Single gold trimers and 3D superstructures exhibit a polarization-independent SERS response†
Dennis
Steinigeweg
,
Max
Schütz
and
Sebastian
Schlücker
*
University of Osnabrück, Department of Physics, Barbarastr. 7, 49069 Osnabrück, Germany. E-mail: sebastian.schluecker@uos.de; Fax: +49 541 969 13592; Tel: +49 541 969 3592
First published on 20th September 2012
Abstract
Dimers of metal nanospheres are well-known for their characteristic anisotropic optical response. Here, we demonstrate in single-particle SERS experiments that individual gold trimers and 3D superstructures exhibit a polarization-independent SERS response. This optical behavior of single particle clusters provides constant SERS signals, independent of the mutual orientation of the incident laser polarization and the plasmonic nanostructure, which is desired or even required in many SERS applications.
Introduction
Metal nanoparticles have fascinating physical and chemical properties,1,2 which are exploited in numerous and diverse applications including surface-enhanced Raman scattering (SERS).3–5 Raman scattering of molecules adsorbed on the surface of noble metal nanostructures such as Ag or Au colloids is enhanced by several orders of magnitude.3,6 This enhancement is particularly high when the molecules are located in “hot spots”, i.e. very localized regions with extremely high field enhancements.3,6,7 Dimers of metal nanospheres are one of the simplest systems in which such “hot spots” occur. Several groups have simulated and measured the scattering spectra of dimers,6–13 trimers13–15 and superstructures.16 The optical response of a dimer (D∞h symmetry) is highly polarization-dependent,6,17–20 similar to nanorods,21,22 particle–nanowire systems,23,24 and nanoparticle arrays.25,26 In contrast, this polarization dependence does not occur for spherical particles.27,28In a dimer, the magnitude of scattering depends on the relative orientation of the polarization of the incident laser radiation and the dimer axis, characterized by an angle θ. Maximum signals occur for a parallel orientation when the longitudinal localized plasmon resonance is most effectively excited, while a perpendicular orientation results in no signal. In between, a characteristic cos2θ dependency is observed. Simulations and theoretical considerations predict a nearly polarization-independent behavior for nanoparticle trimers29 and superstructures.16 However, initial polarization-dependent SERS experiments on an asymmetrical trimer did not show the theoretically expected result.30
Experimental
Gold dimers and trimers were prepared and functionalized with the Raman reporter molecule 2-bromo-4-mercaptobenzoic acid.31 For the preparation of the 3D plasmonic superstructure, 80 nm gold cores were functionalized with 5,5′-dithiobis(2-nitrobenzoic acid)-diethylene glycol-NH2 (DTNB-DEG-NH2) to form a complete self-assembled monolayer (SAM) on the particle surface. After washing the SAM-functionalized colloid, monodisperse 20 nm silver nanoparticles32 were added. A detailed description of the chemical synthesis of the rationally designed Raman probe molecule (DTNB–DEG–NH2) will be presented in a separate manuscript. The extinction spectrum and SERS spectrum of Au/Ag core/satellite 3D superstructures in a colloidal suspension together with a transmission electron microscopy (TEM) image and the localized surface plasmon resonance (LSPR) spectrum of a single Au/Ag superstructure are shown in Fig. S1 and S2 in the ESI.†SERS measurements were performed with a modified WiTec Alpha 300 R microscope (Zeiss 50× objective, bright- and dark-field, NA = 0.7) coupled to a grating monochromator (30 cm focal length, 600 grooves per mm grating) equipped with an EMCCD (Andor, Newton DU-970N-BV). For SERS measurements, the polarization of the laser beam (HeNe laser, 632.8 nm; output power: 5 mW; integration time: 100 ms) was rotated by a Fresnel rhomb. Fig. S3† shows the laser output power on the sample surface. By adding a polarization filter in front of the power meter, it is clearly observable that the laser beam is linearly polarized.
Scanning electron microscopy measurements were performed with a Zeiss Supra 50 microscope with a field emission cathode. The colloid was dried on a silicon wafer.
Results and discussions
In this contribution, we report on the polarization-dependent SERS properties of three different clusters at the single-particle level: a single glass-coated dimer of 60 nm Au spheres (SEM image: inset in Fig. 1 top), a single glass-coated trimer of 60 nm Au spheres (SEM image: inset in Fig. 1 middle), and a single self-assembled 3D plasmonic superstructure comprising a 80 nm Au core surrounded by 20 nm Ag satellites (SEM image: inset in Fig. 1 bottom). All particles have a self-assembled monolayer of aromatic thiols as Raman reporter molecules on their surface (for details: please see Experimental section and ESI†).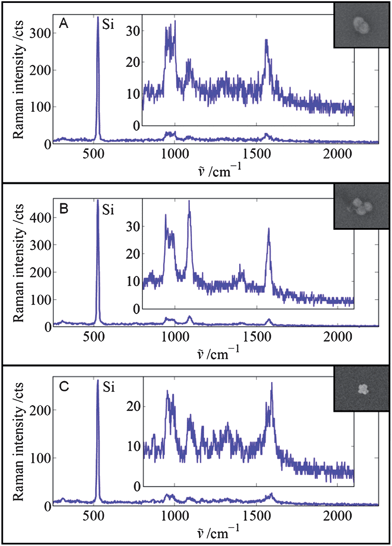 | ||
| Fig. 1 SERS spectra and corresponding SEM images of three different single particles. (A) Glass-coated dimer of 60 nm Au spheres with 2-bromo-4-mercaptobenzoic acid as the Raman reporter molecule, (B) glass-coated trimer of 60 nm Au spheres with 2-bromo-4-mercaptobenzoic acid as the Raman reporter molecule, and (C) Au/Ag superstructure with 80 nm Au core and 20 nm Ag satellites with 2-nitro-5-mercaptobenzoic acid-diethylene glycol-NH2 as the Raman reporter molecule on the Au core. Laser output power: 5 mW. Integration time: 100 ms. | ||
Fig. 1 shows the single-particle SERS spectra of the dimer (A), the trimer (B) and the superstructure (C) deposited on a silicon wafer. The polarization of the laser beam was optimized for maximum SERS enhancement (cf.Fig. 2). The spectra are dominated by the intense first-order phonon Raman peak of Si at ∼520 cm−1. The broader and much weaker peak below ∼1000 cm−1 is the second-order phonon peak of Si.
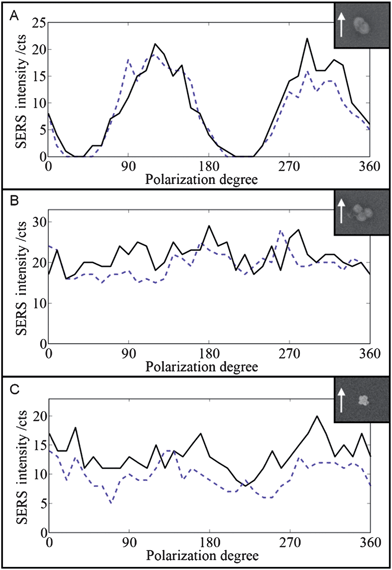 | ||
| Fig. 2 SEM images and SERS polarization dependence (black line) of the single Au dimer (A), the single Au trimer (B), and the Au/Ag 3D superstructure (C). Laser output power: 5 mW. Integration time: 100 ms. The measurements were repeated (blue dashed line) after the polarization was rotated through 360° in 10° intervals. The arrows indicate the polarization direction (0°). | ||
All three individual particles (A–C) exhibit a detectable SERS signal within only 100 ms acquisition time. In the case of the dimer (A) and trimer (B), 2-bromo-4-mercaptobenzoic acid was employed as the Raman reporter molecule, while for the synthesis of the 3D superstructure (C) a derivative of 2-nitro-5-mercaptobenzoic acid was rationally designed and synthesized (please see Experimental section and ESI† for details). All three aryl thiols exhibit characteristic Raman bands at ∼1080 cm−1 and ∼1580 cm−1 due to phenyl ring modes.33 The nitro compound on the superstructure (C) exhibits an additional characteristic peak at ∼1340 cm−1, which is assigned to the symmetric nitro stretching vibration.33
We first measured and reproduced the known characteristic polarization-dependent SERS response from a single Au dimer (Fig. 2A) in two subsequent measurements in order to test our experimental setup and, more importantly, to use it as a reference for comparison with the performance of the single Au trimer and the single Au/Ag 3D superstructure. As expected for the dimer from theory and experiment, the parallel orientation results in maximum signals (22 cts), while no SERS signal is detectable for the perpendicular orientation (0 cts). The gap between the spheres affects the LSPR and SERS properties of a dimer.6 In our case the dimer comprises two coalesced gold cores (see HR-TEM image in Fig. S4 in the ESI†) and exhibits the theoretically expected anisotropy.
The maximum signal of 22 cts in the first measurement (solid black line) is slightly higher than the 19 cts in the subsequent second measurement (dashed blue line). We attribute this to “fatigue” effects due to the long and intense optical illumination of the single plasmonic nanostructure (2 × 36 angles = 72 measurements total). The experimentally observed polarization dependence of the dimer in Fig. 2A matches perfectly with the results reported earlier by other groups.
Fig. 2B shows the results for the single Au trimer. The SERS intensity varies between 16 and 29 cts in the first measurement and between 15 and 28 cts in the second measurement. Results from two other trimers are shown in Fig. S5 in the ESI.† In all cases, no polarization dependency is observed. The trimer in Fig. 2B has nearly D3h symmetry. Plasmon hybridization in combination with group theory predicts that the bright in-plane modes have E′ symmetry and that in-plane plasmon modes exhibit no azimuthal dependence,15 which agrees with our experimental observation in Fig. 2B.
Fig. 2C shows the polarization dependence of the single Au/Ag 3D superstructure. The SERS intensity is about 8–20 cts for the first measurement and 5–14 cts for the second measurement. Results from two other superstructures are shown in Fig. S7.† Overall, the SERS signal of the three single Au/Ag superstructures seems to be slightly more polarization-dependent compared with the trimer. However, the absolute SERS signals obtained at the single-particle level are probably too weak to draw definite quantitative conclusions.
A direct and fair comparison of the maximum SERS signals obtained from the three different types of plasmonic nanostructures – dimer, trimer, and superstructure – requires an internal standard since several parameters such as the laser power at the sample and the focusing conditions may slightly differ from sample to sample. We therefore normalized the maximum SERS intensities of the plasmonic nanostructures to the polarization-averaged Raman signal of the underlying Si wafer (see ESI, Fig. S8†). The normalized results of the first measurement are shown in Table 1. The maximum SERS intensities (IMAX) for all three nanostructures are comparable, confirming earlier studies which reported very similar SERS intensities for individual glass-coated SERS-active dimers and trimers.13 The direct comparison of the Au dimer and Au trimer with the single Au/Ag superstructure should not be overstated due to its hybrid composition (Au core, Ag satellites) and the different Raman reporter molecule compared with the Au dimer and Au trimer. The polarization-averaged SERS intensity (IAVG) of the trimer is twice as large compared with the dimer. This is expected since the mean value of cos2θ averaged over all polarizations (0–360°) is 0.5, i.e. the mean SERS intensity of the dimer is only half of its maximum intensity (cf.Fig. 2A).
| I MAX | I AVG | EFMAX | EFAVG | |
|---|---|---|---|---|
| Au dimer | 1.00 | 1.00 | 7.7 × 108 | 2.8 × 108 |
| Au trimer | 1.16 | 2.18 | 6.9 × 108 | 4.6 × 108 |
| Au/Ag superstructure | 1.12 | 2.04 | 9.7 × 108 | 6.5 × 108 |
The calculated maximum and average enhancement factors (EFMAX and EFAVG, respectively) for the dimer, trimer, and the superstructure (see ESI† for details) are comparable to the results reported from earlier single-particle SERS studies on glass-coated nanoantennas.13 The superstructure with a 80 nm core and 20 nm satellites exhibits the highest enhancement factor, but a lower absolute SERS signal due to the smaller number of Raman reporter molecules on its 80 nm core compared to the dimer and trimer (two and three 60 nm spheres, respectively).
Conclusions
In summary, we presented experimental results on individual nanoantennas which demonstrate the quasi-polarization-independent SERS response of a single Au trimer and a single 3D Au/Ag core/satellite superstructure. By measuring also the polarization-dependent behavior for a single Au dimer combined with internal referencing to the Raman signal of the underlying Si wafer, we were able to directly compare their SERS responses at the single-particle level. Overall, SERS intensities are comparable in all three cases, but the average SERS signal is larger for the trimer and the superstructure due to their quasi-polarization-independent response. This behavior is important for single-particle SERS applications, in which the SERS response should be constantly high, i.e. independent of the particle orientation. One example is SERS microscopy for tissue diagnostics using SERS-labeled ligands for target recognition.5,34 In case the single dimers are oriented in the “wrong” perpendicular orientation, no SERS signal will be observed, yielding false-negative results. However, this issue is less critical for SERS assays due to orientational averaging in colloidal suspensions. Nevertheless, trimers are still expected to exhibit an orientation-averaged SERS response larger by a factor of ca. 2 compared with the corresponding dimers (see Table 1).Acknowledgements
We thank Bernd Walkenfort for technical assistance with SEM measurements. Financial support by the German Research Foundation (INST 190/128-1) and the University of Osnabrück is acknowledged.Notes and references
- C. F. Bohren and D. P. Huffman, Absorption and Scattering of Light by Small Particles, Wiley, 1998 Search PubMed.
- M.-C. Daniel and D. Astruc, Chem. Rev., 2004, 104, 293 CrossRef CAS.
- E. C. Le Ru and P. G. Etchegoin, Principles of Surface-enhanced Raman Spectroscopy; Elsevier, 2009 Search PubMed.
- Surface Enhanced Raman Spectroscopy, ed. S. Schlücker, Wiley-VCH, 2011 Search PubMed.
- S. Schlücker, ChemPhysChem, 2009, 10, 1344 CrossRef.
- H. Xu, J. Aizpurua, M. Käll and P. Apell, Phys. Rev. E, 2000, 62, 4318 CrossRef CAS.
- J. M. McMahon, A.-I. Henry, K. L. Wustholz, M. J. Natan, R. G. Freeman, R. P. Van Duyne and G. C. Schatz, Anal. Bioanal. Chem., 2009, 394, 1819 CrossRef CAS.
- M. P. Busson, B. Rolly, B. Stout, N. Bonod, E. Larquet, A. Polman and S. Bidault, Nano Lett., 2011, 11, 5060 CrossRef CAS.
- N. J. Halas, S. Lal, W.-S. Chang, S. Link and P. Nordlander, Chem. Rev., 2011, 111, 3913 CrossRef CAS.
- L. V. Brown, H. Sobhani, J. B. Lassiter, P. Nordlander and N. J. Halas, ACS Nano, 2010, 4, 819 CrossRef CAS.
- J. B. Lassiter, J. Aizpurua, L. I. Hernandez, D. W. Brandl, I. Romero, S. Lal, J. H. Hafner, P. Nordlander and N. J. Halas, Nano Lett., 2008, 8, 1212 CrossRef CAS.
- E. C. Talley, J. B. Jackson, C. Oubre, N. K. Grady, C. Hollars, S. M. Lane, T. R. Huser, P. Nordlander and N. J. Halas, Nano Lett., 2005, 5, 1569 CrossRef.
- K. L. Wustholz, A.-I. Henry, J. M. McMahon, R. G. Freeman, N. Valley, M. E. Piotti, M. J. Natan, G. C. Schatz and R. P. Van Duyne, J. Am. Chem. Soc., 2010, 132, 10903 CrossRef CAS.
- J. Alegret, T. Rindzevicius, T. Pakizeh, Y. Alaverdyan, L. Gunnarsson and M. Käll, J. Phys. Chem. C, 2008, 112, 14313 CAS.
- D. W. Brandl, N. A. Mirin and P. Nordlander, J. Phys. Chem. B, 2006, 110, 12302 CrossRef CAS.
- M. Gellner, D. Steinigeweg, S. Ichilmann, M. Salehi, M. Schütz, K. Kömpe, M. Haase and S. Schlücker, Small, 2011, 7, 3445 CrossRef CAS.
- H. Xu and M. Käll, ChemPhysChem, 2003, 4, 1001 CrossRef CAS.
- C. L. Du, M. X. Yang, Y. M. You, T. Chen, H. Y. Chen and Z. X. Shen, Chem. Phys. Lett., 2009, 473, 317 CrossRef CAS.
- H.-Y. Lin, C.-H. Huang, C.-H. Chang, Y.-C. Lan and H.-C. Chui, Opt. Express, 2010, 18, 165 CrossRef CAS.
- W. Li, P. H. C. Camargo, X. Lu and Y. Xia, Nano Lett., 2009, 9, 485 CrossRef CAS.
- J. Tao, Y. Lu, J. Chen, D. Lu, C. Chen, P. Wang and H. Ming, Plasmonics, 2011, 6, 785 CrossRef CAS.
- J. Jiao, X. Wang, F. Wackenhut, A. Horneber, L. Chen, A. V. Failla, A. J. Meixner and D. Zhang, ChemPhysChem, 2012, 13, 952 CrossRef CAS.
- H. Wei, F. Hao, Y. Huang, W. Wang, P. Nordlander and H. Xu, Nano Lett., 2008, 8, 2497 CrossRef CAS.
- S. J. Lee, J. M. Baik and M. Moskovits, Nano Lett., 2008, 8, 3244 CrossRef CAS.
- C. Tian, C. Ding, S. Liu, S. Yang, X. Song, B. Ding, Z. Li and J. Fang, ACS Nano, 2011, 5, 9442 CrossRef CAS.
- W. Luo, W. van der Veer, P. Chu, D. L. Mills, R. M. Penner and J. C. Hemminger, J. Phys. Chem. C, 2008, 112, 11609 CAS.
- H. Liang, Z. Li, W. Wang, Y. Wu and H. Xu, Adv. Mater., 2009, 21, 4614 CrossRef CAS.
- B. Zhang, P. Xu, X. Xie, H. Wei, Z. Li, N. H. Mack, X. Han, H. Xu and H.-L. Wang, J. Mater. Chem., 2011, 21, 2495 RSC.
- P. G. Etchegoin, C. Galloway and E. C. Le Ru, Phys. Chem. Chem. Phys., 2006, 8, 2624 RSC.
- T. Shegai, Z. Li, T. Dadosh, Z. Zhang, X. Xu and G. Haran, Proc. Natl. Acad. Sci. U. S. A., 2008, 105, 16448 CrossRef CAS.
- D. Steinigeweg, M. Schütz, M. Salehi and S. Schlücker, Small, 2011, 7, 2443 CrossRef CAS.
- D. Steinigeweg and S. Schlücker, Chem. Commun., 2012, 48, 8682 RSC.
- G. Varsanyi, Assignments for Vibrational Spectra of Seven Hundred Benzene Derivatives, Wiley, 1974 Search PubMed.
- S. Schlücker, B. Küstner, A. Punge, A. Marx and P. Ströbel, J. Raman Spectrosc., 2006, 37, 719 CrossRef.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c2nr31982a |
| This journal is © The Royal Society of Chemistry 2013 |
