Anion-directed self-assembly of Cu(II) coordination compounds with tetrazole-1-acetic acid: syntheses in ionic liquids and crystal structures†
Jun
Chen
ab,
Shuai-Hua
Wang
ab,
Zhi-Fa
Liu
ab,
Mei-Feng
Wu
c,
Yu
Xiao
ab,
Fa-Kun
Zheng
*a,
Guo-Cong
Guo
a and
Jin-Shun
Huang
a
aState Key Laboratory of Structural Chemistry, Fujian Institute of Research on the Structure of Matter, Chinese Academy of Sciences, Fuzhou, Fujian 350002, P. R. China. E-mail: zfk@fjirsm.ac.cn; Fax: +86 591-8371-4946; Tel: +86 591-8370-4827
bUniversity of Chinese Academy of Sciences, Beijing 100039, P. R. China
cSchool of Environmental and Chemical Engineering, Nanchang Hangkong University, Nanchang, Jiangxi 330063, P. R. China
First published on 25th October 2013
Abstract
Reaction of tetrazole-1-acetic acid (1-Htza) with a Cu(II) salt in ionic liquids with different anions, [BMIM]X (BMIM = 1-butyl-3-methylimidazolium; X = Br−, BF4−, NTf2− (NTf2− = bis((trifluoromethyl)sulfonyl)amide)), afforded five Cu(II) coordination compounds, [Cu2(1-tza)4]Br·H3O·1/3H2O (1), [Cu2(1-tza)4]BF4·H3O·H2O (2), [Cu(μ2-Cl)(1-tza)(1-Htza)(H2O)]·0.5H2O (3), [CuCl(μ2-Cl)(1-Htza)2(H2O)]·H2O (4), and [CuCl2(1-Htza)2]·H2O (5). Single-crystal X-ray diffraction analyses reveal that 1–5 display various structures, and the 1-tza− ligand exhibits diverse coordination modes. Compounds 1 and 2 possess higher dimensional structures (a 2-D neutral Kagomé topology network for 1 and a 3-D lvt-type topology framework for 2) with fully deprotonated 1-tza− ligands. Compounds 3–5 display lower dimensional structures (1-D, 1-D and 0-D for 3, 4 and 5, respectively) with partly or fully protonated 1-Htza. The anions of ionic liquids have significant influences on the final molecular architectures, which arise from different water miscibility of ionic liquids.
Introduction
The exploration of coordination compounds has attracted significant interest in recent decades, because of their fascinating structures1 and potential applications in molecular magnetism,2 photoluminescence,3 ferroelectric devices,4 ion exchange,5 gas storage,6 and catalysis.7 However, there are still challenges in employing traditional synthetic methods to obtain functional metal complexes with expected structures and properties. Therefore, it is quite necessary to develop new synthetic approaches in the pursuit of novel coordination compounds. Ionic liquids (ILs) can serve as reaction media instead of conventional water or organic solvents.8 Distinct from molecular solvents, ILs are composed entirely of ions with organic cations and inorganic (or organic) anions,9 and they usually possess many special properties, such as a low melting point, a wide liquid range, good thermal stabilities, suitable viscosity, high solubility for both polar and non-polar organic and inorganic substances, negligible vapour pressure and non-flammability,10 which make ILs ideal solvents widely used in the field of inorganic or inorganic–organic hybrid materials synthesis,11,12 especially in the metal–organic frameworks (MOFs) synthesis.13–15 In addition, ILs involved in MOFs synthesis act as charge compensating species, particularly potential templates and/or structural directing agents.13–15 The cation and anion species of ILs can individually or cooperatively influence the resulting structures of coordination compounds.14,15 As many examples demonstrate, IL cations are usually incorporated in the final molecular architectures, and they also have potential abilities to tune the structures of the final compounds by changing their sizes. For example, Young-Uk Kwon's group showed that varying the length of the alkyl group on the imidazolium cation has significant effects on the reaction products of Zn(NO3)2·6H2O and H3BTC.14 Meanwhile, IL anions also have the ability to direct the self-assembly of coordination compounds,15 which is different from the “templating effect” of IL cations. Moreover, the anions are not commonly contained in the final structures and their effect can be termed as the “induction effect”.13a One example is that Morris' group has used an enantiopure anion as one component of the IL to induce homochirality in a nickel(II) complex constructed entirely using achiral building blocks, but the IL anions are not included in the product.15a However, investigations on IL anions controlling the preparation of coordination compounds still remain less developed.In this study, we employed [BMIM]X (BMIM = 1-butyl-3-methylimidazolium; X = Br−, BF4−, NTf2− (NTf2− = bis((trifluorom-ethyl)sulfonyl)amide)), whose miscibility with water can be altered using different anions,16 as reaction solvents in designing Cu(II) coordination compounds. We selected bifunctional tetrazole-1-acetic acid (1-Htza) as an organic ligand, which simultaneously possesses carboxylate and tetrazolate groups. As is well known, carboxylate O atoms and tetrazolyl ring N atoms have good coordination capacities.17 Therefore, 1-Htza could exhibit versatile coordination modes (Scheme S1, ESI†) to construct transition metal,18 rare earth metal19 or heterometallic transition-rare earth metal complexes20 with diverse structures from 0-D to 3-D and interesting physical performances in magnetism,18a,b,20 photoluminescence18d and ion exchange.18c In this paper, five Cu-1-tza coordination compounds, [Cu2(1-tza)4]Br·H3O·1/3H2O (1), [Cu2(1-tza)4]BF4·H3O·H2O (2), [Cu(μ2-Cl)(1-tza)(1-Htza)(H2O)]·0.5H2O (3), [CuCl(μ2-Cl)(1-Htza)2(H2O)]·H2O (4), [CuCl2(1-Htza)2]·H2O (5), have been successfully synthesized in [BMIM]+ ILs with varied anions (Scheme 1). Flexible coordination modes of 1-Htza have been found in 1–5 (Scheme 2). The effects of IL anions on the final structural assembly have been discussed. As far as we know, ILs have first been employed in the syntheses of tetrazolate–carboxylate complexes.18–21
 | ||
| Scheme 2 Three coordination modes of the tetrazole-1-acetic acid ligand in compounds 1–5 with mode a observed in 1, b in 1, 2 and 3, c in 3, 4 and 5. | ||
Experimental section
Materials and instruments
All chemicals were commercially available sources of analytical grade and used without further purification. The used ILs in this study were purchased from Centre for Green Chemistry and Catalysis, LICP, CAS with the grade of purity of 99%. In order to avoid absorbing moisture, the ILs were stored in a vacuum desiccator before use. The elemental analyses of C, H and N were carried out using a Vario EL III elemental analyzer. The FT-IR spectra were recorded in the 4000–400 cm−1 range on a Perkin–Elmer Spectrum One Spectrometer using KBr pellets. Thermogravimetric analysis (TGA) experiments were done using a NETZSCH STA 449C Jupiter thermogravimetric analyzer in flowing nitrogen with the sample heated in an Al2O3 crucible at a heating rate of 5 K min−1. All powder X-ray diffraction data were collected on a Rigaku Miniflex II diffractometer using Cu-Kα radiation (λ = 1.540598 Å) at 40 kV and 40 mA in the range 5.00° ≤ 2θ ≤ 65.00°.Syntheses of 1–5
Single crystal structure determination
Single crystals of 1–5 suitable for X-ray analyses were sticked to a fiberglass. Data collections were performed on a Rigaku Saturn-724 CCD diffractometer for 3 at 293 K, and a Rigaku Saturn-70 CCD diffractometer for the remaining compounds at 293 K. All diffractometers were equipped with graphite-monochromated Mo-Kα radiation (λ = 0.71073 Å). The intensity data sets were collected using the ω scan technique and reduced using CrystalClear software.22 The structures were solved by direct methods using the SHELXTL (version 5) crystallographic software package23 and refined by full-matrix least-squares refinement on F2. Non-hydrogen atoms were located by using difference Fourier maps and subjected to anisotropic refinement. The hydrogen atoms of water molecules except those belonging to disordered ones were located in difference Fourier syntheses and refined with O–H distances restrained to a target value of 0.85 Å and Uiso(H) = 1.5Ueq(O), and other hydrogen atoms were added according to theoretical models. Pertinent crystal data and structure refinement results for 1–5 are summarized in Table 1, and selected bonds lengths and angles are listed in Table S1 (ESI†).| 1 | 2 | 3 | 4 | 5 | |
|---|---|---|---|---|---|
| a R 1 = ∑(Fo − Fc)/∑Fo. b wR2 = [∑w(Fo2 − Fc2)2/∑w(Fo2)2]1/2. | |||||
| Formula | C12H15.67BrCu2N16O9.33 | C12H19BCu2F4N16O10 | C6H10ClCuN8O5.5 | C6H12Cl2CuN8O6 | C6H10Cl2CuN8O5 |
| M r (g mol−1) | 740.41 | 761.32 | 381.22 | 426.66 | 408.66 |
| Space group |
R![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) |
I41/a |
P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) |
P212121 | Cc |
| a/Å | 16.5929(16) | 24.074(4) | 6.537(2) | 8.8662(6) | 26.882(16) |
| b/Å | 16.5929(16) | 24.074(4) | 9.314(3) | 10.1720(6) | 6.802(3) |
| c/Å | 33.137(5) | 5.1021(16) | 11.282(4) | 16.6340(11) | 7.981(7) |
| α/° | 90 | 90 | 108.282(3) | 90 | 90 |
| β/° | 90 | 90 | 99.043(4) | 90 | 103.38(3) |
| γ/° | 120 | 90 | 98.410(2) | 90 | 90 |
| V/Å3 | 7901.1(16) | 2957.0(12) | 630.0(4) | 1500.17(17) | 1419.7(16) |
| Z | 9 | 4 | 2 | 4 | 4 |
| D c/g cm−3 | 1.400 | 1.710 | 2.010 | 1.889 | 1.912 |
| μ/mm−1 | 2.406 | 1.537 | 1.990 | 1.857 | 1.953 |
| F(000) | 3305 | 1528 | 384 | 860 | 820 |
| Flack | 0.040(18) | 0.00(4) | |||
| Total reflns | 19![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 763 763 |
8629 | 6171 | 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 030 030 |
4818 |
| Unique reflns | 3255 | 1289 | 2854 | 3409 | 2250 |
| R int | 0.0535 | 0.0582 | 0.0253 | 0.0532 | 0.0382 |
| GOF | 1.024 | 1.095 | 1.078 | 1.005 | 1.010 |
| R 1[I > 2σ(I)]a | 0.0496 | 0.0666 | 0.0299 | 0.0392 | 0.0334 |
| wR2(all data)b | 0.1779 | 0.1875 | 0.0958 | 0.0809 | 0.0864 |
| CCDC | 942747 | 942748 | 942949 | 942750 | 942751 |
Results and discussion
Syntheses and thermal stability
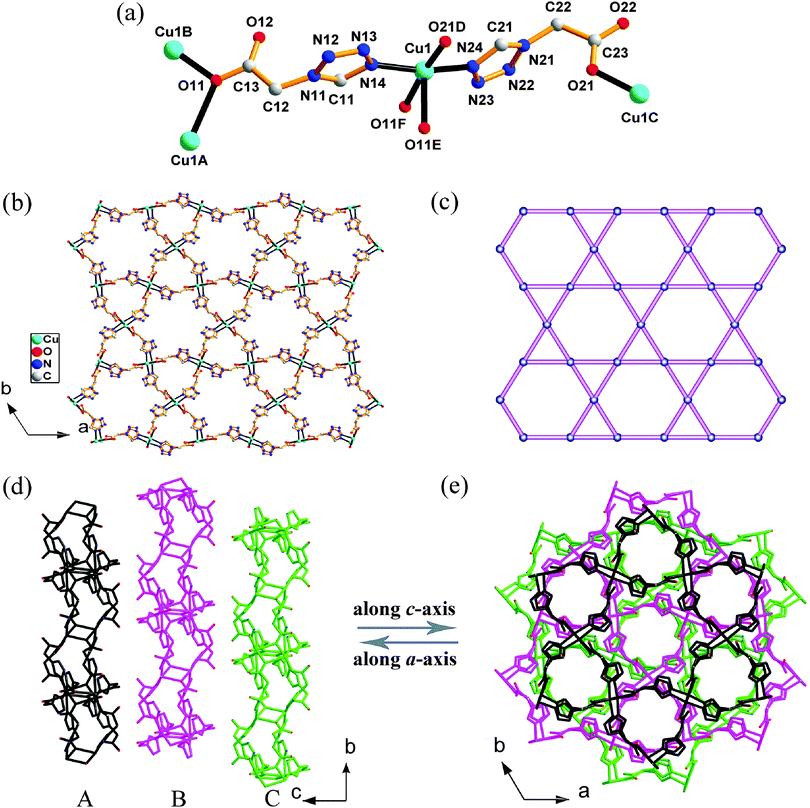
In order to investigate the effects of IL anions on the formation of Cu-1-tza coordination compounds, we chose anhydrous CuCl2 and 1-Htza as starting materials in [BMIM]X ILs (X = Br−, BF4−, NTf2−) with different water miscibility (Scheme 1). Compounds 1–4 were synthesized under the same reaction conditions. Compounds 1 and 2, obtained from [BMIM]Br and [BMIM]BF4, respectively, show higher dimensional structures (2-D for 1, and 3-D for 2, respectively) with both carboxylate and tetrazolate groups of the fully deprotonated 1-tza− ligand participating in coordination (Scheme 2), while both 3 and 4 achieved from [BMIM]NTf2 exhibit a lower dimensional structure (1-D) with partially or fully protonated 1-Htza. Only the tetrazolate group of protonated 1-Htza takes part in metal complexation. The main reason is that water miscibility of ILs is distinct from each other with anions being altered. A well-known function of IL anions is their ability to control the amount of water present in ILs. [BMIM]Br and [BMIM]BF4 are hygroscopic and can absorb a certain quantity of water when exposed to air, while [BMIM]NTf2 is hydrophobic and only permits little water in it.16a For this reason, the reaction mixture of 1, 2, 3 and 4 would absorb different amounts of water from air mainly determined by the hygroscopic property of the [BMIM]+ ILs. As an active proton acceptor, water can dramatically increase the degree of dissociation of an acid in a IL.16c Therefore, 1-Htza in hydrophilic [BMIM]Br or [BMIM]BF4 is more easily deprotonated than in hydrophobic [BMIM]NTf2. Deprotonated 1-tza− comprises more coordination sites and exhibits more diverse connectivity modes than protonated 1-Htza, and tends to facilitate higher dimensional products emerging. Moreover, protonated water involved in 1 and 2 also confirms the role of water miscibility of ILs. On the other hand, a closed environment may permit less amount of water as the reaction progresses. Compound 5 was synthesized in a sealed Teflon-lined stainless-steel autoclave, and only protonated 1-Htza and neutral water exist in 5, which further denotes the importance of water in affecting the degree of dissociation of the 1-Htza ligand. Thermogravimetric analysis (TGA) curves (Fig. S1, ESI†) show that 1 is stable up to 146 °C. Further heating led to a rapid weight loss arising from the decomposition of the organic ligand. For 2, the weight loss of 4.35% before 181 °C occurs in accordance with the release of the lattice water molecule (calcd: 4.64%), and then the framework sharply breaks down above 181 °C. The weight loss of 7.21% from 68 to 135 °C in 3 corresponds to the release of a half of lattice water molecule and one coordination water molecule (calcd: 7.09%). With continuous heating, the organic ligand was decomposed. The experimental PXRD patterns of 1–3 agree well with the simulated ones based on the single-crystal X-ray data (Fig. S2, ESI†), which implies that 1–3 are in a pure phase.Structural descriptions of 1–5
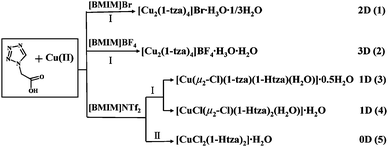
![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) and features a 2-D neutral framework constructed by 1-tza− ligands and centrosymmetric [Cu–(μ2-O)2–Cu] units. In each asymmetric unit of 1, there is one-third of the Br1 atom on a threefold axis and one-sixth of the Br2 atom at the site with bar-3 symmetry, one-third of the water oxygen O1W on a threefold axis is assigned 0.5-occupancy and one other water oxygen O2W in a general position is also assigned 0.5-occupancy. The unique Cu1 atom and the two 1-tza ligands (labeled as LI and LII) are in general positions with unit-occupancy. The LI ligand exhibits a μ3-κN14:κO11:κO11 coordination mode (mode a in Scheme 2), while the LII ligand adopts a μ2-κN24:κO21 coordination style (mode b in Scheme 2). Every Cu(II) atom is five-coordinated by two tetrazolate nitrogen atoms (N14, N24) and three carboxylate oxygen atoms (O21D, O11E, O11F) from two symmetry-related LI and one symmetry-related LII ligands (Fig. 1a). The pentacoordinated Cu(II) atom displays a distorted square pyramidal geometry with τ5 = 0.11 (τ5 = (β − α)/60, where α and β are the two biggest bond angles around the Cu(II) center; τ5 = 0 for an ideal square pyramid, and τ5 = 1 for an ideal trigonal bipyramid).24 The N14, N24, O21D and O11F atoms constitute the equatorial plane, and O11E occupies the apical position. As the Jahn–Teller effect of Cu2+, the axial Cu–O bond length of 2.425(3) Å is significantly longer than those of Cu–N/O in the equatorial positions ranging from 1.934(2) to 2.025(3) Å. Interestingly, two Cu(II) atoms are doubly bridged by carboxylate O11 atoms to give rise to an ideal parallelogram centrosymmetric [Cu–(μ2-O)2–Cu] unit with a Cu(II)⋯Cu(II) distance of 3.4803(11) Å and Cu1–O11–Cu1 angle of 105.06(10)°. The paratactic LI and LII ligands, acting as double linkers, bridge [Cu–(μ2-O)2–Cu] units to generate a 2-D network parallel to the ab plane (Fig. 1b). The shortest Cu(II)⋯Cu(II) distance between adjacent dinuclear [Cu–(μ2-O)2–Cu] units is equal to 7.7070(11) Å. If each “double-linker” is considered as a bridge, and each [Cu–(μ2-O)2–Cu] unit is regarded as a four-connected node, compound 1 features a perfect 2-D Kagomé topology (Fig. 1c), which comprises a planar array of equilateral triangles and regular hexagons. Every triangle is corner-shared with three other neighboring triangles and edge-shared with three adjacent hexagons, which is also the same for every hexagon in the Kagomé network.25 Although the 1-tza-based complexes have been studied extensively,18–20 to the best of our knowledge, this kind of 2-D Kagomé array has been first observed in the reported 1-tza-based coordination compounds, which may be owing to two different kinds of coordination modes of 1-tza− ligands coexisting in the framework of 1. Furthermore, 2-D layers stack over each other in an –ABC–ABC– fashion (viewed along the a-axis in Fig. 1d) to generate open channels viewed along the c-axis (Fig. 1e). Bromine anions and lattice water molecules as guests are located in these voids. However, there is still approximately 32% void volume calculated using PLATON software.26
and features a 2-D neutral framework constructed by 1-tza− ligands and centrosymmetric [Cu–(μ2-O)2–Cu] units. In each asymmetric unit of 1, there is one-third of the Br1 atom on a threefold axis and one-sixth of the Br2 atom at the site with bar-3 symmetry, one-third of the water oxygen O1W on a threefold axis is assigned 0.5-occupancy and one other water oxygen O2W in a general position is also assigned 0.5-occupancy. The unique Cu1 atom and the two 1-tza ligands (labeled as LI and LII) are in general positions with unit-occupancy. The LI ligand exhibits a μ3-κN14:κO11:κO11 coordination mode (mode a in Scheme 2), while the LII ligand adopts a μ2-κN24:κO21 coordination style (mode b in Scheme 2). Every Cu(II) atom is five-coordinated by two tetrazolate nitrogen atoms (N14, N24) and three carboxylate oxygen atoms (O21D, O11E, O11F) from two symmetry-related LI and one symmetry-related LII ligands (Fig. 1a). The pentacoordinated Cu(II) atom displays a distorted square pyramidal geometry with τ5 = 0.11 (τ5 = (β − α)/60, where α and β are the two biggest bond angles around the Cu(II) center; τ5 = 0 for an ideal square pyramid, and τ5 = 1 for an ideal trigonal bipyramid).24 The N14, N24, O21D and O11F atoms constitute the equatorial plane, and O11E occupies the apical position. As the Jahn–Teller effect of Cu2+, the axial Cu–O bond length of 2.425(3) Å is significantly longer than those of Cu–N/O in the equatorial positions ranging from 1.934(2) to 2.025(3) Å. Interestingly, two Cu(II) atoms are doubly bridged by carboxylate O11 atoms to give rise to an ideal parallelogram centrosymmetric [Cu–(μ2-O)2–Cu] unit with a Cu(II)⋯Cu(II) distance of 3.4803(11) Å and Cu1–O11–Cu1 angle of 105.06(10)°. The paratactic LI and LII ligands, acting as double linkers, bridge [Cu–(μ2-O)2–Cu] units to generate a 2-D network parallel to the ab plane (Fig. 1b). The shortest Cu(II)⋯Cu(II) distance between adjacent dinuclear [Cu–(μ2-O)2–Cu] units is equal to 7.7070(11) Å. If each “double-linker” is considered as a bridge, and each [Cu–(μ2-O)2–Cu] unit is regarded as a four-connected node, compound 1 features a perfect 2-D Kagomé topology (Fig. 1c), which comprises a planar array of equilateral triangles and regular hexagons. Every triangle is corner-shared with three other neighboring triangles and edge-shared with three adjacent hexagons, which is also the same for every hexagon in the Kagomé network.25 Although the 1-tza-based complexes have been studied extensively,18–20 to the best of our knowledge, this kind of 2-D Kagomé array has been first observed in the reported 1-tza-based coordination compounds, which may be owing to two different kinds of coordination modes of 1-tza− ligands coexisting in the framework of 1. Furthermore, 2-D layers stack over each other in an –ABC–ABC– fashion (viewed along the a-axis in Fig. 1d) to generate open channels viewed along the c-axis (Fig. 1e). Bromine anions and lattice water molecules as guests are located in these voids. However, there is still approximately 32% void volume calculated using PLATON software.26
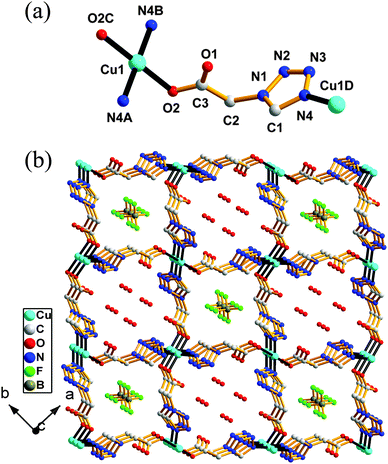
![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) and features a 1-D chain structure. The asymmetric unit of 3 consists of one Cu(II) center, one protonated 1-Htza ligand (labeled as LI), one deprotonate 1-tza− ligand (labeled as LII), one Cl− ion, one coordinated water molecule and half a lattice water molecule. The coordination geometry around the Cu(II) atom can be described as a distorted octahedron. As illustrated in Fig. 3a, two nitrogen atoms (N14, N24) from two different 1-Htza ligands, one oxygen atom (O1W) and a μ2-bridging chloride ion (Cl1) constitute a equatorial plane, while the axial positions are filled by a carboxylate O21B atom and another symmetry-related chloride ion (Cl1A) with the axial angle Cl1A–Cu1–O21B of 176.95(5)°, deviating the ideal angle of 180°. The axial Cu–Cl/O bond lengths (Cu1–Cl1A (2.8204(10) Å) and Cu1–O21B (2.3949(19) Å)) are significantly longer than those in the equatorial plane (Cu1–Cl1 (2.2961(8) Å) and Cu1–O1W (2.0300(19) Å)), which may be owing to Jahn–Teller distortion of the Cu(II) ion. However, the Cu–N bond lengths are in the normal range with 2.011(2) and 1.997(2) Å for Cu1–N14 and Cu1–N24, respectively. Each Cl− ion shows a μ2-bridging mode connecting two Cu(II) atoms into a centrosymmetric parallelogram unit [Cu–(μ2-Cl)2–Cu] with a Cu(II)⋯Cu(II) distance of 3.7041(11) Å and a Cu1–Cl1–Cu1A angle of 92.18(3)°. Then, two symmetry-related LII ligands with a μ2-κN4:κO1 coordination mode (mode b in Scheme 2) acting as double linkers bridge [Cu–(μ2-Cl)2–Cu] units into a 1-D chain along the b direction (Fig. 3b). As carboxylate in the LI ligand is protonated and the LI ligand displays a monodentate terminal coordination mode via its N4 atom (mode c in Scheme 2), the structure of 3 cannot be further extended. However, three kinds of O–H⋯O hydrogen bonds (Table S2, ESI†) connect the 1-D chain into a 3-D supramolecular framework (Fig. S3, ESI†).
and features a 1-D chain structure. The asymmetric unit of 3 consists of one Cu(II) center, one protonated 1-Htza ligand (labeled as LI), one deprotonate 1-tza− ligand (labeled as LII), one Cl− ion, one coordinated water molecule and half a lattice water molecule. The coordination geometry around the Cu(II) atom can be described as a distorted octahedron. As illustrated in Fig. 3a, two nitrogen atoms (N14, N24) from two different 1-Htza ligands, one oxygen atom (O1W) and a μ2-bridging chloride ion (Cl1) constitute a equatorial plane, while the axial positions are filled by a carboxylate O21B atom and another symmetry-related chloride ion (Cl1A) with the axial angle Cl1A–Cu1–O21B of 176.95(5)°, deviating the ideal angle of 180°. The axial Cu–Cl/O bond lengths (Cu1–Cl1A (2.8204(10) Å) and Cu1–O21B (2.3949(19) Å)) are significantly longer than those in the equatorial plane (Cu1–Cl1 (2.2961(8) Å) and Cu1–O1W (2.0300(19) Å)), which may be owing to Jahn–Teller distortion of the Cu(II) ion. However, the Cu–N bond lengths are in the normal range with 2.011(2) and 1.997(2) Å for Cu1–N14 and Cu1–N24, respectively. Each Cl− ion shows a μ2-bridging mode connecting two Cu(II) atoms into a centrosymmetric parallelogram unit [Cu–(μ2-Cl)2–Cu] with a Cu(II)⋯Cu(II) distance of 3.7041(11) Å and a Cu1–Cl1–Cu1A angle of 92.18(3)°. Then, two symmetry-related LII ligands with a μ2-κN4:κO1 coordination mode (mode b in Scheme 2) acting as double linkers bridge [Cu–(μ2-Cl)2–Cu] units into a 1-D chain along the b direction (Fig. 3b). As carboxylate in the LI ligand is protonated and the LI ligand displays a monodentate terminal coordination mode via its N4 atom (mode c in Scheme 2), the structure of 3 cannot be further extended. However, three kinds of O–H⋯O hydrogen bonds (Table S2, ESI†) connect the 1-D chain into a 3-D supramolecular framework (Fig. S3, ESI†).
Structural discussions of 1–5
As discussed above, compounds 1–5, obtained in [BMIM]X ILs (X = Br−, BF4−, NTf2−), possess distinct structures, and the 1-Htza ligand exhibits diverse connectivity modes (summarized in Scheme 2). Mode a is observed in 1, b in 1, 2 and 3, c in 3, 4 and 5. Modes b and c have been reported in previous literatures.18–20 However, mode a is first observed in 1-Htza-based coordination compounds. It is well known that Cu(II) ions usually display various coordination numbers, and four/five/six-coordinated Cu(II) centers have been found in 1–5. The different coordination modes of 1-Htza linkers and varied coordination numbers of Cu2+ ions offer the possibility of forming coordination compounds with different structures. Notably, the tetrazolate groups of 1-Htza ligands in 1–5 connect Cu(II) atoms with the N4-position atom, probably due to the N4-site having minimum steric hindrance compared with N2/N3-atoms. This fact has been observed in many other 1-Htza-based transition metal compounds.18 On the other hand, different guest molecules are located in 1–5. Although all the title compounds possess neutral structures, protonated water molecules and a corresponding amount of anions (Br−, BF4−) mainly from ILs are located in voids of 1 and 2, respectively, while only neutral water molecules are incorporated into 3–5. This shows that water molecules, as active proton acceptors, can effectively capture protons from 1-Htza in the medium of hygroscopic [BMIM]Br or [BMIM]BF4.Conclusion
In summary, we have first employed ionic liquids with different water miscibility, [BMIM]X (X = Br−, BF4−, NTf2−), as reaction media for synthesizing five new Cu-1-tza coordination compounds. Flexible coordination modes of the 1-Htza ligand and various coordination environments of Cu(II) atoms afford an opportunity to construct 1-Htza-based Cu(II) coordination compounds with different structures. Water molecules can act as active proton acceptors and ionic liquids have significant impacts on the deprotonation degree of 1-Htza with anions being altered. [BMIM]Br and [BMIM]BF4 are hygroscopic, and fully deprotonated 1-tza− exists in 1 and 2. However, [BMIM]NTf2 is hydrophobic, and partly or fully protonated 1-Htza exists in 3–5.Acknowledgements
This work was financially supported by 973 Program (2011CBA00505) and National Nature Science Foundation of China (21201099 and 21371170).References
- (a) M. Eddaoudi, D. B. Moler, H. L. Li, B. L. Chen, T. M. Reineke, M. O'Keeffe and O. M. Yaghi, Acc. Chem. Res., 2001, 34, 319–330 CrossRef CAS PubMed; (b) S. Natarajan and P. Mahata, Chem. Soc. Rev., 2009, 38, 2304–2318 RSC.
- (a) Z. M. Wang, Y. J. Zhang, T. Liu, M. Kurmoo and S. Gao, Adv. Funct. Mater., 2007, 17, 1523–1536 CrossRef CAS; (b) W. Ouellette, A. V. Prosvirin, K. Whitenack, K. R. Dunbar and J. Zubieta, Angew. Chem., Int. Ed., 2009, 48, 2140–2143 CrossRef CAS PubMed.
- (a) Y. Li, G. Xu, W. Q. Zou, M. S. Wang, F. K. Zheng, M. F. Wu, H. Y. Zeng, G. C. Guo and J. S. Huang, Inorg. Chem., 2008, 47, 7945–7947 CrossRef CAS PubMed; (b) M. D. Allendorf, C. A. Bauer, R. K. Bhaktaa and R. J. T. Houka, Chem. Soc. Rev., 2009, 38, 1330–1352 RSC; (c) M. S. Wang, S. P. Guo, Y. Li, L. Z. Cai, J. P. Zou, G. Xu, W. W. Zhou, F. K. Zheng and G. C. Guo, J. Am. Chem. Soc., 2009, 131, 13572–13573 CrossRef CAS PubMed; (d) Z. F. Liu, M. F. Wu, S. H. Wang, F. K. Zheng, G. E. Wang, J. Chen, Y. Xiao, A. Q. Wu, G. C. Guo and J. S. Huang, J. Mater. Chem. C, 2013, 1, 4634–4639 RSC.
- (a) W. Zhang, H. Y. Ye and R. G. Xiong, Coord. Chem. Rev., 2009, 253, 2980–2997 CrossRef CAS PubMed; (b) W. Zhang and R. G. Xiong, Chem. Rev., 2012, 112, 1163–1195 CrossRef CAS PubMed.
- (a) Y. Wang, P. Cheng, Y. Song, D. Z. Liao and S. P. Yan, Chem.–Eur. J., 2007, 13, 8131–8138 CrossRef CAS PubMed; (b) Q. X. Yao, J. L. Sun, J. K. Li, J. Su, M. V. Peskova and X. D. Zou, Dalton Trans., 2012, 41, 3953–3955 RSC.
- (a) M. Eddaoudi, J. Kim, N. Rosi, D. Vodak, J. Wachter, M. O'Keeffe and O. M. Yaghi, Science, 2002, 295, 469–472 CrossRef CAS PubMed; (b) J. R. Li, R. J. Kuppler and H. C. Zhou, Chem. Soc. Rev., 2009, 38, 1477–1504 RSC.
- (a) J. Y. Lee, O. K. Farha, J. Roberts, K. A. Scheidt, S. B. T. Nguyen and J. T. Hupp, Chem. Soc. Rev., 2009, 38, 1450–1459 RSC; (b) Y. Liu, W. M. Xuan and Y. Cui, Adv. Mater., 2010, 22, 4112–4135 CrossRef CAS PubMed.
- (a) E. R. Cooper, C. D. Andrews, P. S. Wheatley, P. B. Webb, P. Wormald and R. E. Morris, Nature, 2004, 430, 1012–1016 CrossRef CAS PubMed; (b) F. H. Aidoudi, D. W. Aldous, R. J. Goff, A. M. Z. Slawin, J. P. Attfield, R. E. Morris and P. Lightfoot, Nat. Chem., 2011, 3, 801–806 CrossRef CAS PubMed.
- (a) T. Welton, Chem. Rev., 1999, 99, 2071–2083 CrossRef CAS PubMed; (b) R. D. Rogers and K. R. Seddon, Science, 2003, 302, 792–793 CrossRef PubMed; (c) Ionic Liquids in Synthesis, ed. P. Wasserscheid and T. Welton, Wiley-VCH, Weinheim, 2008 Search PubMed.
- (a) N. Prondzinski, J. Cybinska and A. V. Mudring, Chem. Commun., 2010, 46, 4393–4395 RSC; (b) E. Boros, M. J. Earle, M. A. Gilea, A. Metlen, A. V. Mudring, F. Rieger, A. J. Robertson, K. R. Seddon, A. A. Tomaszowska, L. Trusov and J. S. Vyle, Chem. Commun., 2010, 46, 716–718 RSC; (c) R. Giernoth, Angew. Chem., Int. Ed., 2010, 49, 2834–2839 CrossRef CAS PubMed.
- (a) Z. Ma, J. Yu and S. Dai, Adv. Mater., 2010, 22, 261–285 CrossRef CAS PubMed; (b) D. Freudenmann, S. Wolf, M. Wolff and C. Feldmann, Angew. Chem., Int. Ed., 2011, 50, 11050–11060 CrossRef CAS PubMed; (c) E. Ahmed and M. Ruck, Coord. Chem. Rev., 2011, 255, 2892–2903 CrossRef CAS PubMed; (d) A. Taubert and Z. Li, Dalton Trans., 2007, 723–727 RSC; (e) W. W. Xiong, J. R. Li, B. Hu, B. Tan, R. F. Li and X. Y. Huang, Chem. Sci., 2012, 3, 1200–1204 RSC; (f) E. Ahmed and M. Ruck, Angew. Chem., Int. Ed., 2012, 51, 308–309 CrossRef CAS PubMed; (g) B. T. Yonemoto, Z. J. Lin and F. Jiao, Chem. Commun., 2012, 48, 9132–9134 RSC.
- (a) K. Jin, X. Y. Huang, L. Pang, J. Li, A. Appel and S. Wherland, Chem. Commun., 2002, 2872–2873 RSC; (b) D. N. Dybtsev, H. Chun and K. Kim, Chem. Commun., 2004, 1594–1595 RSC; (c) J. H. Liao, P. C. Wu and W. C. Huang, Cryst. Growth Des., 2006, 6, 1062–1063 CrossRef CAS; (d) Z. J. Lin, D. S. Wragg and R. E. Morris, Chem. Commun., 2006, 2021–2023 RSC; (e) E. R. Parnham and R. E. Morris, Acc. Chem. Res., 2007, 40, 1005–1013 CrossRef CAS PubMed; (f) J. Zhang, T. Wu, S. Chen, P. Feng and X. Bu, Angew. Chem., Int. Ed., 2009, 48, 3486–3490 CrossRef CAS PubMed; (g) L. Xu, E. Y. Choi and Y. U. Kwon, Inorg. Chem. Commun., 2008, 11, 150–154 CrossRef CAS PubMed; (h) W. X. Chen, Y. P. Ren, L. S. Long, R. B. Huang and L. S. Zheng, CrystEngComm, 2009, 11, 1522–1525 RSC.
- (a) R. E. Morris, Chem. Commun., 2009, 2990–2998 RSC; (b) G. A. V. Martins, P. J. Byrne, P. Allan, S. J. Teat, A. M. Z. Slawin, Y. Li and R. E. Morris, Dalton Trans., 2010, 39, 1758–1762 RSC; (c) H. Fu, Y. G. Li, Y. Lu, W. L. Chen, Q. Wu, J. X. Meng, X. L. Wang, Z. M. Zhang and E. B. Wang, Cryst. Growth Des., 2011, 11, 458–465 CrossRef CAS; (d) H. Fu, C. Qin, Y. Lu, Z. M. Zhang, Y. G. Li, Z. M. Su, W. L. Li and E. B. Wang, Angew. Chem., Int. Ed., 2012, 51, 7985–7989 CrossRef CAS PubMed; (e) L. M. Li, K. Cheng, F. Wang and J. Zhang, Inorg. Chem., 2013, 52, 5654–5656 CrossRef CAS PubMed.
- (a) L. Xu, E. Y. Choi and Y. U. Kwon, Inorg. Chem., 2007, 46, 10670–10680 CrossRef CAS PubMed; (b) L. Xu, E. Y. Choi and Y. U. Kwon, Inorg. Chem., 2008, 47, 1907–1909 CrossRef CAS PubMed.
- (a) Z. J. Lin, A. Z. Slawin and R. E. Morris, J. Am. Chem. Soc., 2007, 129, 4880–4881 CrossRef CAS PubMed; (b) Z. J. Lin, D. S. Wragg, J. E. Warren and R. E. Morris, J. Am. Chem. Soc., 2007, 129, 10334–10335 CrossRef CAS PubMed; (c) L. Xu, S. Yan, E. Y. Choi, J. Y. Lee and Y. U. Kwon, Chem. Commun., 2009, 3431–3433 RSC; (d) Q. Y. Liu, W. L. Xiong, C. M. Liu, Y. L. Wang, J. J. Wei, Z. J. Xiahou and L. H. Xiong, Inorg. Chem., 2013, 52, 6773–6775 CrossRef CAS PubMed.
- (a) P. Wasserscheid and W. Keim, Angew. Chem., Int. Ed., 2000, 39, 3772–3789 CrossRef CAS; (b) J. G. Huddleston, A. E. Visser, W. M. Reichert, H. D. Willauer, G. A. Broker and R. D. Rogers, Green Chem., 2001, 3, 156–164 RSC; (c) D. R. MacFarlane, J. M. Pringle, K. M. Johansson, S. A. Forsyth and M. Forsyth, Chem. Commun., 2006, 1905–1917 RSC.
- (a) C. N. R. Rao, S. Natarajan and R. Vaidhyanathan, Angew. Chem., Int. Ed., 2004, 43, 1466–1496 CrossRef CAS PubMed; (b) H. Zhao, Z. R. Qu, H. Y. Ye and R. G. Xiong, Chem. Soc. Rev., 2008, 37, 84–100 RSC; (c) D. J. Tranchemonotagne, J. L. Mendoza-Cortes, M. O'Keeffe and O. M. Yaghi, Chem. Soc. Rev., 2009, 38, 1257–1283 RSC; (d) G. Aromí, L. A. Barrios, O. Roubeau and P. Gamez, Coord. Chem. Rev., 2011, 255, 485–546 CrossRef PubMed.
- (a) Q. Yu, X. Q. Zhang, H. D. Bian, H. Liang, B. Zhao, S. P. Yan and D. Z. Liao, Cryst. Growth Des., 2008, 8, 1140–1146 CrossRef CAS; (b) W. W. Dong, J. Zhao and L. Xu, Cryst. Growth Des., 2008, 8, 2882–2886 CrossRef CAS; (c) H. Y. Yang, L. K. Li, J. Wu, H. W. Hou, B. Xiao and Y. T. Fan, Chem.–Eur. J., 2009, 15, 4049–4056 CrossRef CAS PubMed; (d) K. L. Zhang, Z. C. Pan, Y. Chang, W. L. Liu and N. S. Weng, Mater. Lett., 2009, 63, 2136–2138 CrossRef CAS PubMed; (e) X. Q. Zhang, Q. Yu, H. D. Bian, X. G. Bao and H. Liang, J. Coord. Chem., 2009, 62, 2108–2117 CrossRef CAS; (f) L. Ma, G. Peng, J. B. Cai, H. Deng and M. Z. M. Zeller, Z. Anorg. Allg. Chem., 2011, 637, 755–758 CrossRef CAS.
- (a) X. Q. Zhang, Q. Yu, H. D. Bian, S. P. Yan, D. Z. Liao, W. Gu and H. Liang, Aust. J. Chem., 2008, 61, 303–309 CrossRef CAS; (b) D. G. Ding, M. L. Zhang, L. C. Li, Y. T. Fan and H. W. Hou, J. Coord. Chem., 2009, 62, 2675–2681 CrossRef CAS.
- F. He, M. L. Tong, X. L. Yu and X. M. Chen, Inorg. Chem., 2005, 44, 559–565 CrossRef CAS PubMed.
- (a) Z. R. Qu, H. Zhao, X. S. Wang, Y. H. Li, Y. M. Song, Y. J. Liu, Q. O. Ye, R. G. Xiong, B. F. Abrahams, Z. L. Xue and X. Z. You, Inorg. Chem., 2003, 42, 7710–7712 CrossRef CAS PubMed; (b) Q. Ye, Y. M. Song, G. X. Wang, K. Chen, D. W. Fu, P. W. H. Chan, J. S. Zhu, S. D. Huang and R. G. Xiong, J. Am. Chem. Soc., 2006, 128, 6554–6555 CrossRef CAS PubMed; (c) A. Rodriguez-Dieguez, A. J. Mota, J. Cano, J. Ruiz, D. Choquesillo-Lazarte and E. Colacio, Dalton Trans., 2009, 6335–6344 RSC; (d) P. Pachfule, Y. F. Chen, S. C. Sahoo, J. W. Jiang and R. Banerjee, Chem. Mater., 2011, 23, 2908–2916 CrossRef CAS; (e) M. F. Wu, M. S. Wang, S. P. Guo, F. K. Zheng, H. F. Chen, X. M. Jiang, G. N. Liu, G. C. Guo and J. S. Huang, Cryst. Growth Des., 2011, 11, 372–381 CrossRef CAS; (f) S. H. Wang, F. K. Zheng, M. F. Wu, Z. F. Liu, J. Chen, G. C. Guo and A. Q. Wu, CrystEngComm, 2013, 15, 2616–2623 RSC.
- CrystalClear, version 1.35; Software User's Guide for the Rigaku R-Axis and Mercury and Jupiter CCD Automated X-ray Imaging System, Rigaku Molecular Structure Corporation, UT, 2002 Search PubMed.
- SHELXTL Reference Manual, version 5, Siemens Energy & Automation Inc., Madison, WI, 1994 Search PubMed.
- L. Yang, D. R. Powell and R. P. Houser, Dalton Trans., 2007, 955–964 RSC.
- (a) Y. L. Liu, V. C. Kravtsov, D. A. Beauchamp, J. F. Eubank and M. Eddaoudi, J. Am. Chem. Soc., 2005, 127, 7266–7267 CrossRef CAS PubMed; (b) S. K. Pati and C. N. R. Rao, Chem. Commun., 2008, 4683–4693 RSC; (c) C. Valkringer, M. Meddouri, T. Loiseau, N. Guillou, J. Marrot, G. Férey, M. Haouas, F. Taulelle, N. Audebtand and M. Latroche, Inorg. Chem., 2008, 47, 11892–11901 CrossRef PubMed; (d) F. H. Aidoudi, D. W. Aldous, R. J. Goff, A. M. Z. Slawin, J. P. Attfield, R. E. Morris and P. Lightfoot, Nat. Chem., 2011, 3, 801–806 CrossRef CAS PubMed; (e) A. C. Wibowo, M. D. Smith and H. C. zur Loye, Chem. Commun., 2011, 47, 7371–7373 RSC.
- A. L. Spek, PLATON, A Multipurpose Crystallographic Tool, Utrecht University, The Netherlands, 2005 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: X-ray crystallographic files in CIF format for 1–5, selected bond lengths and angles, hydrogen-bonds, coordination modes of 1-Htza, thermochemical properties, PXRD patterns, additional structural plots and FT-IR spectra. CCDC 942747–942751. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c3nj01198d |
| This journal is © The Royal Society of Chemistry and the Centre National de la Recherche Scientifique 2014 |