 0.379 |
| MEA-modified PVAm | 4.230 | 0.378 |
| MC-modified PVAm | 4.330 | 0.378 |
Where R′ may be an H or another group. The reaction between CO2 and amine carriers is defined by the zwitterion mechanism in formulas (1) to (4). Firstly, CO2 reacts with primary or secondary amines to form zwitterions as an intermediate. Then, the zwitterions are deprotonated by amine or H2O to form the carbamate ions. The carbamate ions of the amine carrier are unstable and could react with H2O to form bicarbonate ions. From the formulas of (1) to (4), the amine carrier can react with CO2 to form zwitterions, protonated-amines, carbamate ions and bicarbonate ions. The amine carriers can adsorb CO2 molecules by the CO2-carrier complex forms of carbamate and bicarbonate. And the reactions between amine groups and CO2 are reversible.
Compared with the pure PVAm sample, the concentration of amine groups in the SMA-modified PVAm samples has been greatly improved, which will result in great increases in the CO2 adsorption capacity and selectivity simultaneously. The amine group concentrations of the PIP-, MEA- or MC-modified PVAm samples are higher than that of the EDA-modified PVAm sample due to EDA having a volatile structure.
The crystallinity has an effect on the selectivity and adsorption capacity of CO2 because the crystalline region is not beneficial for CO2 adsorption.29,30 The crystallinity of the pure PVAm and SMA-modified PVAm samples was investigated by using an XRD in reflection mode with 2θ scanned between 5° and 80° under an 8 kW power. Table 1 shows the crystallinity of the SMA-modified PVAm samples. As shown in Table 1, the EDA-modified PVAm sample shows the highest crystallinity due to EDA having a regular and symmetric structure. Compared with the pure PVAm sample, the crystallinity of the PIP-, MEA- or MC-modified PVAm samples does not increase due to PIP, MEA or MC having an asymmetric structure.
In addition, though the amine group concentration of the PIP-, MEA- or MC-modified PVAm samples is almost the same, the reaction ability of the amine groups in the PIP-, MEA- or MC-modified PVAm samples is different. The reaction of the amine group with CO2 is a reaction gaining protons. The ability to gain protons depends on the electronegativity of the nitrogen atom of the amine groups.31 The electronegativity of the nitrogen atom included in MEA or MC with polar groups is higher than that of the nitrogen included in PIP. Therefore, the reaction activity of amine groups in the MEA- or MC-modified PVAm with CO2 is higher than that in the PIP-modified PVAm.
The CO2, N2, CH4 and H2 adsorption capacities of the pure PVAm and SMA-modified PVAm samples were characterized by Brunauer–Emmett–Teller measurement using the single component of CO2, N2, CH4 and H2 gases at low pressure region (below 1 bar). The ideal adsorption solution theory (IAST) of Myers and Prausnitz has been reported to predict binary gas mixture adsorption.32–34 To judge the merit of the SMA-modified PVAm samples for CO2/N2, CO2/CH4 and CO2/H2 separation, the CO2/N2, CO2/CH4 and CO2/H2 selectivities were predicted by IAST. The adsorption selectivity [defined as Sads = (q1/q2)/(P1/P2), where qi is the amount of i adsorbed and Pi is the partial pressure of i in the mixture] of the pure PVAm and SMA-modified PVAm samples for CO2 over N2 in flue gas (typically 15% CO2 and 85% N2), CO2 over CH4 in natural gas (typically 10% CO2 and 90% CH4) and CO2 over H2 in synthesis gas (typically 40% CO2 and 60% H2) were estimated from the experimental single-component isotherms. As shown in Fig. 2 and 3, the SMA-modified PVAm samples not only displayed high CO2 adsorption capacities, but also displayed high CO2/N2, CO2/CH4 and CO2/H2 selectivity. Because CO2 can react with amine groups contained in the SMA-modified PVAm samples, the CO2 adsorption capacity is much higher than the N2, CH4 and H2 adsorption capacities. As shown in Fig. 3, compared with the pure PVAm sample, the SMA-modified PVAm samples showed higher CO2 adsorption capacity and selectivity at various pressures. Compared with EDA- or PIP-modified PVAm samples, MEA- or MC-modified PVAm samples showed much higher CO2 adsorption capacity and selectivity due to MEA or MC having nonvolatile and asymmetric structures and containing amine groups with strong electronegativity. And thus SMAs such as MEA and MC are the most suitable to modify PVAm. The CO2 adsorption performance of the MC-modified PVAm sample is slightly higher than that of the MEA-modified PVAm sample due to the MC-modified PVAm sample having a higher amine group concentration.
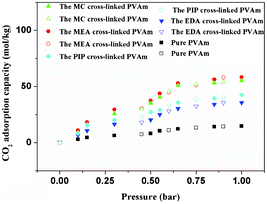 | ||
| Fig. 2 CO2 adsorption–desorption experiments at 50 °C. Filled and open symbols represent adsorption and desorption branches, respectively. | ||
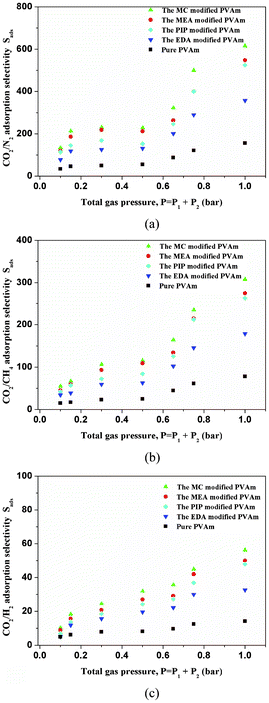 | ||
| Fig. 3 IAST-predicted CO2 adsorption selectivity of the pure PVAm and SMA-modified PVAm samples, (a) CO2/N2, (2) CO2/CH4, (3) CO2/H2. | ||
Furthermore, because the MC-modified PVAm sample shows the highest CO2 adsorption selectivity, according to the literature,35,36 the recovery and purity of the product was calculated. This could be achieved by using the MC-modified PVAm sample in the two-stage adsorption process with a recycle stream for the syngas and natural gas purification and the CO2 capture from flue gas. The separation targets of H2 recovery of 99.1% with H2 purity of 99.9% in the syngas purification, CH4 recovery of 99.8% with CH4 purity of 98% in the natural gas purification and CO2 recovery of 95% with CO2 purity of 96.3% in the flue gas CO2 capture can be achieved. The results indicate that the MC-modified PVAm sample shows promising application in CO2 separation processes.
Conclusions
It is suggested that polymers modified by small molecules with other functional groups besides amine groups, such as carboxylate groups and metal ions, also can increase the functional group concentration and densify the molecular aggregation of the polymer simultaneously. In conclusion, it has been demonstrated that using small molecules with functional groups to modify the polymer can greatly increase CO2 adsorption capacity and selectivity. Also, the SMA-modified PVAm has good stability. Polymers modified by small molecules with functional groups can be applied as new functional materials in CO2 separation. Moreover, like CO2, H2S and SO2 are both acidic gases, which can make interaction with functional groups contained in small molecules used to modify polymers. Therefore, to get high H2S and SO2 adsorption capacity and selectivity, polymers modified by small molecules with functional groups could be applied in the adsorption process for H2S and SO2 separation from gas mixtures with N2, CH4 and H2. In addition, small molecules with functional groups might be used to modify other gas separation materials such as metal–organic frameworks and molecular sieves to increase the gas adsorption capacity and selectivity.Acknowledgements
This research is supported by the Natural Science Foundation of China (No. 20836006), the National Basic Research Program (No. 2009CB623405), the National High-tech Research and Development Project (No. 2012AA03A611), the Science & Technology Pillar Program of Tianjin (No. 10ZCKFSH01700), the Programme of Introducing Talents of Discipline to Universities (No. B06006), and the Cheung Kong Scholar Program for Innovative Teams of the Ministry of Education (No. IRT0641).References
- (a) R. E. Morris and P. S. Wheatley, Angew. Chem., Int. Ed., 2008, 47, 4966–4981 CrossRef CAS; (b) C. Marichy, M. Bechelany and N. Pinna, Adv. Mater., 2012, 24, 1017–1032 CrossRef CAS.
- F. M. Orr, Energy Environ. Sci., 2009, 2, 449–458 CAS.
- G. P. Hao, W. C. Li, D. Qian and A. H. Lu, Adv. Mater., 2010, 22, 853–857 CrossRef CAS.
- Q. Wang, J. Z. Luo, Z. Y. Zhong and A. Borgna, Energy Environ. Sci., 2011, 4, 42–55 CAS.
- N. MacDowell, N. Florin, A. Buchard, J. Hallett, A. Galindo, G. Jackson, C. S. Adjiman, C. K. Willians, N. Shah and P. Fennell, Energy Environ. Sci., 2010, 3, 1645–1669 CAS.
- (a) N. P. Patel, A. C. Miller and R. J. Spontak, Adv. Mater., 2003, 15, 729–733 CrossRef CAS; (b) S. Li, J. L. Falconer and R. D. Noble, Adv. Mater., 2006, 18, 2601–2603 CrossRef CAS; (c) M. A. Carreon, S. Li, J. L. Falconer and R. D. Noble, Adv. Mater., 2008, 20, 729–732 CrossRef CAS.
- R. Banerjee, H. Furukawa, D. Britt, C. Knobler, M. O'Keeffe and O. M. Yaghi, J. Am. Chem. Soc., 2009, 131, 3875–3877 CrossRef CAS.
- J. An and N. L. Rosi, J. Am. Chem. Soc., 2010, 132, 5578–5579 CrossRef CAS.
- Z. R. Herm, J. A. Swisher, B. Smit, R. Krishna and J. R. Long, J. Am. Chem. Soc., 2011, 133, 5664–5667 CrossRef CAS.
- S. Couck, J. F. M. Denayer, G. A. Baron, T. Remy, J. Gascon and F. Kapteijn, J. Am. Chem. Soc., 2009, 131, 6326–6327 CrossRef CAS.
- B. S. Ghanem, N. B. Mckeown, P. M. Budd, J. D. Selbie and D. Fritsch, Adv. Mater., 2008, 20, 2766–2771 CrossRef CAS.
- H. P. Brack, C. Padeste, M. Slaski, S. Alkan and H. H. Solak, J. Am. Chem. Soc., 2004, 126, 1004–1005 CrossRef CAS.
- E. D. Bates, R. D. Mayton, I. Nati, Jr and J. H. Davis, J. Am. Chem. Soc., 2002, 124, 926–927 CrossRef CAS.
- C. M. White, B. R. Strazisar, E. J. Granite, J. S. Hoffman and H. W. Pennline, J. Air Waste Manage. Assoc., 2003, 53, 645–715 CAS.
- D. M. D. Alessandro, B. Smit and J. R. Long, Angew. Chem., Int. Ed., 2010, 49, 6058–6082 CrossRef.
- H. S. Choi and M. P. Suh, Angew. Chem., Int. Ed., 2009, 48, 6865–6869 CrossRef CAS.
- P. Bernardo, E. Drioli and G. Golemme, Ind. Eng. Chem. Res., 2009, 48, 4638–4663 CrossRef CAS.
- T. J. Kim, B. Li and M. B. Hägg, J. Polym. Sci., Part B: Polym. Phys., 2004, 43(23), 4326 CrossRef.
- Z. Qizo, Z. Wang, C. Zhang, S. Yuan, Y. Zhu, J. Wang and S. Wang, AIChE Journal, 2012 DOI:10.1002/aic.13781.
- http://www.kema.com/news/articles/2011/inauguration-nanoglowa.aspx.
- M. Wang, Z. Wang, J. Wang, Y. Zhu and S. Wang, Energy Environ. Sci., 2011, 4, 3955–3959 CAS.
- K. Yao, Z. Wang, J. Wang and S. Wang, Chem. Commun., 2012, 48, 1766–1768 RSC.
- I. Mochida, S. Yatsunami, Y. Kawabachi and Y. Nakayama, Carbon, 1995, 33, 1611–1619 CrossRef CAS.
- W. J. Koros and R. Mahajan, J. Membr. Sci., 2000, 175, 181–196 CrossRef CAS.
- N. B. MaKeown and P. M. Budd, Macromolecules, 2010, 43, 5163–5176 CrossRef.
- P. J. E. Harlick and A. Sayari, Ind. Eng. Chem. Res., 2006, 45, 3248–3255 CrossRef CAS.
- Y. Cai, Z. Wang, C. H. Yi, Y. H. Bai, J. X. Wang and S. C. Wang, J. Membr. Sci., 2008, 310, 184–196 CrossRef CAS.
- H. Matsuyama, A. Terada and T. Nakagawara, J. Membr. Sci., 1999, 163, 221–227 CrossRef CAS.
- K. Binder, J. Baschnagle, M. Muller, W. Paul and F. Rampf, Macromol. Symp., 2006, 237, 128–138 CrossRef CAS.
- Y. H. Jhon, M. Cho, H. R. Jeon, I. Park, R. Chang, J. L. C. Rowsell and J. Kim, J. Phys. Chem. C, 2007, 111, 16618–16625 CAS.
- (a) F. A. Carey, Organic Chemistry (4th edition). McGraw-Hill Higher Education, New York: 2000, 86[4] Search PubMed; (b) F. A. Carey, Organic Chemistry (4th edition). McGraw-Hill Higher Education, New York: 2000, 863–864 Search PubMed.
- W. G. Lu, D. Q. Yuan, J. Sculley, D. Zhao, R. Krishna and H. C. Zhou, J. Am. Chem. Soc., 2011, 133, 18126–18129 CrossRef CAS.
- W. G. Lu, J. Sculley, D. Q. Yuan, R. Krishna, Z. G. Wei and H. C. Zhou, Angew. Chem., Int. Ed., 2012, 51, 7480–7484 CrossRef CAS.
- J. A. Mason, K. Sumida, Z. R. Herm, R. Krishna and J. R. Long, Energy Environ. Sci., 2011, 4, 3030–3040 CAS.
- D. X. Yang, Z. Wang, J. X. Wang and S. C. Wang, Ind. Eng. Chem. Res., 2009, 48, 9013–9022 CrossRef CAS.
- L. Zhao, R. Menzer, E. Riensche, L. Blum and D. Stolten, Energy Procedia. GHGT 9., 2009, 1, 269–278 CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Supplementary Information including the S1 (the intermolecular hydrogen bonds in the SMA-modified PVAm). See DOI: 10.1039/c2ra21769d |
| This journal is © The Royal Society of Chemistry 2013 |
