Mussel-inspired functionalization of graphene for synthesizing Ag-polydopamine-graphene nanosheets as antibacterial materials†
Zhe
Zhang
,
Jing
Zhang
,
Bailin
Zhang
and
Jilin
Tang
*
State Key Laboratory of Electroanalytical Chemistry, Changchun Institute of Applied Chemistry, Chinese Academy of Sciences, Changchun 130022, P. R. China. E-mail: jltang@ciac.jl.cn; Fax: +86 431-85262734; Tel: +86 431-85262734
First published on 23rd October 2012
Abstract
Mussels have been shown to attach to virtually all types of inorganic and organic surfaces via their adhesive proteins. The adhesive proteins secreted by mussels contain high concentrations of catechol and amine functional groups, which have similar functional groups with polydopamine (PDA). Inspired by mussels, a mild and environmentally friendly method was used to synthesize Ag nanoparticles (Ag NPs) on functionalized PDA-graphene nanosheets (PDA-GNS) with uniform and high dispersion. First, a uniform layer of PDA was coated on graphene oxide (GO) by polymerizing dopamine (DA) at room temperature. During the process GO was reduced by the DA. The PDA layer on the surface of GNS can be used as a nanoscale guide to form uniform Ag NPs on the surface of PDA-GNS. The obtained Ag-PDA-GNS hybrid materials are characterized by atomic force microscopy, transmission electron microscopy, UV–vis spectroscopy, Raman spectroscopy, X-ray photo-electron spectroscopy, X-ray diffraction, and thermal gravimetric analysis. The resultant Ag-PDA-GNS hybrid materials exhibited strong antibacterial properties to both Gram-negative and Gram-positive bacteria due to the synergistic effect of GNS and Ag NPs.
1. Introduction
Ag nanoparticles (Ag NPs) have been extensively used as antibacterial agents in recent years.1,2 The bactericidal mechanism of Ag NPs has been well studied and understood. It has been shown that Ag ions released from Ag NPs can inactivate the cells of a microorganism by destroying its cell membrane and DNA replication ability.3,4 In addition, Ag NPs themselves can directly incorporate into the cell membrane and subsequently form permeable pits, leading to an osmotic collapse and a release of intracellular materials.5,6 However, the practical applications are often limited by the problems of the ease of oxidization and aggregation of Ag NPs, which are important parameters for determining the antibacterial activity of Ag NPs. To resolve these problems, different materials, including silicon nanowires,7 multi-walled carbon nanotubes,8 ZnO NPs,9 and carbonaceous nanospheres,10 have been employed to enhance the stability and antibacterial activity of Ag NPs.Graphene nanosheets (GNS) are atomic layers of carbon atoms with sp2-bound carbon atoms, arranged in a honeycomb lattice. Since the experimental discovery of single layers by Geim in 2004,11 GNS has drawn significant attention because of its fascinating electrical,12 thermal,13 mechanical,14,15 and optical16 properties and potential applications in energy storage,17 transparent electrodes,18 and chemical sensors.19 Until now, the combination of GNS and various NPs as a new kind of hybrid materials has aroused extensive interest due to the synergistic enhancement in the electronic, catalytic, and optical properties.20,21 Nevertheless, in most of the reported methods, high temperatures and highly toxic reductants are required. Recently, our group has fabricated Ag-graphene composite nanosheets (AGCN) using poly(N-vinyl-2-pyrrolidone) as reductant and stabilizer at 60 °C. The obtained AGCN substrate is extremely suitable for surface enhanced Raman spectroscopy.22
On the other hand, materials in nature inspire us to exploit unique, effective strategies to fabricate functional materials with intricate structures and properties. Indeed, many features of living beings, such as the brick-and-mortar structure of nacre,23–26 the adhesion ability of the gecko,27 the self-cleaning lotus leaf,28 the antifogging eyes of a mosquito,29 and the color of butterfly wings,30etc. have been studied for developing smart materials and functional devices. Recently, the Messersmith group identified 3,4-dihydroxy-L-phenylalanine (DOPA) and lysine peptides in Mytilus edulis foot protein 5 (Mefp-5) of mussel's adhesive threads as the origins of the extraordinarily strong adhesion.31 Dopamine (DA), a biomolecule that contains catechol and amine functional groups, can self-polymerize at alkaline pH values and spontaneously deposit polydopamine (PDA) conformal films on virtually any surface, and has similar properties to the adhesive proteins secreted by mussels. Inspired by the versatile adhesion capability of PDA, here we report a mild method to prepare hybrid materials of Ag-PDA-GNS using DA as reductant at room temperature.
2. Experimental
2.1. Chemicals
Graphite was purchased from Alfa Aesar (325 mesh). 3-Hydroxytyramine hydrochloride was obtained from Acros Organics. Silver nitrate was purchased from the Beijing Chemical Reagent Factory. Other reagents were of analytical grade and were used as received without further purification. All aqueous solutions were prepared with Milli-Q water (18.2 MΩ cm).2.2. Apparatus
Atomic force microscopic (AFM) images were recorded by using an SPI3800N microscope (Seiko Instruments, Inc. Japan) operating in the tapping mode with standard silicon tips (NANOSENSORS, Switzerland) at room temperature under ambient conditions. The samples were drop-cast on freshly cleaved mica and dried at room temperature. Infrared spectra were collected on a VERTEX 70 Fourier transform infrared spectrometer (Bruker, Germany) over a range from 400 to 4000 cm−1. UV–vis detection was carried out on a Cary 500 UV–vis–NIR spectrophotometer (Varian, U.S.A.). Raman spectra were acquired on a Renishaw (Renishaw, United Kingdom) 2000 model confocal microscopy Raman spectrometer with 514.5 nm wavelength incident laser light. X-Ray photoelectron spectroscopy (XPS) measurement was performed on an ESCALAB-MKII spectrometer (VG Co., United Kingdom) with Al Kα X-ray radiation as the X-ray source for excitation. The sample for XPS characterization was deposited onto an Si slide. X-Ray diffraction spectra were obtained using a D8 ADVANCE (Germany) using Cu Kα (1.5406 Å) radiation. Transmission electron microscopy (TEM) and high-resolution TEM (HRTEM) images were obtained with a TECNAI G2 high-resolution transmission electron microscope (Holland) with an accelerating voltage of 200 kV and Hitachi H600 electron microscope (Japan) with an accelerating voltage of 100 kV. The sample for TEM characterization was prepared by placing a drop of prepared solution on carbon-coated copper grid and drying at room temperature. Thermogravimetric analysis (TGA) of sample was performed on a Pyris Diamond TG/DTA thermogravimetric analyzer (Perkin–Elmer Thermal Analysis). The sample was heated under an air atmosphere from room temperature to 915 °C at 10 °C min−1.2.3. Preparation of graphene oxide
The graphite oxide was synthesized from graphite according to the literature.20 Then, exfoliation of graphite oxide into graphene oxide (GO) was achieved by sonication of the dispersion for 60 min (80 W, 90% amplitude). The obtained brown dispersion was then subjected to centrifugation at 3000 rpm for 30 min with a rotor radius of 8 cm in order to remove any unexfoliated graphite oxide. Finally, a homogeneous aqueous GO (about 0.5 mg mL−1) was obtained.2.4. Synthesis of PDA-GNS
In a typical preparation, 12 mL of GO dispersion (about 0.5 mg mL−1) was added into 30 mL of Tris-buffer (10 mM, pH 8.5) under magnetic stirring. Then 6 mg DA was added in and the mixture was kept stirring at room temperature for 24 h. The obtained PDA-GNS composite was collected by centrifugation at 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm for 30 min and dissolved in 6 mL of Milli-Q water.
000 rpm for 30 min and dissolved in 6 mL of Milli-Q water.
2.5. Synthesis of Ag-PDA-GNS
In a typical procedure for synthesis of Ag-PDA-GNS, the resulting homogeneous dispersion of PDA-GNS was mixed with 20 mL of Milli-Q water and 50 μL of ammonia solution (28 wt% in water) under magnetic stirring. Subsequently, 50 mg of AgNO3 dissolved in 1 mL of Milli-Q water was added to the dispersion with stirring for 1 h. Then, 1 mL of DA aqueous solution (2 mg mL−1) was added to the above dispersion and stirred for 30 min to ensure reduction of AgNO3 to Ag NPs. Three exemplary composite nanosheets were synthesized at different PDA-GNS/AgNO3 ratios, denoted as Ag-PDA-GNS-X, where X corresponds to the amount of AgNO3 in milligrams. The product was centrifuged at 5000 rpm for 20 min and washed with Milli-Q water. The obtained Ag-PDA-GNS was redispersed into 5 mL Milli-Q water for further use.2.6. Bacterial culture
E. coli and B. subtilis were selected as model Gram-negative and Gram-positive bacteria, respectively. Bacteria cells were grown overnight in Luria–Bertani (LB) medium (Luria–Bertani broth, Sangon) at 37 °C and then harvested at the exponential growth phase.2.7. LB–agar plates
Ag-PDA-GNS composite nanosheets dispersion was mixed with molten LB–agar at final concentrations of 1% (v/v). E. coli and B. subtilis suspensions were then spread onto solidified LB agar plates. Finally, the plates were observed after 24 hours incubated at 37 °C.2.8. Bacterial kinetic test
For the kinetic test, 1% (v/v) the active substances of Ag-PDA-GNS was prepared in each sterilized flask and inoculated with an equivalent volume of E. coli or B. subtils suspension. The initial time of adding Ag-PDA-GNS to LB bacteria suspension was taken as zero, and 0.5 mL aliquots were withdrawn from the flasks at set time intervals. Immediately, these aliquots were maintained at 4 °C to inhibit further bacterial cell division. After 48 hours, all of the solution was diluted to an appropriate concentration, and the OD600 was determined to count thebacterial colonies.3. Results and discussion
The overall synthesis procedure of Ag-PDA-GNS is shown in Fig. 1. First, the PDA coating was obtained by polymerizing DA on GO in 10 mM Tris-buffer (pH 8.5) at room temperature. During the process GO was reduced by the DA.32 | ||
| Fig. 1 Illustration of the procedure for preparing Ag-PDA-GNS. | ||
Second, the PDA layer on the surface of GNS can be used as a nanoscale guide to form uniform Ag NPs on the surface of PDA-GNS with additional added DA as reducing agent. The surface morphology of Ag-PDA-GNS was first examined by AFM. Fig. 2a shows a typical AFM image of GO, where the cross-sectional view indicates that the average thickness of nanosheets is about 0.9 nm. Compared to the smooth and flat surface of GO nanosheets, densely packed PDA is clearly observed across the surface of the GNS with a thickness of 3.8 nm (Fig. 2b). After loading Ag NPs, the thickness of Ag-PDA-GNS increases to about 15 nm (Fig. 2c), which suggests that the Ag NPs are successfully adsorbed on the surface of the PDA-GNS. The formation of Ag-PDA-GNS was further characterized by XRD (Fig. 2d). Compared with the pristine graphite (Fig. 2d, trace 1) and graphite oxide (Fig. 2d, trace 2), the XRD pattern of Ag-PDA-GNS (Fig. 2d, trace 3) shows distinct diffraction peaks from the reflections of the face-centered cubic (fcc) structure of the metal Ag, revealing the crystalline nature of the Ag NPs.
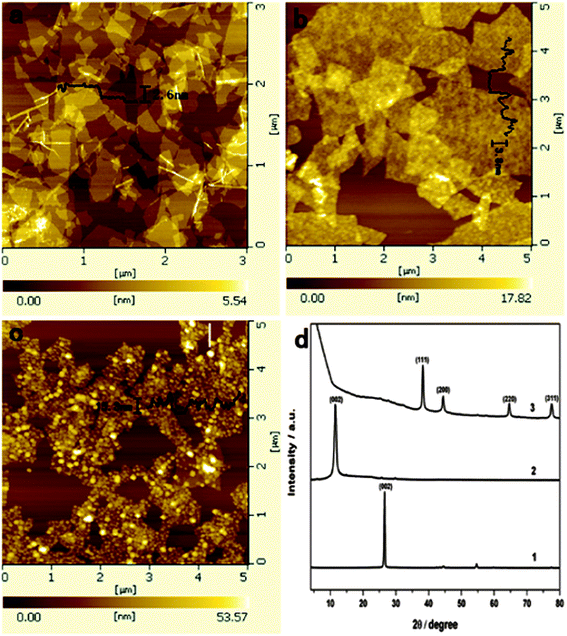 | ||
| Fig. 2 AFM images of GO (a), PDA-GNS (b), and Ag-PDA-GNS-50 (c). XRD pattern (d) of pristine graphite (1), graphite oxide (2), and Ag-PDA-GNS-50 (3). | ||
To further validate the formation of Ag NPs and map their distribution on the surface of PDA-GNS, TEM was used to observe directly the morphologies of the Ag-PDA-GNS hybrid materials. As shown in the TEM images of Ag-PDA-GNS at different magnification (Fig. 3a and b), small Ag NPs are uniformly and densely distributed on the surface of PDA-GNS. The edge of PDA-GNS and the nanostructure of the Ag NPs can be clearly observed in the higher magnification image of Fig. 3b. An HRTEM image (Fig. 3c) of the Ag NPs shows that the interplanar spacing of the particle lattice is 0.23 nm, which agrees well with the (111) lattice spacing of fcc Ag. The corresponding energy-dispersive X-ray spectroscopy (Fig. 3d) of Ag-PDA-GNS shows the peaks corresponding to C, N, and Ag elements, which further confirm the existence of Ag NPs on the surface of PDA-GNS. More importantly, the density of the Ag NPs on the surface of PDA-GNS can be easily changed by changing the weight ratio between AgNO3 and PDA-GNS (Fig. S4†). TGA of Ag-PDA-GNS, as shown in Fig. S10,† further illustrates that the Ag NPs content increases from 77.79% to 84.49% with increasing the weight ratio between AgNO3 and PDA-GNS. In addition, we also tried to use the PDA layer on the surface of GNS as a reducing agent.31,33,34 However, the resultant product is disappointing. As shown in Fig. S5,† Ag NPs on the surface of PDA-GNS have a low loading density and a wide particle size distribution.
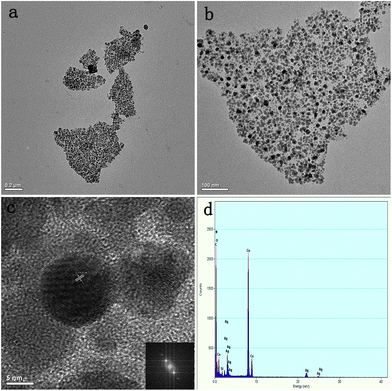 | ||
| Fig. 3 TEM images of Ag-PDA-GNS-50 (a and b) at different magnifications. HRTEM image and corresponding FFT analysis (inset) of Ag NPs (c). EDX pattern of Ag-PDA-GNS-50 (d). | ||
Fig.4a shows the UV–vis absorption spectra of aqueous dispersion of GO and the resulting Ag-PDA-GNS. As shown in Fig. 4a (trace 1), GO displays a maximum absorption peak centered at 230 nm and a shoulder peak at about 300 nm. After loading Ag NPs on the surface of PDA-GNS, there is a clear peak at about 436 nm (Fig. 4a, trace 2). This is characteristic of Ag NPs due to surface plasmon absorption, which implies the formation of Ag-PDA-GNS. Raman spectroscopy as a powerful nondestructive technique has been widely used to distinguish ordered and disordered crystal structures of carbon. As shown in Fig. 4b, compared to the Raman spectrum of graphite, the G band of graphite oxide is broadened and blue shifts to 1602 cm−1 due to the presence of isolated double bonds that resonate at higher frequencies. In addition, the D band at 1350 cm−1 becomes prominent, indicating the reduction in size of the in-plane sp2 domains due to extensive oxidation.35 After reduction by DA, it is found that PDA-GNS and Ag-PDA-GNS have a decreased D/G intensity ratio compared to graphite oxide. This change suggests an increase in the average size of the crystalline graphene domains in our products.36 XPS was also used to prove the reduction of GO through the removal of oxygen functional groups. XPS measurement performed on the PDA-GNS (Fig. S9†) and Ag-PDA-GNS (Fig. 4c) show a significant drop in C–O relative to C–C bonds, indicating that the GO is reduced by DA. Furthermore, the appearance of the C–N peak component at the binding energy of 285.5 eV in the C 1s core-level spectrum of PDA-GNS and Ag-PDA-GNS is consistent with the presence of a PDA layer on the surface of GNS. The XPS spectra of Ag-PDA-GNS also shows a significant Ag 3d signal, corresponding to a binding energy of Ag (Fig. 4d), which further supports the conclusion that Ag NPs have been effectively assembled on the surface of PDA-GNS.
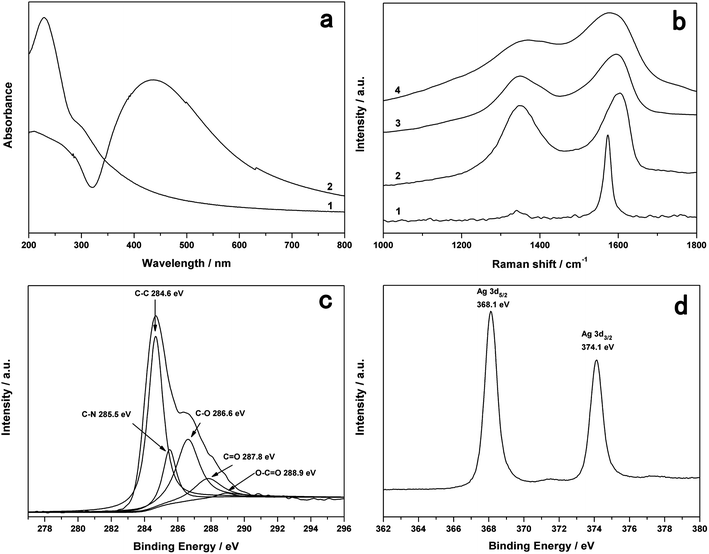 | ||
| Fig. 4 UV–vis absorption spectra (a) of GO (1) and Ag-PDA-GNS-50 (2). Raman spectra (b) of pristine graphite (1), graphite oxide (2), PDA-GNS (3), and Ag-PDA-GNS-50 (4). C 1s XPS spectra (c) and Ag 3d XPS spectra (d) of Ag-PDA-GNS-50. | ||
Silver is well known for its biocidal activity for a wide range of bacteria. In this work, E. coli and B. subtilis were used as model bacteria to investigate the antimicrobial properties of the Ag-PDA-GNS. When bacteria are cultured in LB liquid media without active substances for 24 h, the mixture becomes turbid (Fig. 5a and c, left column), which suggests that bacteria in such media rapidly proliferate.
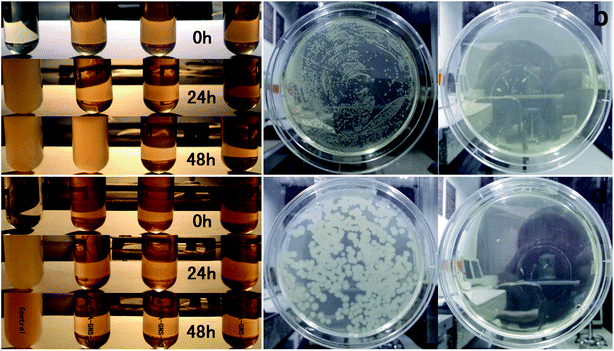 | ||
| Fig. 5 LB liquid medium turbidity assays (a and c) and LB–agar plate tests (b and d) were employed to evaluate antibacterial activities toward Gram-negative bacteria E. coli cells (a and b) and Gram-positive bacteria B. subtilis (c and d). | ||
However, the media containing 1% (v/v) active substances of Ag-PDA-GNS remain pellucid, indicating that the Ag-PDA-GNS are effective to inhibit bacterial growth. After 48 h, the mixed medium containing Ag-PDA-GNS-30 with a low density of Ag NPs become turbid for E. coli, however the other mixture mediums containing higher density of Ag NPs still remain pellucid. The antibacterial efficacy of Ag-PDA-GNS was also evaluated by LB–agar plates containing 1% (v/v) Ag-PDA-GNS-50 hybrid materials. The suspension of bacteria was spread onto the LB–agar plates. When the LB–agar plates without active substances are cultured at 37 °C for 24 h, it is shown from photographs that the LB–agar plates have a robust growth of bacteria for both strains (Fig. 5b and d, left column). Nevertheless, when the LB–agar plates with Ag-PDA-GNS-50 are cultured at the same conditions, the formation of colonies for both strains is fully inhibited. We further quantitatively evaluated the antibacterial activity of the Ag-PDA-GNS by studying the bacteria growth kinetics in LB liquid media. The bacterial proliferation was monitored by the optical density at 600 nm (OD600) based on the turbidity of the cell suspension within 48 h. As shown in Fig. S11,† the growth of both E. coli and B. subtilis were completely inhibited during the whole 48 h culture period when the concentration of the Ag-PDA-GNS was 1% (v/v). The above results showed that the Ag-PDA-GNS can prevent both Gram-negative and Gram-positive bacterial growth.
4. Conclusions
We present an environmentally friendly, mussel-inspired method to prepare Ag-PDA-GNS hybrid materials using the pre-synthesis PDA layer as a nanoscale guide and DA as reducing reagent to form uniform Ag NPs on the surface of PDA-GNS. It is shown that the obtained Ag-PDA-GNS have strong antibacterial properties to both Gram-negative and Gram-positive bacteria. We believe that the Ag-PDA-GNS hybrid materials have great potential for clinical and environmental applications.Acknowledgements
This work was supported by the National Basic Research Program of China (973 Program no. 2011CB935800).References
- H. Y. Lee, H. K. Park, Y. M. Lee, K. Kim and S. B. Park, Chem. Commun., 2007, 2959–2961 RSC.
- A. Kumar, P. K. Vemula, P. M. Ajayan and G. John, Nat. Mater., 2008, 7, 236–241 CrossRef CAS.
- V. Alt, T. Bechert, P. Steinrucke, M. Wagener, P. Seidel, E. Dingeldein, E. Domann and R. Schnettler, Biomaterials, 2004, 25, 4383–4391 CrossRef CAS.
- W. K. Jung, H. C. Koo, K. W. Kim, S. Shin, S. H. Kim and Y. H. Park, Appl. Environ. Microbiol., 2008, 74, 2171–2178 CrossRef CAS.
- A. B. Smetana, K. J. Klabunde, G. R. Marchin and C. M. Sorensen, Langmuir, 2008, 24, 7457–7464 CrossRef CAS.
- A. Panacek, L. Kvitek, R. Prucek, M. Kolar, R. Vecerova, N. Pizurova, V. K. Sharma, T. Nevecna and R. Zboril, J. Phys. Chem. B, 2006, 110, 16248–16253 CrossRef CAS.
- M. Lv, S. Su, Y. He, Q. Huang, W. B. Hu, D. Li, C. H. Fan and S. T. Lee, Adv. Mater., 2010, 22, 5463–5467 CrossRef CAS.
- P. Gunawan, C. Guan, X. H. Song, Q. Y. Zhang, S. S. J. Leong, C. Y. Tang, Y. Chen, M. B. Chan-Park, M. W. Chang, K. A. Wang and R. Xu, ACS Nano, 2011, 5, 10033–10040 CrossRef CAS.
- S. Ghosh, V. S. Goudar, K. G. Padmalekha, S. V. Bhat, S. S. Indi and H. N. Vasan, RSC Adv., 2012, 2, 930–940 RSC.
- J. H. Cui, C. F. Hu, Y. H. Yang, Y. J. Wu, L. F. Yang, Y. L. Wang, Y. L. Liu and Z. Y. Jiang, J. Mater. Chem., 2012, 22, 8121–8126 RSC.
- K. S. Novoselov, A. K. Geim, S. V. Morozov, D. Jiang, Y. Zhang, S. V. Dubonos, I. V. Grigorieva and A. A. Firsov, Science, 2004, 306, 666–669 CrossRef CAS.
- A. K. Geim and K. S. Novoselov, Nat. Mater., 2007, 6, 183–191 CrossRef CAS.
- A. A. Balandin, S. Ghosh, W. Z. Bao, I. Calizo, D. Teweldebrhan, F. Miao and C. N. Lau, Nano Lett., 2008, 8, 902–907 CrossRef CAS.
- C. Lee, X. D. Wei, J. W. Kysar and J. Hone, Science, 2008, 321, 385–388 CrossRef CAS.
- J. S. Bunch, S. S. Verbridge, J. S. Alden, A. M. van der Zande, J. M. Parpia, H. G. Craighead and P. L. McEuen, Nano Lett., 2008, 8, 2458–2462 CrossRef CAS.
- S. Pisana, M. Lazzeri, C. Casiraghi, K. S. Novoselov, A. K. Geim, A. C. Ferrari and F. Mauri, Nat. Mater., 2007, 6, 198–201 CrossRef CAS.
- E. Yoo, J. Kim, E. Hosono, H. Zhou, T. Kudo and I. Honma, Nano Lett., 2008, 8, 2277–2282 CrossRef CAS.
- S. Bae, H. Kim, Y. Lee, X. F. Xu, J. S. Park, Y. Zheng, J. Balakrishnan, T. Lei, H. R. Kim, Y. I. Song, Y. J. Kim, K. S. Kim, B. Ozyilmaz, J. H. Ahn, B. H. Hong and S. Iijima, Nat. Nanotechnol., 2010, 5, 574–578 CrossRef CAS.
- C. H. Lu, H. H. Yang, C. L. Zhu, X. Chen and G. N. Chen, Angew. Chem., Int. Ed., 2009, 48, 4785–4787 CrossRef CAS.
- G. Williams, B. Seger and P. V. Kamat, ACS Nano, 2008, 2, 1487–1491 CrossRef CAS.
- X. Q. Lu, H. T. Qi, X. F. Zhang, Z. H. Xue, J. Jin, X. B. Zhou and X. H. Liu, Chem. Commun., 2011, 47, 12494–12496 RSC.
- Z. Zhang, F. G. Xu, W. S. Yang, M. Y. Guo, X. D. Wang, B. L. Zhanga and J. L. Tang, Chem. Commun., 2011, 47, 6440–6442 RSC.
- Z. Y. Tang, N. A. Kotov, S. Magonov and B. Ozturk, Nat. Mater., 2003, 2, 413–418 CrossRef CAS.
- Q. F. Cheng, M. Z. Li, L. Jiang and Z. Y. Tang, Adv. Mater., 2012, 24, 1838–1843 CrossRef CAS.
- J. F. Wang, Q. F. Cheng and Z. Y. Tang, Chem. Soc. Rev., 2012, 41, 1111–1129 RSC.
- Y. Liu, W. S. Ma, W. W. Liu, C. Li, Y. L. Liu, X. Y. Jiang and Z. Y. Tang, J. Mater. Chem., 2011, 21, 19214–19218 RSC.
- H. Lee, B. P. Lee and P. B. Messersmith, Nature, 2007, 448, 338–341 CrossRef CAS.
- F. Xia and L. Jiang, Adv. Mater., 2008, 20, 2842–2858 CrossRef CAS.
- X. F. Gao, X. Yan, X. Yao, L. Xu, K. Zhang, J. H. Zhang, B. Yang and L. Jiang, Adv. Mater., 2007, 19, 2213–2217 CrossRef CAS.
- P. Vukusic and J. R. Sambles, Nature, 2003, 424, 852–855 CrossRef CAS.
- H. Lee, S. M. Dellatore, W. M. Miller and P. B. Messersmith, Science, 2007, 318, 426–430 CrossRef CAS.
- L. Q. Xu, W. J. Yang, K. G. Neoh, E. T. Kang and G. D. Fu, Macromolecules, 2010, 43, 8336–8339 CrossRef CAS.
- B. Fei, B. T. Qian, Z. Y. Yang, R. H. Wang, W. C. Liu, C. L. Mak and J. H. Xin, Carbon, 2008, 46, 1795–1797 CrossRef CAS.
- S. M. Kang, S. Park, D. Kim, S. Y. Park, R. S. Ruoff and H. Lee, Adv. Funct. Mater., 2011, 21, 108–112 CrossRef CAS.
- C. Z. Zhu, S. J. Guo, Y. X. Fang and S. J. Dong, ACS Nano, 2010, 4, 2429–2437 CrossRef CAS.
- H. L. Wang, J. T. Robinson, X. L. Li and H. J. Dai, J. Am. Chem. Soc., 2009, 131, 9910–9911 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c2nr32092d |
| This journal is © The Royal Society of Chemistry 2013 |
