Surfactant modification of aggregation-induced emission material as biocompatible nanoparticles: Facile preparation and cell imaging†
Xiqi
Zhang‡
a,
Xiaoyong
Zhang‡
a,
Shiqi
Wang
a,
Meiying
Liu
b,
Lei
Tao
*a and
Yen
Wei
*a
aDepartment of Chemistry and the Tsinghua Center for Frontier Polymer Research, Tsinghua University, Beijing, 100084, P. R. China. E-mail: leitao@mail.tsinghua.edu.cn; weiyen@tsinghua.edu.cn
bBeijing National Laboratory for Molecular Sciences (BNLMS), Key Laboratory of Organic Solids, Laboratory of New Materials, Institute of Chemistry, Chinese Academy of Sciences, Beijing 100190, P. R. China
First published on 5th November 2012
Abstract
Water soluble and biocompatible fluorescent organic nanoparticles based on aggregation-induced emission (AIE) material were facilely prepared by mixing AIE material and surfactant. The utilization of such fluorescent organic nanoparticles for cell imaging applications was further explored.
Fluorescent (FL) bioprobes have attracted increasing attention in recent years due to their excellent ability for imaging and monitoring biological species and processes in living systems.1–12 FL probes used for biomedical applications should be water-dispersible, nontoxic, resistant to photobleaching, highly luminescent, and stable to environmental conditions (e.g., pH). Various fluorescent materials including organic dyes, fluorescent proteins, fluorescent inorganic/organic nanoparticles have been widely used as FL probes.13–21 However, most of the previously used FL probes have inherent disadvantages.22 For example, semiconductor quantum dots (QDs) exhibit excellent optical properties which are attractive for cell imaging applications. However, such materials suffer from high cytotoxicity due to their heavy metal composition. Other fluorescent inorganic nanoparticles such as carbon dots, silicon quantum dots, metal clusters are normally not degradable and have potential toxicity due to their accumulation in the reticuloendothelial system (RES) after intravenous injection.23–27 On the other hand, organic dyes, fluorescent proteins and conventional fluorescent organic nanoparticles show poor membrane permeability and are easily photo-bleached.28,29 Novel fluorescent materials with good cytocompatibility, high fluorescence and low cytotoxicity are still urgently needed. Organic dyes are prone to aggregate in aqueous media due to their poor solubility in water, resulting in aggregation-caused quenching (ACQ).30,31 Although incorporation of cationic or anionic groups into organic dyes can improve their miscibility in biological media, the dyes are still easy to aggregate since the hydrophobic conjugated groups, can also cause PL quenching.
In 2001, Tang et al.32 reported on aggregation-induced emission (AIE) materials, an important class of anti-ACQ materials that emit more efficiently while in an aggregated state rather than in a dispersed state. Since then, AIE materials have attracted more and more research attention for their potential application in various fields, such as organic light-emitting devices and chemosensors.33–43 Although the introduction of AIE can solve the problem of fluorescent quenching, the limitation of poor cytocompatibility due to their poor water-dispersity still remains. Modification of AIE materials with ions improves their solubility in cell media, but the electric charges of the highly concentrated ionized dyes may affect intracellular physiology and sometimes even kill the cells.44
Herein we report a new FL bioprobe system based on nanoaggregates combining AIE-based organic fluorogens An18 (derivatized from 9,10-distyrylanthracene with an alkoxyl endgroup) and surfactant Pluronic F127 (Scheme 1). The surfactant can surround the An18 to achieve An18–F127 fluorescent organic nanoparticles. F127 can change the hydrophobic surface of fluorescent nanoparticles to hydrophilic in character, thus the An18–F127 composite exhibits good water dispersibility and a novel AIE property. To exploit the potential biomedical applications of such AIE-based fluorescent organic nanoparticles (An18–F127), its biocompatibility as well as cell imaging applications were further investigated. In comparison to the QDs or ordinary ionized organic dyes, this AIE probe could be facilely prepared, and the obtained bioprobe is easy to handle and use, friendly to live cells at high concentrations so making it possible to obtain very emissive stained cells.
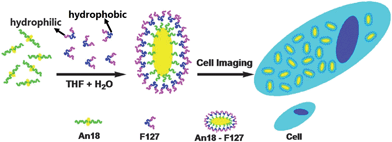 | ||
| Scheme 1 Schematic showing transformation of An18 from hydrophobic to hydrophilic fluorescent nanoparticles with Pluronic F127 and their use as cell imaging probes. | ||
The AIE fluorogens An18 were prepared following the synthetic route shown in Scheme S1 (ESI).† Its structure was characterized and confirmed by standard spectroscopic methods (see ESI†). An18 is not water-soluble and emits strong yellow–greenish fluorescence in the solid state. When An18 is dissolved in THF, it has weak fluorescence in the dispersed state. After being modified by surfactant Pluronic F127, An18 could be dispersed well in water under the assistance of F127, meanwhile, the fluoregen is still in the aggregated state in the An18–F127 nanoparticles, thus the An18–F127 composite emitted strong fluorescence in water with significantly enhanced FL intensity compared with that in a pure THF solution (Fig. 1A). As shown in the inset of Fig. 1A, after surface modification with surfactant (Pluronic F127), the surfactant modified nanoparticles An18–F127 were readily dispersed in water, suggesting the successful formation of hydrophilic AIE-based nanoparticles (NPs). FT-IR spectra showed that a peak centered at 1108 cm−1 which corresponds to the stretching vibration of C–O was significantly enhanced in An18–F127 NPs, demonstrating the successful modification of Pluronic F127 with An18 dyes (Fig. S1†). The transmission electron microscopy (TEM) image further confirmed the formation of AIE-based nanoparticles (NPs). A lot of small organic spheres which were aggregated into large nanoparticles with diameters ranging from 400 to 600 nm were observed (Fig. 1B). The formation of such nanostructures is likely due to the strong interactions between the alkyl chain of An18 and the hydrophobic segments of F127, however, the hydrophilic segments of Pluronic F127 covered the spheres to render them water dispersible.
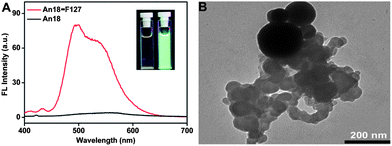 | ||
| Fig. 1 (A) FL spectra of An18 in THF and surfactant modified nanoparticles (An18–F127) dispersed in water. Inset: FL image of An18 in THF and An18–F127 in water taken at 365 nm UV light; (B) TEM image of An18–F127 NPs dispersed in water, scale bar = 200 nm. | ||
The biocompatibility of An18–F127 was subsequently evaluated.45,46Fig. 2A–C showed the morphology of A549 cells when they were incubated with different concentrations of An18–F127 NPs for 24 h, 0 μg mL−1 (A, control cells), 20 μg mL−1 (B), 100 μg mL−1 (C). It can be seen that all the cells still kept their normal morphology, which preliminarily proves the An18–F127 NPs are biocompatible with cells. Cell viability results from cell counting kit-8 (CCK-8) assay further demonstrated the excellent biocompatibility of the above mentioned NPs. As shown in Fig. 2D, no obvious cytotoxicity is observed via cell viability examination even at concentrations of NPs as high as 100 μg mL−1. To eliminate interference of An18–F127 on cell viability measurement, A549 cells were thoroughly washed with phosphate buffered saline (PBS) to remove the NPs adsorbed on the cell surface. Therefore, the small increase of cell viability of NPs incubated samples is likely due to the interference of An18–F127 which was taken up by cells. Taken together, both cell morphology observation and cell viability examination suggested that An18–F127 is biocompatible with A549 cells, which should be valuable for its biological imaging applications.
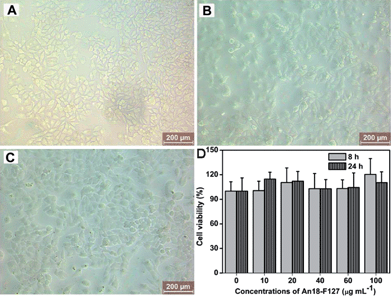 | ||
| Fig. 2 Biocompatibility of An18–F127. Optical microscopy of cells incubated with different concentrations of An18–F127 nanoparticles, (A) control cells, (B) 20 μg mL−1, (C) 100 μg mL−1, (D) time- and concentration-dependent cytotoxicity of An18–F127. | ||
To exploit the potential biomedical applications of An18–F127 NPs, the cell imaging of An18–F127 NPs using confocal laser scanning microscopy (CLSM) was examined.47,48 As shown in Fig. 3, bright yellow fluorescence were observed when cells were incubated with 40 μg mL−1 of An18–F127 for 3 h. From CLSM images, many dark areas were also found within the cells, which may be the locations of cell nuclei. The above results further suggest that An18–F127 could easily translocate into A549 cells and locate at cytoplasm. It is noticeable that the concentration of NPs (40 μg mL−1) used for cell imaging is much lower than that for cell viability testing (100 μg mL−1), suggesting their potential for biological imaging applications.
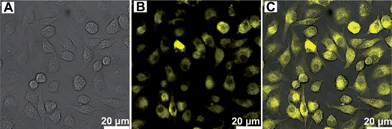 | ||
| Fig. 3 Cell imaging of An18–F127 nanoparticles using CSLM (A) bright field, (B) 405 nm excitations, (C) overlay of image A and B. | ||
In summary, a facile method for the preparation of AIE-based fluorescent organic nanoparticles (FONs) via mixing of AIE units (An18) and a commercial surfactant F127 was developed for the first time. Due to the hydrophobic interactions between An18 and F127, water soluble An18–F127 NPs with diameters of about 400–600 nm were prepared and subsequently utilized for cell imaging applications. Our results demonstrated that such AIE-based NPs were biocompatible with cells and could be easily observed in cells. Given their good water solubility, excellent biocompatibility and easy preparation approach, the surfactant modified AIE-based FONs would be novel and promising dyes for biological imaging applications.
Acknowledgements
This research was supported by the National Science Foundation of China (no. 21104039, 21134004, 21201108), and the National 973 Project (no. 2011CB935700), China Postdoctoral Science Foundation (2011M500280).References
- B. Dubertret, P. Skourides, D. J. Norris, V. Noireaux, A. H. Brivanlou and A. Libchaber, Science, 2002, 298, 1759–1762 CrossRef CAS.
- E. Betzig, G. H. Patterson, R. Sougrat, O. W. Lindwasser, S. Olenych, J. S. Bonifacino, M. W. Davidson, J. Lippincott-Schwartz and H. F. Hess, Science, 2006, 313, 1642–1645 CrossRef CAS.
- X. Michalet, F. Pinaud, L. Bentolila, J. Tsay, S. Doose, J. Li, G. Sundaresan, A. Wu, S. Gambhir and S. Weiss, Science, 2005, 307, 538–544 CrossRef CAS.
- N. L. Rosi and C. A. Mirkin, Chem. Rev., 2005, 105, 1547–1562 CrossRef CAS.
- X. Feng, L. Liu, S. Wang and D. Zhu, Chem. Soc. Rev., 2010, 39, 2411–2419 RSC.
- A. Burns, H. Ow and U. Wiesner, Chem. Soc. Rev., 2006, 35, 1028–1042 RSC.
- B. N. G. Giepmans, S. R. Adams, M. H. Ellisman and R. Y. Tsien, Science, 2006, 312, 217–224 CrossRef CAS.
- E. M. Nolan and S. J. Lippard, J. Am. Chem. Soc., 2007, 129, 5910–5918 CrossRef CAS.
- E. L. Que, D. W. Domaille and C. J. Chang, Chem. Rev., 2008, 108, 1517–1549 CrossRef CAS.
- E. M. Nolan and S. J. Lippard, Acc. Chem. Res., 2009, 42, 193–203 CrossRef CAS.
- G. Sun, M. Y. Berezin, J. Fan, H. Lee, J. Ma, K. Zhang, K. L. Wooley and S. Achilefu, Nanoscale, 2010, 2, 548–558 RSC.
- M. Nyk, R. Kumar, T. Y. Ohulchanskyy, E. J. Bergey and P. N. Prasad, Nano Lett., 2008, 8, 3834–3838 CrossRef CAS.
- V. Sokolova and M. Epple, Nanoscale, 2011, 3, 1957–1962 RSC.
- J. L. Bridot, A. C. Faure, S. Laurent, C. Rivière, C. Billotey, B. Hiba, M. Janier, V. Josserand, J. L. Coll and L. Vander Elst, J. Am. Chem. Soc., 2007, 129, 5076–5084 CrossRef CAS.
- J. Kim, J. E. Lee, S. H. Lee, J. H. Yu, J. H. Lee, T. G. Park and T. Hyeon, Adv. Mater., 2008, 20, 478–483 CrossRef CAS.
- W. C. Law, K. T. Yong, I. Roy, G. X. Xu, H. Ding, E. J. Bergey, H. Zeng and P. N. Prasad, J. Phys. Chem. C, 2008, 112, 7972–7977 CAS.
- J. Kim, Y. Piao and T. Hyeon, Chem. Soc. Rev., 2009, 38, 372–390 RSC.
- R. N. Mitra, M. Doshi, X. Zhang, J. C. Tyus, N. Bengtsson, S. Fletcher, B. D. G. Page, J. Turkson, A. J. Gesquiere and P. T. Gunning, Biomaterials, 2011, 33, 2969–2978 Search PubMed.
- I. Díez and R. H. A. Ras, Nanoscale, 2011, 3, 1963–1970 RSC.
- Y. P. Ho and K. W. Leong, Nanoscale, 2010, 2, 60–68 RSC.
- Z. Cai, Z. Ye, X. Yang, Y. Chang, H. Wang, Y. Liu and A. Cao, Nanoscale, 2011, 3, 1974–1976 RSC.
- R. Jin, Nanoscale, 2010, 2, 343–362 RSC.
- X. Wang, S. Xu and W. Xu, Nanoscale, 2011, 3, 4670–4675 RSC.
- X. Wu, X. He, K. Wang, C. Xie, B. Zhou and Z. Qing, Nanoscale, 2010, 2, 2244–2249 RSC.
- S. Chandra, P. Das, S. Bag, D. Laha and P. Pramanik, Nanoscale, 2011, 3, 1533–1540 RSC.
- A. Shiohara, S. Prabakar, A. Faramus, C. Y. Hsu, P. S. Lai, P. T. Northcote and R. D. Tilley, Nanoscale, 2011, 3, 3364–3370 RSC.
- A. Bhirde, J. Xie, M. Swierczewska and X. Chen, Nanoscale, 2011, 3, 142–153 RSC.
- K. Li, J. Pan, S. S. Feng, A. W. Wu, K. Y. Pu, Y. Liu and B. Liu, Adv. Funct. Mater., 2009, 19, 3535–3542 CrossRef CAS.
- A. M. Smith, H. Duan, A. M. Mohs and S. Nie, Adv. Drug Delivery Rev., 2008, 60, 1226–1240 CrossRef CAS.
- S. W. I. Thomas, G. D. Joly and T. M. Swager, Chem. Rev., 2007, 107, 1339–1386 CrossRef CAS.
- M. Kumar and S. J. George, Nanoscale, 2011, 3, 2130–2133 RSC.
- J. Luo, Z. Xie, J. W. Y. Lam, L. Cheng, H. Chen, C. Qiu, H. S. Kwok, X. Zhan, Y. Liu, D. Zhu and B. Z. Tang, Chem. Commun., 2001, 1740–1741 RSC.
- Z. Yang, Z. Chi, T. Yu, X. Zhang, M. Chen, B. Xu, S. Liu, Y. Zhang and J. Xu, J. Mater. Chem., 2009, 19, 5541–5546 RSC.
- B. Xu, Z. Chi, X. Zhang, H. Li, C. Chen, S. Liu, Y. Zhang and J. Xu, Chem. Commun., 2011, 47, 11273–11275 RSC.
- X. Zhang, Z. Chi, H. Li, B. Xu, X. Li, S. Liu, Y. Zhang and J. Xu, J. Mater. Chem., 2011, 21, 1788–1796 RSC.
- Z. Chi, X. Zhang, B. Xu, X. Zhou, C. Ma, Y. Zhang, S. Liu and J. Xu, Chem. Soc. Rev., 2012, 41, 3878–3896 RSC.
- Z. Zhao, S. Chen, X. Shen, F. Mahtab, Y. Yu, P. Lu, J. W. Y. Lam, H. S. Kwok and B. Z. Tang, Chem. Commun., 2010, 46, 686–688 RSC.
- Y. Hong, J. W. Y. Lam and B. Z. Tang, Chem. Soc. Rev., 2011, 40, 5361–5388 RSC.
- X. Zhang, Z. Yang, Z. Chi, M. Chen, B. Xu, C. Wang, S. Liu, Y. Zhang and J. Xu, J. Mater. Chem., 2010, 20, 292–298 RSC.
- B. K. An, D. S. Lee, J. S. Lee, Y. S. Park, H. S. Song and S. Y. Park, J. Am. Chem. Soc., 2004, 126, 10232–10233 CrossRef CAS.
- X. Zhang, Z. Chi, J. Zhang, H. Li, B. Xu, X. Li, S. Liu, Y. Zhang and J. Xu, J. Phys. Chem. B, 2011, 115, 7606–7611 CrossRef CAS.
- X. Zhang, Z. Chi, H. Li, B. Xu, X. Li, W. Zhou, S. Liu, Y. Zhang and J. Xu, Chem.–Asian J., 2011, 6, 808–811 CrossRef CAS.
- H. Li, X. Zhang, Z. Chi, B. Xu, W. Zhou, S. Liu, Y. Zhang and J. Xu, Org. Lett., 2011, 13, 556–559 CrossRef CAS.
- Y. Yu, C. Feng, Y. Hong, J. Liu, S. Chen, K. M. Ng, K. Q. Luo and B. Z. Tang, Adv. Mater., 2011, 23, 3298–3302 CrossRef CAS.
- X. Zhang, W. Hu, J. Li, L. Tao and Y. Wei, Toxicol. Res., 2012, 1, 62–68 RSC.
- X. Zhang, H. Qi, S. Wang, L. Feng, Y. Ji, L. Tao, S. Li and Y. Wei, Toxicol. Res., 2012, 1, 201–205 RSC.
- X. Zhang, S. Wang, L. Xu, Y. Ji, L. Feng, L. Tao, S. Li and Y. Wei, Nanoscale, 2012, 4, 5581–5584 RSC.
- X. Zhang, S. Wang, C. Fu, L. Feng, Y. Ji, L. Tao, S. Li and Y. Wei, Polym. Chem., 2012, 3, 2716–2719 RSC.
Footnotes |
| † Electronic supplementary information (ESI) available: Detailed information about 1H NMR spectra of An18 and FT-IR spectra of An18–F127. See DOI: 10.1039/c2nr32698a |
| ‡ These authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2013 |
