Highly monodisperse rattle-structured nanomaterials with gold nanorod core–mesoporous silica shell as drug delivery vehicles and nanoreactors†
Eunjin
Choi
a,
Minjeong
Kwak
a,
Byungchul
Jang
a and
Yuanzhe
Piao
*ab
aGraduate School of Convergence Science and Technology, Seoul National University, Seoul 151-742, Korea. E-mail: parkat9@snu.ac.kr
bAdvanced Institutes of Convergence Technology, Seoul National University, Suwon 443-270, Korea
First published on 7th November 2012
Abstract
Rattle-structured nanomaterials composed of a gold nanorod in a mesoporous silica nanocapsule (AuNR@mSiO2) were prepared by a novel solution-based consecutive process. The drug-loading properties of the nanomaterial and regrowth control of the core nanoparticles were also studied.
Over the past two decades, hollow-structured nanomaterials have gained increasing interest owing to potential applications as nanoreactors and in nanocatalysis and the biomedical field.1 Recently, hollow-structured nanomaterials with a movable core, which are referred to as “rattle-structured nanomaterials,” have attracted tremendous attention because they allow the optical, electric, and magnetic properties of the core material to be added to the nanomaterial.2 Until recently, there have been a variety of approaches for the preparation of rattle-structured nanomaterials. Sönnichsen et al. reported gold nanorattles obtained through the Kirkendall effect.3 Further, a Au@SiO2 core–shell nanostructure could be transferred into a rattle-shaped one by a further selective core-etching process.2b In addition, a template-assisted etching approach,4 the Ostwald ripening process,5 and a water-in-oil microemulsion method6 have also been used to prepare rattle-structured nanomaterials.
Of the various rattle-type mesoporous nanomaterial systems, the rattle-structured nanomaterial composed of Au NRs in the mesoporous silica nanocapsule is expected to attract a great deal of interest. On the one hand, the mesoporous silica shell possesses the advantages of low cytotoxicity, uniform size, high stability, easy functionalization, high pore volumes, and low cost.7 On the other hand, Au NRs offer fascinating tuned surface plasmon resonance (SPR) properties,8 which make them well suited for diverse biomedical applications such as diagnosis, photothermal therapy, bio-imaging, and biological sensing.9 Herein, we report a novel solution-based consecutive process for the fabrication of a highly monodisperse rattle-structured nanomaterial composed of Au NRs in mesoporous silica nanocapsules. The resulting nanostructured materials were highly uniform in morphology and showed good dispersibility properties, similar to the initial Au NRs. Drug loading was studied using the material as a drug carrier and doxorubicin hydrochloride (DOX) as a model. In addition, Au NRs inside the confined mesoporous silica nanocapsules were further regrown in a controlled manner by simply adding a gold precursor and ascorbic acid (AA).
The synthetic procedure for the rattle-structured AuNR@mSiO2, as shown in Scheme 1, is performed in three steps. In the first step, the Au NRs are homogeneously coated with Ag using AA as a reducing agent (AuNR@Ag).8 Next, a mesoporous silica shell is deposited by the sol–gel method on the cetyltrimethylammonium bromide (CTAB)-stabilized AuNR@Ag core–shell nanoparticles (AuNR@Ag@mSiO2). In the final step, Ag is selectively removed by the addition of excess hydrogen peroxide (H2O2).10
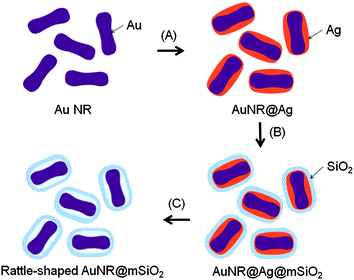 | ||
| Scheme 1 Schematic illustration of the procedure for preparing the rattle-structured Au NRs encapsulated within a mesoporous silica nanocapsule. Three main steps are (A) homogeneous coating of silver on the surface of Au NRs, (B) deposition of a mesoporous silica shell on the AuNR@Ag nanoparticles, and (C) selective etching of the silver inner layer using H2O2 as an oxidant of Ag. | ||
The changes in the shapes of the obtained nanomaterials at each step have been fully characterized using transmission electron microscopy (TEM). The as-prepared Au NRs were shaped similar to dog bones, being thicker at each end in comparison with the thin middle portion (Fig. 1A). They were synthesized by using a seed-mediated growth approach in aqueous surfactant solution with the addition of sufficient AA available to reduce gold ions.11
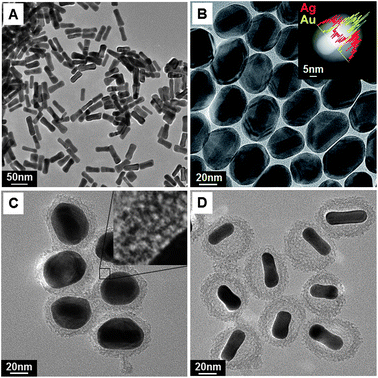 | ||
| Fig. 1 TEM images corresponding to all the steps for the fabrication of rattle-structured AuNR@mSiO2: (A) Au NRs, (B) AuNR@Ag core–shell (inset: STEM-EDS line scan analysis), (C) AuNR@Ag@mSiO2 core–shell–shell (inset: expanded image of the silica shell), and (D) the rattle-structured AuNR@mSiO2 core–void–shell. | ||
AuNR@Ag was prepared by the chemical reduction of silver nitrate using AA as the reducing agent under alkaline conditions.
At a pH of 7, AA exists as ascorbate anions, which are in the monobasic form.12 The ascorbate anion is the reducing species, and the concentration of ascorbate anions increases at high pH values.13 For this reason, silver ions could be reduced by increasing the reducing power of AA, which is achieved by elevating the pH over 10 by adding NaOH.14 The color of the Au NR solution turned from purple to orange within several minutes of the addition of silver nitrate and AA. Typical TEM and scanning transmission electron microscopy (STEM) images show the epitaxial growth of the Ag nanoshell on Au NRs (Fig. 1B). Moreover, as shown in the inset of Fig. 1B, the STEM-energy-dispersive X-ray spectroscopy (EDS) line scan through the center of a single nanoparticle clearly shows strong Ag signals at the particle edge and strong Au signals in the particle center, which reveals a typical core–shell nanostructure. Xie et al. reported that a Ag shell, shaped as an orange slice, on a Au NR could be synthesized by eliminating the various ions present in the solution using purification and by adding only CTAB aqueous solution.15 Here, we demonstrate that oval-shaped AuNR@Ag nanoparticles can be generated by directly adding a silver precursor to the as-prepared Au NR solution without any further washing step. The shell thickness of silver was varied from 5 nm to 11 nm by varying the amount of silver nitrate from 0.001 mmol to 0.01 mmol, respectively (Fig. S1†).
Mesoporous silica shells have been successfully deposited on AuNR@Ag nanoparticles through a single-step procedure without any intermediate coating (Fig. 1C). The formation of mesopores is strongly influenced by the CTAB molecules, which were used as the organic template to establish mesopores in the sol–gel reaction and also as the stabilizing agent in the aqueous phase.16 The inset in Fig. 1C shows an expanded section of the silica shell, where the gray areas indicate condensed silica and the white dots indicate mesopores that are aligned in a disorderly manner. The silica shell thickness of the AuNR@Ag@mSiO2 nanoparticles was modified from 8 nm to 21 nm by adjusting the amount of tetraethyl orthosilicate from 0.04 mmol to 0.08 mmol (Fig. S2†).
To selectively remove the silver layer, H2O2 was used as an oxidant of Ag. H2O2 can be either an oxidant or a reductant depending upon the pH value of the solution. The standard redox potential of H2O2 is given by:
| H2O2 + 2H+ + 2e → 2H2O, E0 = 1.763 V |
| H2O2 + 2e → 2OH−, E0 = 0.867 V |
Under basic conditions such as those used in these experiments, H2O2 is an ideal etchant for the selective removal of Ag because the standard reduction potential of H2O2 (E0 = 0.867 V) is higher than that of Ag+/Ag (E0 = 0.799 V) and lower than that of Au3+/Au (E0 = 1.51 V).10,17 In addition, this reaction involves a color change from orange to cloudy purple, which can provide a facile colorimetric assay. The resulting color of cloudy purple can be ascribed not only to the original color of the Au NRs but also to the white color of the coated silica nanoshell. After removing the Ag nanoshell, the final rattle-structured Au@mSiO2 (Fig. 1D) is readily dispersible in water, affording a transparent suspension (Fig. S4†). The Au@mSiO2 is found to be stable in water over several months without adding any stabilizing reagent.
The synthetic strategy described in this work has several advantages. First, although several steps are required to prepare the rattle-structured nanomaterial, washing steps are not needed between each of the steps; therefore, the complete synthesis is a consecutive process that can be finished in one pot. Second, this strategy provides easy and reproducible control of the hollow space as well as the thickness of the mesoporous silica layer. Third, the route via selective removal of the silver layer shows good feasibility for synthesizing rattle-structured nanomaterials that maintain the original morphology of the cores.
The growth and removal of Ag on Au NRs drastically affects the UV-vis absorption spectra, as illustrated in Fig. 2. Au NRs have two absorption peaks in the visible region of the spectrum, corresponding to the transverse plasmon resonances at around 530 nm and the longitudinal plasmon resonances at around 730 nm. When the silver atoms are deposited onto the Au NRs, the spectrum is drastically blue-shifted and its absorption intensity is increased. As noted in the previous reports, this blue shift is mainly due to the decrease in the aspect ratio (AR).15,18 While an average AR of the as-prepared Au NRs is 3.2 ± 0.4 (50.8 ± 6.7 × 16.1 ± 1.3 nm), the AR decreases to 1.3 ± 0.1 (51.8 ± 5.4 × 38.4 ± 1.9 nm) after Ag coating. However, the particles maintain the rod-like morphology, and their extinction spectrum still has two absorption peaks. The increase in the absorption intensity can be attributed to the increase in the particle volume. The two absorption peaks at ∼400 nm and ∼520 nm (Fig. 2B) might be attributed to the differences between the dielectric functions of Ag and Au, as previously reported.18 When the surface of the AuNR@Ag nanoparticles is coated with a silica shell of ∼9 nm thickness, the slight red shift of both peaks is related to the change in the refractive index of silica and water.19 After eliminating silver, the rattle-structured AuNR@mSiO2 has an almost identical absorption band of SPR with pure Au NRs.
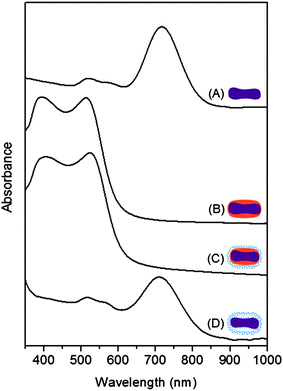 | ||
| Fig. 2 UV-vis absorption spectra of (A) Au NR, (B) AuNR@Ag core–shell, (C) AuNR@Ag@mSiO2 core–shell–shell, and (D) the rattle-structured AuNR@mSiO2 core–void–shell nanoparticles. | ||
The UV-vis absorption spectra recorded before and after loading the DOX into the rattle-structured AuNR@mSiO2 are shown in Fig. 3. The DOX loading efficiency, which can be evaluated through a comparison between the original DOX solution (0.046 mg mL−1) and the residual DOX solution in the supernatant after loading into the rattle-structured AuNR@mSiO2 (0.72 mg), reaches up to 71.2%, and the drug loading content was 59.7 μg of DOX per 1 mg of support. These results show that the rattle-structured nanomaterial could be used for drug loading because of its large cavities, which include both the inner hollow space and mesopore channels.
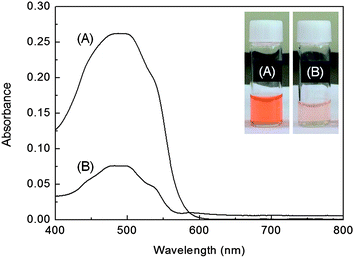 | ||
| Fig. 3 UV-vis absorption spectra of (A) the DOX solution before being loaded into the rattle-structured AuNR@mSiO2 and (B) the DOX solution after being loaded into the nanoparticles, interacting with them, and then being unloaded from the nanoparticles. Inset: photograph of vials containing DOX solutions corresponding to the spectra in (A) and (B). The more deeply colored solutions faded to light orange, which indicates that the DOX was loaded in the rattle-structured nanomaterials. | ||
Rattle-structured AuNR@mSiO2 can also be used as a reaction chamber in which chemical reactions are performed. Au NRs inside the mesoporous silica capsules were further grown by adding gold(III) chloride hydrate(HAuCl4) and AA into the rattle-structured nanomaterials dispersed in aqueous solution.
Considerable regrowth of Au occurred inside the confined silica capsules rather than outside. By changing the HAuCl4 concentrations from 0.03 mM to 0.15 mM and 0.6 mM, the size of the further-grown gold cores in the silica nanocapsules was made to gradually increase (Fig. 4A–F). The shape of the gold cores was converted from the original rod morphology to a truncated octahedron shape. To derive the crystal structures of the gold core in silica nanocapsules, high-resolution TEM (HR-TEM) analysis of gold nanoparticles was performed. In Fig. 4G and H, lattice fringes with a spacing of d = 2.03 Å and 2.35 Å are clearly visible, which can be attributed to the (200) and (111) planes of gold, respectively. This indicates that the structure of the Au nanoparticles is a truncated octahedron, which corresponds to both the {111} and {100} facets.20 When Au NRs are regrown without silica nanocapsules, the nanoparticles are extremely aggregated (Fig. S5†). The rattle structure can provide shielding from activation between the core nanoparticles as well as a space where the reaction can occur within the hollow nanostructure.
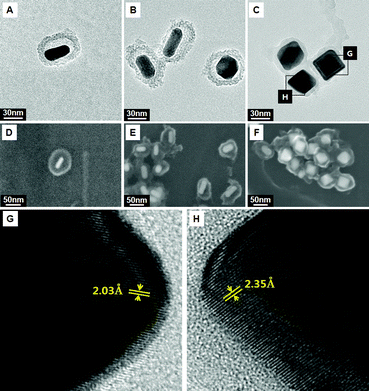 | ||
| Fig. 4 Shape evolution of gold cores grown in rattle-structured AuNR@mSiO2. TEM and field-emission scanning electron microscopy (FE-SEM) images obtained by changing the HAuCl4 concentrations to (A and D) 0.03 mM, (B and E) 0.15 mM, and (C and F) 0.6 mM. (G and H) HR-TEM images of the edge of the regrown Au core. | ||
In summary, we introduced the rattle-structured AuNR@mSiO2 nanostructured material with a well-controlled morphology. The rattle-structured nanomaterial dispersed well in aqueous solution, and the Au NRs inside the mesoporous silica shell showed a strong absorption peak in the long-wavelength region, which was the same as that of the Au NRs themselves. Examining the drug-loading properties of the nanomaterial showed that its DOX loading content was 59.7 μg DOX per mg of the rattle-structured AuNR@mSiO2. Additionally, the as-prepared rattle-structured nanomaterial was used to controllably regrow the core nanoparticles. The results indicate that the rattle-structured nanomaterials with a well-controlled particle size and morphology have great potential for biomedical applications and as nanoreactors. The current process is highly reproducible and is expected to be extended to other nanomaterials.
This work was supported by the Korea Research Council of Fundamental Science and Technology (KRCF) through the project of ‘Development of Characterization Techniques for Nano-materials Safety’ and a grant of the Korea Healthcare Technology R&D Project, Ministry for Health, Welfare & Family Affairs, Republic of Korea (A092145). This work was also supported by the grant from the Industrial Source Technology Development Program (10041041) of the Ministry of Knowledge Economy (MKE) of Korea.
Notes and references
- (a) F. Caruso, R. A. Caruso and H. Möhwald, Science, 1998, 282, 1111 CrossRef CAS; (b) S. W. Kim, M. Kim, W. Y. Lee and T. Hyeon, J. Am. Chem. Soc., 2002, 124, 7642 CrossRef CAS; (c) D. Grosso, C. Boissière, B. Smarsly, T. Brezesinski, N. Pinna, P. A. Albouy, H. Amenitsch, M. Antonietti and C. Sanchez, Nat. Mater., 2004, 3, 787 CrossRef CAS; (d) X. W. D. Lou, L. A. Archer and Z. Yang, Adv. Mater., 2008, 20, 3987 CrossRef CAS.
- (a) S.-H. Hu, Y.-Y. Chen, T.-C. Liu, T.-H. Tung, D.-M. Liu and S.-Y. Chen, Chem. Commun., 2011, 47, 1776 RSC; (b) J. Lee, J. C. Park and H. Song, Adv. Mater., 2008, 20, 1523 CrossRef CAS; (c) S. Buathong, D. Ung, T. J. Daou, C. Ulhaq-Bouillet, G. Pourroy, D. Guillon, L. Ivanova, I. Bernhardt, S. Bégin-Colin and B. Donnio, J. Phys. Chem. C, 2009, 113, 12201 CrossRef CAS.
- Y. Khalavka, J. Becker and C. Sönnichsen, J. Am. Chem. Soc., 2009, 131, 1871 CrossRef CAS.
- P. M. Arnal, M. Comotti and F. Schüth, Angew. Chem., Int. Ed., 2006, 118, 8404 CrossRef.
- H. Li, Z. Bian, J. Zhu, D. Zhang, G. Li, Y. Huo and Y. Lu, J. Am. Chem. Soc., 2007, 129, 8406 CrossRef CAS.
- S. H. Wu, C. T. Tseng, Y. S. Lin, C. H. Lin, Y. Hung and C. Y. Mou, J. Mater. Chem., 2010, 21, 789 RSC.
- (a) I. I. Slowing, B. G. Trewyn, S. Giri and V. S. Y. Lin, Adv. Funct. Mater., 2007, 17, 1225 CrossRef CAS; (b) Q. Zhan, J. Qian, X. Li and S. He, Nanotechnology, 2010, 21, 055704 CrossRef; (c) N. Andersson, P. C. A. Alberius, J. Skov Pedersen and L. Bergström, Microporous Mesoporous Mater., 2004, 72, 175 CrossRef CAS.
- B. Nikoobakht and M. A. El-Sayed, Chem. Mater., 2003, 15, 1957 CrossRef CAS.
- (a) A. M. Alkilany, L. B. Thompson, S. N. Boulos, P. N. Sisco and C. J. Murphy, Adv. Drug Delivery Rev., 2012, 64, 190 CrossRef CAS; (b) X. Huang, S. Neretina and M. A. El-Sayed, Adv. Mater., 2009, 21, 4880 CrossRef CAS; (c) X. Yang, X. Liu, Z. Liu, F. Pu, J. Ren and X. Qu, Adv. Mater., 2012, 24, 2890 CrossRef CAS; (d) Z. Zhang, L. Wang, J. Wang, X. Jiang, X. Li, Z. Hu, Y. Ji, X. Wu and C. Chen, Adv. Mater., 2012, 24, 1418 CrossRef CAS; (e) H. Tang, S. Shen, J. Guo, B. Chang, X. Jiang and W. Yang, J. Mater. Chem., 2012, 22, 16095 RSC.
- Q. Zhang, C. M. Cobley, J. Zeng, L.-P. Wen, J. Chen and Y. Xia, J. Phys. Chem. C, 2010, 114, 6396 CAS.
- (a) L. Gou and C. J. Murphy, Chem. Mater., 2005, 17, 3668 CrossRef CAS; (b) T. I. Yang, R. N. C. Brown, L. C. Kempel and P. Kofinas, J. Nanopart. Res., 2010, 12, 2967 CrossRef CAS.
- V. Vasantha and S. M. Chen, J. Electroanal. Chem., 2006, 592, 77 CrossRef CAS.
- A. Ambrosi, A. Morrin, M. R. Smyth and A. J. Killard, Anal. Chim. Acta, 2008, 609, 37 CrossRef CAS.
- T. Pal, S. De, N. R. Jana, N. Pradhan, R. Mandal, A. Pal, A. Beezer and J. Mitchell, Langmuir, 1998, 14, 4724 CrossRef CAS.
- Y. Xiang, X. Wu, D. Liu, Z. Li, W. Chu, L. Feng, K. Zhang, W. Zhou and S. Xie, Langmuir, 2008, 24, 3465 CrossRef CAS.
- I. Gorelikov and N. Matsuura, Nano Lett., 2007, 8, 369 CrossRef.
- P. G. Tratnyek and D. L. Macalady, Handbook of Property Estimation Methods for Chemicals: Environmental and Health Sciences, 2000 Search PubMed.
- A. Sánchez-Iglesias, E. Carbó-Argibay, A. Glaria, B. Rodríguez-González, J. Pérez-Juste, I. Pastoriza-Santos and L. M. Liz-Marzán, Chem.–Eur. J., 2010, 16, 5558 Search PubMed.
- Y. Lu, Y. Yin, Z.-Y. Li and Y. Xia, Nano Lett., 2002, 2, 785 CrossRef CAS.
- B. Lim, M. Jiang, J. Tao, P. H. C. Camargo, Y. Zhu and Y. Xia, Adv. Funct. Mater., 2009, 19, 189 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental section and additional SEM and TEM images. See DOI: 10.1039/c2nr32885b |
| This journal is © The Royal Society of Chemistry 2013 |
