Gold nanorod-covered kanamycin-loaded hollow SiO2 (HSKAurod) nanocapsules for drug delivery and photothermal therapy on bacteria
Bo Hu, Li-Pei Zhang, Xu-Wei Chen* and Jian-Hua Wang*
Research Center for Analytical Sciences, Northeastern University, Box 332, Shenyang 110004, China. E-mail: chenxuwei@mail.neu.edu.cn; jianhuajrz@mail.neu.edu.cn; Fax: +86 24 83676698; Tel: +86 24 83688944
First published on 25th October 2012
Abstract
A hybrid bactericidal material, gold nanorod-covered kanamycin-loaded hollow SiO2 (HSKAurod) nanocapsules, is constructed. The hybrid material combines the features of a chemical drug with photothermal physical sterilization which decreases the dosage of broad-spectrum antibiotic and the physical damage of biological systems. Hollow SiO2 nanocapsules are used as carriers for drug delivery. The nanocapsules load a model drug, kanamycin, and are covered with gold nanorods to avoid drug leakage and realize photothermal treatment. The sterilizing effect on the bacterial strain is investigated by incubating E. coli BL21 with the hybrid nanocapsules and irradiating under near-infrared light (NIR) for 20 min. A bactericidal effect, i.e., a sterilizing rate of 53.47%, is achieved for the HSKAurod nanocapsules under NIR irradiation, with respect to a net sum sterilizing rate of 34.49% for the individual components of the HSKAurod nanocapsules, e.g., carrier nanocapsules, chemical sterilization of kanamycin and physical sterilization due to the gold nanorods under NIR irradiation. It is demonstrated that the combination of chemical drug and physical sterilization results in an obvious synergistic effect and makes the sterilization more effective. This novel hybrid has great potential as an adjuvant therapeutic alternative material for sterilization or even for the control of disease.
1 Introduction
The microbes in air and water are closely related to human health. It is thus very important to perform research into aspects of sterilization, prevention, disinfection etc., to control disease. Presently, sterilization is generally based on chemical drugs. Broad-spectrum antibiotics have the ability to kill certain types of bacteria and restrain viruses. However, the long-term use of such antibiotics has the potential to cause adverse drug reactions or even increase the risk of deep fungal infection. On the other hand, the wide application of broad-spectrum antibiotics may also confer drug resistance, which is becoming a serious problem in clinics. In this respect, physical sterilization approaches, e.g., hot/moist-heat sterilization, ultraviolet sterilization, radiation and microwave based sterilization, are useful alternatives. But these approaches tend to cause damage to the biological system. In this context, a composite material combining the features of a chemical drug and physical sterilization is highly called for in order to provide a favorable sterilization capability, while at the same time avoiding the above limitations.As a promising and versatile material, mesoporous silica has recently attracted extensive attention.1,2 It has a very high surface area and a controllable morphology/tunable size as well as pore structure/pore size.3–5 It also has favorable biocompatibility and the capacity to carry molecular drugs within the porous core.6 In order to achieve a larger carrying capacity for drugs, solid mesoporous silica nanospheres are usually etched to obtain a hollow structure by sacrificial templates, e.g., polymer beads and micelles, emulsion droplets and metals.7 This is usually a complex process with certain risks, due to the use aqua regia as an etching agent. As a substitute, a more simple and effective method is available to drive the spontaneous transformation of silica colloids in NaBH4 solution8 and produce high morphological fidelity hollow structures. The obtained hollow SiO2 nanocapsule is most suitable for serving as a drug carrier.
Due to their excellent photothermal properties, NIR-activated gold nanoparticles have been widely used in thermotherapy by local heating in a wide variety of shapes, e.g., half-shell nanoparticles,9–11 nanoshell particles,12–14 nanocages15,16 and nanorods.17,18 These Au nanomaterials absorb near-infrared light and then realize NIR photothermal ablation. Among these nanoparticles, Au nanorods have emerged as an excellent photothermal therapy agent due to a more efficient photothermal energy conversion than most other nanostructures,18–20 in addition to simple preparation protocols21 and tunable aspect ratios.18,22,23 Moreover, it has two characteristic absorptions, e.g., the transverse surface plasmon resonance, which is driven to blue shift by the raised aspect ratio, and the longitudinal surface plasmon known as the resonance and NIR-responsive. Recently, Au nanorods have been reported to convert to particles by selectively shortening the nanorods by oxidation,24 near-infrared irradiation,25 thermal reshaping26 and external reagent stimuli.27 That is to say, upon exposure to appropriate NIR light, Au nanorods can not only convert heat, but also undergo shape changes. As a photothermal material, Au nanorods loaded on the surface of hollow SiO2 nanocapsules can potentially prevent drug molecules escaping from the hollow nanocapsules and can aid the slow or controlled release of drug.
Hollow SiO2 nanocapsules are chosen as a drug carrier and Au nanorods as a photothermal treatment agent to present a hybrid bactericidal material, i.e., Au nanorod-covered kanamycin-loaded hollow-SiO2 nanocapsules. The hybrid material can realize chemical drug/physical photothermal sterilization and drug release. A broad-spectrum antibiotic kanamycin is used as the chemical drug model and E. coli BL21 as the target bacterial strain to demonstrate the sterilizing effect. It is expected that combining the drug kanamycin and photothermal therapy may reduce the risk of abuse of broad-spectrum antibiotic and biological system damage by physical sterilization. In addition, the synergetic effect of chemical drug and physical sterilization brings an improvement on the sterilization effect of the Au nanorod-covered kanamycin-loaded hollow SiO2 nanocapsules.
2 Experimental
2.1 Instrumentation
UV-vis absorption spectra are obtained with a U-3900 UV-vis spectrophotometer (Hitachi Ltd, Japan) with a 1 cm quartz cell and a bandwidth setting of 2 nm at a scan speed of 1200 nm min−1.FT-IR spectra are carried out by a Nicolet-6700 FT-IR spectrometer (Thermo Ltd, USA) within 1500–700 cm−1.
The transmission electron microscopy (TEM) images are acquired with a Tecnai G2 20 microscope (Philips, Holland), operated at 200 kV.
A Zetasizer Nano ZS/ZEN3690 (Malvern, UK) is used to measure the size and the zeta potential of the nanoparticles.
The survival of Escherichia coli BL21 (E. coli BL21) after near infrared (NIR) treatment with a 785 nm emitting diode laser (120 mW) is obtained by using a FUJIFILM FinePix F50fd camera (Japan).
Other instruments used include: IKA vortex 3 (IKA Ltd, China), SW-CJ-1FD air clean bench (Antai Air Tech Co, Suzhou, China), TQ2-312 HZQ-X (Jing Hong Laboratory Instruments, Shanghai, China), LDZX-40BI stainless steel vertical pressure steam sterilizer (Shenan Medical Devices, Shanghai, China).
2.2 Reagents
Chloroauric acid tetrahydrate (HAuCl3·HCl·4H2O, AR), cetyltrimethylammonium bromide (CTAB, AR), sodium tetrahydroborate (NaBH4, 96%), polyvinylpyrrolidone (PVP, K-30), silver nitrate (AgNO3, AR), kanamycin sulfate and sodium tungstophosphate (AR) are purchased from Sinopharm Chemical Reagent Co, China. Tetraethyl orthosilicate (TEOS), ethanol, L-ascorbic acid (AR) and hydrochloric acid (HCl, 1.0 M) are obtained from Tianjin Damao Chemicals. Ammonium hydroxide (NH3·H2O, 25%) is received from Tianjin Kermel Reagent Co. The Luria–Bertani (LB) nutrient solution (pH 7.3–7.5) is prepared by dissolving sodium chloride (3.0 g), TSB (3.0 g) and yeast extract (1.5 g) in 300 mL DI water. The pH value is adjusted by NaOH and the mixture is further treated with high temperature disinfection. TSBY solid medium is obtained by adding granulated agar (4.5 g). Phosphate buffer (pH 7.4) is obtained by mixing 0.2 M of Na2HPO4 and 0.2 M of NaH2PO4 and is controlled by an Orion 818 pH meter (Thermo Fisher Scientific, USA). Wahaha pure water and DI water of 18 MΩ cm is used throughout.2.3 Preparation of the gold nanorods
CTAB-stabilized gold nanorods are prepared by a seed-mediated and silver(I) assisted growth method28 with slight modifications. Specifically, the seed solution is prepared by rapidly adding a freshly prepared ice cold NaBH4 solution (0.01 M, 0.6 mL) into a mixture containing 0.25 mL HAuCl4 (0.01 M) and 9.75 mL CTAB (0.1 M), followed by strong stirring for 3 min then kept at 30 °C for 1.5–2 h before use.Au nanorods are obtained by injecting 0.24 mL of the seed solution into a growth solution (pH 1–2) containing 5 × 10−4 M HAuCl4, 1 × 10−4 M AgNO3 and 8 × 10−4 M AA in 100 mL CTAB (0.1 M). This solution is gently stirred for 5 min and then left undisturbed overnight. The obtained Au nanorods solution is centrifuged before use at 8000 rpm for 10 min (twice) to remove any excess CTAB surfactant.
2.4 Preparation of hollow SiO2 nanocapsules (HS)
SiO2 nanospheres are obtained by mixing 9.0 mL TEOS in ethanol (91.0 mL) with 8.0 mL NH3·H2O, 30.8 mL H2O and 71.2 mL ethanol. SiO2 nanospheres are formed under magnetic stirring after ca. 10 min. The reaction mixture is continuously stirred for 6 h to ensure complete hydrolysis of TEOS. The oyster white SiO2 nanospheres are washed with ethanol and DI water alternately for three times. The supernatant is then discarded and the precipitate is re-dispersed in DI water at a concentration of 192.5 mg mL−1.Hollow SiO2 nanocapsules are prepared based on a modified approach.8 SiO2 nanosphere solution (1.56 mL) is first dispersed in aqueous PVP (8.44 mL, 3%). After supersonic dispersing, 0.6 g NaBH4 is added into the mixture and the solution is heated to 51 °C under stirring for 6 h. The HS nanocapsules are washed to neutral and re-dispersed in DI water. The final concentration of the hollow SiO2 nanocapsules is 143 mg mL−1.
2.5 Kanamycin-loading on hollow SiO2 nanocapsules
To prepare the drug-loaded nanocapsules (HSK), 1.0 mL of HS nanocapsules is injected to 29.0 mL of kanamycin aqueous solution (0.3 mg mL−1, ultimately). The obtained mixture is vortex vibrated for 16 h and then centrifuged at 8000 rpm for 5 min (twice) to remove the excess kanamycin. The obtained HSK is redispersed in DI water at 150 mg mL−1.2.6 Au nanorod-covered HSKAurod nanocapsules
Au nanorod-covered HSKAurod nanocapsules are prepared by mixing 3.0 mL of the above Aurod solution and 1.0 mL of HSK solution and the mixture is vortex vibrated and left overnight. The obtained precipitate is washed and re-dispersed in 1.0 mL DI water and diluted with PBS (0.1 M, sterile) to the desired concentration.To examine the photothermal response properties of the hybrid material, HSKAurod nanocapsules (100 μL, 2.0 mg mL−1) are irradiated by an emitting diode laser (785 nm, 120 mW) at a 1 cm distance for 90 min with temperature monitoring. In order to evaluate the kanamycin release behavior of the HSKAurod nanocapsules, 500 μL of the HSKAurod nanocapsule aqueous solution at 143 mg mL−1 is irradiated for various periods of time. The sample solution is vortex vibrated for 20 min, and then centrifuged at 8000 rpm for 10 min. The content of kanamycin in the supernatant is derived by measuring the UV-vis absorption at 256 nm.
2.7 Photothermal treatment of bacteria by the HSKAurod nanocapsules
E. coli BL21 are collected with inoculating loops from agar slant culture medium, inoculated in LB broth and grown at 37 °C for 10 h, then stored at 4 °C for use.To evaluate the bactericidal property of the HSKAurod nanocapsules, E. coli BL21 (1 × 109 cfu mL−1, cfu = colony forming units) are diluted with broth to 1 × 105 cfu mL−1, and stored as original bacteria solution. The as-prepared E. coli BL21 solution (50 μL) is mixed with HSKAurod nanocapsules (1.43 mg mL−1, 30 μL) and diluted with LB broth to 500 μL. Three sets of the same solution are prepared in parallel. The obtained bacteria mixture is irradiated under NIR laser for 20 min and then shaked for 10 min. Then, 100 μL of the bacteria solution is placed on TSBY solid medium and cultured at 37 °C for 14–16 h before counting the number of bacterial colonies. A control experiment is performed in parallel, i.e., the bacteria diluted with LB broth in the absence of HSKAurod nanocapsules. To illustrate the combined bactericidal effect of chemical drug and physical photothermal sterilization, the net sterilizing effects in the presence of solely kanamycin or Au nanorods under NIR irradiation are investigated in parallel following the previously described procedures.
3 Results and discussion
3.1 Fabrication of the HSKAurod nanocapsules
Fig. 1 illustrated a four-step process for the preparation of Au nanorod-covered kanamycin-loaded hollow SiO2 nanocapsules. The hydrolysis of TEOS results in uniform SiO2 nanospheres, which is followed by etching with NaBH4 to form hollow structure nanocapsules. The hollow structure brings a large cavity in the interior and nanopores on the surface of hollow SiO2 nanocapsules. This makes it convenient for the drug to pass freely through the pores into the hollow cavity and at the same time increases the drug loading capacity. A widely used microbicide, i.e., kanamycin, is selected as the model drug to be loaded into the hollow SiO2 nanocapsules to form the HSK hybrid material. Afterwards, Au nanorods are immobilized physically on the surface of the HSK hybrid. Au nanorods prevent the leakage of kanamycin from the hollow structure and meanwhile serve as a physical bactericidal material due to their excellent photothermal properties. In addition, the loaded Au nanorods could serve as an optical tag on a strong light scattering by dark-field microscopy as well as spectroscopic detection of bacteria by SERS.29 The morphology of the hybrid product is characterized by TEM.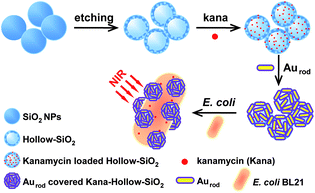 | ||
| Fig. 1 Schematic of the fabrication of gold nanorod-covered kanamycin-loaded hollow SiO2 nanocapsules. | ||
3.2 Characterization of the HSKAurod nanocapsules
CTAB-stabilized Au nanorods are obtained by the seed-mediated and silver-assisted growth method. TEM images of the Au nanorods are shown in Fig. 2A. The nanorods have an average aspect ratio (length/diameter) of 4.6 ± 0.7, resulting in an average longitudinal surface plasmon resonance wavelength of 770 nm and a weak transverse plasmon band at 512 nm.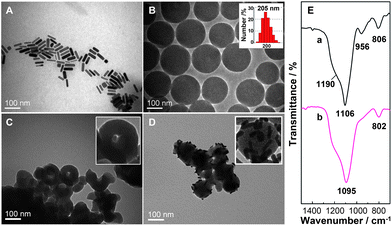 | ||
| Fig. 2 TEM images of (A) Au nanorods, (B) SiO2 nanospheres, (C) hollow SiO2 nanocapsules and (D) Au nanorod-covered hollow SiO2 nanocapsules. (E) FT-IR spectra of (a) the as-prepared SiO2 nanospheres, and (b) the hollow SiO2 nanocapsules after reacting with 0.06 g mL−1 NaBH4 at 51 °C for 6 h. | ||
Spherical silica nanoparticles are prepared by TEOS hydrolysis in ethanol in the presence of ammonia, based on the Stöber process with a little modification.30,31 By regulating the volume of ammonia at fixed TEOS and ethanol/DI water concentrations, monodispersed SiO2 nanospheres are obtained with an average hydrodynamic size of ca. 205 nm (Fig. 2B) measured by dynamic light scattering (DLS). TEM images of these nanoparticles indicate that they are well monodispersed with uniform size distribution.
Hollow SiO2 nanocapsules are obtained by etching with NaBH4 at 51 °C for 6 h. In Fig. 2C, TEM images of the hollow SiO2 nanocapsules illustrate well-defined hollow nanostructures with smooth SiO2 shells and discernable pores on the surface, which allow kanamycin to pass through and load onto the SiO2 shells. In the etching process, PVP is added to prevent aggregation of the hollow spheres8 and serves as a bridging agent between the hollow SiO2 nanocapsules and Au nanorods.
FT-IR spectra for the SiO2 nanoparticles before and after NaBH4 etching are shown in Fig. 2E. For the SiO2 nanospheres, the absorptions at 1190, 1106, 956 and 806 cm−1 (curve a) arising from the longitudinal-optical and transverse-optical modes of the Si–O–Si asymmetric bond stretching vibration, the Si–OH stretching vibration, and the network Si–O–Si symmetric bond stretching vibration are clearly identified.32 After etching in NaBH4–PVP solution, the SiO2 nanospheres exhibit an obvious solid-to-hollow structure conversion. A red shift is observed within the broad absorption region of 1106–1095 cm−1 (curve b), which is recorded for the Si–O–Si asymmetric stretching vibration. This absorption makes the Si–OH stretching vibration band at 956 cm−1 unobservable because of overlap. In addition, the Si–O–Si symmetric stretching vibration at 806 cm−1 shifts to 802 cm−1. These shifts are attributed to a more open SiO2 network structure with lower internal stress in the newly formed silica shells.32–34
The drug-loading is performed by incubating the hollow SiO2 nanocapsules in a 300 μg mL−1 kanamycin sulfate solution for 16 h. After incubation, the zeta potential of the hollow SiO2 nanocapsules increased from −52.7 to −20.7 mV. This partly illustrates the successful loading of kanamycin into the nanocapsules and the mesoporous silica coating. As an aminoglycosides antibiotic, kanamycin can form a copper–ammine (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) complex at pH 6.5–8.8. By adding CuSO4 into kanamycin aqueous solution, the obtained dark blue complex has a maximum absorption at 256 nm, which can be used for the quantification of kanamycin.35Fig. 3A shows the absorption spectra of kanamycin solution before and after loading. The decrease of kanamycin concentration in the supernatant illustrates its storage into the cavities and the mesoporous silica coating of hollow SiO2 nanocapsules. The difference of kanamycin concentrations in the original solution and the supernatant gives rise to the kanamycin-loading efficiency. Further studies derived a loading capacity of 8.34 mg g−1 for kanamycin.
1) complex at pH 6.5–8.8. By adding CuSO4 into kanamycin aqueous solution, the obtained dark blue complex has a maximum absorption at 256 nm, which can be used for the quantification of kanamycin.35Fig. 3A shows the absorption spectra of kanamycin solution before and after loading. The decrease of kanamycin concentration in the supernatant illustrates its storage into the cavities and the mesoporous silica coating of hollow SiO2 nanocapsules. The difference of kanamycin concentrations in the original solution and the supernatant gives rise to the kanamycin-loading efficiency. Further studies derived a loading capacity of 8.34 mg g−1 for kanamycin.
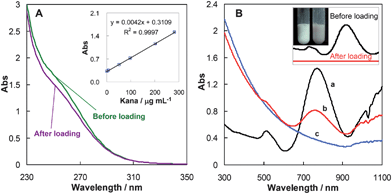 | ||
| Fig. 3 (A) UV-vis spectra of kanamycin (300 μg mL−1) with Cu(II) and that of the residual kanamycin in the supernatant after incubated in hollow SiO2 nanocapsules. (B) UV-vis spectra of (a) Au nanorods, (b) Au nanorod-covered hollow SiO2 nanocapsules, and (c) hollow SiO2 nanocapsules. The inset reveals the spectra of the Au nanorods before and after being covered on the hollow SiO2 nanocapsules with images of the solutions. | ||
After drug-loading, Au nanorods are attached onto the kanamycin-loaded hollow SiO2 nanocapsules surface. Fig. 3B clearly identified the characteristic absorptions for Au nanorods at 512 and 770 nm in the UV-vis spectra of Au nanorod-coated kanamycin-loaded hollow SiO2 nanocapsules with a small blue shift. The color of the hybrid nanocapsules and the decrease of the absorptions of Au nanorods further demonstrate the successful preparation of Au nanorod-covered kanamycin-loaded hollow SiO2 nanocapsules (HSKAurod).
3.3 In vitro photothermal bactericidal properties of the HSKAurod nanocapsules
In order to demonstrate the suitable amount of the HSKAurod nanocapsules for further bactericidal study, the bactericidal properties of Au nanorods and kanamycin are evaluated separately. Fig. 4A indicated that at an Au nanorod concentration of 180 μmol L−1, more than 85% of E. coli BL21 is still viable. On the other hand, the increase of kanamycin concentration within 0–100 μg mL−1 results in an improvement on the sterilizing effect, and the viability of E. coli BL21 decreases to 11% at 100 μg mL−1 (Fig. 4B). For the purpose of studying the bactericidal ability of the hybrid HSKAurod nanocapsules, it is necessary to maintain a viability of >85% for E. coli BL21. In this respect, the concentration of HSKAurod nanocapsules should be chosen such that the corresponding contents of Au nanorods and kanamycin are <180 μmol L−1 and <30 μg mL−1, respectively.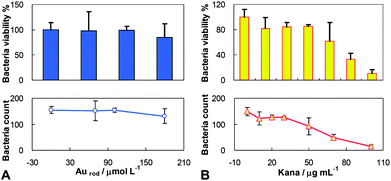 | ||
| Fig. 4 The dependence of viability of E. coli BL21 on the concentration of (A) Au nanorods and (B) kanamycin. E. coli BL21: 1 × 105 cfu mL−1; Au nanorods: 0, 70, 100, 180 μmol L−1; kanamycin: 0, 10, 20, 30, 50, 70, 100 μg mL−1. The data were averaged by 3 parallel assays. | ||
It is known that heat-induced NIR irradiation depends closely on the NIR intensity and the irradiation time, as well as the concentration of the photothermal response materials.36 In this case, when fixing the dosage of the hybrid HSKAurod nanocapsules and the intensity of the NIR laser, the irradiation depends solely on the irradiation time. As illustrated in Fig. 5 (curve a), the temperature increases rapidly from ambient temperature, i.e., ca. 21 °C, to ca. 50 °C within an irradiation time of 30 min, and afterwards the temperature remains virtually constant when further increasing the irradiation time upto 90 min. At this temperature, the E. coli BL21 may be inactive. Generally the generation of E. coli BL21 occurs within ca. 20 min. By considering both the photothermal temperature and the bacteria generation time, the irradiation time should be better chosen at 20–25 min.
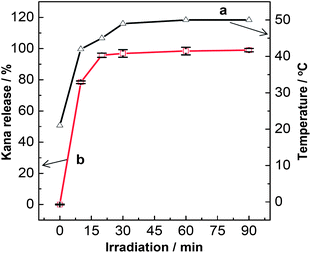 | ||
| Fig. 5 Photothermal properties of the hybrid HSKAurod nanocapsules (a) and the drug-release behavior from the HSKAurod under NIR irradiation, 785 nm, 120 mW (b). | ||
Fig. 5b depicts the kanamycin release behavior from the hybrid HSKAurod nanocapsules along with an increase of the irradiation time. It shows that the release of kanamycin is temperature-dependent, and its release can be regulated by manipulating the NIR irradiation and vortex vibration. When exposing the hybrid HSKAurod nanocapsules to NIR irradiation for 20 min and then vortex vibration for 5 min, more than 90% release of the drug is recorded, which indicates the expected drug release ability of the HSKAurod nanocapsules.
The bactericidal effect of the HSKAurod nanocapsules under NIR irradiation is investigated by incubating them with bacteria E. coli BL21 followed by measuring the bacteria viability. For comparison, the bacteria blank (control), the bacteria viability with physical sterilization by Au nanorods under NIR irradiation, the chemical drug sterilization by kanamycin and carrier hollow SiO2, respectively, are also performed under the same experimental conditions. The survival fractions of the bacteria after treatment are given in Table 1. The histogram (bottom) and the photos of the culture growth of E. coli BL21 on the solid medium (top) are illustrated in Fig. 6.
| Bacterial strain | Bacteria (blank) | Aurod-NIR | Kanamycin | HS | HSKAurod (NIR) |
|---|---|---|---|---|---|
| E. coli BL21 | 100.00 ± 4.42 | 89.53 ± 4.94 | 90.16 ± 2.54 | 85.82 ± 0.03 | 46.53 ± 1.74 |
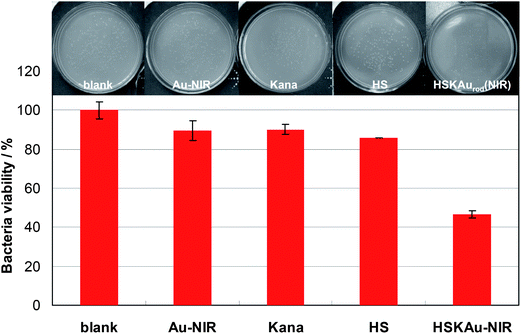 | ||
| Fig. 6 Histogram presenting the viability of E. coli BL21 (1 × 105 cfu mL−1) after treatment with various materials. The viability is acquired by using the plate counting method. The upper part shows the plate sample. The hybrid HSKAurod nanocapsules: 1.4 mg mL−1; Au nanorods: 70 μmol L−1; kanamycin: 1 μg mL−1. | ||
The viabilities of bare E. coli BL21, or after exposure to Au nanorods under NIR irradiation, or incubation with bare kanamycin and hollow SiO2 nanocapsules, are (100.00 ± 4.42), (89.53 ± 4.94), (90.16 ± 2.54) and (85.82 ± 0.03)%, respectively. The bacteria viability is significantly decreased to (46.53 ± 1.74)% after incubating with the Au nanorod-covered kanamycin-loaded hollow nanocapsules and exposing to NIR irradiation for 20 min (780 nm, 120 mW). These results clearly indicate that a bactericidal effect, i.e., sterilizing rate of 53.47%, is achieved for the HSKAurod nanocapsules under NIR irradiation. In comparison, a net sum of the sterilizing rate of 34.49% is recorded for the physical sterilization (10.47%), chemical sterilization by kanamycin (9.84%) and carrier hollow SiO2 nanocapsules (14.18%). This clearly demonstrates that there exists a synergistic effect among each component of the hybrid HSKAurod nanocapsules in the bactericidal effect, giving rise to a much improved sterilization performance on the bacteria E. coli BL21. It is considered that the synergistic effect may be attributed to the local thermal ablation provided by the Au nanorods under NIR radiation. This can destroy the bacteria surface structure and make it easier for kanamycin to penetrate into the bacteria and therein inhibit protein synthesis by binding to a site on the 30S ribosomal subunit. Benefited by the synergistic effect, the dosage of the drug kanamycin is obviously decreased, in addition to the reduction of the sterilization time, thus avoiding the unnecessary injury of the biological systems.
Fig. 7(a) and (b) illustrate the TEM images of the native bacteria E. coli BL21 and that after incubation with the hybrid HSKAurod nanocapsules. It can be seen that after incubation, the surface of the bacteria E. coli BL21 is obviously covered by many HSKAurod nanocapsules. The interaction might involve electrostatic interactions between the positively charged hybrid HSKAurod nanocapsules and the negatively charged surface of the bacteria E. coli BL21. In addition, as demonstrated in Fig. 7(c) and (d) the viability of the bacteria E. coli BL21 can readily be controlled at a very low level, i.e., <10%, by increasing the content of the hybrid HSKAurod nanocapsules to ca. 10 mg mL−1.
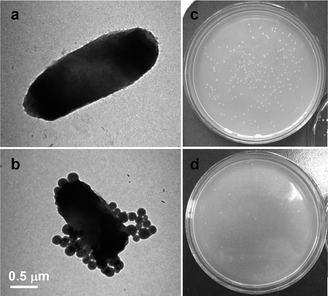 | ||
| Fig. 7 TEM images and the plate sample photographs of (a and c) the native bacteria E. coli BL21 and (b and d) those incubated with the hybrid HSKAurod nanocapsules. | ||
4 Conclusions
We fabricated gold nanorod-covered kanamycin-loaded hollow SiO2 nanocapsules (HSKAurod). The hybrid nanomaterial has the ability to simultaneously exhibit drug delivery and heat-treatment sterilization. When incubated with bacteria E. coli BL21, the HSKAurod composites accumulate on the surface of the bacteria. Upon NIR irradiation, the Au nanorods exhibit excellent photothermal properties and the therapeutic effect of the HSKAurod nanocapsules has significantly improved. Compared with drug and photothermal treatment alone, the sterilizing rate by combined treatment with HSKAurod nanocapsules is much higher than the net sum of the individual sterilizing rate by each of the single components. This demonstrates a synergistic effect between each component of the hybrid HSKAurod nanocapsules on the bacteria, which reduces the dosage of the drug kanamycin and shortens the treatment time.Acknowledgements
This work is financially supported by Natural Science Foundation of China (21075013, 21275027, 21235001), the SRFDP program (20100042120027) and Fundamental Research Funds for Central Universities (N110605001, N100305003, N110705002 and N110805001).References
- N. Singh, A. Karambelkar, L. Gu, K. Lin, J. S. Miller, C. S. Chen, M. J. Sailor and S. N. Bhatia, J. Am. Chem. Soc., 2011, 133, 19582–19585 CrossRef CAS.
- F. Muhammad, M. Y. Guo, W. X. Qi, F. X. Sun, A. Wang, Y. J. Guo and G. S. Zhu, J. Am. Chem. Soc., 2011, 133, 8778–8781 CrossRef CAS.
- M. Vallet-Regi, F. Balas and D. Arcos, Angew. Chem., Int. Ed., 2007, 46, 7548–7558 CrossRef CAS.
- B. G. Trewyn, I. I. Slowing, S. Giri, H. T. Chen and V. S. Y. Lin, Acc. Chem. Res., 2007, 40, 846–853 CrossRef CAS.
- Y. J. Wang, A. D. Price and F. J. Caruso, Mater. Chem. Phys., 2009, 19, 6451–6464 RSC.
- (a) C. E. Ashley, E. C. Carnes, G. K. Phillips, D. Padilla, P. N. Durfee, P. A. Brown, T. N. Hanna, J. W. Liu, B. Phillips, M. B. Carter, N. J. Carroll, X. M. Jiang, D. R. Dunphy, C. L. Willman, D. N. Petsev, D. G. Evans, A. N. Parikh, B. Chackerian, W. Wharton, D. S. Peabody and C. Brinker, Nat. Mater., 2011, 10, 476 CrossRef CAS; (b) I. I. Slowing, B. G. Trewyn, S. Giri and V. S. Y. Lin, Adv. Funct. Mater., 2007, 17, 1225–1236 CrossRef CAS; (c) I. I. Slowing, J. L. Vivero-Escoto, B. G. Trewyn and V. S. Y. Lin, J. Mater. Chem., 2010, 20, 7924–7937 RSC; (d) S. H. Wu, Y. S. Lin, Y. Hung, Y. H. Chou, Y. H. Hsu, C. Chang and C. Y. Mou, ChemBioChem, 2008, 9, 53–57 CrossRef CAS; (e) J. L. Vivero-Escoto, I. I. Slowing, B. G. Trewyn and V. S. Y. Lin, Small, 2009, 6, 1952–1967 CrossRef; (f) E. Tasciotti, X. Liu, R. Bhavane, K. Plant, A. D. Leonard, B. K. Price, M. M.-C. Cheng, P. Decuzzi, J. M. Tour, F. Robertson and M. Ferrari, Nat. Nanotechnol., 2008, 3, 151–157 CrossRef CAS.
- (a) J. G. Wang, Q. Xiao, H. J. Zhou, P. C. Sun, Z. Y. Yuan, B. H. Li, D. T. A. Ding, C. Shi and T. H. Chen, Adv. Mater., 2006, 18, 3284–3288 CrossRef CAS; (b) Z. W. Deng, M. Chen, S. X. Zhou, B. You and L. M. Wu, Langmuir, 2006, 22, 6403–6407 CrossRef CAS; (c) H. Djojoputro, X. F. Zhou, S. Z. Qiao, L. Z. Wang, C. Z. Yu and G. Q. Lu, J. Am. Chem. Soc., 2006, 128, 6320–6321 CrossRef CAS; (d) M. Chen, L. M. Wu, S. X. Zhou and B. You, Adv. Mater., 2006, 18, 801–806 CrossRef CAS; (e) B. Tan, H. J. Lehmler, S. M. Vyas, B. L. Knutson and S. E. Rankin, Adv. Mater., 2005, 17, 2368–2371 CrossRef CAS; (f) Q. Y. Sun, P. J. Kooyman, J. G. Grossmann, P. H. H. Bomans, P. M. Frederik, P. C. M. M. Magusin, T. P. M. Beelen, R. A. van Santen and N. A. J. M. Sommerdijk, Adv. Mater., 2003, 15, 1097–1100 CrossRef CAS; (g) K. J. C. van Bommel, J. H. Jung and S. Shinkai, Adv. Mater., 2001, 13, 1472–1476 CrossRef CAS; (h) Y. S. Li, J. L. Shi, Z. L. Hua, H. R. Chen, M. L. Ruan and D. S. Yan, Nano Lett., 2003, 3, 609–612 CrossRef CAS.
- T. R. Zhang, J. P. Ge, Y. X. Hu, Q. Zhang, S. Aloni and Y. D. Yin, Angew. Chem., Int. Ed., 2008, 47, 5806–5811 CrossRef CAS.
- K. S. Kim, S. J. Park, M. Y. Lee, K. G. Lim and S. K. Hahn, Macromol. Res., 2012, 20, 277–282 CrossRef CAS.
- S. M. Lee, H. Park and K. H. Yoo, Adv. Mater., 2010, 22, 4049–4053 CrossRef CAS.
- H. Park, J. Yang, J. Lee, S. Haam, I. H. Choi and K. H. Yoo, ACS Nano, 2009, 3, 2919–2926 CrossRef CAS.
- C. Loo, A. Lowery, N. J. Halas, J. West and R. Drezek, Nano Lett., 2005, 5, 709–711 CrossRef CAS.
- L. R. Hirsh, R. J. Stafford, J. A. Bankson, S. R. Sersher, B. R. Rivera, E. Price, J. D. Hazle, N. J. Halas and J. L. West, Proc. Natl. Acad. Sci. U. S. A., 2003, 100, 13549–13554 CrossRef.
- S. R. Sershen, S. L. Westcott, N. J. Halas and J. L. West, J. Biomed. Mater. Res., 2000, 51, 293–298 CrossRef CAS.
- J. Chen, F. Saeki, B. J. Wiley, H. Chang, M. J. Cobb, Z. Y. Li, L. Au, H. Zhang, M. B. Kimmey, X. Li and Y. Xia, Nano Lett., 2005, 5, 473–477 CrossRef CAS.
- J. Y. Chen, D. L. Wang, J. F. Xi, L. Au, A. Siekkinen, A. Warsen, Z. Y. Li, H. Zhang, Y. N. Xia and X. D. Li, Nano Lett., 2007, 7, 1318–1322 CrossRef CAS.
- L. Tong, Y. Zhao, T. B. Huff, M. N. Hansen, A. Wei and J. X. Cheng, Adv. Mater., 2007, 19, 3136–3141 CrossRef CAS.
- X. H. Huang, I. H. El-Sayed, W. Qian and M. A. El-Sayed, J. Am. Chem. Soc., 2006, 128, 2115–2120 CrossRef CAS.
- R. S. Norman, J. W. Stone, A. Gole, C. J. Murphy and T. L. Sabo-Attwood, Nano Lett., 2008, 8, 302–306 CrossRef CAS.
- D. Pissuwan, S. M. Valenzuela, C. M. Miller and M. B. Cortie, Nano Lett., 2007, 7, 3808–3812 CrossRef CAS.
- (a) C. C. Chen, Y. P. Lin, C. W. Wang, H. C. Tzeng, C. H. Wu, Y. C. Chen, C. P. Chen, L. C. Chen and Y. C. Wu, J. Am. Chem. Soc., 2006, 128, 3709–3715 CrossRef CAS; (b) T. B. Huff, L. Tong, Y. Zhao, M. N. Hansen, J. X. Cheng and A. Wei, Nanomedicine, 2007, 2, 125–132 CrossRef CAS; (c) C. G. Wang, Z. F. Ma, T. T. Wang and Z. M. Su, Adv. Funct. Mater., 2006, 16, 1673–1678 CrossRef CAS; (d) C. X. Yu, H. Nakshatri and J. Irudayaraj, Nano Lett., 2007, 7, 2300–2306 CrossRef CAS; (e) C. G. Wang, Y. Chen, T. T. Wang, Z. F. Ma and Z. M. Su, Adv. Funct. Mater., 2008, 18, 355–361 CrossRef CAS; (f) C. X. Yu and J. Irudayaraj, Anal. Chem., 2007, 79, 572–579 CrossRef CAS; (g) C. G. Wang, Y. Chen, T. T. Wang, Z. F. Ma and Z. M. Su, Chem. Mater., 2007, 19, 5809–5811 CrossRef CAS; (h) K. M. Mayer, S. Lee, H. W. Liao, B. C. Rostro, A. Fuentes, P. T. Scully, C. L. Nehl and J. H. Hafner, ACS Nano, 2008, 2, 687–692 CrossRef CAS.
- B. Nikoobakht and M. A. El-Sayed, Chem. Mater., 2003, 15, 1957–1962 CrossRef CAS.
- L. F. Gou and C. J. Murphy, Chem. Mater., 2005, 17, 3668–3672 CrossRef CAS.
- C. K. Tsung, X. Kou, Q. Shi, J. Zhang, M. H. Yeung, J. Wang and G. D. Stucky, J. Am. Chem. Soc., 2006, 128, 5352–5353 CrossRef CAS.
- S. Link, C. Burda, B. Nikoobakht and M. A. El-Sayed, J. Phys. Chem. B, 2000, 104, 6152–6163 CrossRef CAS.
- M. B. Mohamed, K. Z. Ismail, S. Link and M. A. El-Sayed, J. Phys. Chem. B, 1998, 102, 9370–9374 CrossRef CAS.
- T. S. Sreeprasad, A. K. Samal and T. Pradeep, Langmuir, 2007, 23, 9463–9471 CrossRef CAS.
- (a) W. H. Ni, X. S. Kou, Z. Yang and J. F. Wang, ACS Nano, 2008, 2, 677–686 CrossRef CAS; (b) T. Ming, X. S. Kou, H. J. Chen, T. Wang, H. L. Tam, K. W. Cheah, J. Y. Chen and J. F. Wang, Angew. Chem., Int. Ed., 2008, 47, 9685–9690 CrossRef CAS; (c) M. Z. Liu and P. Guyot-Sionnest, J. Phys. Chem. B, 2005, 109, 22192–22200 CrossRef CAS.
- T. T. Wang, F. Chai, C. G. Wang, L. Li, H. Y. Liu, L. Y. Zhang, Z. M. Su and Y. Liao, J. Colloid Interface Sci., 2011, 358, 109–115 CrossRef CAS.
- W. Stöber, A. Fink and E. A. Bohn, J. Colloid Interface Sci., 1968, 26, 62–69 CrossRef.
- P. A. Buining, L. M. Liz-Marzan and A. P. Philipse, J. Colloid Interface Sci., 1996, 179, 318–321 CrossRef CAS.
- S. Bruynooghe, F. Bertin, A. Chabli, J. C. Gay, C. B. Blanchard and M. Couchaud, Thin Solid Films, 1998, 313, 722–726 CrossRef.
- H. Zhu, Y. G. Ma, Y. G. Fan and J. C. Shen, Thin Solid Films, 2001, 397, 95–101 CrossRef CAS.
- G. S. Pappas, P. Liatsi, I. A. Kartsonakis, I. Danilidis and G. J. Kordas, J. Non-Cryst. Solids, 2008, 354, 755–760 CrossRef CAS.
- C. Yao, L. N. Xu, Z. P. Wang and D. R. Yang, Anal. Lab., 1999, 18, 16–18 CAS.
- H. Park, J. Yang, J. Lee, S. Haam, I. H. Choi and K. H. Yoo, ACS Nano, 2009, 3, 2919–2926 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2013 |
