One-pot hydrothermal synthesis of lanthanide ions doped one-dimensional upconversion submicrocrystals and their potential application in vivo CT imaging†
Guo
Gao
*,
Chunlei
Zhang
,
Zhijun
Zhou
,
Xin
Zhang
,
Jiebing
Ma
,
Chao
Li
,
Weilin
Jin
* and
Daxiang
Cui
*
Department of Bio-Nano-Science and Engineering, Key Laboratory for Thin Film and Microfabrication of Ministry of Education, Institute of Micro-Nano Science and Technology, Shanghai Jiao Tong University, Shanghai, 200240, China. E-mail: gaogaoguoguo@yahoo.com.cn; weilinjin@sjtu.edu.cn; dxcui@sjtu.edu.cn; Fax: +86 21 34206886; Tel: +86 21 34206962
First published on 2nd November 2012
Abstract
Multi-functional rare-earth Yb3+ and Ln3+ (Ln = Er, Tm and Ho) ions doped one-dimensional (1-D) upconversion submicrocrystals (NaYF4 and NaGdF4) possessing upconversion luminescence, biocompatibility and magnetic properties have been synthesized by a one-pot hydrothermal method. Rare-earth Yb3+ and Ln3+ ions doped NaYF4 microrods (∼1 μm in diameter, 3–5 μm in length) exhibit porous properties, and the average pore sizes are ∼28.2 nm. They show paramagnetism in the magnetic range of −60 to −2 kOe and 2 to 60 kOe at 300 K, and exhibit near superparamagnetic behaviour at the magnetic range of −2 to 2 kOe. Saturation magnetization was ∼12.1 emu g−1 at 2 K. The Yb3+ and Ln3+ ions doped NaGdF4 submicrocrystals (∼100 nm in diameter, 200–300 nm in length) show paramagnetism at 300 K, and exhibit superparamagnetic behaviour with a saturation magnetization of 129.2 emu g−1 at 2 K. The magnetic properties of Yb3+ and Ln3+ ions doped 1-D upconversion submicrocrystals indicate they can be used for drug targeting under a magnetic field. Their unique upconversion emission (green for Yb3+/Er3+ and blue for Yb3+/Tm3+) under 980 nm laser excitation indicate that they could be used for specific luminescent immunolabeling and imaging. MTT assays reveal that 1-D upconversion submicrocrystals have satisfactory bio-affinity, where the viability keeps in good state even at a concentration of 500 μg mL−1, which is much higher than the concentration usually used in cell labelling. Luminescent microscopy images show that the morphologies of the cytoskeleton and cell nucleus are well maintained after incubating different concentrations of 1-D upconversion submicrocrystals. After injecting upconversion submicrocrystals into the mice (tumor sites or back normal tissue), a clearly distinguished CT signal was observed, indicating the synthesized 1-D submicrocrystals are effective for CT imaging in vivo.
1. Introduction
In the past decades, rare-earth ions doped upconversion nano- and micro-crystals (UNs) have attracted considerable attention because of their intriguing merits (e.g., narrow emission peak, long lifetimes, large Stokes shifts, high quantum yields and low toxicity) for optical imaging probes, biological luminescent labels and drug delivery carriers.1 Compared with organic dyes and quantum dots (QDs), UNs can convert near-infrared light into visible light, which makes them promising as biological probes with deep tissue penetration, low autofluorescence background, sharp emission bandwidths and high resistance to photobleaching.2 The upconversion luminescence is much stronger than that of QDs. Furthermore, the ratio of signal-to-noise and sensitivity of detection can be improved evidently. It is also suggested that the rare-earth UNs have lower toxicity than QDs elements. The 50% lethal dosage (LD50) is about a thousand times higher than that of QDs.3 On the other hand, QDs and organic dyes will cause damage (refer to toxicity) to biological samples as well as strong autofluorescence. Recently, the imaging penetration depth has been reported to be as thick as 1.6 cm by Li et al.4a and 3.2 cm by Han et al.4b Rare-earth-based host nano- and micro-crystals (NaYF4 or NaGaF4) doped with Yb3+/Er3+, Yb3+/Tm3+ and Yb3+/Ho3+ have shown excellent upconversion luminescence under continuous-wave excitation of 980 nm.5 The introduction of Yb3+ ions as a sensitizing center can greatly enhance the upconversion luminescence of the Tm3+, Er3+ and Ho3+ activators. The properties of UNs are affected not only by their composition, but also by their size, morphology and structure. Thus, designing and synthesizing UNs with desired shapes are important for exploring their next applications.Among down- and up-conversion luminescent nanomaterials with different structures, one-dimensional (1-D) nano- and micro-sized materials stimulated great attention due to their unique physical/chemical properties.6 Up to now, great efforts have been devoted to fabricate rare-earth-doped UNs, and the synthetic methods include hydro/solvothermal, microemulsion, coprecipitation, thermal decomposition and microwave-assisted methods.7 Li et al. reported a hydrothermal method to prepare NaYF4:Yb3+/Tm3+ nanorods.8 The synthesized UNs can improve upconversion efficiency of rare-earth-doped phosphors. Wang et al. reported a facile microemulsion method for the synthesis of BaSiF6:Yb3+/Tm3+ nanorods.9 Qu et al. suggested that the macroporous ZrO2:Yb3+/Er3+ upconversion nanocrystals can be obtained using polystyrene spheres as soft templates.10 Wang et al. reported the one-pot synthesis of poly(acrylic acid)-functionalized YF3:Yb3+/Er3+ nanocrystals with strong near-infrared upconversion luminescence in a mixed solution containing ethylene glycol, ethanol and poly (acrylic acid).11a Recently, our group has successfully synthesized uniform lanthanide-doped NaGdF4 nanocrystals in an oleic acid–ionic liquid system.12a Although great achievements have been made in the synthesis of UNs with different host/activator combinations, it still exhibits some drawbacks such as the use of hazardous precursors, coordinating solvents, rigorous reaction conditions and complex surface modification. Exploring novel ‘green’ approaches based on low-cost, mild reaction conditions and non-toxic precursors for synthesizing UNs is currently a hot-topic subject for material scientists.
Herein, we report a facile one-pot hydrothermal method for controlled-synthesis of rare-earth Yb3+ and Ln3+ (Ln = Er, Tm and Ho) ions codoped NaGdF4 submicrocrystals (∼100 nm in diameter, 200–300 nm in length) and porous NaYF4 microrods (∼1 μm in diameter, 3–5 μm in length) only using ethylene diamine tetraacetic acid (EDTA) as a template. Porous NaYF4:Yb3+/Er3+, NaYF4:Yb3+/Tm3+ and NaYF4:Yb3+/Ho3+ microrods, NaGdF4:Yb3+/Er3+, NaGdF4:Yb3+/Tm3+ and NaGdF4:Yb3+/Ho3+ submicrocrystals were synthesized according to the above-mentioned hydrothermal method. The advantages of this method are: (1) rare-earth Yb3+ and Ln3+ (Ln = Er, Tm and Ho) ions doped NaGdF4 submicrocrystals and NaYF4 microrods can be prepared in a large scale. (2) Only EDTA was used as template, so this method is low-cost, non-toxic and avoids the use of hazardous precursors. Furthermore, EDTA can endow plenty of COO− groups to the surface of UNs, which facilitates their biological applications. (3) The prepared submicrocrystals have been demonstrated to be effective for CT imaging in vivo.
2. Experimental
2.1 Synthesis of rare-earth Yb3+ and Ln3+ (Ln = Er, Tm and Ho) ions doped 1-D NaYF4 and NaGdF4 microcrystals
All the chemical reagents were of analytical grade and were used without further purification. In a typical synthesis procedure, 2.1 g NaF was dissolved in a mixed solution of 20 mL deionized water and 30 mL ethanol. Then, 16 mL YCl3 (0.2 M), 340 μL YbCl3 (0.2 M) and 60 μL ErCl3 (0.2 M) were gradually added into the mixed solution. When the mixture was stirred for 30 min, 20 mL EDTA (0.2 M) was added. The obtained solution was transferred into a stainless-lined autoclave and heated at 320 °C for 10 h. The elevation of temperature speed is 5 °C per min. After reaction, NaYF4:Yb3+/Er3+ microrods can be obtained. NaYF4:Yb3+/Tm3+ and NaYF4:Yb3+/Ho3+ microrods, well-defined NaGdF4:Yb3+/Er3+, NaGdF4:Yb3+/Tm3+ and NaGdF4:Yb3+/Ho3+ submicrocrystals can also be synthesized according to the above-mentioned procedure via varying the type of chemical reagents. The obtained 1-D upconversion microcrystals were washed by ethanol and deionized water three times, and freeze-dried for 24 h.2.2 Characterization of the synthesized 1-D upconversion microcrystals
The crystal structure and sizes were characterized by scanning electron microscopy (SEM, FEI-Sirion 200), transmission electron microscopy (TEM, JEM-2010), high-resolution transmission microscopy (HRTEM) and selected area electron diffraction (SAED). The samples compositions were characterized by an X-ray diffractometer (XRD, Rigaku, Japan) using Cu Kα radiation at 1.5418 Å at a scanning rate of 7° min−1. Upconversion luminescence spectra of the samples were carried out on a Fluorolog-3 spectrofluorometer (Jobin Yvon, France) at room temperature. The specific surface area was calculated by the Brunauer–Emmett–Teller (BET) method. The pore size distribution was obtained by the Barret–Joyner–Halenda (BJH) method. The X-ray photoelectron spectra were measured using an AXIS ULTRA DLD (Kratos). Magnetic properties of 1-D upconversion microcrystals were evaluated by a magnetic analyzer (PPMS-9T (EC-II), Quantum Design).2.3 In vitro cells experiment
Healthy human gastric cells GES-1 were seeded into 96-well plates at a density of 104 in 100 μL of medium per well and grown in RPMI 1640 cells medium supplemented with 10% NCBS, 100 units per mL penicillin and 0.1 mg mL−1 streptomycin under standard conditions in a conventional incubator with 5% CO2 at 37 °C overnight. The 1-D upconversion submicrocrystals were dispersed in cell culture medium after sterilization and obtained different concentrations of submicrocrystals. The cells were treated with different concentrations of 1-D submicrocrystals respectively with 100 μL per well and allowed for 36 h incubation.2.4 In vivo animal experiment
Male nude mice (∼24 g) were purchased from Shanghai SLAC Laboratory Animal Co. Ltd (Shanghai, China). All animal procedures were in agreement with institutional animal use and care regulations. In vivo CT imaging, the NaGdF4:Yb3+/Er3+ aqueous solution (100 μL 20 mg mL−1 per site) in PBS was subcutaneously injected into the back of mice, and was intramuscularly injected into the tumor sites of mice, respectively. All CT scans were performed using a clinical CT (SIEMENS Spirit CT 31159, German).3. Results and discussion
The formation process of rare-earth Yb3+ and Ln3+ (Ln = Er, Tm and Ho) ions codoped NaGdF4 submicrorods (∼100 nm in diameter, 200–300 nm in length) and porous NaYF4 microrods (∼1 μm in diameter, 3–5 μm in length) is shown in Scheme 1. The whole process can be divided into three steps: (a) rare earth chloride, NaF and EDTA were mixed together in aqueous solution at room temperature. The rare earth ions encountered and reacted with Na and F ions to form the small precursor particles. EDTA molecules are strong complexing agents, and they may preferentially adsorb on the six nonpolar crystalline facets [e.g., (![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) 011), (
011), (![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) 101), (01
101), (01![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) 1), (10
1), (10![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) 1), (1
1), (1![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) 01) and (0
01) and (0![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) 11)] of precursors and inhibit the growth of precursors along these orientations, resulting in the formation of the initial shape of UNs with hexahedral structure. (b) The adsorption difference between EDTA and each crystalline plane of the precursor may promote the epitaxial growth of nuclei along these orientations. In the current system, the adsorption capability of EDTA molecules on the z-axis may probably be weaker than the other six nonpolar crystalline planes. When the temperature was elevated, the homogeneous reaction system is destroyed. EDTA molecules that originally adsorb on the z-axis were gradually released, resulting in the growth of precursors along the z-axis. (c) A lot of small precursors slowly grow with the increase of reaction time. When the nutrients in the reaction system were consumed completely, 1-D upconversion submicrorods were formed.
11)] of precursors and inhibit the growth of precursors along these orientations, resulting in the formation of the initial shape of UNs with hexahedral structure. (b) The adsorption difference between EDTA and each crystalline plane of the precursor may promote the epitaxial growth of nuclei along these orientations. In the current system, the adsorption capability of EDTA molecules on the z-axis may probably be weaker than the other six nonpolar crystalline planes. When the temperature was elevated, the homogeneous reaction system is destroyed. EDTA molecules that originally adsorb on the z-axis were gradually released, resulting in the growth of precursors along the z-axis. (c) A lot of small precursors slowly grow with the increase of reaction time. When the nutrients in the reaction system were consumed completely, 1-D upconversion submicrorods were formed.
 | ||
| Scheme 1 Formation process of rare-earth Yb3+ and Ln3+ (Ln = Er, Tm and Ho) ions doped NaGdF4 and NaYF4 microcrystals. | ||
Fig. 1(a)–(c) show the scanning electron microscopy (SEM, FEI-Sirion 200) images of NaYF4:Yb3+/Er3+, NaYF4:Yb3+/Tm3+ and NaYF4:Yb3+/Ho3+ microrods, respectively. It can be seen that the rare-earth Yb3+ and Ln3+ (Ln = Er, Tm and Ho) ions codoped NaYF4 microrods have a hexahedral architecture with porous structures. Fig. 1(a) shows the diameter of NaYF4:Yb3+/Er3+ submicrocrystals is about 880 nm, and length is ∼3.2 μm (Fig. S1†). The higher resolution SEM image (Fig. S2†) reveals that the surface of NaYF4:Yb3+/Er3+ submicrocrystals is covered by numerous uniform nanoparticles (30–50 nm in diameter). High-resolution transmission electron microscopy (HRTEM, JEM-2010) image (Fig. 1(g)) shows the clear and continuous lattice fringes, which indicates high crystallinity. The selected area electron diffraction (SAED) pattern (Fig. 1(h)) taken from the edge of the submicrocrystals exhibits sharp diffraction spots, suggesting the prepared NaYF4:Yb3+/Er3+ microcrystals have a single-crystal nature. The measured lattice spacing is 0.256 nm which is close to the (200) lattice plane (0.258 nm) of hexagonal NaYF4 structure (JCPDS no. 28-1192). As for NaYF4:Yb3+/Tm3+ submicrocrystals, the sizes have increased (Fig. 1(b)). The diameter is found to be 1.7 μm, and length is ∼4.5 μm (Fig. S3†). As for NaYF4:Yb3+/Ho3+ submicrocrystals (Fig. 1(c)), the diameter is about 810 nm, and length is ∼2.5 μm (Fig. S4†). More details about the Er3+, Tm3+ and Ho3+ codoped NaYF4 microrods can be seen in Fig. S5.†Fig. 1(d)–(f) show the TEM images of Yb3+ and Ln3+ doped NaGdF4 submicrocrystals. Rare-earth Yb3+ and Ln3+ (Ln = Er, Tm and Ho) ions codoped NaGdF4 submicrocrystals have a smooth surface, and the diameter is about 100 nm and length is 200–300 nm. For NaGdF4 submicrocrystals, there are no small nanoparticles on their surface. The samples of NaGdF4 submicrorods are composed by a lot of 1-D hexahedral structure and a few small-sized hexahedral particles. More details about the rare-earth Yb3+ and Ln3+ doped NaGdF4 submicrorods can be seen in Fig. S6.† Formation mechanism of 1-D upconversion submicrocrystals can be explained by a synergic effect of EDTA assisted orientation-assembly and Ostwald ripening process. In order to verify the formation mechanism, the growth process (using NaYF4:Yb3+/Er3+ as an example) has been followed at different reaction stages. Obviously, the EDTA in the hydrothermal system plays a key role for the formation of 1-D upconversion submicrocrystals functionalized by COO− groups. The presence of EDTA may cause a significant difference in the relative growth rates of various crystallographic planes. It is known that Y3+, Yb3+ and Er3+ ions can be chelated with EDTA molecules to form the complex. When NaF solution was added, NaYF4:Yb3+/Er3+ is nucleated and encapsulated by EDTA molecules. Before the hydrothermal process, the initial structures for hexahedral NaYF4:Yb3+/Er3+ microrods are the quasi-hexahedral aggregates with a diameter of 100 nm (Fig. S7†), and their surface is rather rough. EDTA molecules are more preferentially adsorbed to the six nonpolar crystalline planes of growing NaYF4:Yb3+/Er3+ particles, hindering the crystal growth along this plane. When increasing the reaction temperature to 200 °C, hexahedral NaYF4:Yb3+/Er3+ microrods with relatively smooth surfaces and sharp edges are formed (Fig. S8†). Also, we observe that there are few small cavities on the surface of NaYF4:Yb3+/Er3+ microrods. It is known that the ionic strength of solution will be dramatically increased at higher temperatures. Cl− ions are the most aggressive anions. An increase in temperature will accelerate the rates of migration and diffusion of the Cl− ions and microrods substrates into and from the cavities. When the temperature increases from 200 °C to 320 °C, Cl− ions will continuously corrode the NaYF4 microrods substrates, leading to the formation of porous structures. As for NaGdF4 submicrorods, their substrates are more stable than NaYF4 substrates, which are related to the natural physical/chemical properties of the substrates. NaGdF4 substrates have good tolerance to the aggressive Cl− ions at high temperature. So, the rare-earth Yb3+ and Ln3+ doped NaGdF4 submicrorods have smooth surfaces.
 | ||
| Fig. 1 SEM and TEM images of the 1-D upconversion submicrocrystals: (a) NaYF4:Yb3+/Er3+, (b) NaYF4:Yb3+/Tm3+, (c) NaYF4:Yb3+/Ho3+, (d) NaGdF4:Yb3+/Er3+, (e) NaGdF4:Yb3+/Tm3+ and (f) NaGdF4:Yb3+/Ho3+, HRTEM image (g) and SAED pattern (h) of NaYF4:Yb3+/Er3+ microcrystals. Each kind of upconversion microcrystal was synthesized according to the same procedure three times in order to verify the repeatability. | ||
Typical SEM images suggest that the rare-earth Yb3+ and Ln3+ (Ln = Er, Tm and Ho) ions codoped NaYF4 microrods have a porous structure whereas there are no cavities for NaGdF4 submicrorods. In order to obtain more information concerning the porous NaYF4 microrods, further surface analysis techniques were used to provide insight into the shape and structure of the samples. The N2 adsorption/desorption isotherms of NaYF4:Yb3+/Er3+ microrods are shown in Fig. 2(a). The typical IV-type isotherms between 0.2 and 0.6 P/P0, indicate that the samples possess many pore defects and fractures on the microcrystalline surface. The type H4 loop with parallel branches at relative pressures between 0.85 and 1.0 P/P0 is attributed to the hollow cavity space.12d The pore-size distribution curve obtained through the Barrett–Joyner–Halenda (BJH) method (Fig. 2(b)) indicates that average pore defect sizes are ∼28.2 nm. The Brunauer–Emmett–Teller (BET) surface area and pore volume are 2.02 m2 g−1 and 0.047 m3 g−1, respectively. The porous structure of NaYF4:Yb3+/Er3+ microrods were also evaluated by atomic force microscopy (AFM). AFM images were examined on a Multi-Mode Nanoscope V scanning probe microscopy system (Veeco). The samples were prepared by dropping 1 mL aqueous suspensions containing NaYF4:Yb3+/Er3+ microrods (0.01 mg mL−1) on a freshly cleaved mica surface. The AFM image in Fig. 2(c) shows that there are some larger hollow cavities in the substrates of NaYF4:Yb3+/Er3+ microrods. The shape of hollow cavities is irregular. We also observe that some small particles are covered on the surface of NaYF4:Yb3+/Er3+ microcrystals. Line profiles indicate that the surface of NaYF4:Yb3+/Er3+ microrods are rather rough. As for the two line profiles, the maximum depth is about 150 nm, which is located at the position of the hollow cavity. Fig. 2(d) shows the typical images of porous pores on the surface of NaYF4:Yb3+/Er3+ microrods. The depth of pore defects changes from 14 to 18 nm. The leaf teeth shape exhibited in the two line profiles suggested that a lot of pores and fractures on the surface of NaYF4:Yb3+/Er3+ microrods are in the gestation and growing stage. FTIR analysis was carried out between 500 and 4000 cm−1. In the FTIR spectra of NaYF4:Yb3+/Er3+ microrods (Fig. 2(e)), the absorption band at 3459 cm−1 can be assigned to the O–H stretch of EDTA. The peak at 1646 cm−1 is attributed to the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching vibrations from carboxylic groups. The absorption bands located at 1125 and 527 cm−1 can be assigned to the C–N and C–Hx bonds respectively. FTIR results confirm the successful carboxyl functionalization of UNs. The carboxyl groups on the surface of UNs not only confer hydrophilicity of these submicrocrystals, but also provide numerous favourable sites for grafting various biomolecules for drug and gene delivery.12c Thermogravimetric analysis (TGA) was used to evaluate the loaded amount of EDTA on the surface of UNs. Fig. 2(f) shows the TGA curve of NaYF4:Yb3+/Er3+ microrods at the temperature range from 20 to 900 °C under N2 gas protection. From Fig. 2(f), we can see that there is nearly no weight loss at 20–300 °C, indicating that the samples are desiccated and no adsorbed water is on its surface. The dip in the TGA spectra may be attributed to the slight fluctuation of the instrument during the experiment. Within the temperature region of 300–900 °C, the weight decreased quickly from 99.9 to 94.1%. This evolution is attributed to the removal of EDTA molecules from NaYF4:Yb3+/Er3+ microrods. The 5.8% weight loss indicates that the surface of NaYF4:Yb3+/Er3+ microrods have plenty of COO− groups, which facilitate their biological applications.
O stretching vibrations from carboxylic groups. The absorption bands located at 1125 and 527 cm−1 can be assigned to the C–N and C–Hx bonds respectively. FTIR results confirm the successful carboxyl functionalization of UNs. The carboxyl groups on the surface of UNs not only confer hydrophilicity of these submicrocrystals, but also provide numerous favourable sites for grafting various biomolecules for drug and gene delivery.12c Thermogravimetric analysis (TGA) was used to evaluate the loaded amount of EDTA on the surface of UNs. Fig. 2(f) shows the TGA curve of NaYF4:Yb3+/Er3+ microrods at the temperature range from 20 to 900 °C under N2 gas protection. From Fig. 2(f), we can see that there is nearly no weight loss at 20–300 °C, indicating that the samples are desiccated and no adsorbed water is on its surface. The dip in the TGA spectra may be attributed to the slight fluctuation of the instrument during the experiment. Within the temperature region of 300–900 °C, the weight decreased quickly from 99.9 to 94.1%. This evolution is attributed to the removal of EDTA molecules from NaYF4:Yb3+/Er3+ microrods. The 5.8% weight loss indicates that the surface of NaYF4:Yb3+/Er3+ microrods have plenty of COO− groups, which facilitate their biological applications.
 | ||
| Fig. 2 N2 adsorption–desorption isotherms of NaYF4:Yb3+/Er3+ microrods (a) and pore size distribution calculated from the adsorption branch (b), AFM images of NaYF4:Yb3+/Er3+ microrods (c and d), FTIR spectra of NaYF4:Yb3+/Er3+ microrods (e) and TGA analysis of NaYF4:Yb3+/Er3+ microrods (f). | ||
The element composition of the synthesized upconversion microcrystals is also confirmed by energy dispersive spectrum (EDS) analysis. The characteristic elements of Na, Y, F, Yb, Er, Tm, Ho and Gd are found in the spectrum. All the samples were also characterized by an X-ray diffractometer (XRD, Rigaku, Japan) using Cu Kα radiation at 1.5418 Å at a scanning rate of 7° min−1. The synthesized upconversion microcrystals could be well indexed as hexagonal NaYF4 (JCPDS no. 28-1192) structure for NaYF4:Yb3+/Er3+, NaYF4:Yb3+/Tm3+ and NaYF4:Yb3+/Ho3+ (Fig. S9†), and hexagonal NaGdF4 (JCPDS no. 27-699) structure for NaGdF4:Yb3+/Er3+, NaGdF4:Yb3+/Tm3+ and NaGdF4:Yb3+/Ho3+ (Fig. S10†).
The upconversion luminescence spectrum of Yb3+ and Ln3+ codoped NaYF4 and NaGdF4 microcrystals is shown in Fig. 3. As for the upconversion process, the upconversion emission intensity (I) is relative to the excitation power (P) according to the power equation I ∝ Pn, where n is the number of pump photons absorbed per upconverted photon emitted. Luminescence spectra of the synthesized 1-D microcrystals were obtained on an Fluorolog-3 spectrofluorometer with a continuous wave laser (106 to 109 W cm−2). As for NaYF4:Yb3+/Er3+ microcrystals (Fig. 3(a)), the dominant green emission peaks at 519 and 538 nm can be assigned to 2H11/2 and 4S3/2 → 4I15/2 transitions. The red emission peak at 652 nm is attributed to 4F9/2 → 4I15/2 of Er3+. As for NaYF4:Yb3+/Tm3+ (Fig. 3(b)), the weak peak at 449 nm (violet) and strongest peak at 474 nm (blue) correspond to the relaxation of 1D2 → 3F4 and 1G4 → 3H6, respectively. The red emission peak at 644 nm was assigned to the 1G4 → 3F4 transition. Fig. 3(c) shows the luminescence spectrum of NaYF4:Yb3+/Ho3+. The green emission in the range from 550 to 560 nm is assigned to the 5S2 → 5I8 transition while the emission at 643 nm corresponded to the 5F2 → 5I8 transition.13 For NaGdF4:Yb3+/Er3+ submicrocrystals (Fig. 3(d)), the emission peaks at around 522, 539 and 652 nm are assigned to the 2H11/2 → 4I15/2, 4S3/2 → 4I15/2 and 4F9/2 → 4I15/2 respectively. Fig. 3(e) shows the luminescence spectrum of NaGdF4:Yb3+/Tm3+. Four emission peaks at 449, 473, 644 and 724 nm were attributed to the 1D2 → 3F4, 1G4 → 3H6, 1G4 → 3F4 and 3F3 → 3H6 transitions of Tm3+. The strong emission peak at 800 nm was assigned to the 3H4 → 3H6 transition which is much stronger than the other peaks, suggesting the radiative deactivation of upconversion energy predominantly occurs through near-infrared luminescence. As for the NaGdF4:Yb3+/Ho3+ submicrocrystals (Fig. 3(f)), the blue emission at 484 nm is assigned to the 5F3 → 5I8 transition. The strongest green emission at 538 nm is attributed to the 5F4, 5S2 → 5I8 transition. The peaks at 643 and 748 nm were ascribed to the 5F5 → 5I8 and 5F4, 5S2 → 5I7 transitions.14 Generally, upconversion processes involve the excited state absorption, energy transfer upconversion and photon avalanche. All these processes include the sequential absorption of two or more photons. Nanoscale designing and manipulating upconversion submicrocrystals may lead to the important modification of their optical properties in excited state dynamics, emission profiles and upconversion efficiency.
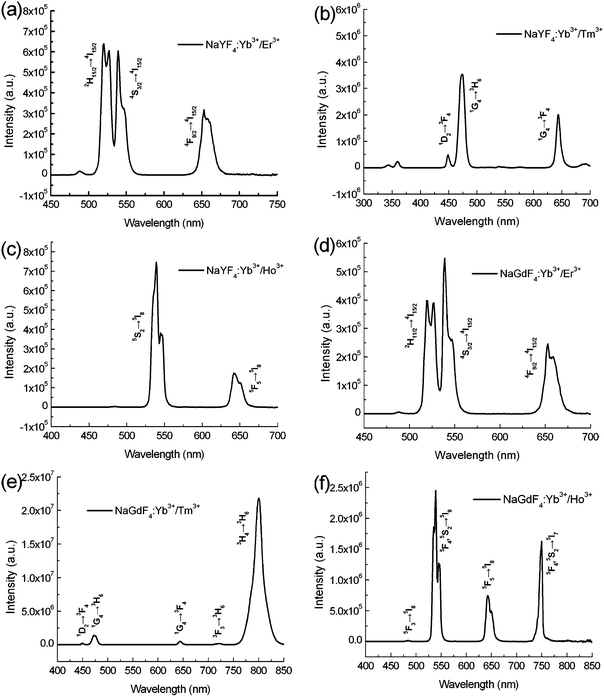 | ||
| Fig. 3 Upconversion luminescence spectra of (a) NaYF4:Yb3+/Er3+, (b) NaYF4:Yb3+/Tm3+, (c) NaYF4:Yb3+/Ho3+, (d) NaGdF4:Yb3+/Er3+, (e) NaGdF4:Yb3+/Tm3+ and (f) NaGdF4:Yb3+/Ho3+ submicrocrystals. | ||
X-Ray photoelectron spectroscopy (XPS) was used to further determine the successful incorporation of rare earth ions into the NaYF4 and NaGdF4 substrates. Typically, the NaGdF4:Yb3+/Er3+ submicrorods and NaYF4:Yb3+/Tm3+ microrods were selected for XPS analysis. Fig. 4 shows the XPS spectra of NaGdF4:Yb3+/Er3+ submicrorods. The characteristic peak at 1071.8 eV can be assigned to the binding energy of Na 1s (Fig. 4(a)). Peak at 685.0 eV is attributed to the binding energy of F 1s (Fig. 4(b)). Peaks located at 199.4 and 173.1 eV can be assigned to the binding energy of Yb 4d and Er 4d, respectively (Fig. 4(c)). Fig. 4(d) shows two strongest peaks at 144.1 and 149.4 eV correspond to binding energy of Gd 2p3/2 and Gd 2p5/2, respectively.15a The peak located at 284.8 eV can be attributed to the binding energy of C 1s (Fig. 4(e)). Carbon components, which correspond to C–C/C–H, are originated from the EDTA molecules. As for NaYF4:Yb3+/Tm3+ microrods (Fig. S11†), the binding energy of Na (1s, 1071.6 eV), F (1s, 684.6 eV), Y (2p3/2, 160.8 eV)(2p1/2, 158.7 eV), Yb (4d, 198.1 eV) and Tm (4d, 171.7 eV).
 | ||
| Fig. 4 XPS spectra of NaGdF4:Yb3+/Er3+ submicrorods, the Na 1s region (a), F 1s region (b), Yb 4d and Er 4d region (c), Gd 4d region (d) and C 1s region (e). | ||
To determine the magnetic property of the synthesized rare-earth ions doped UNs, its magnetization was measured as a function of the applied magnetic field. Fig. 5(a) shows that the NaYF4:Yb3+/Tm3+ microrods exhibited paramagnetism in the magnetic range of −60 to −2 kOe and 2 to 60 kOe at room temperature (300 K). Fig. 5(b) shows that the NaYF4:Yb3+/Tm3+ microrods presented a near superparamagnetic behaviour at the magnetic range of −2 to 2 kOe. From Fig. 5(a), we can see that the magnetization curve is non-linear in nature. At −2 to 2 kOe, there is an evident jump. One of the possible reasons is that the magnetic moments of NaYF4:Yb3+/Tm3+ are changed because Yb3+ ions (86.8 pm) have a simple electronic configuration and its crystal ionic radii is closely to that of Y3+.15c At 2 K, NaYF4:Yb3+/Tm3+ showed superparamagnetic behaviour (Fig. 5(c)) and no coercivity or remanence is observed. The saturation magnetization value of the NaYF4:Yb3+/Tm3+ microrod was found to be ∼12.1 emu g−1. Fig. 5(d) illustrates that NaGdF4:Yb3+/Er3+ submicrorods exhibited paramagnetism at room temperature (300 K), and the magnetization at 30 kOe was 2.8 emu g−1, whereas the NaGdF4:Yb3+/Er3+ submicrorods at low temperature (2 K) exhibited superparamagnetic behaviour with a saturation magnetization of 129.2 emu g−1 (Fig. 5(e)). As for both NaYF4:Yb3+/Tm3+ microrods and NaGdF4:Yb3+/Er3+ submicrorods, the saturation magnetization has an increasing trend at low temperature. Generally, the increase of magnetic susceptibility at low temperature can be attributed to the reduction in thermal fluctuation which is the typical behaviour of paramagnetic materials described by Curie's law.15b NaYF4 is the most efficient matrix for green and blue upconversion energy transfer phenomena. The matrix of NaGdF4 is very similar to NaYF4. However, the advantage of the NaGdF4 matrix is that through substituting Y by Gd to reduce the size of UNs doped by lanthanide ions, new magnetic properties are introduced and optical markers become multifunctional. The superparamagnetic behaviour of nanomaterials is crucial for applications in biomedicine and biotechnology.11b,11c,12b Furthermore, the ionic radius of Gd (93.8–101 pm) is much bigger than Y (90 pm), and therefore Gd ions can distort the lattice more and make the lattice even less centrosymmetric. The magnetic property of Gd ions arises from the seven unpaired inner 4f electrons which are closely bound with the nucleus and shielded by the outer closed-shell electrons 5s25p6 from the crystal field. Therefore, the rare-earth Yb3+ and Ln3+ (Ln = Er, Tm and Ho) ions codoped NaGdF4 submicrorods were selected for further biocompatible evaluation in vitro and biological imaging applications in vivo.
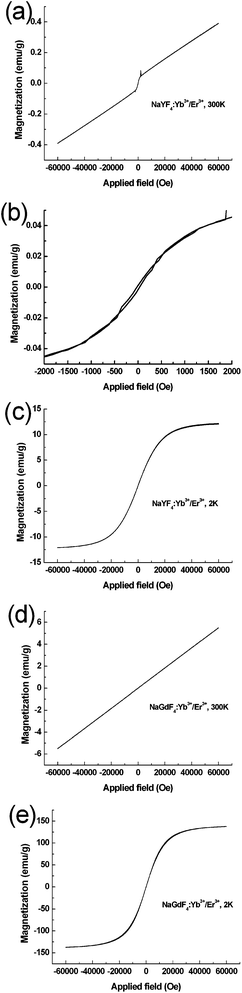 | ||
| Fig. 5 Magnetization curves of NaYF4:Yb3+/Tm3+ microrods measured at 300 K (a), local magnification image (b) and at 2 K (c), and NaGdF4:Yb3+/Er3+ submicrorods measured at 300 K (d) and 2 K (e), respectively. | ||
To investigate the biocompatibility of the UNs, GES-1 cells were employed to test the cytotoxicities of UNs (using NaGdF4:Yb3+/Er3+ submicrorods as example) after co-incubation with these UNs for 36 h. Mitochondrial function was evaluated spectrophotometrically by measuring the degree of mitochondrial reduction of the tetrazolium salt 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetra zolium bromide (MTT) to formazan by succinic dehydrogenase as described elsewhere. In vitro cells study, The NaGdF4:Yb3+/Er3+ submicrocrystals were dissolved in cell culture medium after sterilization and obtained different concentration of NaGdF4:Yb3+/Er3+ submicrocrystals (31.25, 62.5, 125, 250 and 500 μg mL−1). From Fig. 6, the NaGdF4:Yb3+/Er3+ show satisfactory biocompatibility, where the viability keeps in good state even at a concentration of 500 μg mL−1, which is much higher than the concentration usually used in cell labeling. Owing to the GES-1 cells keeping a good viability at 500 μg mL−1, we expect that the cells may keep good viability even at a higher concentration of 1000 μg mL−1. Also, this trend has been demonstrated by other researchers.1c–e
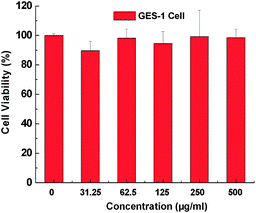 | ||
| Fig. 6 Cell viability of GES-1 cells incubated with different concentrations of NaGdF4:Yb3+/Er3+ submicrocrystals. The viability of cells remains over 85% or so at test dose. | ||
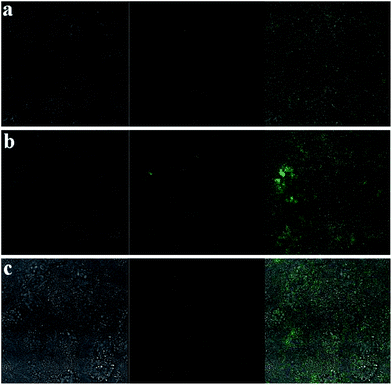
In order to confirm the influence of NaGdF4:Yb3+/Er3+ submicrocrystals on the GES-1 cells, the cells were observed by a laser confocal fluorescence microscope (FV 1000) after incubation with different concentrations of NaGdF4:Yb3+/Er3+ submicrocrystals after 2 days. The results for fluorescence imaging are shown in Fig. 7. From Fig. 7(a), we can see that the GES-1 cell cytoskeleton showed bright green fluorescence in dark field, and the cell nucleus showed blue fluorescence in dark field. Cells incubated with 20 μg mL−1 NaGdF4:Yb3+/Er3+ submicrocrystals also show evident fluorescence, as shown in Fig. 7(b). The results showed that the incubation of NaGdF4:Yb3+/Er3+ submicrocrystals has no influence on the cell viability and growth. Increasing the concentration of NaGdF4:Yb3+/Er3+ submicrocrystals from 20 to 50 (Fig. 7(c)) and 100 μg mL−1 (Fig. 7(d)), the morphology of the cell nucleus is well maintained and no reduction phenomenon is observed. The morphology of cytoskeleton and cell nucleus was also monitored in real time by a fluorescence microscopy (LEICA, DFC300 FX), as shown in the Fig. S12.† The results showed that the synthesized NaGdF4:Yb3+/Er3+ submicrocrystals have no evident toxicity for GES-1 cells, but have a slight inhibition for cells proliferation. As for our investigated GES-1 cells, the average size of cells is about 20–30 μm. So, the synthesized 1-D microcrystals are relatively easy to be endocytosed by cells, especially for small-sized NaGdF4 submicrorods (∼100 nm in diameter, 200–300 nm in length).
 | ||
| Fig. 7 Fluorescence imaging of GES-1 cells before and after incubation with different concentration of NaGdF4:Yb3+/Er3+ submicrocrystals after 2 days, control cells (a), with 20 μg mL−1 NaGdF4:Yb3+/Er3+ nanocrystals (b), with 50 μg mL−1 NaGdF4:Yb3+/Er3+ (c) and with 100 μg mL−1 NaGdF4:Yb3+/Er3+ (d). Note that: left column, fluorescent images of cell skeleton in green field; middle column, fluorescent images of cells nucleus in blue field; right column, overlay of left and middle columns. Cytoskeleton was staining by acetyl-2-tubulin, and cell nucleus was staining by DAP2. | ||
To assess the feasibility of synthesized 1-D submicrocrystals for upconversion microscopy imaging, we have investigated the influence of submicrocrystals concentrations on the upconversion microscopy imaging. Luminescent imaging of GES-1 cells after incubation with different concentration of upconversion submicrocrystals (NaGdF4:Yb3+/Tm3+ was selected as an example) after 1 day. The luminescent image is shown in Fig. 8. The results indicate that a few submicrocrystals will be endocytosed by the cells. As for the cell toxicity evaluation, we also studied the influence of surface charge of 1-D submicrocrystals on the cell viability. After NaGdF4:Yb3+/Tm3+ submicrocrystals were transferred into positively ligand (typically, 0.1 g UNs were dispersed in 20 mL deionized water, and then 100 μL 1,2-ethanediamine was gradually added. After mixing for 6 h, the obtained samples were washed by ethanol and deionized water three times, and freeze-dried for 24 h), the upconversion microscopy imaging has been shown in Fig. 9. The results indicate that much more positively ligand modified 1-D submicrocrystals will be internalized by the cells. Furthermore, the positive ligand 1-D submicrocrystals have some toxicity on GES-1 cells. Our MTT studies also show that the viability of cells remains less than 80% at a higher concentration of positively ligand 1-D submicrocrystals (Fig. 10).
 | ||
| Fig. 8 Upconversion luminescence imaging of GES-1 cells after incubation with different concentration of NaGdF4:Yb3+/Tm3+ submicrocrystals after 1 day (a) 20 μg mL−1, (b) 50 μg mL−1 and (c) 100 μg mL−1. Left image, bright field; middle image, luminescent image; right image, overlay image. | ||
 | ||
| Fig. 9 Upconversion luminescence imaging of GES-1 cells after incubation with different concentration of positively ligand NaGdF4:Yb3+/Tm3+ submicrocrystals after 1 day (a) 20 μg mL−1, (b) 50 μg mL−1 and (c) 100 μg mL−1. Left image, bright field; middle image, luminescent image; right image, overlay image. | ||
 | ||
| Fig. 10 Cell viability of GES-1 cells incubated with different concentrations of positively ligand NaGdF4:Yb3+/Tm3+ submicrocrystals. The viability of cells keeps less 80% at higher concentration. | ||
In order to demonstrate the feasibility of the synthesized upconversion submicrocrystals for in vivo computed tomography (CT) imaging, we performed tests on back normal tissue and tumor tissue of male nude mice by the direct injection of water-soluble NaGdF4:Yb3+/Er3+ submicrorods dispersed in PBS solution. UNs agents have excellent prolonged time during the whole blood circulation, which make them potential contrast agents for CT imaging.16 In the present study, we found that our proposed one-pot hydrothermal method is suitable for the large scale synthesis of 1-D rare-earth Yb3+ and Ln3+ ions doped NaGdF4 and NaYF4 microcrystals. Since the size of NaYF4 microrods is relatively big, so the small-sized NaGdF4 submicrorods were used for in vivo imaging. Our next work will be focused on the optimization of hydrothermal parameters and shortening the length and diameter of 1-D upconversion crystals. NaGdF4 submicrorods were water-soluble, they can be well dispersed in water (Fig. S13†) and could act as potential contrast agent for in vivo imaging.
Fig. 11(a) shows CT images of NaGdF4:Yb3+/Er3+ submicrorods at different concentrations (0.25–20 mg mL−1). It is clearly seen that the CT signal intensity increased with the increase of NaGdF4:Yb3+/Er3+ submicrorods concentrations. The injection sites of mice were shown in Fig. 11(b). For in vivo CT imaging, 100 μL (20 mg mL−1) NaGdF4:Yb3+/Er3+ were injected into the mice. After injection (Fig. 11(c)), the corresponding injection sites (tumor sites and back normal tissue) displayed a clearly distinguished CT signal, indicating the NaGdF4:Yb3+/Er3+ submicrocrystals could act as potential contrast agents for CT imaging. After 2 h, the attenuation values (HU) for tumor sites increases from 379 to 541 whereas the HU values for back normal sites decrease from 3402 to 3198 (Fig. 11(d)). After 4 h, the HU values for tumor sites and back normal tissues decrease significantly (Fig. 11(e)). The decrease of HU values indicates that partial NaGdF4:Yb3+/Er3+ submicrocrystals gradually penetrated into the adjacent soft tissues of mice body. In our previous work, we reported an OA–IL two phase system for the synthesis of ultra-small size Ln3+ doped NaGdF4 nanoparticles (∼5 nm), and found that the synthesized NaGdF4 nanoparticles are suitable for in vivo CT imaging.12a However, there exist two problems to be resolved. One is the synthetic scale, and another is to further improve the HU values in CT imaging. Therefore, in this work, we developed a hydrothermal method for the synthesis of 1-D upconversion submicrocrystals in order to resolve the above mentioned problems. When the 1-D NaGdF4:Yb3+/Er3+ were used as contrast agents for CT imaging, the intensity of CT signal was about six times greater than the NaGdF4 nanoparticles (∼5 nm). This means that 1-D NaGdF4:Yb3+/Er3+ submicrocrystals are more suitable for bioimaging.
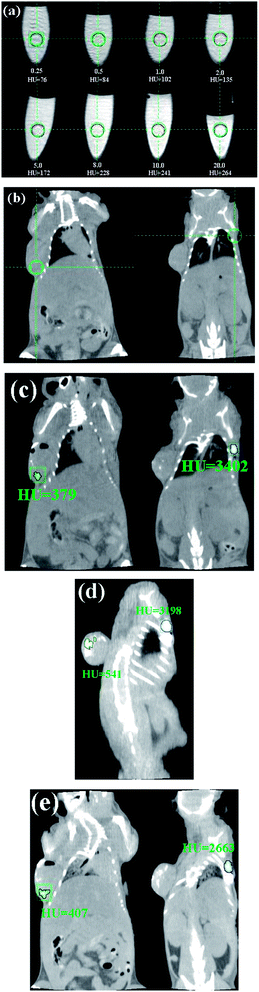 | ||
| Fig. 11 In vitro CT imaging of NaGdF4:Yb3+/Er3+ submicrocrystals suspended in PBS solution (a), in vivo CT imaging of mouse before injection (b), after injection (c), after 2 h injection (d) and after 4 h injection (e). As for the investigated nude mice, tumor sites are located in the left side of back, and normal tissues are located in the right side of back. Nude mice loaded with gastric cancer MGC803 cells was selected as the animal model. The tumor was implanted and grown in nude mice to an appropriate size (3–5 mm in diameter). | ||
Although the synthesized NaGdF4:Yb3+/Er3+ submicrocrystals were water-soluble, their particle sizes (∼100 nm in diameter, 200–300 nm in length) are still relatively bigger. In vitro CT tests, the NaGdF4:Yb3+/Er3+ submicrocrystals were first dispersed in solution, and then laid up for 30 min before it was used. We think that some bigger-sized NaGdF4:Yb3+/Er3+ particles were deposited on the bottom of V-shape tube. So, the 80 folds denser solution could only increase the HU to 264. In vivo, the possible reasons for the increase of the HU value in tumor site after 2 h injection may involve three reasons: (1) the position and pose of nude mice is some different with the after injection; (2) the calculated area for HU values between after injection and after 2 h injection has slight difference; (3) the agglomeration of NaGdF4:Yb3+/Er3+ particles in tumor site will also result in the increase of HU values. The injection site at back normal tissue showed an extremely high HU value (above 3000) including: (1) partial HU values are originated from the bone tissues. Generally, the HU value of bone tissues is about 1000; (2) at a certain position, the overlay of mice rib and chine will also contribute to the increase of total HU values; (3) after the injection of submicrocrystals, the agglomeration and deposition of particles will also increase the HU values.
We have carried out a time-dependent dynamic light scattering experiment (Fig. 12), and found the synthesized upconversion submicrocrystals are relatively stable in PBS solution. With the increase of time, the average size of submicrocrystals increases gradually. After 10 h, the agglomeration behavior of particles is evident. When the PBS solution containing upconversion submicrocrystals was left for 24 h, white sediments were found. So, when the upconversion submicrocrystals were applied for bioimaging or in vivo applications, controlling the test time within 6 h is necessary. In our next work, we will focus on decreasing the size of 1-D upconversion particles and improving their water-solubility.
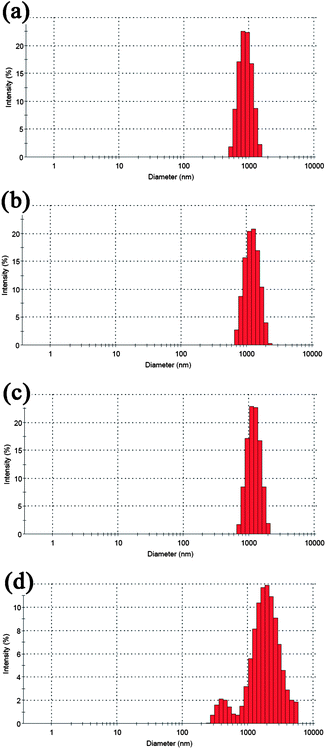 | ||
| Fig. 12 Particle sizes distribution of the synthesized upconversion submicrocrystals (NaGdF4:Yb3+/Er3+ was selected as an example) when dispersing in PBS buffer after different times (a) after 1 h, average size is 1016 nm, (b) after 2 h, average size is 1245 nm, (c) after 6 h, average size is 1406 nm and (d) after 10 h, average size is 1562 nm. | ||
4. Conclusions
In summary, a facile ethylenediaminetetraacetic acid (EDTA)-assisted hydrothermal method for controlled-synthesis of rare-earth Yb3+ and Ln3+ (Ln = Er, Tm and Ho) ions doped 1-D NaGdF4 submicrorods (∼100 nm in diameter, 200–300 nm in length) and porous NaYF4 microrods (∼1 μm in diameter, 3–5 μm in length) has been demonstrated. Formation mechanism of 1-D upconversion submicrocrystals is attributed to the synergic effect of EDTA assisted orientation-assembly and the Ostwald ripening process. Rare-earth Yb3+ and Ln3+ doped 1-D NaYF4 and NaGdF4 microcrystals exhibit paramagnetism behaviour at 300 K whereas NaYF4 microcrystals present a near superparamagnetic behaviour at the magnetic range of −2 to 2 kOe. Both 1-D UNs exhibit superparamagnetic behaviour at 2 K. Their saturation magnetizations were 12.1 and 129.2 emu g−1 for NaYF4 and NaGdF4 crystals, respectively. In addition, we have demonstrated that the synthesized 1-D UNs have good biocompatibility and strong CT signal intensity when they were applied for CT imaging in vivo. The high quality of 1-D UNs possessing upconversion luminescence, bio-affinity and magnetic properties showed great applications for colour displays, luminescent immunolabeling and imaging.Acknowledgements
We thank the financial support by National Natural Science Foundation of China (no. 81225010, 81101169, 31100717, 31170961), Chinese 973 Project (2010CB933901 and 2011CB933100), New Century Excellent Talent of Ministry of Education of China (NCET-08-0350), Shanghai Science and Technology Fund (1052nm04100), Important National Science & Technology Specific Projects (2009ZX10004-311). Authors are also grateful for the Instrumental Analytical Center of Shanghai Jiao Tong University.Notes and references
- (a) F. Wang and X. Liu, Chem. Soc. Rev., 2009, 38, 976 RSC; (b) C. Li and J. Lin, J. Mater. Chem., 2010, 20, 6831 RSC; (c) Z. L. Wang, J. Hao, H. L. Chan, G. L. Law, W. T. Wong, K. L. Wong, M. B. Murphy, T. Su, Z. H. Zhang and S. Q. Zeng, Nanoscale, 2011, 3, 2175 RSC; (d) S. Zeng, M. K. Tsang, C. F. Chan, K. L. Wong, B. Fei and J. Hao, Nanoscale, 2012, 4, 5118 RSC; (e) Z. L. Wang, J. Hao, H. L. W. Chan, W. T. Wong and K. L. Wong, Small, 2012, 8, 1863 CrossRef CAS; (f) J. Hao, Y. Zhang and X. Wei, Angew. Chem., Int. Ed., 2011, 50, 6876 CrossRef CAS; (g) M. L. Gou, K. Men, J. Zhang, Y. H. Li, J. Song, S. Luo, H. S. Shi, Y. J. Wen, G. Guo, M. J. Huang, X. Zhao, Zh. Y. Qian and Y. Q. Wei, ACS Nano, 2010, 4, 5573 CrossRef CAS; (h) X. M. Duan, P. Wang, Ke. Men, X. Gao, M. J. Huang, M. L. Gou, L. J. Chen, Z. Y. Qian and Y. Q. Wei, Nanoscale, 2012, 4, 2400 RSC; (i) M. L. Gou, K. Men, H. S. Shi, M. L. Xiang, J. Zhang, J. Song, J. L. Long, Y. Wan, F. Luo, X. Zhao and Z. Y. Qian, Nanoscale, 2011, 3, 1558 RSC.
- (a) F. Wang and X. Liu, J. Am. Chem. Soc., 2008, 130, 5642 CrossRef CAS; (b) F. Wang, L. D. Sun, J. Gu, Y. F. Wang, W. Feng, Y. Yang, J. F. Wang and C. H. Yan, Angew. Chem., Int. Ed., 2012, 51, 8796 CrossRef CAS.
- (a) S. Jiang, M. K. Gnanasammandhan and Y. Zhang, J. R. Soc. Interface, 2010, 7, 3 CrossRef CAS; (b) R. J. Palmer, J. L. Butenhoff and J. B. Stevens, Environ. Res., 1987, 43, 142 CrossRef CAS; (c) D. R. Larson, W. R. Zipfel, R. M. Williams, S. W. Clark, M. P. Bruchez, F. W. Wize and W. W. Webb, Science, 2003, 300, 1434 CrossRef CAS; (d) Z. Bao, Z. Sun, M. Xiao, H. Chen, L. Tian and J. Wang, J. Mater. Chem., 2011, 21, 11527 Search PubMed; (e) H. Bao, Z. H. Sun, Z. F. Li, L. W. Tian, T. Ngai and J. F. Wang, Langmuir, 2011, 27, 5071 CrossRef.
- (a) Q. Liu, Y. Sun, T. Yang, W. Feng, C. Li and F. Li, J. Am. Chem. Soc., 2011, 133, 17122 CrossRef CAS; (b) G. Chen, J. Shen, T. Y. Ohulchanskyy, N. J. Patel, A. Kutikov, Z. Li, J. Song, R. K. Pandey, H. Agren, P. N. Prasad and G. Han, ACS Nano, 2012, 6, 8280 CrossRef CAS.
- G. Wang, Q. Peng and Y. Li, Chem.–Eur. J., 2010, 16, 4923 CrossRef CAS.
- L. Wang, P. Li and Y. Li, Adv. Mater., 2007, 19, 3304 CrossRef CAS.
- (a) C. X. Li and J. Lin, J. Mater. Chem., 2010, 20, 6831 RSC; (b) C. Mi, Z. Tian, C. Cao, Z. Wang, C. Mao and S. Xu, Langmuir, 2011, 27, 14632 CrossRef CAS.
- Z. Li, S. Xiao, X. Yang, J. W. Ding, G. Y. Tan and X. H. Yan, Phys. B, 2012, 407, 2584 CrossRef CAS.
- G. Wang, W. Qin, D. Zhang, G. Wei, K. Zheng, L. Wang and F. Ding, J. Fluorine Chem., 2009, 130, 755 CrossRef CAS.
- X. S. Qu, H. W. Song, X. Bai, G. H. Pan, B. Dong, H. F. Zhao, F. Wang and R. F. Qin, Inorg. Chem., 2008, 47, 9654 CrossRef CAS.
- (a) L. Wang, Y. Zhang and Y. Zhu, Nano Res., 2010, 3, 317 CrossRef CAS; (b) G. Gao, P. Huang, Y. Zhang, K. Wang, W. Qin and D. Cui, CrystEngComm, 2011, 13, 1782 RSC; (c) F. Hu, L. Wei, Z. Zhou, Y. Ran, Z. Li and M. Gao, Adv. Mater., 2006, 18, 2553 CrossRef CAS.
- (a) M. He, P. Huang, C. Zhang, H. Hu, C. Bao, G. Gao, R. He and D. Cui, Adv. Funct. Mater., 2011, 21, 4470 CrossRef CAS; (b) M. He, P. Huang, C. Zhang, F. Chen, C. Wang, J. Ma, R. He and D. Cui, Chem. Commun., 2011, 47, 9510 RSC; (c) J. Kim, J. E. Lee, J. Lee, J. H. Yu, B. C. Kim, K. An, Y. Hwang, C.-H. Shin, J.-G. Park, J. Kim and T. Hyeon, J. Am. Chem. Soc., 2006, 128, 688 CrossRef CAS; (d) P. J. Kooyman, M. J. Verhoef and E. Prouzet, Stud. Surf. Sci. Catal., 2000, 129, 535 CrossRef CAS.
- (a) J. Shan, X. Qin, N. Yao and Y. Ju, Nanotechnology, 2007, 18, 445607 CrossRef; (b) H. Zhang, Y. Li, Y. Lin, Y. Huang and X. Duan, Nanoscale, 2011, 3, 963 RSC; (c) J. Fang, R. S. Pillai, M. Saunders, J. Zou, D. Lorenser, D. D. Sampson, Y. Guo, G. Lu and K. S. Iyer, Chem. Commun., 2011, 47, 10043 RSC.
- (a) S. Zeng, J. Xiao, Q. Yang and J. Hao, J. Mater. Chem., 2012, 22, 9870 RSC; (b) L. Wang, P. Li and Y. Li, Adv. Mater., 2007, 19, 3304 CrossRef CAS; (c) F. Wang and X. Liu, Chem. Soc. Rev., 2009, 38, 976 RSC.
- (a) J. P. Zhou, C. L. Chai, S. Y. Yang, Z. K. Liu, S. L. Song, Y. L. Li and N. F. Chen, J. Cryst. Growth, 2004, 270, 21 CrossRef CAS; (b) H. T. Wong, F. Vetrone, R. Naccache, H. L. W. Chan, J. Hao and J. A. Capobianco, J. Mater. Chem., 2011, 21, 16589 RSC; (c) L. M. Engelhardt and B. N. Figgis, J. Chem. Soc. A, 1970, 415 RSC.
- S. M. Janib, A. S. Moses and J. A. MacKay, Adv. Drug Delivery Rev., 2010, 62, 1052 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c2nr32850j |
| This journal is © The Royal Society of Chemistry 2013 |
