Synthesis of ultrafine layered double hydroxide (LDHs) nanoplates using a continuous-flow hydrothermal reactor†
Qiang
Wang
ab,
Selina Vi Yu
Tang
c,
Edward
Lester
cd and
Dermot
O'Hare
*b
aCollege of Environmental Science and Engineering, Beijing Forestry University, Qinghua East Road, Haidian District, Beijing 100083, China
bChemistry Research Laboratory, Department of Chemistry, University of Oxford, 12 Mansfield Road, Oxford, OX1 3TA, UK. E-mail: dermot.ohare@chem.ox.ac.uk; Tel: +44 (0)1865 272686
cDepartment of Chemical and Environmental Engineering, Faculty of Engineering, University of Nottingham, University Park, Nottingham NG7 2RD, UK
dPromethean Particles Ltd, 6 Faraday Building, Nottingham Science Park, University Boulevard, Nottingham, NG7 2QP, UK
First published on 30th October 2012
Abstract
We report a novel continuous-flow hydrothermal method for the synthesis of layered double hydroxide (LDH) nanoplates. The precursor solutions may be fed to the reactor so that the production of LDHs occurs in a continuous mode. By control of the synthesis temperature, pressure and contact time, the synthesis of LDH nanoplates can be tuned with constant and consistent product quality. This very general and simple approach shows high potential for commercial scale-up.
Layered double hydroxides (LDHs) are a class of synthetic anionic clays and have been widely used in many different fields including catalysts,1–4 ion exchange hosts,5–7 fire retardant additives,8,9 cement additives,10 polymer–LDH nanocomposites,11,12 CO2 capture,13–15 drug delivery,16 luminescent materials,17,18 and magnetic materials.19,20 LDHs are generally synthesised by the conventional co-precipitation method, which involves feeding a solution containing the cations and a solution containing the precipitating agent into a mechanically stirred reactor at either constant or variable pH. However, it has been widely accepted that the co-precipitation method possesses several major drawbacks. The first being a considerably long process for solution addition, making it difficult to control the particle size and distribution of LDHs. The nuclei formed at the very beginning of the addition procedure have a much longer period of time to undergo aging than those formed at the end of the solution adding process, during which crystal growth, agglomeration, breakage and Ostwald ripening may occur. Ultimately, the formation of nuclei and aging take place simultaneously during the prolonged solution adding process. The inevitable consequence is that very big LDH particles with a wide particle size distribution are commonly obtained. In order to avoid this problem, Duan et al.21 developed a novel synthesis method named separate nucleation and aging steps (SNAS), which involves a very rapid mixing and nucleation process in a colloid mill (rotating at 3000 rpm and mixing for 2 min), followed by a separate aging process (100 °C for 13 h). A further drawback of the co-precipitation method is that it is operated as a batch process. The discontinuous operation makes it more difficult to produce a constant product quality throughout the whole precipitation process, which is often an essential product requirement.22 In order to tackle this problem, Pérez-Ramírez and Abelló22 developed a novel in-line dispersion–precipitation (ILDP) method, which is accomplished by performing the precipitation in continuous mode using a vigorously stirred microreactor with in-line pH control at fixed residence time.
In the present work, we report a novel continuous-flow hydrothermal method for the synthesis of LDH nanoplates. The nucleation and aging time can be very short (e.g. 4 s), which prevents the growth of the LDH nanoplates. The synthesis pressure and temperature of the process can be controlled within a wide range, typically 0–240 bar and 0–400 °C respectively, which allows us to optimise the process parameters and in particular the crystallinity of the isolated LDH. Since the feeding of precursor solutions and the production of LDH are continuous and at steady-state, a constant product quality can be achieved.23–26 The key feature of this new method is that it possesses the advantages of both SNAS and ILDP technologies.
The continuous flow nozzle reactor used in this work has been previously described in detail, and an image of the reactor setup is shown in Fig. 1.23,27,28 For the synthesis of LDHs, the base aqueous solution was pumped (20 ml min−1) through the pre-heater before flowing into the inner tube (down-flow) of the nozzle reactor. Simultaneously, the metal precursor aqueous solution mixture was pumped (10 ml min−1) at ambient temperature into the reactor (up-flow), to meet the down-flow. The retention time for all synthesis was adjusted to ca. 4 s. A matrix of samples was created by employing four different reaction temperatures, and two different system pressures; the pre-heater was set at 75, 150, 250 or 400 °C while the system pressure was maintained at 50 or 240 bar by the back pressure regulator. After the reaction point, the products were immediately cooled using cooling water.
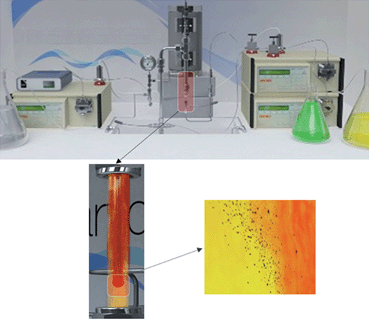 | ||
| Fig. 1 Animated images depicting the continuous hydrothermal rig. The reactor section is highlighted, showing the heated downflow (red) mixing with the ambient upflow (yellow), leading to nanoplate formation. | ||
The formation of LDHs synthesised using the continuous-flow hydrothermal reactor was first characterized by X-ray diffraction (XRD) analysis, as shown in Fig. 2. The characteristic Bragg reflections of both [Ca2Al(OH)6](NO3) (Ca2Al–NO3) and [Mg3Al1(OH)8](CO3)0.5 (Mg3Al–CO3) LDHs were clearly seen; the positions of (00l) Bragg reflections correspond to the basal spacing reported for the nitrate and carbonate phases respectively.29–31 Mg3Al–CO3 has R3m rhombohedral space group symmetry with lattice parameters of a = 3.057 Å and c = 23.502 Å, and Ca2Al–NO3 has the rhombohedral space group R![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) c with lattice parameters of a = 5.731 Å and c = 48.32 Å. The increase in synthesis temperature from 75 to 250 °C and pressure from 50 to 240 bar seems to have little effect on the XRD patterns for Mg3Al–CO3. The full width at half maximum (FWHM) of the 003 Bragg reflections vary between 0.7 and 0.9° which equates to a Scherrer crystallinity of 10 nm (±1 nm). For Ca2Al–NO3 the FWHM varies from 0.13 to 0.32° (Table S1†), here the crystallinity is high but more sensitive, with crystallite size ranging from 25–62 nm (Table S1†). The data show that LDHs can be synthesised over a very wide range of temperatures and pressures. However, when the temperature was raised to 400 °C, some reflections are observed due to impurity phases. We believe this is due to the dissociation/decomposition of LDHs and the formation of AlOOH or Al(OH)3.32,33 The XRD result suggests that this continuous-flow hydrothermal synthesis method could be a very general and highly efficient method for the continuous production of LDHs.
c with lattice parameters of a = 5.731 Å and c = 48.32 Å. The increase in synthesis temperature from 75 to 250 °C and pressure from 50 to 240 bar seems to have little effect on the XRD patterns for Mg3Al–CO3. The full width at half maximum (FWHM) of the 003 Bragg reflections vary between 0.7 and 0.9° which equates to a Scherrer crystallinity of 10 nm (±1 nm). For Ca2Al–NO3 the FWHM varies from 0.13 to 0.32° (Table S1†), here the crystallinity is high but more sensitive, with crystallite size ranging from 25–62 nm (Table S1†). The data show that LDHs can be synthesised over a very wide range of temperatures and pressures. However, when the temperature was raised to 400 °C, some reflections are observed due to impurity phases. We believe this is due to the dissociation/decomposition of LDHs and the formation of AlOOH or Al(OH)3.32,33 The XRD result suggests that this continuous-flow hydrothermal synthesis method could be a very general and highly efficient method for the continuous production of LDHs.
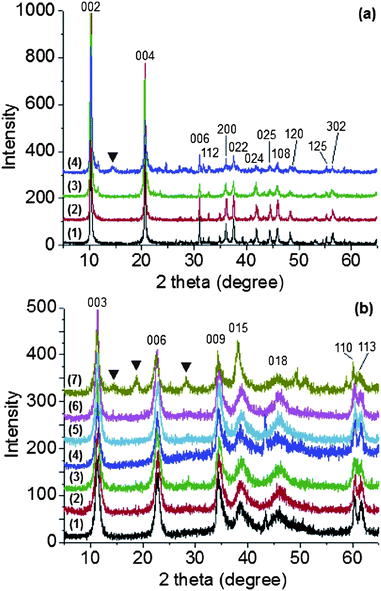 | ||
| Fig. 2 (a) XRD patterns of Ca2Al–NO3 LDHs synthesised at various pressures and temperatures: (1) 50 bar, 75 °C, (2) 240 bar, 75 °C, (3) 240 bar, 150 °C, and (4) 240 bar, 400 °C. (b) XRD patterns of Mg3Al–CO3 LDHs synthesised at various pressures and temperatures: (1) 50 bar, 75 °C, (2) 50 bar, 150 °C, (3) 50 bar, 250 °C, (4) 240 bar, 75 °C, (5) 240 bar, 150 °C, (6) 240 bar, 250 °C and (7) 240 bar, 400 °C. (▼) AlOOH or Al(OH)3 impurity. | ||
The particle size and morphology of synthesised LDHs were examined using transmission electron microscopy (TEM) analysis. Fig. 3(a) and (b) show the TEM images of Ca2Al–NO3 synthesised at 50 bar and 75 °C, from which a typical nanoplate-like morphology was clearly observed. Although some hexagonal shaped nanoplates can be seen, most of the particles have irregular shapes. Most dramatic is the observation that the edges of all these nanoplates are surrounded by primary nanoparticles. This strongly suggests that the nanoplates were still growing due to the very short contact time (nucleation and aging time) and this growing process was suddenly quenched by the rapid cooling by cold water. We believe this is the first time that growing LDHs nanoparticles (primary seeds) are observed by TEM analysis, which is a very important observation for our understanding of the intrinsic formation mechanism of LDHs. Furthermore, the isolated Ca2Al–NO3 nanoplates are so thin that they can be easily imaged through several layers.
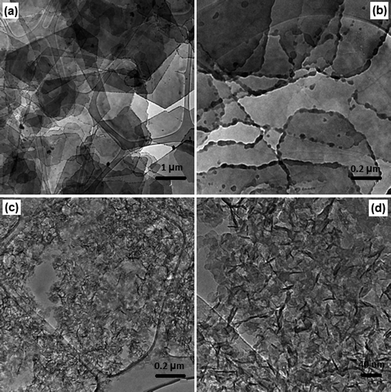 | ||
| Fig. 3 (a) and (b) TEM images of Ca2Al–NO3 LDH synthesised at 50 bar and 75 °C, (c) and (d) TEM images of Mg3Al–CO3 LDH synthesised at 50 bar and 75 °C. | ||
The very thin aspect of these particles was further confirmed by AFM analysis as shown in Fig. 4(a); the thickness of these nanoplates was measured to be around 10 nm. Fig. 3(c) and (d) show the TEM images of Mg3Al–CO3 synthesised at the same condition (50 bar and 75 °C). Unlike Ca2Al–NO3, much smaller nanoplatelets with a lateral width dimension of ca. 30–40 nm were observed. Some vertically aligned particles can also been seen and it indicates that the thickness of nanoplatelets is ca. 4–5 nm, which is consistent with the AFM analysis in Fig. 4(b). Due to their very high aspect ratio, LDHs synthesised using the novel continuous-flow hydrothermal method is promising in many important applications such as drug delivery,34 and polymer–LDH nanocomposites.35,36
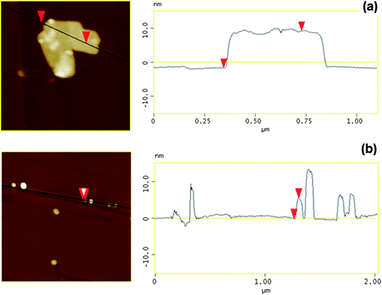 | ||
| Fig. 4 AFM images of (a) Ca2Al–NO3 and (b) Mg3Al–CO3 LDHs synthesised at 50 bar and 75 °C. | ||
The influence of synthesis temperature and pressure on the LDH morphology and particle size were further investigated using TEM analysis. Fig. 5 shows the TEM images of Ca2Al–NO3 and Mg3Al–CO3 LDHs synthesised at same pressure (50 bar) but different temperatures (150 or 250 °C).
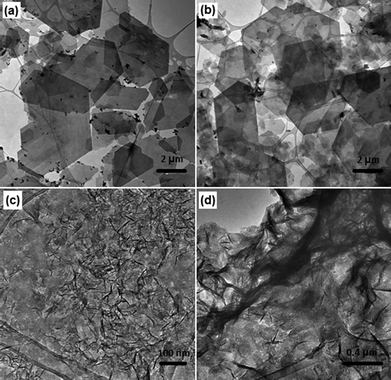 | ||
| Fig. 5 TEM images of (a) Ca2Al–NO3 (50 bar, 150 °C), (b) Ca2Al–NO3 (50 bar, 250 °C), (c) Mg3Al–CO3 (50 bar, 150 °C), and (d) Mg3Al–CO3 (50 bar, 250 °C) LDHs. | ||
Compared with Fig. 3, significant differences were observed with both Ca2Al–NO3 and Mg3Al–CO3 LDHs by increasing the temperature from 75 to 150 °C. For Ca2Al–NO3, the nanoplates became more regular and no primary nanoparticles can be seen on the edges. In contrast, some other-type impurity particles start to appear on the surface of nanoplates. TEM images in Fig. S3† show that shuttle-like particles with a width of ca. 50 nm and a length of ca. 200 nm were formed. According to the literature, these impurity particles might be the primary precursor for AlOOH.32,33 Since it can not be detected by XRD analysis, probably because the crystallinity degree is very low or the amount is too low to be detected. At 150 °C, the lateral width of Mg3Al–CO3 nanoplatelets became much bigger, ca. 50–70 nm. These results indicate that high temperature favours the growth of LDHs, particularly along the 00l direction. However, when the synthesis temperature is too high (e.g. 250 °C), Ca2Al–NO3 LDH starts to dissociate and decompose into small pieces (Fig. 5(b)), while Mg3Al–CO3 LDH continues to grow to very long nanowires (Fig. 5(d)). At this condition, the control of particle size and shape becomes difficult. Particularly when the synthesis temperature reached 400 °C, a big portion of AlOOH/Al(OH)3 impurities can be clearly found in Ca2Al–NO3 LDH sample, see Fig. S4.† Therefore, to obtain a fairly pure LDH with controllable particle size and morphology, a lower synthesis temperature is preferred.
Fig. S5† shows the TEM image of Ca2Al–NO3 and Mg3Al–CO3 synthesised at 240 bar and 75 °C. Compared with the images in Fig. 3, some differences were also observed by increasing synthesis pressure from 50 to 240 bar. For Ca2Al–NO3, the crystallinity is higher at this pressure and so no primary nanoparticles were found on the edges of the nanoplates. For Mg3Al–CO3, nanoplatelets with a lateral width dimension of 40–50 nm were obtained. The morphology is similar to the sample synthesised at 50 bar, but particle size is slightly bigger. The particle size distribution of Ca2Al–NO3 and Mg3Al–CO3 LDHs synthesized at same pressure (50 bar) but different temperatures (75 and 150 °C) were shown in Fig. S1 and S2.† These results suggest that higher pressure also favours the formation of LDHs, but its influence is weaker than that of temperature.
The Ca/Al and Mg/Al ratios of synthesised LDHs were examined using SEM-EDX analysis, as summarized in Table S2.† For Ca2Al–NO3, the Ca/Al ratios for all samples are around 1.6–1.8, which is close to the theoretical value of 2. With the increase of temperature from 75 to 150 °C, Ca/Al ratio slightly increased from 1.6 to 1.8. However, the Ca/Al ratio then starts to decrease with a further increase of temperature to 250 and 400 °C. At 400 °C, the Ca/Al ratio falls to ca. 1.06, which can be explained by the formation of AlOOH/Al(OH)3. This result is consistent with the XRD and TEM analysis that the Ca2Al–NO3 LDH has higher crystallinity at 150 °C but begins to dissociate and decompose into AlOOH/Al(OH)3 impurities at higher temperatures. For Mg3Al–CO3, the Mg/Al ratios are all close to the theoretical value of 3. The Mg/Al ratio also first increased and then decreased. However, Mg3Al–CO3 seems to have a higher thermal stability than Ca2Al–NO3 because the Mg/Al starts to decrease at a higher temperature of 250 °C.
In conclusion, Ca2Al–NO3 and Mg3Al–CO3 LDHs has been successfully synthesised using a novel continuous-flow hydrothermal method. By varying the synthesis temperature and pressure, ultra-fine LDH nanoplates can be obtained, and the particle size and shape can be controlled. Temperature appeared to have a more significant influence on the final products than pressure. In order to prevent the formation of the AlOOH/Al(OH)3 impurities, a relatively low temperature is preferred. With this novel synthesis method, since the feeding of precursor solutions and the production of LDHs are in a continuous mode, and the synthesis temperature, pressure and contact time can be tuned, a controllable synthesis of LDHs with constant product quality is guaranteed. We also proved that it is a very rapid and simple approach with high potential for commercial application.
Acknowledgements
Q. Wang would like to thank the Fundamental Research Funds for the Central Universities for funding (YX2012-1). Q. Wang and D. O'Hare thank SCG Chemicals, Thailand for financial support. S. Tang and E. Lester thank the EPSRC for financial support.Notes and references
- Q. Wang and D. O'Hare, Chem. Rev., 2012, 112, 4124 CrossRef CAS.
- Y. Zhao, M. Wei, J. Lu, Z. L. Wang and X. Duan, ACS Nano, 2009, 3, 4009 CrossRef CAS.
- J. L. Gunjakar, T. W. Kim, H. N. Kim, I. Y. Kim and S. J. Hwang, J. Am. Chem. Soc., 2011, 133, 14998 CAS.
- C. G. Silva, Y. Bouizi, V. Fornes and H. Garcia, J. Am. Chem. Soc., 2009, 131, 13833 CrossRef.
- F. Millange, R. I. Walton, L. Lei and D. O'Hare, Chem. Mater., 2000, 12, 1990 CrossRef CAS.
- I. C. Chisem and W. Jones, J. Mater. Chem., 1994, 4, 1737 RSC.
- A. M. Fogg, J. S. Dunn, S. G. Shyu, D. R. Cary and D. O'Hare, Chem. Mater., 1998, 10, 351 CrossRef CAS.
- C. Nyambo, P. Songtipya, E. Manias, M. M. Jimenez-Gasco and C. A. Wilkie, J. Mater. Chem., 2008, 18, 4827 RSC.
- C. Manzi-Nshuti, J. M. Hossenlopp and C. A. Wilkie, Polym. Degrad. Stab., 2009, 94, 782 Search PubMed.
- J. Plank, D. Zhimin, H. Keller, F. v. Hössle and W. Seidl, Cem. Concr. Res., 2010, 40, 45 Search PubMed.
- F. Leroux and J. P. Besse, Chem. Mater., 2001, 13, 3507 CrossRef CAS.
- Q. Wang, X. Zhang, J. Zhu, Z. Guo and D. O'Hare, Chem. Commun., 2012, 48, 7450 RSC.
- Q. Wang, J. Luo, Z. Zhong and A. Borgna, Energy Environ. Sci., 2011, 4, 42 RSC.
- Q. Wang, H. H. Tay, D. J. W. Ng, L. Chen, Y. Liu, J. Chang, Z. Zhong, J. Luo and A. Borgna, ChemSusChem, 2010, 3, 965 CrossRef CAS.
- Q. Wang, H. H. Tay, Z. Zhong, J. Luo and A. Borgna, Energy Environ. Sci., 2012, 5, 7526 RSC.
- A. C. S. Alcantara, P. Aranda, M. Darder and E. Ruiz-Hitzky, J. Mater. Chem., 2010, 20, 9495 RSC.
- D. P. Yan, J. Lu, J. Ma, S. H. Qin, M. Wei, D. G. Evans and X. Duan, Angew. Chem., Int. Ed., 2011, 50, 7037 CrossRef CAS.
- D. P. Yan, J. Lu, J. Ma, M. Wei, D. G. Evans and X. Duan, Angew. Chem., Int. Ed., 2011, 50, 720 CrossRef CAS.
- E. Coronado, C. Marti-Gastaldo, E. Navarro-Moratalla, A. Ribera, S. J. Blundell and P. J. Baker, Nat. Chem., 2010, 2, 1031 CrossRef CAS.
- E. Coronado, J. R. Galan-Mascaros, C. Marti-Gastaldo and A. Ribera, Chem. Mater., 2006, 18, 6112 CrossRef CAS.
- Y. Zhao, F. Li, R. Zhang, D. G. Evans and X. Duan, Chem. Mater., 2002, 14, 4286 CrossRef CAS.
- S. Abelló and J. Pérez-Ramírez, Adv. Mater., 2006, 18, 2436 CrossRef CAS.
- E. Lester, P. Blood, J. Denyer, D. Giddings, B. Azzopardi and M. Poliakoff, J. Supercrit. Fluids, 2006, 37, 209 CrossRef CAS.
- S. A. Morin, M. J. Bierman, J. Tong and S. Jin, Science, 2010, 328, 476 CrossRef CAS.
- S. Jin, M. J. Bierman and S. A. Morin, J. Phys. Chem. Lett., 2010, 1, 1472 Search PubMed.
- F. Meng, S. A. Morin and S. Jin, J. Am. Chem. Soc., 2011, 133, 8408 CrossRef CAS.
- H. Hobbs, S. Briddon and E. Lester, Green Chem., 2009, 11, 484 RSC.
- E. Lester, G. Aksomaityte, J. Li, S. Gomez, J. Gonzalez-Gonzalez and M. Poliakoff, Prog. Cryst. Growth Charact. Mater., 2012, 58, 3 CrossRef CAS.
- X. Gao, L. Lei, C. Lv, Y. Sun, H. Zheng and Y. Cui, J. Solid State Chem., 2008, 181, 1776 CrossRef CAS.
- Q. Wang, Z. Wu, H. H. Tay, L. Chen, Y. Liu, J. Chang, Z. Zhong, J. Luo and A. Borgna, Catal. Today, 2011, 164, 198 CrossRef CAS.
- Q. Wang, H. H. Tay, Z. Guo, L. Chen, Y. Liu, J. Chang, Z. Zhong, J. Luo and A. Borgna, Appl. Clay Sci., 2012, 55, 18 CrossRef CAS.
- W. Cai, J. Yu and S. Mann, Microporous Mesoporous Mater., 2009, 122, 42 CrossRef CAS.
- W. Kim and F. Saito, Powder Technol., 2000, 113, 109 Search PubMed.
- Z. An, S. Lu, J. He and Y. Wang, Langmuir, 2009, 25, 10704 CrossRef CAS.
- F. Leroux and C. T. Gueho, J. Mater. Chem., 2005, 15, 3628 RSC.
- C. Taviot-Gueho and F. Leroux, Struct. Bonding, 2006, 119, 121 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental details and more characterisations. See DOI: 10.1039/c2nr32568c |
| This journal is © The Royal Society of Chemistry 2013 |
