A retrospective analysis of real-life practice of off-label photodynamic therapy using methyl aminolevulinate (MAL-PDT) in 20 Italian dermatology departments. Part 1: Inflammatory and aesthetic indications†
Pier Giacomo
Calzavara-Pinton
*a,
Maria Teresa
Rossi
a,
Erica
Aronson
b,
Raffaella
Sala
a and
The Italian Group for Photodynamic Therapy‡
c
aDepartment of Dermatology, University of Brescia, Brescia, Italy. E-mail: fototerapia@spedalicivili.brescia.it; Fax: +39 030 3995015; Tel: +39 030 3995307
bThe University of Chicago Medical Center, Chicago, Illinois
c20 Dermatology Departments, Italy
First published on 2nd August 2012
Abstract
Experimental investigations have demonstrated that photodynamic therapy (PDT) with methyl aminolevulinate (MAL) may be a useful treatment in several inflammatory skin disorders and aesthetic indications. To assess the effectiveness, tolerability and safety of off-label MAL-PDT in daily clinical practice in 20 Italian hospital centers, a retrospective observational study of medical records of patients treated for off-label inflammatory and aesthetic indications was carried out. In all patients standard treatment options had been either ineffective, unacceptably toxic, or medically contraindicated. Clinical data regarding 221 patients affected by 22 different diseases were collected. The most common off-label indication was acne vulgaris, with >75% improvement in 72.8% of patients. Other disorders of the sebaceous gland, i.e. acne rosacea, hidradenitis suppurativa and sebaceous hyperplasia, were less responsive. Alopecia areata did not show any improvement. Granuloma annulare and necrobiosis lipoidica showed marked or moderate response in the majority of treated patients. The rate of patients with complete remission was lower for inflammatory skin disorders with hyperkeratosis, i.e. psoriasis (6/17) and porokeratosis (3/16). The efficacy for lichenoid dermatoses was dependent on the clinical variant (erosive and scleroatrophic were more responsive than hypertrophic). Only 1 of 6 patients with Zoon balanitis had a marked improvement. MAL-PDT of venous leg ulcers, photo-aging and hypertrophic scars led to a marked remission in 3/5, 3/6 and 5/8 patients, respectively. The treatment had to be interrupted because of strong pain and burning in 24 patients. Long term adverse events were not registered. Most patients with marked improvement had lasting remission with overall excellent cosmetic outcomes. The present findings demonstrate a high interest in off-label uses of MAL-PDT for inflammatory skin disorders. According to the observed clinical responses, safety, and favorable cosmetic results, MAL-PDT seems to have a potential therapeutic role for the treatment of granulomatous dermal disorders and follicular inflammatory diseases whereas results in other conditions are less encouraging.
Introduction
More than 20 years after the first clinical report,1 topical photodynamic therapy (PDT) with aminolevulinic acid (ALA) or its methyl esther (methyl aminolevulinate, MAL) can no longer be considered a “novel” treatment modality in dermatology. At this time, a cream (Metvix®, Galderma, F) containing 160 mg g−1 of MAL has been approved for the treatment of actinic keratosis, basal cell carcinoma and Bowen's disease in the European Union (EU) and the United States (US).In addition to anti-tumor activities, experimental studies on PDT have demonstrated a variety of pro- and anti-inflammatory effects and immunological activities. The final outcome stimulated by this treatment modality was mainly dependent on the dosage of light and drug. Evidence of the effects of PDT on keratinocytes, fibroblasts, mast cells, sebaceous glands and hair follicles as well as the anti-microbial activity associated with the procedure has recently been demonstrated.2
Both ALA and MAL have been investigated for the treatment of a multitude of inflammatory skin disorders and aesthetic indications.3–5 However, most clinical studies describe individual patients or small case series and they have significant differences in patient selection criteria. ALA treatment variables such as drug concentration, type of vehicle, light spectrum and dosage are inconsistent between studies as well. In addition, results are usually assessed visually without unbiased parameters, such as histopathology or non-invasive imaging techniques. Furthermore, almost all publications report successful outcomes. This may be due to authors choosing not to submit negative observations, or, if they do, risk not being accepted for publication. On the other hand, the few randomized blind clinical trials (RCTs) often enrolled a limited number of patients and had a short follow-up period. Therefore most off-label clinical indications must be considered to be in an exploratory stage.
We report here a retrospective analysis of medical records of 221 patients affected by inflammatory skin disorders and aesthetic indications who were treated with MAL-PDT in the period 2005–2010 by a network of 20 Italian Dermatology Departments that routinely use this therapy. The present findings may be useful in providing information on effectiveness of the technique performed in everyday practice and, therefore, it may be different from efficacy that is assessed with RCTs which have more stringent criteria for patient inclusion, often excluding those patients with milder or more complicated disease and they often do not allow for alteration of the number and frequency of treatments according to the individual patient's response and needs. In addition, in RCTs the assessment of efficacy, tolerability and safety is scored according to rigid scales whereas, in clinical practice, the assessment is subjective. Furthermore, in our retrospective analysis, the follow-up of patients with a complete remission or a marked improvement was often prolonged and therefore it may be useful for the assessment of safety because RCTs often include only short term follow-up.
Finally, the present findings are useful for a more reliable assessment of the technique in the treatment of disease processes for which there are no available RCTs because we included all of the treated patients, regardless of the clinical outcome.
Materials and methods
Patients
This retrospective, observational, open study was performed by collecting previously unpublished clinical records of patients treated for off-label indications of MAL-PDT in 20 Italian hospital departments. In all patients, conventional topical or systemic therapies were not effective or were discontinued because of the development of adverse events, or were contraindicated because of co-morbidities, concurrent therapies, or a high hazard of toxicity. All subjects gave written informed consent prior to being treated.The diagnosis was assessed visually in most patients and a biopsy for histological confirmation was taken only in selected cases.
In all centers, contraindications to the treatment were pregnancy or lactation, any active systemic infectious disease, other inflammatory, infectious or neoplastic skin diseases in the area treated, allergy to MAL, other topical photosensitizers or ingredients of the cream, history of photosensitivity, use of immunosuppressive or photosensitizing drugs, and history or indicators of poor compliance.
Treatment protocol
In all patients, a 1 mm thick layer of Metvix® cream was applied for 3–4 hours under an occlusive and opaque dressing. After the removal of the cream, lesions were irradiated with 37 J cm−2 of red (635 ± 18 nm) light from a diode lamp (Aktilite CL128, PhotoCure ASA, Oslo, Norway) or equivalent light doses from other polychromatic light sources (PDT S-630 lamp, Alpha Strumenti Srl, Melzo, Italy in 2 centers; PDT 1200L, Waldmann GmbH, Villingen-Schwenningen, Germany in 1 center). Any use of pretreatment such as curettage or keratolytics was documented as well. The number and frequency of treatments were chosen by the investigators according to their experience and patients’ needs.Study procedures
A standardized data collection form was sent to the participating centers to collect the following data:– Pre-treatment information: patients’ demographic data, baseline lesion characteristics and severity, histopathological findings (if present), and prior therapies and efficacy.
– Treatment parameters: MAL application time, light source, light dose, number of treatments and time interval between treatments.
– Adverse events: local reaction was considered moderate if temporary erythema was seen and marked if persistent erythema with edema and/or erosions developed. Lack of local reaction was registered as absent. Pain and/or burning sensation were rated according to the following score: mild (therapy continued); moderate (strength of irradiation was fractionated); intense (irradiation was stopped).
– Clinical results were assessed 7–15 days post-treatment and the degree of improvement from baseline was rated as follows: marked (complete remission or >75% improvement), moderate (50–75% improvement) and poor/absent (less than 50% improvement). The cosmetic outcome was assessed only in patients with a marked improvement and it was scored as excellent if no scarring, atrophy, induration or change in pigmentation was seen.
– Post-treatment patient information: patients with a marked improvement (complete remission or >75% improvement) did not undergo further treatments and the clinical result at the last follow-up visit was scored according to the above-mentioned criteria and recorded.
Data are reported as mean ± standard deviation (m ± SD).
Results
Fully evaluable data collection forms of the clinical records of 221 patients treated with MAL-PDT were received and analyzed. We have not included records of 47 additional patients treated with ALA because of differences in treatment variables such as drug concentration, type of vehicle, light spectrum and dosage.For those diseases with at least 5 treated patients, their demographic information, the number of treatments and the interval between them, are summarized in Table 1.
| No of patients | Gender (M/F) | Age (years) | Number of treatments | Interval between treatments (days) | |
|---|---|---|---|---|---|
| Acne | 92 | 36/56 | 24 ± 6.2 | 3.3 ± 1.7 | 19.6 ± 7.7 |
| Hidradenitis suppurativa | 6 | 1/5 | 34 ± 10.9 | 4.8 ± 2.9 | 18.8 ± 8.4 |
| Sebaceous hyperplasia | 5 | 3/2 | 56.6 ± 10.9 | 2.6 ± 0.5 | 37.2 ± 13.0 |
| Rosacea | 7 | 3/4 | 56.6 ± 15.6 | 5.7 ± 5.1 | 19.6 ± 7.7 |
| Alopecia areata | 7 | 2/5 | 39.9 ± 8.53 | 2.7 ± 1.1 | 17.3 ± 10.8 |
| Psoriasis | 17 | 9/8 | 42.5 ± 13.0 | 3.6 ± 1.1 | 9.9 ± 5.6 |
| Porokeratosis (all variants) | 16 | 7/9 | 61.9 ± 18.25 | 2.1 ± 0.9 | 14.0 ± 11.7 |
| Zoon balanitis | 6 | 6/0 | 65.5 ± 9.5 | 3.2 ± 1.0 | 19.8 ± 9.3 |
| Lichenoid dermatoses (all variants) | 12 | 9/3 | 62.8 ± 12.9 | 2.7 ± 1.2 | 26.1 ± 12.2 |
| Granuloma annulare | 13 | 5/8 | 50.8 ± 9.8 | 2.8 ± 1.4 | 23.5 ± 6.5 |
| Necrobiosis lipoidica | 8 | 0/8 | 35 ± 16.9 | 10.0 ± 7.5 | 18.0 ± 12.0 |
| Venous leg ulcers | 5 | 1/4 | 79.8 ± 10.2 | 2.4 ± 0.9 | 18.2 ± 8.0 |
| Photoaging | 6 | 1/5 | 63.2 ± 12.9 | 3.7 ± 2.1 | 15.2 ± 13.6 |
| Scars | 8 | 1/7 | 49.6 ± 15.4 | 4.0 ± 3.2 | 13.1 ± 2.5 |
The mean number of treatments was similar ranging from 2 to 4 for most indications and only patients with rosacea (5.7 ± 5.1) and necrobiosis lipoidica (10.0 ± 7.5) received more treatments.
The mean interval between treatments ranged from 2 to 4 weeks for most indications. The intervals were longer for chronic disorders of the sebaceous glands (acne, rosacea, hidradenitis suppurativa and sebaceous hyperplasia) and shorter for psoriasis.
With skin penetration as a goal, the MAL cream was consistently applied under occlusion. Curettage was not used and keratolytic creams were applied for a few days before treatment only for patients with psoriasis. For pain or burning that developed during the light therapy, the treatment area was cooled with a fan or ice water was sprayed during and immediately after treatment. In addition, cool-packs were commonly utilized but topical or regional anesthesia was not typically used. If erythema with or without edema and erosion developed, antiseptic soaks and an antibiotic cream were applied until complete recovery was achieved.
Results of treatment of disorders of the pilosebaceous unit are summarized in Table 2.
| Disease | No of patients | Clinical response [n, %] | Local reaction [n, %] | Pain or burning during the treatment [n, %] | Excellent cosmesis and marked response [n, %] | Follow-up (months, m ± SDa) | Persistent marked response at follow-up [n, %] | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No/poor (<50%) | Moderate (50–75%) | Marked (>75%) | Absent | Moderate | Marked | No | Mild | Moderate | Marked | |||||
| a m ± SD: mean ± standard deviation. | ||||||||||||||
| Acne | 92 | 9 | 12 | 71 | 23 | 68 | 1 | 17 | 40 | 21 | 14 | 67 | 2.1 ± 1.8 | 67 |
| 10% | 13% | 77% | 25% | 74% | 1% | 18% | 44% | 23% | 15% | 73% | 73% | |||
| Hidradenitis suppurativa | 6 | 1 | 3 | 2 | 1 | 5 | 0 | 0 | 2 | 4 | 0 | 2 | 2.6 ± 0.55 | 1 |
| 17% | 50% | 33% | 17% | 83% | 0% | 0% | 33% | 67% | 0% | 33% | 17% | |||
| Sebaceous hyperplasia | 5 | 1 | 2 | 2 | 0 | 5 | 0 | 0 | 2 | 3 | 0 | 2 | 7.6 ± 4.3 | 2 |
| 20% | 40% | 40% | 0% | 100% | 0% | 0% | 40% | 60% | 0% | 40% | 40% | |||
| Rosacea | 7 | 1 | 3 | 3 | 4 | 3 | 0 | 1 | 3 | 2 | 1 | 3 | 6.8 ± 4.9 | 3 |
| 14% | 43% | 43% | 57% | 43% | 0% | 14% | 43% | 29% | 14% | 43% | 43% | |||
| Alopecia areata | 7 | 6 | 1 | 0 | 4 | 2 | 1 | 0 | 6 | 1 | 0 | 0 | 4.0 ± 2.0 | 0 |
| 88% | 14% | 0% | 57% | 29% | 14% | 0% | 88% | 14% | 0% | 0% | 0% | |||
The overall improvement of acne was rated as marked in 71 (77.2%) patients (Fig. 1), moderate in 12 (13. 0%) and poor in 9 (9.8%). Sixty seven patients (72.8%) had an excellent cosmetic result and four with skin type IV had transiently hyper-pigmented areas. The marked response was maintained at follow-up (2.1 ± 1.8 months) by 67 patients (72.8%).
 | ||
| Fig. 1 (a) Acne vulgaris of the face and neck before MAL-PDT. (b) Acne vulgaris of the face and neck after 2 MAL-PDT treatment sessions. | ||
Papulopustular and cystic lesions were seen in 54 and 38 patients, respectively, and similar rates of marked improvement were seen of 77.7% and 76.4%, respectively. Worsening of the lesions was not registered. Patients with comedonal acne alone were not treated. The majority (73.9%) of patients developed a moderate local reaction soon after treatment and only one patient had a marked reaction with erythema, edema and erosions. Because of pain or burning sensation, exposure was discontinued in 15.2% of patients and fractionated in 22.8%.
Other inflammatory diseases of the pilosebaceous unit, i.e. hidradenitis suppurativa, sebaceous hyperplasia and rosacea, had marked and moderate improvement in 2 and 3 out of 6, 2 and 2 out of 5, and 3 and 3 out of 7 patients, respectively. All patients with a marked response had excellent cosmetic outcome and, at follow-up (2.6 ± 0.5, 7.6 ± 4.3. 6.8 ± 4.9 months, respectively), only one patient with rosacea relapsed. No patient developed severe erythema or erosions and exposure was discontinued in only one patient with rosacea. A 19 year old male with lupus miliaris of the face underwent 2 treatment sessions at monthly intervals. Therapy was well tolerated and he had a complete improvement that was maintained at 12 months follow-up. Six out of 7 patients with alopecia areata remained unchanged and only one had moderate improvement (Table 2).
Psoriatic plaques were treated in 17 patients, 4 (23.5%) of which developed a marked inflammatory reaction and 5 patients (29.4%) with severe pain and/or burning sensation. Six (35.3%) patients had marked improvement but transient hyperpigmentation developed in one of the patients. Psoriatic lesions treated with PDT worsened in 2 patients (11.8%) suggestive of Koebner's phenomenon. All patients with a marked response relapsed during a 2.8 ± 0.7 months’ follow-up period (Table 3).
| Disease | No of patients | Clinical response [n, %] | Local reaction [n, %] | Pain or burning during the treatment [n, %] | Excellent cosmesis and marked response [n, %] | Follow-up (months, m ± SDb) | Persistent marked response at follow-up [n, %] | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No/poor (<50%) | Moderate (50–75%) | Marked (> 75%) | Absent | Moderate | Marked | Absent | Mild | Moderate | Marked | |||||
| a 2 psoriatic patients (11.8%) had a worsening of their lesions. b m ± SD: mean ± standard deviation. | ||||||||||||||
| Psoriasisa | 17 | 3 | 6 | 6 | 2 | 11 | 4 | 4 | 4 | 4 | 5 | 5 | 2.8 ± 0.7 | 0 |
| 18% | 35% | 35% | 12% | 65% | 24% | 24% | 24% | 24% | 29% | 29% | 0% | |||
| Porokeratosis | 16 | 10 | 3 | 3 | 2 | 13 | 1 | 2 | 10 | 3 | 1 | 3 | 9.6 ± 12.2 | 2 |
| 63% | 19% | 19% | 12% | 81% | 6% | 13% | 63% | 19% | 6% | 19% | 13% | |||
| Zoon balanitis | 6 | 2 | 3 | 1 | 2 | 3 | 1 | 0 | 4 | 2 | 0 | 1 | 48 | 0 |
| 33% | 50% | 17% | 33% | 50% | 17% | 0% | 67% | 33% | 0% | 17% | 0% | |||
| Lichenoid dermatoses | 12 | 4 | 0 | 8 | 3 | 7 | 2 | 1 | 6 | 3 | 2 | 7 | 6.6 ± 5.0 | 8 |
| 33% | 0% | 67% | 25% | 58% | 17% | 8% | 50% | 25% | 17% | 58% | 67% | |||
| Granuloma annulare | 13 | 4 | 2 | 7 | 1 | 12 | 0 | 1 | 9 | 3 | 0 | 7 | 7.3 ± 8.4 | 7 |
| 31% | 15% | 54% | 8% | 92% | 0% | 8% | 69% | 23% | 0% | 54% | 54% | |||
| Necrobiosis lipoidica | 8 | 2 | 3 | 3 | 2 | 6 | 0 | 3 | 5 | 0 | 0 | 3 | 9.4 ± 13.3 | 2 |
| 25% | 38% | 38% | 25% | 75% | 0% | 38% | 63% | 0% | 0% | 38% | 25% | |||
| Venous leg ulcers | 5 | 1 | 1 | 3 | 0 | 4 | 1 | 0 | 3 | 1 | 1 | 3 | 3.2 ± 0.4 | 3 |
| 20% | 20% | 60% | 0% | 80% | 20% | 0% | 60% | 20% | 20% | 60% | 60% | |||
Porokeratosis was treated in 16 patients (13 with disseminated actinic porokeratosis, one with the linear variety and two with Mibelli's type) and good improvement was seen in three (18.8%) of the patients with actinic porokeratosis. A marked local reaction with strong pain developed in one patient (6.3%) (Table 3).
Twelve patients with lichen planus (5 with erosive lesions of the genitalia and lips, 3 with hypertrophic lesions of the legs and 4 with scleroatrophic lesions of the genitalia) were treated and the clinical improvement was marked in 8 of the patients. All patients with hypertrophic lesions and one with scleroatrophic lesions were unresponsive although they reported improvement in pruritus with the therapy. Local inflammation with intense pain or burning was seen in two patients with erosive lesions (Table 3).
Six patients with Zoon balanitis were treated. Exposures were well tolerated but efficacy was poor. Only one patient had a marked improvement and a relapse was seen after 48 months.
Three patients with Hailey–Hailey disease were each treated with two MAL-PDT sessions. All three patients reported the treatment to be very painful and followed by intense erythema and erosions. At the end of the treatment cycle, two patients had worsening of the lesions and the third was unresponsive.
Granuloma annulare (GA) and necrobiosis lipoidica diabeticorum (NLD) were treated in 13 and 8 patients, respectively. The rate of patients with marked improvement (all with an excellent cosmesis) was higher for GA (7/13) (Fig. 2) than NLD (3/8), although the GA patients received fewer treatment sessions: 2.8 ± 1.4 and 10.0 ± 7.5, respectively. At follow-up (7.3 ± 8.4 and 9.4 ± 13.3 months, respectively), 7 patients with GA and 2 patients with NLD were still in remission. The treatment was well tolerated and marked local inflammation and symptoms were not documented (Table 3).
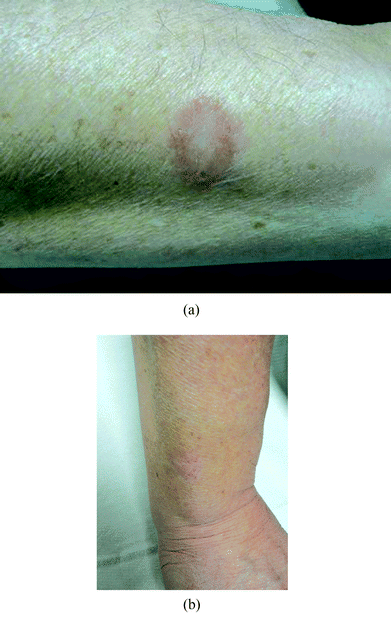 | ||
| Fig. 2 (a) Granuloma annulare of the right forearm before 2 MAL-PDT sessions. (b) Granuloma annulare of the right forearm after 2 MAL-PDT sessions. | ||
A 63 year old male with an actinic granuloma of O'Brien and a 49 year old man with granuloma faciale were unresponsive to 2 and 3 MAL-PDT sessions, respectively. A 70 year old male patient with cutaneous sarcoidosis had a complete and persistent (after 12 months’ follow-up) remission following 12 very well tolerated MAL-PDT sessions over one-week intervals. Two MAL-PDT sessions were delivered to two female patients, aged 31 and 50 years, both with morphea, who achieved opposite results: the former did not improve and the second had a complete remission that was persistent at 12 months’ follow-up.
A 58 year old male patient and a 66 year old female patient with discoid lupus erythematosus (DLE) were treated with 4 and 6 MAL-PDT sessions, respectively, with moderate improvement. Both patients treated for DLE relapsed after 1 and 3 months, respectively. The light treatments were well tolerated but both patients developed hyperpigmentation of the treated areas.
A 43 year old male patient with a chronic radiodermatitis of the face was treated successfully with 2 MAL-PDT sessions with a very good cosmetic result. The patient tolerated the treatment well and remission was maintained at the 2 month follow up.
Mid-dermal elastolysis was not responsive to 3 MAL-PDT sessions at 4 week intervals in a 31 year old woman. The treatments did not cause skin inflammation or symptoms.
Venous leg ulcers were treated in 5 patients and the improvement was good in 3 of those patients, with two achieving complete recovery and one patient with partial remission. In two patients, the exposures needed to be fractionated and the only patient who did not respond at all was the individual who discontinued the treatment due to intense pain (Table 3).
Six patients were treated with PDT for photoaging. The improvement was rated as marked in three patients, moderate in one and poor in two. In two of the patients, the marked improvement was maintained at the 10.3 ± 18.5 months’ follow-up. None of the patients experienced severe pain or burning and only one described moderate pain.
Treatment of hypertrophic scars resulted in marked improvement in five of eight patients. The treatment was well tolerated but in one patient the exposures needed to be fractionated (Table 4). All of the patients with significant improvement seen for follow-up were monitored for long-term adverse events and none were recorded.
| Disease | No of patients | Clinical response [n, %] | Local reaction [n, %] | Pain or burning during the treatment [n, %] | Follow-up (months, m ± SDa) | Persistent marked response at follow-up [n, %] | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No/poor (<50%) | Moderate (50–75%) | Marked (>75%) | Absent | Moderate | Marked | Absent | Mild | Moderate | Strong | ||||
| a m ± SD: mean ± standard deviation. | |||||||||||||
| Photoaging | 6 | 2 | 1 | 3 | 1 | 5 | 0 | 1 | 4 | 1 | 0 | 10.3 ± 18.5 | 2 |
| 33% | 17% | 50% | 17% | 83% | 0% | 17% | 67% | 17% | 0% | 33% | |||
| Hypertrophic scars | 8 | 1 | 2 | 5 | 4 | 4 | 0 | 5 | 2 | 1 | 0 | 14.1 ± 21.0 | 5 |
| 13% | 25% | 63% | 50% | 50% | 0% | 63% | 25% | 13% | 0% | 63% | |||
Discussion
The present retrospective analysis shows that the interest for the exploration of off-label uses of MAL-PDT is high, because it was used in the treatment of 221 patients in 20 hospital institutions for a broad spectrum of inflammatory and adnexal skin disorders and aesthetic indications. Some indications were explored in a good number of patients and results provide general information on effectiveness, tolerability and safety in everyday practice when only the so-called “difficult” patients who were not candidates for, or not responsive to, standard therapies can be selected for an off-label treatment. Therefore, the present results are not generalizable to all patients with a given disease and they may be different from results of RCTs with stringent criteria of selection of patients and centers.Response rates in small and heterogeneous case series or isolated case reports are of minor value. However, our findings demonstrate that in “real life” an attempt at PDT of these conditions is done for unresponsive or recalcitrant cases although data from RCTs are not available and only case reports or studies of small case series are available. In addition, the present findings help to clarify feasibility, safety and tolerability for these uncommon indications because we included both successful and negative results, and the last ones are not usually reported in the medical literature.
As a general rule, effectiveness of photodynamic therapy did not seem to correlate with the intensity of the local inflammatory reaction that, instead, was generally proportional to the degree of pain and burning experienced by the patient. The strategy of Italian dermatologists to manage inflammation and pain was fairly uniform with discontinuation or fractionation of the exposure. In addition, various techniques for cooling the treated skin were used but oral analgesics and topical or regional anesthesia were not administered.
Despite the limitation of only visual clinical assessment, we found that effectiveness and tolerability were quite similar among groups of diseases with similar pathologic features.
MAL-PDT was highly effective with a moderate or marked improvement in the majority of patients, with few relapses at follow-up for the treatment of disorders characterized by enlarged and inflamed sebaceous glands with colonization by Propionibacterium acnes and/or other microorganisms, i.e. acne, hidradenitis suppurativa, sebaceous hyperplasia and rosacea.
The rate of significant improvement of acne was higher than reported in two previous RCTs.6,7 However, the patients treated for acne and included in this study received a greater number of treatments: 3.3 ± 1.7 versus 2.6,7 The severity of the acne lesions did not influence treatment outcomes and similar results were found in cystic as well as papulo-pustular variants of the disease. Of note, comedonal acne was not considered a therapeutic target of MAL-PDT.
Our results for rosacea and sebaceous hyperplasia were similar to those previously reported with MAL-PDT8 and ALA-PDT.9–11
The present findings in hidradenitis suppurativa (2 out of 6 patients with marked improvement) conflict with the results of three previously published papers examining PDT in this disease process. Those previous publications outlined five cases of hidradenitis suppurativa in which ALA was ineffective12 and two cases in which a complete clearance was achieved in patients treated with MAL.13,14
The present case series includes findings of a patient with lupus miliaris faciei who was treated successfully. This was the first time that a patient with this disorder was treated.
Consistent with two previous investigations,15,16 alopecia areata proved unresponsive to PDT, suggesting a lack of penetration of MAL through the normal epidermis into the deeper portions of the thick dermis of the scalp where the lymphocytic infiltrate around the terminal hair bulbs is located.
Disorders with chronic granulomatous inflammation and/or degenerative changes of collagen were highly responsive to PDT and very well tolerated with low pain and little local inflammation. A possible explanation is that the overlying normal or slightly atrophic epidermis hindered the penetration of MAL leading to low skin sensitization and low pain. However the reaction was enough to modulate the dermal granulomatous processes.17 The overall rates of moderate and marked improvement of GA (9/13 patients) and NLD (6/8 patients) were similar but the rate of marked improvement was higher for GA than for NLD. This is likely due to the fact that eradication of the granulomatous inflammation leads to complete recovery of the skin in GA but not in necrobiosis NLD where cicatricial and atrophic areas often remain.
Our findings in GA are slightly better than those of a previous study that reported a complete response or a marked improvement in four of seven patients treated with ALA-PDT.18 Another study from an Italian center reported three cases with long-standing generalized GA responding to MAL-PDT.19
Our findings in NLD are not as successful as two previous case reports that described the clearance of both treated patients,20,21 but they also do not correlate with a recent multicenter retrospective study that demonstrated poor response to treatment in patients with NLD: one complete and six partial improvements out of 18 treated patients.22
The only patient we treated with sarcoidosis had a very good result, which was consistent with the literature.23,24 In our experience, MAL-PDT was ineffective for the treatment of three uncommon skin disorders with dermal inflammatory infiltrate: actinic granuloma of O'Brien, mid-dermal elastolysis and granuloma faciale. There are no case reports of these diseases treated with PDT in the literature with which to compare our data.
We found that one patient with morphea was cleared of disease while another was unresponsive to treatment. This data mirrors the unpredictability of results of PDT as demonstrated in two studies of morphea, one with 6 patients25 that did not improve and an earlier study with positive findings in 5 patients.26
Good results were recorded in the treatment of patients with hypertrophic scars, another disease process with abnormalities of collagen metabolism (but without sclerosis as opposed to morphea) which confirms the findings of previous investigations with MAL-PDT.27,28 These results suggest that the activity of dermal fibroblasts is modulated by MAL-PDT despite the fact that a poor local reaction to the treatment suggests a low level of epidermal photosensitization.
A therapeutic effect on dermal cells is supported by the effectiveness of MAL-PDT for photorejuvenation in the present retrospective study and previous investigations,29,30 and chronic radiodermatitis.31 MAL-PDT was well tolerated and the relatively low number of patients treated for photorejuvenation can be explained by the hospital setting of the participating centers.
The activity of MAL-PDT on dermal fibroblasts, together with antimicrobial properties, could explain the complete recovery of venous leg ulcers in 3 out of 5 treated patients, confirming previous findings.32,33 However, in these previous studies the pain was described as minimal compared to the significant reaction seen in our patient population.
PDT for Hailey–Hailey disease was described as very painful with lesions improving only partially or even worsening in the present and previous investigation.34 These data conflict with earlier findings that described the disappearance of symptoms (pain, pruritus) and the achievement of clinical clearance of lesions in two patients with Hailey–Hailey disease.35 Our findings in patients with leg ulcers and Hailey–Hailey disease demonstrate that skin may become highly photosensitive upon MAL exposure in diseases in which the skin barrier is damaged.36
The outcome of MAL-PDT for inflammatory skin disorders with a predominantly mononuclear and lymphocytic infiltrate seems dependent largely on the thickness of the epidermis, specifically the stratum corneum. The thicker the corneal layer and epidermis, the less effective MAL was at penetrating the top layers of skin,36 leading to a sub-therapeutic response to treatment. This is clearly demonstrated by the results obtained in the treatment of lichenoid dermatoses. Ulcerative and sclero-atrophic variants had a much higher rate of marked responses along with higher incidence of reported pain and burning, as well as local inflammatory response when compared to hypertrophic lesions.
The present results are in general agreement with previously reported case reports or studies of small case series that were previously reported.37–40
The thick hyperkeratotic layer was likely responsible also for the overall poor results observed in the treatment of porokeratosis in the present retrospective analysis and in two previous studies of small case series,41,42 although MAL-PDT was found highly effective in two single case reports.43,44
Another skin disorder with thick hyperkeratosis, DLE, was partially responsive with quick relapse in both treated patients. Again, findings of previous case reports are conflicting: two patients did not improve and could not tolerate ALA-PDT,45 whereas one patient had lasting remission with MAL-PDT.46 Of note, in this and other disease processes characterized by the presence of hyperkeratosis and scales as prominent features, Italian dermatologists did not use curettage, possibly to avoid bleeding and additional pain in the patients.
The overall results of MAL-PDT in psoriasis (70.6% with moderate and marked improvement) were superior to findings of previous reports using ALA at different concentrations and various treatment protocols.47,48 However, marked pain or burning sensation leading to discontinuation of the exposures and marked local inflammatory response with erythema, edema and erosion were quite common and two cases of worsening of the disease due to Koebner’s phenomenon were recorded.
Results of MAL-PDT of Zoon balanitis have not been reported so far. Although the treatment was well tolerated, the therapeutic results were disappointing. A poor sensitization of plasma cells as well as the non-uniform delivery of light and/or MAL cream due to the irregular tridimensional shape of this peculiar anatomical area must be taken into account.
No matter the skin disorder for which they were receiving the treatment, almost all patients with significant improvement in their disease also had an excellent cosmetic result including lasting remission at follow-up. Although we enrolled a large number of patients, one of the few short term adverse events recorded was transient hyperpigmentation, which developed in a handful of patients with psoriasis and discoid lupus erythematosus. Long term adverse events were never registered.
In conclusion, real life utilization of MAL-PDT has demonstrated a potential therapeutic role for the treatment of granulomatous dermal disorders and follicular inflammatory diseases due to its effectiveness, safety and favorable cosmetic result.
References
- J. C. Kennedy, R. H. Pottier and D. C. Pross, Photodynamic therapy with endogenous protoporphyrin IX: basic principles and present clinical experience, J. Photochem. Photobiol., B, 1990, 6, 143–148 CrossRef CAS.
- B. Ortel, C. R. Shea and P. G. Calzavara-Pinton, Molecular mechanisms of photodynamic therapy, Front. Biosci., 2009, 14, 4157–4172 CrossRef CAS.
- P. G. Calzavara-Pinton, M. Arisi, E. Sereni and B. Ortel, A critical reappraisal of off-label indications for topical photodynamic therapy with aminolevulinic acid and methylaminolevulinate, Rev. Recent Clin. Trials, 2010, 5, 112–116 CrossRef CAS.
- A. Klein, P. Babilas, S. Karrer, M. Landthaler and R. M. Szeimies, Photodynamic therapy in dermatology – an update, J. Dtsch. Dermatol. Ges., 2008, 6, 839–845 Search PubMed.
- C. A. Morton, K. E. McKenna and L. E. Rhodes, Guidelines for topical photodynamic therapy: update, Br. J. Dermatol., 2008, 159, 1245–1266 CrossRef CAS.
- S. R. Wiegell and H. C. Wulf, Photodynamic therapy of acnes vulgaris using methyl aminolaevulinate: a blinded, randomized, controlled trial, Br. J. Dermatol., 2006, 154, 969–976 CrossRef CAS.
- R. Bissonnette, C. Maari, S. Nigen, N. Provost and C. Bolduc, Photodynamic therapy with methylaminolevulinate 80 mg g−1 without occlusion improves acnes vulgaris, J. Drugs Dermatol., 2010, 11, 1347–1352 Search PubMed.
- L. E. Bryld and G. B. Jemec, Photodynamic therapy in a series of rosacea patients, J. Eur. Acad. Dermatol. Venereol., 2007, 21, 1199–1202 CAS.
- T. Horio, O. Horio and H. Miyauchi-Hashimoto, Photodynamic therapy of sebaceous hyperplasia with topical 5-aminolevulinic acid and slide projector, Br. J. Dermatol., 2003, 148, 1274–1276 CrossRef CAS.
- M. H. Gold, V. L. Bradshaw, M. M. Boring, T. M. Bridges, J. A. Biron and T. L. Lewis, Treatment of sebaceous gland hyperplasia by photodynamic therapy with 5-aminolevulinic acid and a blue light source or intense pulsed light source, J. Drugs Dermatol., 2004, 6, S6–S9 Search PubMed.
- S. H. Kim, J. E. Do, H. Y. Kang, E. S. Lee and Y. C. Kim, Combination of topical 5-aminolevulinic acid-photodynamic therapy with carbon dioxide laser for sebaceous hyperplasia, J. Am. Acad. Dermatol., 2007, 56, 523–524 CrossRef.
- E. Sotiriou, Z. Apalla, F. Maliamani and D. Ioannides, Treatment of recalcitrant hidradenitis suppurativa with photodynamic therapy: report of five cases, Clin. Exp. Dermatol., 2009, 34, e235–e236 CrossRef CAS.
- R. Saraceno, M. Teoli, C. Casciello and S. Chimenti, Methyl aminolaevulinate photodynamic therapy for the treatment of hidradenitis suppurativa and pilonidal cysts, Photodermatol., Photoimmunol. Photomed., 2009, 25, 164–165 CrossRef CAS.
- A. Guglielmetti, J. Bedoya, M. Acuna, V. Leiva and S. Gonzalez, Successful aminolevulinic acid photodynamic therapy for recalcitrant severe hidradenitis suppurativa, Photodermatol., Photoimmunol. Photomed., 2010, 26, 110–111 CrossRef.
- M. Fernández-Guarino, A. Harto, I. García-Morales, B. Pérez-García, J. M. Arrazola and P. Jaén, Failure to treat alopecia areata with photodynamic therapy, Clin. Exp. Dermatol., 2008, 33, 585–587 CrossRef.
- R. Bissonnette, J. Shapiro, H. Zeng, D. I. McLean and H. Lui, Topical photodynamic therapy with 5-aminolaevulinic acid does not induce hair regrowth in patients with extensive alopecia areata, Br. J. Dermatol., 2000, 143, 1032–1035 CrossRef CAS.
- D. W. C. Hunt and A. H. Chan, Influence of photodynamic therapy on immunological aspects of disease – an update, Expert Opin. Invest. Drugs, 2000, 9, 807–817 CrossRef CAS.
- P. Weisenseel, A. V. Kuznetsov, S. Molin, T. Ruzicka, C. Berking and J. C. Prinz, Photodynamic therapy for granuloma annulare: more than a shot in the dark, Dermatology, 2008, 217, 329–332 CrossRef.
- S. Piaserico, E. Zattra, D. Linder and A. Peserico, Generalized granuloma annulare treated with methylaminolevulinate photodynamic therapy, Dermatology, 2009, 218, 282–284 CrossRef CAS.
- M. Heidenheim and G. B. Jemec, Successful treatment of necrobiosis lipoidica diabeticorum with photodynamic therapy, Arch. Dermatol., 2006, 142, 1548–1550 Search PubMed.
- V. De Giorgi, G. Buggiani, R. Rossi, S. Sestini, M. Grazzini and T. Lotti, Successful topical photodynamic treatment of refractory necrobiosis lipoidica, Photodermatol., Photoimmunol. Photomed., 2008, 24, 332–333 CrossRef.
- C. Berking, J. Hegyi, P. Arenberger, T. Ruzicka and G. B. Jemec, Photodynamic therapy of necrobiosis lipoidica–a multicenter study of 18 patients, Dermatology, 2009, 218, 136–139 CrossRef CAS.
- C. Penrose, S. E. Mercer and H. Shim-Chang, Photodynamic therapy for the treatment of cutaneous sarcoidosis, J. Am. Acad. Dermatol., 2011, 65, e12–e14 CrossRef.
- S. Karrer, C. Abels, M. B. Wimmershoff, M. Landthaler and R. M. Szeimies, Successful treatment of cutaneous sarcoidosis using topical photodynamic therapy, Arch. Dermatol., 2002, 138, 581–584 Search PubMed.
- R. Batchelor, S. Lamb, V. Goulden, G. Stables, M. Goodfield and W. Merchant, Photodynamic therapy for the treatment of morphoea, Clin. Exp. Dermatol., 2008, 33, 661–663 CrossRef CAS.
- S. Karrer, C. Abels, M. Landthaler and R. M. Szeimies, Topical photodynamic therapy for localized scleroderma, Acta Derm.-Venereol., 2000, 80, 26–27 CrossRef CAS.
- Z. Nie, A. Bayat, F. Behzad and L. E. Rhodes, Positive response of a recurrent keloid scar to topical methyl aminolevulinate-photodynamic therapy, Photodermatol., Photoimmunol. Photomed., 2010, 26, 330–332 CrossRef.
- S. M. Campbell, J. Tyrrell, R. Marshall and A. Curnow, Effect of MAL-photodynamic therapy on hypertrophic scarring, Photodiagn. Photodyn. Ther., 2010, 7, 183–188 CrossRef CAS.
- G. Sanclemente, L. Medina, J. F. Villa, L. M. Barrera and H. I. Garcia, A prospective split-face double-blind randomized placebo-controlled trial to assess the efficacy of methyl aminolevulinate + red-light in patients with facial photodamage, J. Eur. Acad. Dermatol. Venereol., 2011, 25, 49–58 CrossRef CAS.
- C. Zane, R. Capezzera, R. Sala, M. Venturini and P. G. Calzavara-Pinton, Clinical and echographic analysis of photodynamic therapy using methylaminolevulinate as sensitizer in the treatment of photodamaged facial skin, Lasers Surg. Med., 2007, 39, 203–209 CrossRef.
- C. Guillen, O. Sanmartin, A. Escudero, R. Botella-Estrada, A. Sevila and P. Castejon, Photodynamic therapy for in situ squamous cell carcinoma on chronic radiation dermatitis after photosensitization with 5-aminolaevulinic acid, J. Eur. Acad. Dermatol. Venereol., 2000, 14, 298–300 CrossRef CAS.
- T. H. Clayton and P. V. Harrison, Photodynamic therapy for infected leg ulcers, Br. J. Dermatol., 2007, 156, 384–385 CrossRef CAS.
- R. M. Strauss, S. Ogden, R. A. Sheehan-Dare and V. Goulden, Leg ulceration after aminolaevulinic acid photodynamic therapy in a patient with peripheral vascular disease, Dermatology, 2003, 207, 85 CrossRef CAS.
- M. Fernández Guarino, A. M. Ryan, A. Harto, B. Pérez-García, J. M. Arrázola and P. Jaén, Experience with photodynamic therapy in Hailey–Hailey disease, J. Dermatol. Treat., 2008, 19, 288–290 CrossRef.
- R. Ruiz-Rodriguez, J. G. Alvarez, P. Jaén, A. Acevedo and S. Córdoba, Photodynamic therapy with 5-aminolevulinic acid for recalcitrant familial benign pemphigus (Hailey–Hailey disease), J. Am. Acad. Dermatol., 2002, 47, 740–742 CrossRef.
- M. M. Kleinpenning, J. H. Kanis, T. Smits, P. E. van Erp, P. van de Kerkhof and R. M. Gerritsen, The effects of keratolytic pretreatment prior to fluorescence diagnosis and photodynamic therapy with aminolevulinic acid-induced porphyrins in psoriasis, J. Dermatol. Treat., 2010, 21, 245–251 CAS.
- S. Vano-Galvan, M. Fernandez-Guarino, S. Beà-Ardebol, B. Perez, A. Harto and P. Jaen, Successful treatment of erosive vulvar lichen sclerosus with methylaminolaevulinic acid and laser-mediated photodynamic therapy, J. Eur. Acad. Dermatol. Venereol., 2009, 23, 71–72 CrossRef CAS.
- E. Sotiriou, D. Panagiotidou and D. Ioannidis, An open trial of 5-aminolevulinic acid photodynamic therapy for vulvar lichen sclerosus, Eur. J. Obstet. Gynecol. Reprod. Biol., 2008, 141, 187–188 CrossRef.
- P. Hillemanns, M. Untch, F. Pröve, R. Baumgartner, M. Hillemanns and M. Korell, Photodynamic therapy of vulvar lichen sclerosus with 5-aminolevulinic acid, Obstet. Gynecol., 1999, 93, 71–74 CrossRef CAS.
- A. Olejek, K. Steplewska, A. Gabriel, I. Kozak-Darmas, A. Jarek, S. Kellas-Sleczka, F. Bydliński, K. Sieroń-Stoltny, S. Horak, A. Chełmicki and A. Sieroń, Efficacy of photodynamic therapy in vulvar lichen sclerosus treatment based on immunohistochemical analysis of CD34, CD44, myelin basic protein, and Ki67 antibodies, Int. J. Gynecol. Cancer, 2010, 20, 879–887 CrossRef.
- F. A. Nayeemuddin, M. Wong, J. Yell and L. E. Rhodes, Topical photodynamic therapy in disseminated superficial actinic porokeratosis, Clin. Exp. Dermatol., 2002, 27, 703–706 CrossRef CAS.
- M. Fernández-Guarino, A. Harto, B. Pérez-Garcia, M. Martin-González, S. Urrutia and P. Jaén, Photodynamic therapy in disseminated superficial actinic porokeratosis, J. Eur. Acad. Dermatol. Venereol., 2009, 23, 176–177 CrossRef.
- S. Cavicchini and A. Tourlaki, Successful treatment of disseminated superficial actinic porokeratosis with methyl aminolevulinate-photodynamic therapy, J. Dermatol. Treat., 2006, 17, 190–191 CrossRef.
- X. García-Navarro, J. R. Garcés, E. Baselga and A. Alomar, Linear porokeratosis. Excellent response to photodynamic therapy, Arch. Dermatol., 2009, 145, 526–527 Search PubMed.
- A. Romero-Maté, E. Castaño-Suárez, C. García-Donoso, C. Martínez-Morán, C. Meseguer-Yebra and J. Borbujo, Treatment of recalcitrant cutaneous discoid lupus erythematosus with photodynamic therapy, Photodermatol., Photoimmunol. Photomed., 2010, 26, 156–158 CrossRef.
- M. Fernández-Guarino, B. Pérez-García, A. Harto and P. Jaén, Discoid lupus erythematosus: good response to treatment with photodynamic therapy, J. Eur. Acad. Dermatol. Venereol., 2008, 22, 1142–1143 CrossRef.
- P. E. Beattie, R. S. Dawe, J. Ferguson and S. H. Ibbotson, Lack of efficacy and tolerability of topical PDT for psoriasis in comparison with narrowband UVB phototherapy, Clin. Exp. Dermatol., 2004, 29, 545–562 CrossRef.
- S. Radakovic-Fijan, U. Blecha-Thalhammer, V. Schleyer, R. M. Szeimies, T. Zwingers, H. Hönigsmann and A. Tanew, Topical aminolevulinic acid-based photodynamic therapy as a treatment option for psoriasis? Results of a randomized, observer-blinded study, Br. J. Dermatol., 2005, 152, 279–283 CrossRef CAS.
Footnotes |
| † This article is published as part of a themed issue on current topics in photodermatology. |
| ‡ The Italian Group for Photodynamic Therapy: Piergiacomo Calzavara-Pinton, Maria Teresa Rossi, Raffaella Sala (Dermatology Department, University of Brescia), Nicola Arpaia (Dermatology Department II, University of Bari), Elena Cleopatra Burtica (Dermatology Department, University of Bologna), Paolo Amerio (Dermatology Department, Hospital SS. Annunziata, Chieti), Annarosa Virgili (Dermatology Department, Hospital S. Anna, Ferrara), Riccardo Rossi, Gionata Buggiani (Dermatology Department, University of Florence), Andrea Zanca (Dermatology Department, A.O. Carlo Poma, Mantua), Leonardo Bugatti (Dermatology Department, ASUR 5, Jesi), Dario Fai (Dermatology Department, Gagliano del Capo Hospital, Lecce), Elisa Cervadoro (Dermatology Department, USL 6, Livorno), Stefano Cavicchini (Dermatology Department I, University of Milan), Fabrizio Fantini (Dermatology Department, University of Modena), Gabriella Fabbrocini (Dermatology Department, University of Naples), Stefano Piaserico (Dermatology Department, University of Padua), Elisa Cervadoro (Dermatology Department, University of Pisa), Miriam Teoli (Dermatology Department, University Tor Vergata, Rome), Laura Eibenschutz (Dermatology Department, San Gallicano Institute, Rome), Fabrizio Arcangeli (Dermatology Department, Hospital S. Maria, Terni), Paolo Broganelli (Dermatology Department, University of Turin), Donatella Schena (Dermatology Department, University of Verona). |
| This journal is © The Royal Society of Chemistry and Owner Societies 2013 |
