A retrospective analysis of real-life practice of off-label photodynamic therapy using methyl aminolevulinate (MAL-PDT) in 20 Italian dermatology departments. Part 2: Oncologic and infectious indications†
Pier Giacomo Calzavara-Pintona, Maria Teresa Rossia, Raffaella Salaa and The Italian Group for Photodynamic Therapy‡b
aPiergiacomo Calzavara-Pinton, Department of Dermatology, University of Brescia, Piazzale Spedali Civili 1, I-25123 Brescia, Italy. E-mail: fototerapia@spedalicivili.brescia.it; Fax: +39 030 3995015; Tel: +39 030 3995307
b20 Dermatology Departments, Italy
First published on 3rd August 2012
Abstract
Photodynamic therapy (PDT) with methyl aminolevulinate (MAL) has been suggested as a useful treatment option in a number of skin tumors, other than approved indications, and infections. However, evidence is poor because it is mainly supported by isolated case reports or small case series, often with conflicting results. To assess the effectiveness, tolerability and safety of off-label MAL-PDT in daily clinical practice in 20 Italian hospitals, a retrospective observational study of medical records of patients treated for off-label oncologic and infectious skin conditions was carried out. In all patients standard treatment options had been either ineffective, unacceptably toxic, or medically contraindicated. Clinical data from 145 patients were analyzed. Actinic cheilitis showed a complete remission (CR) in 27 out of 43 treated patients and CR was maintained at follow-up. CR was registered in 3 of 8, 5 of 8 and 10 of 17 treated patients who were affected by extra-mammary Paget's disease (EMPD), erythroplasia of Queyrat (QD), and invasive squamous cell carcinoma (SCC), respectively. Five out of 19 patients with cutaneous T cell lymphoma had a complete remission. Cutaneous B-cell lymphoma, malignant fibrous histiocytoma, mastocytosis and nevus sebaceous were not responsive. Warts were treated in 30 patients and 15 had a complete remission. However, periungueal and plantar lesions were much more responsive than flat and common lesions. Condylomata showed a CR in 2 out of 5 male patients but treatment was painful. Bowenoid papulosis showed only a partial improvement. Atypical mycobacteriosis and chronic cutaneous leishmaniasis were successfully treated. Submammary candidal intertrigo and interdigital intertrigo with Pseudomonas aeruginosa did not improve. Among off-label oncological uses of MAL-PDT, the therapy of actinic cheilitis was the most investigated and showed the best results. In addition, MAL-PDT was used successfully in the majority of patients with QD, EMPD and invasive SCC. Treatment of specific cutaneous infections was well tolerated and gave a good therapeutic result in a few patients, but it does not seem to give substantial advantages over conventional treatment options.
Introduction
In the 20 years since the first clinical report,1 topical photodynamic therapy (PDT) with aminolevulinic acid (ALA) and its methyl esther (methyl aminolevulinate, MAL) have been shown to be highly effective in the treatment of actinic keratosis, basal cell carcinoma and Bowen's disease. These results led the FDA and the EMA to approve Metvix® (Galderma, F), a cream containing 160 mg g−1 of MAL for these clinical conditions.2 The advantages of PDT are its high efficacy and tolerability coupled with excellent cosmetic outcomes.2 In addition, the potential of PDT has been investigated in a wide range of infective dermatoses as well as in off-label skin cancers.2–4 The aim in the treatment of both infectious and tumoral indications is the same: the lethal cytotoxicity of the target tumor cells/infectious agents and the modulation of immunological reactions without damaging the surrounding healthy skin.5 However, off-label clinical indications for PDT are still at an exploratory stage as clinical evidence for the use of PDT in these indications is generally poor and based on individual case reports or small case series, and the few randomized clinical trials (RCTs) performed enrolled small numbers of patients with short follow-up.2,3 Furthermore, results are generally based on visual assessments without controlled parameters, such as histopathology and/or non-invasive imaging procedures. Finally, interestingly, most clinical investigations have reported positive outcomes, which may be due to negative outcomes not being submitted by investigators for publication or that once submitted were more likely to be rejected for publication.3Here we report a retrospective analysis of the medical records of 145 patients with off-label oncological and infectious indications who were treated with MAL-PDT in the period 2005–2010 by a network of 20 Italian dermatology departments that routinely use this therapy.
The present findings show the interest of Italian dermatologists for off-label uses of this technique. Obviously, they cannot replace results of randomized clinical trials (RCTs) but they may be useful in providing information on effectiveness and tolerability of the technique performed in everyday practice for patients who are unresponsive to standard treatment options or who cannot use those therapies because of contraindications or previous adverse events.
Finally, the present findings are useful for a more reliable assessment of the technique in the treatment of disease processes for which there are no available RCTs and only a few case reports and studies of small case series have been published.
Materials and methods
Patients
This retrospective, observational, open study was performed by analyzing the clinical records of patients treated with off-label MAL PDT for oncologic and infectious indications in 20 Italian hospitals and university departments from 2005 to 2010. We discuss only records of patients who were not part of a clinical trial (published or unpublished). For all patients included in the study, conventional topical or systemic therapies were not effective or were discontinued because of the development of adverse events, or were contraindicated because of co-morbidities, concurrent therapies, or a high risk of toxicity. All subjects gave written informed consent prior to being treated.Diagnosis was performed only by visual assessment in all patients with warts. A biopsy for histological confirmation was taken in the other patients. In all centers, contraindications to the treatment were: pregnancy or lactation; any active systemic infectious disease; other inflammatory, infectious or neoplastic skin diseases in the area treated; known allergy to MAL, other topical photosensitizers or ingredients of the cream; history of photosensitivity, use of immunosuppressive or photosensitizing drugs; and a history or indicators of poor compliance to treatment.
Treatment protocol
In all patients, a 1 mm thick layer of Metvix® (Galderma, Paris, F) cream was applied for 3–4 hours under an occlusive and opaque dressing. After the removal of the cream, lesions were irradiated with 37 J cm−2 of red (635 ± 18 nm) light from a diode lamp (Aktilite CL128, PhotoCure ASA, Oslo, Norway) or equivalent light doses from other polychromatic light sources (PDT S-630 lamp, Alpha Strumenti Srl, Melzo, Italy in 2 centers, PDT 1200L, Waldmann GmbH, Villingen-Schwenningen, Germany in 1 center). Use of pretreatments such as curettage, surgical paring or use of keratolytic topical products was also documented. Dermatologists selected the number of treatments and the time interval between them on the basis of prior experience and the needs of their patients.Study procedures
A standardized data collection form was sent to the participating centers to collect the following data:– Pre-treatment information: patients’ demographic data, baseline lesion characteristics and severity, histopathological findings (if present), and prior therapies and efficacy.
– Treatment parameters: MAL application time, light source, light dose, number of treatments and time interval between treatments.
– Adverse events: local reaction was considered moderate if temporary erythema was observed and marked if persistent erythema with edema and/or erosions developed. Lack of local reaction was registered as absent. Pain and/or burning sensation were rated according to the following score: mild (therapy continued); moderate (strength of irradiation was fractionated); marked (irradiation was stopped).
– Clinical results were assessed 7–15 days after treatment and the degree of improvement from baseline was rated as follows: complete remission (CR; 100% improvement), partial (50–99% improvement) and no/poor (less than 50% improvement). Patients with partial or poor improvement underwent further experimental treatments. The cosmetic outcome was assessed only in patients with a complete remission and was scored as excellent if no scarring, atrophy, induration or change in pigmentation was seen.
– Post-treatment patient information: patients with a marked improvement (complete remission or >75% improvement) did not undergo further treatments and the clinical result at the last follow-up visit was scored according to the above-mentioned criteria and recorded.
Data are reported throughout as mean ± standard deviation (m ± SD).
Results
Fully evaluable data collection forms of the clinical records of 145 patients (105 with malignant and benign skin tumors and 40 with skin infections) treated with MAL-PDT were received and analyzed. We have not included records of 38 other patients treated with ALA because treatment variables such as drug concentration, type of vehicle, light spectrum and dosage were not consistent between patients.For those diseases with at least 5 treated patients, demographic information, the number of treatments and the treatment intervals are summarized in Table 1. For these diseases the mean number of treatments was similar for all indications ranging from 2.3 ± 1.3 with actinic cheilitis to 3.8 ± 2.4 for mycosis fungoides. The interval between treatments was shorter for squamous cell carcinoma (SCC), mycosis fungoides and warts (14.9 ± 5.2, 16.0 ± 9.0 and 16.0 ± 11.8 days, respectively) than for Queyrat's disease (QD) (20.0 ± 9.4) and extramammary Paget's disease (EMPD) (21.9 ± 10.2). Lesions of the transitional epithelium were treated more aggressively (9.6 ± 7.5 days for actinic cheilitis and 8.4 ± 3.1 for condylomata).
| No of patients | Gender (M/F) | Age (years) | Number of treatments | Interval between treatments (days) | |
|---|---|---|---|---|---|
| Actinic cheilitis | 43 | 27/16 | 71 ± 8.0 | 2.3 ± 1.3 | 9.6 ± 7.5 |
| Queyrat | 8 | 8/0 | 57.5 ± 14.1 | 2.4 ± 0.9 | 20.0 ± 9.4 |
| EMPD | 8 | 4/4 | 65.9 ± 14.4 | 3.3 ± 1.2 | 21.9 ± 10.2 |
| SCC | 17 | 13/4 | 73.6 ± 11.8 | 3.3 ± 1.6 | 14.9 ± 5.2 |
| Mycosis fungoides | 19 | 8/11 | 60.6 ± 12.5 | 3.8 ± 2.4 | 16.0 ± 9.0 |
| Warts | 30 | 12/18 | 29.1 ± 13.1 | 2.7 ± 0.8 | 16.0 ± 11.8 |
| Condylomata | 5 | 5/0 | 40.2 ± 8.4 | 2.8 ± 0.5 | 8.4 ± 3.1 |
Forty-three patients (27 males, 16 females; mean age 71 ± 8.0 years) had actinic cheilitis (Fig. 1; Table 2). Of these 62.8% had a CR and remained disease-free for a mean follow-up of 4.2 ± 5.9 months with excellent cosmetic improvements. The local inflammatory reaction was marked in 41.9% of patients but treatment was discontinued due to manifest and unbearable pain in only 5 (11.6%) patients.
 | ||
| Fig. 1 (a) Actinic cheilitis before MAL-PDT. (b) Actinic cheilitis after 2 MAL-PDT sessions treatment. | ||
| Disease | No of patients | Clinical response [n, %] | Local reaction [n, %] | Pain or burning during the treatment [n, %] | Excellent cosmesis and marked response [n, %] | Follow-up (months, m ± SD) | Persistent marked response at follow-up [n, %] | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No/poor (<50%) | Partial (50–99%) | Complete (100%) | Absent | Moderate | Marked | No | Mild | Moderate | Marked | |||||
| Actinic cheilitis | 43 | 3 | 13 | 27 | 15 | 10 | 18 | 3 | 23 | 12 | 5 | 27 | 4.2 ± 5.9 | 27 |
| 7% | 30% | 63% | 35% | 23% | 42% | 7% | 53% | 28% | 12% | 63% | 63% | |||
| Queyrat | 8 | 2 | 1 | 5 | 1 | 1 | 6 | 0 | 3 | 2 | 3 | 5 | 9.5 ± 12.9 | 3 |
| 25% | 13% | 63% | 13% | 13% | 75% | 0% | 38% | 25% | 38% | 63% | 38% | |||
| EMPD | 8 | 1 | 4 | 3 | 0 | 3 | 5 | 0 | 4 | 3 | 1 | 2 | 8.5 ± 7.1 | 3 |
| 13% | 50% | 38% | 0% | 38% | 63% | 0% | 50% | 38% | 13% | 25% | 38% | |||
| SCC | 17 | 1 | 6 | 10 | 8 | 8 | 1 | 3 | 6 | 7 | 1 | 7 | 10.3 ± 7.1 | 8 |
| 6% | 35% | 59% | 47% | 47% | 6% | 18% | 35% | 41% | 6% | 41% | 47% | |||
| Mycosis fungoides | 19 | 6 | 8 | 5 | 6 | 8 | 5 | 6 | 8 | 5 | 0 | 4 | 10.0 ± 10.5 | 3 |
| 32% | 42% | 26% | 32% | 42% | 26% | 32% | 42% | 26% | 0% | 21% | 16% | |||
Eight patients with QD (mean age: 57.5 ± 14.1 years) and 8 patients with EMPD (Fig. 2) (4 males and 4 females; mean age 65.9 ± 14.4 years) of the groin (6 patients) and internatal cleft (2 patients) were included in the study (Table 2). QD seemed more responsive to treatment (5 of 8 patients had a CR, all with an excellent cosmetic outcome) than EMPD (3 of 8 patients with CR, 2 of them with an excellent cosmetic outcome) but at a follow-up of 9.5 ± 12.9 and 8.5 ± 7.1 months, respectively, CR rates were the same in both patient groups (3 patients of both groups). A marked local reaction was recorded in 6 patients with QD and in 5 with EMPD and treatment was stopped because of marked pain in 3 and 1 patients, respectively.
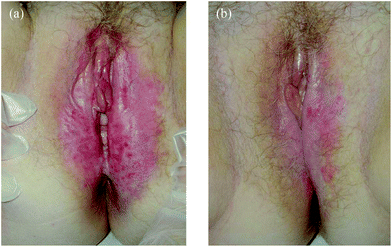 | ||
| Fig. 2 (a) EMPD before MAL-PDT. (b) EMPD after 2 MAL-PDT sessions treatment. | ||
Seventeen patients (13 males, 4 females; mean age: 73.6 ± 11.8 years) with invasive SCC were treated (Table 2). Eleven lesions were on the face, 4 on the scalp and 2 on the penis. SCC was successfully eradicated in 10 cases. However, 2 lesions relapsed at follow-up (10.3 ± 7.1 months follow-up). Treatment was well tolerated and only 1 patient had a marked local reaction with strong pain leading to early discontinuation of treatment.
In total 19 patients (8 males, 11 females; mean age 60.6 ± 12.5 years) with plaque stage mycosis fungoides (9 with unilesional mycosis fungoides and 10 with isolated lesions on the major body folds that could not be exposed to UVA/UVB light) were treated. A marked local reaction was seen in 5 patients but no-one needed to stop the exposure. Five patients had a CR but 2 of them relapsed at follow-up (10.0 ± 10.5 months) (Table 2).Three patients (a 31 and a 38 year old woman and a 54 year old man) with cutaneous follicle center B-cell lymphoma (CBCL) of the face were treated with 1, 3 and 6 sessions, respectively, at monthly intervals. Clinical outcome was disappointing: 1 had a partial improvement and 2 were unresponsive. Pain and local inflammation were rated as moderate.
Nodules and plaques of Kaposi's sarcoma of the legs were treated in 2 male patients (a 42 year old HIV+ immunosuppressed patient and a 54 year old immunocompetent patient) who received 3 treatment sessions at 2 week intervals. Inflammatory local reaction and pain were moderate and both patients had a CR that persisted for the 2 years of follow-up.
An 84 year old male patient with a malignant fibrous histiocytoma of the scalp was treated unsuccessfully with 3 treatment sessions at 2 week intervals. Treatment did not cause any local inflammatory reaction or pain. A 38 year old male with mastocytosis (telangiectasia macularis perstans) of the right leg was treated with 4 weekly treatments without improvement but he did not experience any pain or local inflammation.
Sebaceous nevus was the only benign tumor to be treated. A 73 year old lady and two girls aged 14 and 22 years were treated for lesions. They received 1, 4 and 6 treatment sessions, respectively at monthly intervals. Treatments were well tolerated but lesions did not improve.
The most investigated off-label infectious indications were periungueal, plantar, common and flat warts. The overall number of treated patients was 30 (12 males, 18 females; mean age 29.1 ± 13.1 years) (Table 3). Treatment was well tolerated and 86.7% of patients had no or mild pain and 93.3% had no or moderate inflammatory reaction. A CR was seen in 50% of patients and the CR rate was maintained at follow-up. Warts were periungual in 12 patients, plantar in 7, flat in 8 and common in 3. A complete remission was seen in 8/12, 6/7, 1/8 and 0/3, respectively. Periungual, plantar and common lesions but not flat warts were surgically pared with a curette before treatment. A patient with flat warts had a marked local reaction and a patient with a common wart had both marked local reaction and significant pain. The cosmetic result was considered to be excellent without scar or pigmentary abnormalities.
| Disease | Clinical variant | No of patients | Clinical response [n, %] | Local reaction [n, %] | Pain or burning during the treatment [n, %] | Excellent cosmesis and marked response [n, %] | Follow-up (months, m ± SD) | Persistent marked response at follow-up [n, %] | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No/poor (<50%) | Partial (50–99%) | Complete (100%) | Absent | Moderate | Marked | No | Mild | Moderate | Marked | ||||||
| Warts | Overall | 30 | 10 | 5 | 15 | 19 | 9 | 2 | 17 | 9 | 3 | 1 | 15 | 3.9 ± 3.0 | 17 |
| 33% | 17% | 50% | 63% | 30% | 7% | 57% | 30% | 10% | 3% | 50% | 57% | ||||
| Peri-ungueal | 12 | 0 | 4 | 8 | 0 | 2 | 0 | 0 | 1 | 0 | 0 | 8 | 3 ± 0 | 8 | |
| 0% | 33% | 67% | 0% | 17% | 0% | 0% | 8% | 0% | 0% | 67% | 67% | ||||
| Plantar | 7 | 1 | 0 | 6 | 0 | 5 | 0 | 0 | 6 | 1 | 0 | 6 | 2.7 ± 2.4 | 6 | |
| 14% | 0% | 86% | 0% | 71% | 0% | 0% | 86% | 14% | 0% | 86% | 86% | ||||
| Flat | 8 | 6 | 1 | 1 | 5 | 2 | 1 | 1 | 6 | 1 | 0 | 1 | 6.3 ± 0 | 1 | |
| 75% | 13% | 13% | 63% | 25% | 13% | 13% | 75% | 13% | 0% | 13% | 13% | ||||
| Common | 3 | 3 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 1 | 1 | 0 | 4.0 ± 6.9 | 0 | |
| 100% | 0% | 0% | 0% | 0% | 33% | 33% | 0% | 33% | 33% | 0% | 0% | ||||
| Condylomata | 5 | 0 | 3 | 2 | 1 | 2 | 2 | 0 | 1 | 3 | 1 | 2 | 8.4 ± 9.7 | 2 | |
| 0% | 60% | 40% | 20% | 40% | 40% | 0% | 20% | 60% | 20% | 40% | 40% | ||||
Five patients (all male, mean age 40.2 ± 8.4 years) with condylomata of the glans and penile shaft underwent 2.8 ± 0.5 treatments at intervals of 8.4 ± 3.1 days. Two patients had a CR which was maintained during follow-up (8.4 ± 9.7 months) with excellent cosmetic results. The local inflammatory reaction and pain were marked in 2 patients. A 65 year old male with Bowenoid papulosis had a partial improvement. Local reaction and pain were moderate following 4 MAL-PDT sessions at 3-week intervals.
A 35 year old male with atypical mycobacteriosis by M. marinum of the right hand was treated for 3 sessions at monthly intervals. Therapy was well tolerated and a complete remission was maintained at 12 months of follow-up. An 8 year old girl with cutaneous leishmaniasis of the face was treated with 3 MAL-PDT sessions at 1-week intervals. Local reaction and pain were moderate and she achieved a CR but unfortunately she was lost to follow-up. A partial improvement was seen in a 54 year old male with interdigital recalcitrant pseudomonas infection of the feet and in a 73 year old obese and diabetic woman with submammary candidal intertriginous dermatitis after respectively 3 and 5 well-tolerated weekly sessions of MAL-PDT.
Importantly, in all patients, whatever the clinical diagnosis, cooling of the irradiated area with a fan or by spraying with ice-cold water during and immediately after the treatment was widely used to reduce pain. Oral analgesics and local anesthetics were not administered. Furthermore, in patients with CR, the cosmetic outcome was almost always rated as excellent. The only exceptions were in 1 patient with EMPD, 3 with SCC and 1 with mycosis fungoides who developed hyperpigmented patches that, however, disappeared spontaneously after a few weeks.
Discussion
The present retrospective study enrolled 145 patients from 20 Italian dermatological centers that used MAL-PDT for a broad spectrum of off-label tumoral and infectious conditions. Apart from the interest of clinicians in MAL-PDT, the number of patients treated with a given disease is clearly dependent not only by its incidence but also by the efficacy, tolerability and cosmetic outcome of currently available conventional treatment modalities.This type of study can add to our knowledge of off-label PDT but it is not without its limitations. Unlike RCTs, that, nevertheless, are available only for a few indications, e.g. warts and condylomata, retrospective analysis of medical records cannot determine the efficacy, tolerability and safety of a therapy as there is no fixed protocol and enrolment criteria are not fixed. However, it can provide valuable information on the effectiveness of MAL-PDT in patients unresponsive to prior conventional treatment modalities as well as useful information on practices in real-life clinical experience, as the number of treatment sessions and the interval between them were left to the experience and clinical judgment of the treating physicians, although treatment parameters of single sessions were the same.
Moreover, our findings can help to clarify feasibility, safety and tolerability for uncommon indications for which only small and heterogeneous case series or isolated case reports are available. A possible advantage of our study is that we included both successful and negative results, and the last ones are not usually reported in the medical literature. Finally, we can have information on long-term adverse events because the follow-up was often more prolonged than in RCTs and other previous studies.
Actinic cheilitis of the lower lip was the most investigated (43 patients) indication, suggesting that Italian dermatologists consider that MAL-PDT has potential substantial advantages over standard treatment options. The CR rate with MAL-PDT after 4.2 ± 5.9 months of follow-up was midway (62.8%) between CR rates obtained in two previous small case series that enrolled 3 (CR 100%) patients6 and 15 patients (CR 47% reducing to 38% following a histopathologic analysis).7 These differences are not easily explained as the clinical features of patients and protocol employed were similar: two sessions of MAL-PDT with an interval of 1 week in previous studies6,7 and 2.3 ± 1.3 sessions with 9.6 ± 7.5 days interval in the present study. However, all authors agreed that cosmetic results were excellent and the tolerability was acceptable in the majority of patients even if, in the present retrospective study, local anesthesia was not used. Overall, these results suggest that MAL-PDT is a valuable treatment option for actinic cheilitis and that studies comparing MAL-PDT with standard treatment options, such as cryosurgery, topical chemotherapy, and surgery are required.
QD and EMPD are uncommon skin tumors characterized by an abnormal stratum corneum (and therefore, presumably, an impaired barrier function) and a superficial location of the malignant cells. These characteristics suggest that malignant cells are easily accessible to both MAL and red light. Unfortunately, in the present study, CR rates at follow-up were low (3 out of 8 treated patients). These findings are in general agreement with results of previous reports,8–10 suggesting that these malignant keratinocytes are poorly sensitive to oxidative photodamage. However, MAL-PDT could still have a role in the therapeutic armamentarium because conventional treatments, including surgery, Mohs surgery and CO2 laser treatment, do not provide better results.8,9
MAL-PDT of invasive SCC was successful in approximately half of the patients after a follow-up of 10.3 ± 7.1 months. This rate is in line with results of previous open trials with 20% ALA-PDT,3 and in a prospective, histologically-controlled, clinical trial with MAL-PDT.11 On this basis, current evidence suggests that ALA or MAL-PDT cannot be considered suitable treatment approaches for advanced SCC with a high metastatic potential.2–4,12 However, MAL-PDT was very valuable in patients who were resistant to or could not undergo surgery and radiotherapy and it was curative or at least provided for some palliative benefits in the majority of patients.
MAL can effectively sensitize sebocytes both in vitro and in vivo.13,14 However, in our study we did not observe any improvement in 3 patients with nevus sebaceous in contrast to a previous case report with ALA-PDT13 but in-line with another with patients treated with MAL-PDT.15 The lack of efficacy was accompanied by the lack of inflammatory reaction to the treatment suggesting that nevoid sebocytes are not effectively sensitized by MAL.
In the present study, MAL-PDT was used for mycosis fungoides only if patients had unilesional patches/plaques or lesions of the major body folds that could not be exposed to UV. A possible explanation is that use of MAL-PDT is laborious and time-consuming and much more expensive than phototherapy. The results were disappointing with only 5 patients with a CR after 3.8 ± 2.4 treatment sessions, and the number reduced to 3 at 10.0 ± 10.5 months of follow-up. Previous clinical reports where patients were treated with ALA or MAL reported conflicting results.16,17 However, it should be remembered that up to 9–10 treatments were used in studies reporting the highest CR.2,3,18
Unlike a previous study,19 we have found that MAL-PDT is not effective in primary cutaneous B-cell lymphoma. As both studies enrolled the same number of patients who received a similar number of treatment sessions with similar treatment modalities, the reason for this difference is not entirely clear but may be due to the small number of patients treated (3 in each study). Contrasting results were also obtained with the use of MAL-PDT in tumors with dermal infiltrates of malignant cells underlying a normal epidermis. Kaposi's sarcoma was eradicated in 2 patients whereas one patient with malignant fibrous histiocytoma and another with mastocitosis were refractory. We cannot discuss these results in the view of current evidence, because the treatment of these tumors was never reported before to our knowledge.
The scientific rationale of the use of PDT in a broad range of infectious diseases is that bacteria, yeasts, fungi and some parasites photosensitize and become inactive following exposure to ALA and MAL. In addition, PDT can induce an immune response that contributes to their inactivation. Warts were the most frequent infection to be treated, emphasising the fact that there is an urgent need for new therapy options. It appears from our study that Italian dermatologists focus on plantar, subungueal and flat warts, neglecting common warts. In patients with warts the overall CR rate was 50% (15 patients) at the end of the treatment cycle and after a 3.9 ± 3.0 months follow-up. Tolerability was good. Our findings are similar to those of a previous study from Asia that reported a CR rate of 42.6% and a good tolerability with few patients experiencing mild-to-moderate pain.20 In both studies, lesions were surgically pared but keratolytics were not used before treatment. Much higher CR rates (98%21 and 75%22) were reported by two previous RCTs using 20% ALA-PDT. However, in these studies warts were treated for 1–2 weeks before PDT with topical salicylic acid and lactic acid in a colloidal solution21 or an ointment containing 10% urea and 10% salicylic acid under an occlusive dressing22 before being pared surgically. In one of these studies patients frequently reported marked pain.21 These findings could suggest that outcomes (and tolerability) are dependent on adequate paring and the use of a keratolytic agent pre-PDT. MAL-PDT for penile warts was stopped due to unbearable pain in 2 patients and was fractionated in the remaining 3. Pain could also explain the low number of patients (5) enrolled and the low number (2) of patients who experienced a CR. A low CR rate (3/9 patients treated with four ALA-PDT sessions) was also reported in another study enrolling patients unresponsive to conventional therapies.23 Other open studies enrolling consecutive patients reported improved results, 73%24 and 90%25 of treated lesions in 12 and 16 patients, respectively, suggesting that selection criteria can greatly influence results. Bowenoid papulosis of a patient improved partially after 4 treatment sessions, a finding that contrasts with the only previously reported case history.26
Atypical mycobacteriosis was treated effectively, safely and quickly in a patient. Similarly cutaneous leishmaniasis of the face in a 8 year old girl was treated successfully with an excellent cosmetic result. The low number of patients with these infections obviously reflects that they are extremely uncommon in Italy. While the efficacy of ALA or MAL-PDT for leishmaniasis is well documented,27,28 only 1 patient with atypical mycobacteriosis29 has been reported to date. The use of MAL-PDT in a patient with interdigital intertrigo caused by a pseudomonas and in submammary intertrigo caused by Candida albicans was disappointing. As ALA-PDT was found to be effective against Pseudomonas aeruginosa and Candida albicans and their biofilm cultures in in vitro experiments,30 treatment failure may be related to difficulties involved in obtaining uniform irradiation of body folds.31 Use of PDT in skin infections by Pseudomonas aeruginosa have not been previously reported, whereas disappointing results with Candida intertrigo have already been documented.31
In conclusion, the results of the present retrospective study demonstrate that Italian dermatologists have used MAL-PDT in a broad spectrum of off-label skin tumors and infective indications. In a number of these conditions the use of MAL-PDT has previously been investigated, although RCTs are available only for warts and condylomata, while others were previously unexplored, e.g. Kaposi's sarcoma, mastocytosis, malignant fibrous histiocytoma and skin infections caused by Pseudomonas aeruginosa.
The largest group of patients had actinic cheilitis. This may be due to a number of factors, such as the relative frequency of the disease and the encouraging results (high efficacy and tolerability, excellent cosmetic results and low risk of scarring) in comparison to conventional treatments, e.g. cryotherapy, surgery or radiofrequency ablation. The treatment protocol as well as the number and frequency of treatment sessions for the different clinical indications were quite similar and were not adapted to clinical and pathological features. This therapeutic approach allows us to compare the efficacy of MAL-PDT in different tumors and infections. As general rule, superficial lesions, such as actinic cheilitis, were much more responsive than other skin tumors such as QD, EMPD and advanced SCC. However, in these patients MAL-PDT may be a useful treatment for at least palliation of lesions unresponsive to surgery and other conventional treatment options. Finally, the bias of including ‘only’ patients unresponsive or who cannot undergo conventional treatment options appears to have decreased the CR rates with MAL-PDT in most other indications, including primary cutaneous T- and B-cell lymphoma, warts and condylomata, in comparison to previous reports.
Acknowledgements
Editorial assistance was provided by Siobhan Ward, PhD, and Luca Giacomelli, PhD; this assistance was funded by Galderma, Italy.References
- J. C. Kennedy, R. H. Pottier and D. C. Pross, Photodynamic therapy with endogenous protoporphyrin IX: basic principles and present clinical experience, J. Photochem. Photobiol., B, 1990, 6, 143–148 CrossRef CAS.
- C. A. Morton, K. E. McKenna and L. E. Rhodes, Guidelines for topical photodynamic therapy: update, Br. J. Dermatol., 2008, 159, 1245–1266 CrossRef CAS.
- P. G. Calzavara-Pinton, M. Arisi, E. Sereni and B. Ortel, A critical reappraisal of off-label indications for topical photodynamic therapy with aminolevulinic acid and methylaminolevulinate, Rev. Recent Clin. Trials, 2010, 5, 112–116 CrossRef CAS.
- A. Klein, P. Babilas, S. Karrer, M. Landthaler and R. M. Szeimies, Photodynamic therapy in dermatology – an update, JDDG: J. Deutsch. Dermatol. Ges., 2008, 6, 839–845 Search PubMed.
- B. Ortel, C. R. Shea and P. G. Calzavara-Pinton, Molecular mechanisms of photodynamic therapy, Front. Biosci., 2009, 14, 4157–4172 CrossRef CAS.
- A. Hauschild, S. Lischner, B. Lange-Asschenfeldt and F. Egberts, Treatment of actinic cheilitis using photodynamic therapy with methyl aminolevulinate: report of three cases, Dermatol. Surg., 2005, 31, 1344–1347 CrossRef CAS.
- C. Berking, T. Herzinger, M. J. Flaig, M. Brenner, C. Borelli and K. Degitz, The efficacy of photodynamic therapy in actinic cheilitis of the lower lip: a prospective study of 15 patients, Dermatol. Surg., 2007, 33, 825–830 CrossRef CAS.
- L. Feldmeyer, V. Krausz-Enderlin, B. Töndury, J. Hafner, L. E. French and G. F. Hofbauer, Methylaminolaevulinic acid photodynamic therapy in the treatment of erythroplasia of Queyrat, Dermatology, 2011, 223, 52–56 CrossRef CAS.
- A. A. Nardelli, T. Stafinski and D. Menon, Effectiveness of photodynamic therapy for mammary and extra-mammary Paget's disease: a state of the science review, BMC Dermatol., 2011, 15, 11–13 Search PubMed.
- F. Raspagliesi, R. Fontanelli, G. Rossi, A. Ditto, E. Solima and F. Hanozet, Photodynamic therapy using a methyl ester of 5-aminolevulinic acid in recurrent Paget's disease of the vulva: a pilot study, Gynecol. Oncol., 2006, 103, 581–586 CrossRef CAS.
- P. G. Calzavara-Pinton, M. Venturini, R. Sala, R. Capezzera, G. Parrinello, C. Specchia and C. Zane, Methylaminolaevulinate-based photodynamic therapy of Bowen's disease and squamous cell carcinoma, Br. J. Dermatol., 2008, 159, 137–144 CrossRef CAS.
- C. A. Morton, S. B. Brown, S. Collins, S. Ibbotson, H. Jenkinson, H. Kurwa, K. Langmack, K. McKenna, H. Moseley, A. D. Pearse, M. Stringer, D. K. Taylor, G. Wong and L. E. Rhodes, Guidelines for topical photodynamic therapy: report of a workshop of the British Photodermatology Group, Br. J. Dermatol., 2002, 146, 552–567 CrossRef CAS.
- C. C. Dierickx, M. Goldenhersh, P. Dwyer, A. Stratigos, M. Mihm and R. R. Anderson, Photodynamic therapy for nevus sebaceus with topical delta-aminolevulinic acid, Arch. Dermatol., 1999, 135, 637–640 CAS.
- S. Kosaka, S. Kawana, C. C. Zouboulis, T. Hasan and B. Ortel, Targeting of sebocytes by aminolevulinic acid-dependent photosensitization, Photochem. Photobiol., 2006, 82, 453–457 CrossRef CAS.
- H. S. Kim, J. H. Jun and J. Y. Lee, Photodynamic therapy of facial nevus sebaceous, Photodermatol., Photoimmunol. Photomed., 2010, 26, 98–100 CrossRef.
- C. Zane, M. Venturini, R. Sala and P. G. Calzavara-Pinton, Photodynamic therapy with methylaminolevulinate as a valuable treatment option for unilesional cutaneous T-cell lymphoma, Photodermatol., Photoimmunol. Photomed., 2006, 22, 254–258 CrossRef.
- E. H. Kim, H. Y. Kang, E. S. Lee and Y. C. Kim, Mycosis fungoides showing incomplete response to topical 5-aminolaevulinic acid phototherapy, Eur. J. Dermatol., 2007, 17, 343–345 Search PubMed.
- A. Farkas, L. Kemeny, L. E. French and R. Dummer, New and Experimental Skin-Directed Therapies for Cutaneous Lymphomas, Skin Pharmacol. Physiol., 2009, 22, 322–334 CrossRef CAS.
- M. Mori, P. Campolmi, L. Mavilia, R. Rossi, P. Cappugi and N. Pimpinelli, Topical photodynamic therapy for primary cutaneous B-cell lymphoma: a pilot study, J. Am. Acad. Dermatol., 2006, 54, 524–526 CrossRef.
- Y. S. Wang, Y. K. Tay, C. Kwok and E. Tan, Photodynamic therapy with 20% aminolevulinic acid for the treatment of recalcitrant viral warts in an Asian population, Int. J. Dermatol., 2007, 46, 1180–1184 CrossRef CAS.
- I. M. Stender, R. Na and H. Fogh, Photodynamic therapy with 5-aminolevulinic acid or placebo for recalcitrant foot and hand warts: randomized double blind trial, Lancet, 2000, 355, 963–966 CrossRef CAS.
- G. Fabbrocini, M. P. Di Costanzo, A. M. Riccardo, M. Quarto, A. Colasanti, G. Roberti G and G. Monfrecola, Photodynamic therapy with topical delta-aminolaevulinic acid for the treatment of plantar warts, J. Photochem. Photobiol., B, 2001, 61, 30–34 CrossRef CAS.
- T. Herzinger, R. Wienecke, P. Weisenseel, C. Borelli, C. Berking and K. Degitz, Photodynamic therapy of genital condylomata in men, Clin. Exp. Dermatol., 2006, 31, 51–53 CrossRef CAS.
- I. M. Stefanaki, S. Georgiou, G. C. Themelis, E. M. Vazgiouraki and A. D. Tosca, In vivo fluorescence kinetics and photodynamic therapy in condylomata acuminate, Br. J. Dermatol., 2003, 149, 972–976 CrossRef CAS.
- X. L. Wang, H. W. Wang, H. S. Wang, S. Z. Xu, K. H. Liao and P. Hillemanns, Topical 5-aminolaevulinic acid-photodynamic therapy for the treatment of urethral condylomata acuminata, Br. J. Dermatol., 2004, 151, 880–885 CrossRef CAS.
- C. H. Yang, J. C. Lee, C. H. Chen, C. Y. Hui, H. S. Hong and H. W. Kuo, Photodynamic therapy for bowenoid papulosis using a novel incoherent light-emitting diode device, Br. J. Dermatol., 2003, 149, 1297–1299 CrossRef CAS.
- A. Asilian and M. Davami, Comparison between the efficacy of photodynamic therapy and topical paromomycin in the treatment of Old World cutaneous leishmaniasis: a placebo-controlled, randomized clinical trial, Clin. Exp. Dermatol., 2006, 31, 634–637 CrossRef CAS.
- E. M. Van der Snoek, D. J. Robinson, J. J. van Hellemond and H. A. Neumann, A review of photodynamic therapy in cutaneous leishmaniasis, J. Eur. Acad. Dermatol. Venereol., 2008, 22, 918–922 CrossRef CAS.
- S. R. Wiegell, B. Kongshoj and H. C. Wulf, Mycobacterium marinum infection cured by photodynamic therapy, Arch. Dermatol., 2006, 142, 1241–1242 Search PubMed.
- M. A. Biel, Photodynamic therapy of bacterial and fungal biofilm infections, Methods Mol. Biol., 2010, 635, 175–194 CAS.
- P. G. Calzavara-Pinton, M. Venturini, R. Capezzera, R. Sala and C. Zane, Photodynamic therapy of interdigital mycoses of the feet with topical application of 5-aminolevulinic acid, Photodermatol., Photoimmunol. Photomed., 2004, 20, 144–147 CrossRef CAS.
Footnotes |
| † This article is published as part of a themed issue on current topics in photodermatology. |
| ‡ The Italian Group for Photodynamic Therapy: Piergiacomo Calzavara-Pinton, Maria Teresa Rossi, Raffaella Sala (Dermatology Department, University of Brescia), Nicola Arpaia (Dermatology Department II, University of Bari), Elena Cleopatra Burtica (Dermatology Department, University of Bologna), Paolo Amerio (Dermatology Department, Hospital SS. Annunziata, Chieti), Annarosa Virgili (Dermatology Department, Hospital S. Anna, Ferrara), Riccardo Rossi, Gionata Buggiani (Dermatology Department, University of Florence), Andrea Zanca (Dermatology Department, A.O. Carlo Poma, Mantua), Leonardo Bugatti (Dermatology Department, ASUR 5, Jesi), Dario Fai (Dermatology Department, Gagliano del Capo Hospital, Lecce), Elisa Cervadoro (Dermatology Department, USL 6, Livorno), Stefano Cavicchini (Dermatology Department I, University of Milan), Fabrizio Fantini (Dermatology Department, University of Modena), Gabriella Fabbrocini (Dermatology Department, University of Naples), Stefano Piaserico (Dermatology Department, University of Padua), Elisa Cervadoro (Dermatology Department, University of Pisa), Miriam Teoli (Dermatology Department, University Tor Vergata, Rome), Laura Eibenschutz (Dermatology Department, San Gallicano Institute, Rome), Fabrizio Arcangeli (Dermatology Department, Hospital S. Maria, Terni), Paolo Broganelli (Dermatology Department, University of Turin), Donatella Schena (Dermatology Department, University of Verona). |
| This journal is © The Royal Society of Chemistry and Owner Societies 2013 |
