Multi-emitting properties of hybrid Langmuir–Blodgett films of amphiphilic iridium complexes and the exfoliated nanosheets of saponite clay†
Hisako
Sato
*a,
Kenji
Tamura
b,
Keishi
Ohara
a and
Shin-ichi
Nagaoka
a
aDepartment of Chemistry, Graduate School of Science and Engineering, Ehime University, Matsuyama 790-8577, Japan. E-mail: sato.hisako.my@ehime-u.ac.jp
bNational Institute of Materials Science, Tsukuba 305-0044, Japan
First published on 22nd October 2013
Abstract
Mono- and multi-layered films comprised of amphiphilic cationic iridium(III) complexes hybridized with the exfoliated nanosheets of synthetic saponite were prepared by the modified Langmuir–Blodgett method. Three iridium(III) complexes with different emission maxima (λmax) were used as incorporated complexes: [Ir(dfppy)2(dc9bpy)]+ (λmax = 500 nm) (dfppyH = 2-(4′,6′-difluorophenyl)pyridine; dc9bpy = 4,4′-dinonyl-2,2′-bipyridine) [Ir(ppy)2(dc9bpy)]+ (λmax = 550 nm) (ppyH = 2-phenylpyridine) and [Ir(piq)2(dc9bpy)]+ (λmax = 590 nm) (piqH = 1-phenylisoquinoline) for blue, yellow and red emissions, respectively. Six triple-layered films with different layer sequences were fabricated by layer-by-layer deposition onto quartz substrates. Stationary emission spectra were recorded on the prepared films under vacuum and at various oxygen pressures. Notably the change in the spectral shape at surrounding oxygen pressure depended remarkably on the layer sequence. Quenching by oxygen molecules was analyzed by applying a two-site model to the Stern–Volmer plots. The present nanometer-thick films were regarded as a benchmark for an optical device emitting different visible lights in response to oxygen pressure.
Introduction
Luminescent transition metal complexes have been applied as emitting elements for photo-responsive devices.1–9 Among them, cyclometalated iridium(III) complexes (denoted Ir(III) complexes) are attracting extensive attention due to their high emitting properties in the visible region. Various Ir(III) complexes are explored for tuning emission maxima.10–38 They are used in optical devices such as photo-diodes and oxygen sensors.6,7,13–15 Their functions are based on the properties that energy transfer takes place efficiently from the triplet excited state of an Ir(III) complex to semiconductors or an oxygen molecule in a triplet ground state.13,20The important properties that make them desirable as a sensing device are rapid and reversible responses together with mechanical strength. One approach to achieve these requisites is to fabricate Ir(III) complexes within organic or inorganic host materials such as synthetic polymers or mesoporous silica or alumina.28–37 Such hybridization enhances robustness and reliability as sensing devices. Among inorganic supports, clay minerals are known to be a layered host intercalating photo-responsive molecules.38–47 The extent of energy transfer between donor and acceptor molecules intercalated within the interlayer spaces was estimated previously.45–47
In our recent work, the hybridization of an amphiphilic Ir(III) complex with a montmorillonite clay was performed by means of the modified Langmuir–Blodgett (LB) method.28,47 According to this method, a floating monolayer of an amphiphilic cationic complex was formed on a subphase containing the exfoliated nanosheets of a clay mineral. Hybridization took place electrostatically between a cationic monolayer and negatively charged clay sheets at an air–water interface.28 The floating hybrid film was deposited onto a solid substrate. The nanometer-thick films thus prepared achieved high emission efficiency and sensitivity for oxygen gas.47
The present work employed three amphiphilic Ir(III) complexes with different emission maxima. The monolayer films of these complexes were hybridized with a clay mineral, synthetic sodium saponite. Its elemental composition was stated to be [(Na0.25Mg0.07)(Mg2.98Al0.01)(Si3.6Al0.4)O10(OH)2] with no heavy metal ion acting as a luminescent quencher.48 Triple-layered hybrid films were constructed by the layer-by-layer deposition of floating hybridized monolayers onto quartz substrates. Attention was focused on how the spectral response of emission to oxygen was dependent on the layer sequence of three Ir(III) complexes. This work would be a benchmark to explore multi-emitting sensor films based on photo-responsive metal complexes.
Results and discussion
Preparation of hybrid single- or multi-layered films
The UV-visible spectra of the methanol solutions of the used iridium complexes are shown in the ESI† (Fig. S1). A chloroform solution containing a perchlorate salt of [Ir(ppy)2(dc9bpy)]+ (denoted PPY) or [Ir(dfppy)2(dc9bpy)]+ (denoted DFPPY) or [Ir(piq)2(dc9bpy)]+ (denoted PIQ) was spread onto an aqueous dispersion of a synthetic saponite clay (denoted SAP) at a concentration of 10 mg L−1. The cation-exchange capacity (CEC) of SAP was found to be 80 meq per 100 g. Upon compressing a trough surface, surface pressure started to increase around the molecular area of 1.5–2 nm2. This suggested that the Ir(III) complexes formed a monomolecular film at an air–water interface (Fig. 1). The surface pressure–molecular area curves (denoted π–A curves) were compared with the ones obtained over pure water.28 The monolayers of Ir(III) complexes over a clay dispersion were more expanded and rigid than those over pure water. The results supported the occurrence of hybridization of Ir(III) complexes with clay particles at an air–water interface. The hybrid floating film was transferred by the vertical deposition method onto a hydrophilic quartz plate at the surface pressure of 10 mN m−1. Deposition took place only in the upward direction. The transfer ratio was found to be 0.9 ± 0.1 in all the cases. Six kinds of double- and triple-layered hybrid films with different layer sequences were prepared using the layer-by-layer method as shown in Scheme 1(A) and (B). The details of preparation of multi-layered films are provided in the experimental section. | ||
| Fig. 1 The π–A curves when the perchlorate salts of DFPPY (bold solid), PPY (dotted) and PIQ (broken) were spread onto an aqueous dispersion of SAP (10 mg L−1). | ||
The surfaces of the deposited films were observed by means of atomic force microscopy (AFM). As shown in the ESI† (Fig. S2 and S3), the hybrid films had nanometer-order thickness. There was little difference in the surface morphology among the observed films. The UV-visible spectra of single-, double- and triple-layered hybrid films are also shown in the ESI† (Fig. S4).
Emission properties of single-layered films
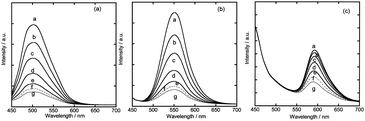
Fig. 2(a)–(c) show the emission spectra of the single-layered films, in which the surface of a quartz plate was modified with a single-layered hybrid film denoted {X/SAP} (X = DFPPY, PPY and PIQ). The films were irradiated at 430 nm either under vacuum or under an oxygen atmosphere. The peak wavelength (λmax) of emission reflected the character of each Ir(III) complex incorporated in the film: λmax = 500 nm, 550 nm, and 590 nm for {DFPPY/SAP}, {PPY/SAP} and {PIQ/SAP}, respectively. In the case of {PIQ/SAP}, the emission was more than ten times weaker than from {PPY/SAP} or {DFPPY/SAP} so that the background overlapped appreciably the emission spectrum in the wavelength region shorter than 500 nm. When oxygen gas was introduced into the cell, the intensity of the emission clearly decreased due to the quenching of excited Ir(III) complexes (denoted X*) by oxygen molecules (Fig. 2).In order to investigate the possibility of energy transfer within a single-layered film, a mixed monolayer containing DFPPY, PPY, and PIQ at a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio was hybridized with clay nanosheets at an air–water interface. The floating hybrid film (denoted {DFPPY, PPY, PIQ/SAP}) was transferred onto a quartz plate. As shown in Fig. S5 in the ESI,† the emission spectrum showed a sharp single peak at 590 nm with low intensity at 550 nm and 500 nm. The results implied that the energy transfer from excited DFPPY (or DFPPY*) and PPY* to PIQ took place efficiently when they were located in the same layer (or intra-layer energy transfer). From the results on the single-layered hybrid films, it was concluded that a nanometer-thick film exhibited high emitting properties characteristic of an adsorbed dye molecule.
1 ratio was hybridized with clay nanosheets at an air–water interface. The floating hybrid film (denoted {DFPPY, PPY, PIQ/SAP}) was transferred onto a quartz plate. As shown in Fig. S5 in the ESI,† the emission spectrum showed a sharp single peak at 590 nm with low intensity at 550 nm and 500 nm. The results implied that the energy transfer from excited DFPPY (or DFPPY*) and PPY* to PIQ took place efficiently when they were located in the same layer (or intra-layer energy transfer). From the results on the single-layered hybrid films, it was concluded that a nanometer-thick film exhibited high emitting properties characteristic of an adsorbed dye molecule.
Emission properties of heterogeneous double-layered films
Six double-layered hybrid films with different layer sequences were prepared as shown in Scheme 1(A). The absorption spectra of these films are shown in the ESI† (Fig. S4). It was confirmed that the absorbance was expressed as the sum of two composite monolayers.Fig. 3(a) and (b) show the emission spectra of the quartz plates modified with (a) {DFPPY/SAP/PPY/SAP} and (b) {DFPPY/SAP/PIQ/SAP}, respectively, when they were irradiated at 430 nm under vacuum or at various oxygen pressures. The results obtained under vacuum showed the emission maximum (λmax) at 550 nm (PPY*) and 590 nm (PIQ*), respectively. Although there was some emission observed at 500 nm from DFPPY*, it was very low in comparison to {DFPPY/SAP}. The results implied that the energy transfer took place efficiently from DFPPY* to PPY and PIQ in the double-layered films. The same conclusion was derived from the emission spectra of other double-layered films as given in Table S1, Fig. S6 and S7 in the ESI.† It was noted that the occurrence of inter-layer energy transfer made PIQ the weakest emitter in a single-layered film and the highly emitting species in the double-layered films.
The intensity of the spectra decreased over the whole wavelength region upon introducing oxygen gas. The results implied that oxygen molecules penetrated both the upper and lower layers, quenching the whole Ir(III) complexes in films.
The above results confirmed that inter-layer energy transfer took place efficiently between the metal complexes located in different layers.
Emission properties of heterogeneous triple-layered hybrid films
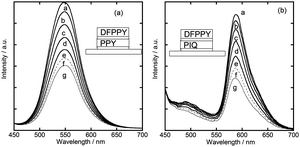
Six triple-layered hybrid films with different layer sequences were prepared as shown in Scheme 1(B). The UV-visible spectra of these films are shown in Fig. S4 in the ESI.†Fig. 4(a)–(f) show the emission spectra, when the films were irradiated at 430 nm either under vacuum or at various oxygen pressures.The following features are noticed concerning the emission profiles under vacuum. For all of the films, no peak was observed at around 500 nm. The results implied that the excitation energy of DFPPY* transferred substantially to PPY and/or PIQ. Such inter-layer energy transfer took place irrespective of the location of {DFPPY/SAP} within the triple-layered films. The ratio of the emission intensity at 590 nm due to PIQ* (denoted I590) to I550 due to PPY* was dependent on the layer sequence remarkably. According to the magnitude of I590/I550, the investigated six films were classified into the following two groups: group A (small I590/I550 values) including (a) and (b) and group B (large I590/I550 values) including (c), (d), (e), and (f) in Scheme 1(B). In the films belonging to group A, {DFPPY/SAP} was located in between {PPY/SAP} and {PIQ/SAP}. Since PPY was remote from PIQ, the inter-layer energy transfer from PPY* to PIQ occurred less efficiently. Thus the emission from PPY* (or I550) remained at a higher level, leading to the small value of I590/I550. In the films belonging to group B, {PPY/SAP} was in direct contact with {PIQ/SAP}. Thus the inter-layer energy transfer from PPY* to PIQ occurred efficiently, lowering the emission at 550 nm with the simultaneous increase of the emission at 590 nm. This resulted in the large value of I590/I550.
The above results on the heterogeneous triple-layered films were compared with the emission behavior of a single-layered mixed film {DFPPY, PPY, PIQ/SAP} (Fig. S5 in the ESI†). The occurrence of selective energy transfer as observed in the former films supported the elucidation of the layer-by-layer structures.
For all the triple-layered films, emission intensity decreased upon introducing oxygen gas. The effect of oxygen molecules on the change in the spectral shape, however, was different among the investigated films. These features are summarized as follows: in the case of (a) {PPY/SAP/DFPPY/SAP/PIQ/SAP}, the emission intensity decreased with the increase of oxygen pressure until the emission peak remained at around 590 nm. PPY* in the uppermost layer was expected to be quenched more efficiently than PIQ* in the lowest layer. Thus the emission from PIQ* (I590) remained at higher oxygen pressure. In the case of (b) {PIQ/SAP/DFPPY/SAP/PPY/SAP} and (d) {PIQ/SAP/PPY/SAP/DFPPY/SAP}, the emission intensity decreased until the emission spectrum exhibited a broad peak at around 550 nm. PIQ* in the uppermost layer was expected to be quenched more efficiently than PPY* in the middle or lowest layer. Thus the emission from PPY* (I550) remained at higher oxygen pressure. In the case of (c) {DFPPY/SAP/PPY/SAP/PIQ/SAP} and (e) {DFPPY/SAP/PIQ/SAP/PPY/SAP}, the emission intensity decreased uniformly with no drastic change in the spectral shape. DFPPY* in the uppermost layer was expected to be quenched most efficiently. The decrease of energy transfer from DFPPY* resulted in the simultaneous lowering of emission from both PPY* (I550) and PIQ* (I590). In the case of (f) {PPY/SAP/PIQ/SAP/DFPPY/SAP}, the emission intensity decreased uniformly with a little change in the spectral shape. The quenching of PPY* in the uppermost layer took place efficiently under the simultaneous energy transfer from DFPPY*. It was difficult to estimate how these two opposite effects affected the whole emission profiles.
The quenching efficiency of oxygen molecules might be dependent on their penetrating depth in the films. As a result, two emitting molecules located in different layers were quenched to different degrees at a given pressure. These situations lead to the appearance of multi-emission behaviour, depending on the oxygen pressure. In the case of (a), for example, the broad emission band (550–600 nm) changed to a sharp peak at 600 nm upon increasing oxygen pressure.
Analysis of energy transfer in triple-layered hybrid films under vacuum
The processes of inter-layer energy transfer in the triple-layered hybrid films were analysed on the basis of the Förster-type mechanism.46 Three paths were assumed as energy transfer routes: DFPPY* to PIQ (route 1), DFPPY* to PPY (route 2), and PPY* to PIQ (route 3). Based on these, the following expressions were derived: | (1) |
The parameters, ηnET, and ϕn (n = 1–3), were determined to make the calculated Ftotal(ν) best fitted to the experimental emission spectra. In reproducing the experimental spectra, the values of ηnET (n = 1 and 2) and ϕ1 were first selected under the condition of η1ET + η2ET + ϕ1 = 1. This assumption was introduced on the basis of the fact that no emission from DFPPY* was observed in the experimental spectra. In the next stage, η3ET and ϕ2 were varied under the condition of η3ET + ϕ2 ≦ 1. This was assumed since the emission from PPY* remained in the observed spectra. The values of these parameters were chosen such that the calculated I590/I550 value was as close as possible to the observed one. Finally the value of ϕ3 was selected so as to adjust the calculated I590 in accord with the observed one. The parameters thus obtained are tabulated in Tables 1(A)–(C). The final simulated curves are shown in the ESI† (Fig. S8).
| (A) | ||
|---|---|---|
| Vacuum | (a) | (b) |
| η 1ET (DFPPY* → PPY) | 0.20 | 0.20 |
| ϕ 1 | 0.48 | 0.48 |
| η 2ET (DFPPY* → PIQ) | 0.32 | 0.32 |
| ϕ 2 | 0.40 | 0.48 |
| η 3ET (PPY* → PIQ) | 0.00 | 0.00 |
| ϕ 3 | −5.10 | −4.50 |
| A df | 0.00017 | 0.00017 |
| A ppy | 0.00096 | 0.00096 |
| A piq | 0.00165 | 0.00165 |
| (B) | ||
|---|---|---|
| Vacuum | (c) | (d) |
| η 1ET (DFPPY* → PPY) | 0.87 | 0.87 |
| ϕ 1 | 0.08 | 0.00 |
| η 2ET (DFPPY* → PIQ) | 0.05 | 0.13 |
| ϕ 2 | 0.60 | 0.60 |
| η 3ET (PPY* → PIQ) | 0.02 | 0.10 |
| ϕ 3 | −5.20 | −3.80 |
| A df | 0.00017 | 0.00017 |
| A ppy | 0.00096 | 0.00096 |
| A piq | 0.00165 | 0.00165 |
| (C) | ||
|---|---|---|
| Vacuum | (e) | (f) |
| η 1ET (DFPPY* → PPY) | 0.00 | 0.00 |
| ϕ 1 | 0.50 | 0.50 |
| η 2ET (DFPPY* → PIQ) | 0.50 | 0.50 |
| ϕ 2 | 0.35 | 0.37 |
| η 3ET (PPY* → PIQ) | 0.37 | 0.37 |
| ϕ 3 | −4.70 | −4.50 |
| A df | 0.00017 | 0.00017 |
| A ppy | 0.00096 | 0.00096 |
| A piq | 0.00165 | 0.00165 |
In the films belonging to group A ((a) and (b) in Fig. 4), the most part of excitation energy in DFPPY* was transferred directly to PPY (∼20%) and to PIQ (∼30%), while the contribution of the energy transfer from PPY* to PIQ was nearly zero. As a result, the emission from PPY* remained to be observed together with the emission from PIQ*. In the films of (c) and (d) in Fig. 4, both of which belonged to group B, the most part of excitation energy in DFPPY* was transferred to PPY (∼90%). The fraction of energy transferred from DFPPY* to PIQ was lower than 10%, probably because {PIQ/SAP} was remote from {DFPPY/SAP}.
When the same parameter set was assigned to the films of (e) and (f), both of which also belonged to group B, the most part of energy in DFPPY* was transferred to PIQ (∼50%). Since {PPY/SAP} was in direct contact with {PIQ/SAP} in this group, the energy transfer from PPY* to PIQ was efficient (∼40%). As a result, the emission from PPY* nearly disappeared in the observed emission spectra.
As for the values of ϕn (n = 1, 2 and 3), which indicated the effects of clay sheets on emission efficiency, ϕn (n = 1 and 2) was positive, while ϕ3 was negative. In other words, the emission was lowered when DFPPY and PPY molecules were in contact with a clay sheet, while the effect was reversed for PIQ. The reason for this difference was unclear. The general features as derived from the analyses were in agreement with the qualitative views stated in the previous section.
Analysis of quenching by oxygen molecules in the case of triple-layered hybrid films
The quenching behavior of triple-layered hybrid films under an oxygen atmosphere was analysed according to the Stern–Volmer plots. In the plots, the total area of the emission in the wavelength region from 450 nm to 700 nm under vacuum or under an oxygen atmosphere was taken to be I0 and I, respectively. The ratio of I0/I was plotted as a function of oxygen pressure (PO2) (Fig. 5). The change of the total emission contained the contribution from oxygen quenching of both PPY* and PIQ*. Thus the results presented in the tables compared the total quenching effects among the triple-layered films with different layer sequences. | ||
| Fig. 5 Stern–Volmer plots for quenching by oxygen molecules in heterogeneous triple-layered films: (a) {PPY/SAP/DFPPY/SAP/PIQ/SAP}, (b) {PIQ/SAP/DFPPY/SAP/PPY/SAP}, (c) {DFPPY/SAP/PPY/SAP/PIQ/SAP}, (d) {PIQ/SAP/PPY/SAP/DFPPY/SAP}, (e) {DFPPY/SAP/PIQ/SAP/PPY/SAP}, and (f) {PPY/SAP/PIQ/SAP/DFPPY/SAP}. Curves were calculated according to eqn (2) by using the parameters in Table 2. | ||
Since the plots curved downward as shown in Fig. 5, the results were analysed in terms of the two-site model as expressed by eqn (2).31 In the equation, two binding sites with different quenching constants (Ksv1 and Ksv2) were assumed:
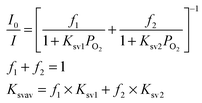 | (2) |
Table 2 shows the parameters determined for six triple-layered hybrid films. According to the results, the films contained strongly quenched (20–60%) and weakly quenched sites (80–40%). The strongly quenching constants were ca. 40–200 times as high as the weakly quenching ones. The overall quenching constants (Ksav) took nearly the same value (∼0.02–0.06) among the six films. In other words, the present triple-layered films exhibited a similar sensitivity for oxygen molecules irrespective of the difference in layer sequence.
| Parameter | (a) | (b) | (c) | (d) | (e) | (f) |
|---|---|---|---|---|---|---|
| K sv1 | 0.004 | 0.001 | 0.003 | 0.002 | 0.002 | 0.004 |
| K sv2 | 0.08 | 0.10 | 0.08 | 0.13 | 0.18 | 0.09 |
| f 1 | 0.37 | 0.78 | 0.58 | 0.81 | 0.76 | 0.47 |
| f 2 | 0.63 | 0.22 | 0.42 | 0.19 | 0.24 | 0.53 |
| K svav | 0.06 | 0.02 | 0.04 | 0.03 | 0.05 | 0.05 |
At present, it was not clear which structure was responsible for the strongly and weakly quenched sites. One possibility was that the strongly and weakly quenched sites corresponded to the edges and basal surfaces of a clay sheet, respectively. The former complexes might be more accessible to oxygen molecules than the latter, leading to a larger quenching constant. Apart from these uncertain aspects, however, the results demonstrated the applicability of the hybrid films organized within nanometer-order thickness as an element for photo-sensing oxygen molecules.
Conclusions
Multi-layered hybrid films consisting of three different kinds of amphiphilic cationic iridium(III) complexes and the exfoliated nanosheets of synthetic saponite were prepared by the modified Langmuir–Blodgett method. The emission spectra were recorded under vacuum or under an oxygen atmosphere. As a result, the spectral response to oxygen pressure was dependent on the layer sequence of iridium(III) complexes remarkably. For one film, for example, the broad emission band (550–600 nm) changed to a sharp peak at 600 nm upon increasing oxygen pressure. The results were rationalized in terms of the mechanism that the quenching by oxygen molecules was dependent on their penetrating depth in the films at a given pressure. The present nanometer-thick films were regarded as a benchmark for an optical device emitting different visible lights in response to oxygen pressure.Experimental section
Materials
[Ir(ppy)2Cl]2 (ppyH = 2-phenylpyridine) was synthesized according to the reported method.5 [Ir(dfppy)2Cl]2 (dfppyH = 2-(4′,6′-difluorophenyl)pyridine) and [Ir(piq)2Cl]2 (piqH = 1-phenylisoquinoline) were purchased from Furuya Metal Co., Ltd, Japan. An amphiphilic cyclometalated Ir(III) complex, [Ir(ppy)2(dc9bpy)]ClO4 (dc9bpy = 4,4′-dinonyl-2,2′-bipyridine) was prepared by refluxing [Ir(ppy)2Cl]2 with an equal amount of dc9bpy in glycerol at 180 °C for 6 hours. The compound was purified chromatographically by being eluted on an HPLC column (CAPCELL PAK C18, MG (Shiseido Inc. Ltd, Japan)) with chloroform. [Ir(dfppy)2(dc9bpy)]ClO4 and [Ir(piq)2(dc9bpy)]ClO4 were prepared in the same way. They were identified by 1H NMR in CDCl3, mass spectra and UV-visible spectra in methanol (ESI,† Fig. S1). Synthetic saponite was purchased from Kunimine Ind. Co., whose elemental composition and cation-exchange capacity (CEC) are stated to be [(Na0.25Mg0.07)(Mg2.98Al0.01)(Si3.6Al0.4)O10(OH)2] and 80 meq per 100 g, respectively.48 The average particle size was reported to be ca. 20 nm.Instruments
1H NMR spectra were recorded using a JNM-AL400 spectrometer (JEOL, Japan). UV-visible electronic spectra of the LB films deposited on quartz substrates were recorded using a Hitachi U-2810 spectrophotometer. Emission spectra were measured using a FP-6500 fluorophotometer (JASCO, Japan). The surface morphologies of hybrid films were investigated using an atomic force microscope (SPM-9600, Shimadzu, Co. Ltd, Japan).Film preparation
Modified LB films were prepared using a LB trough (USI System, Japan). A trough with a surface area of 10.0 cm × 12.0 cm was maintained at 20 °C by circulating water. 0.2 mL of chloroform solution containing a perchlorate salt of iridium(III) complex (ca. 5 × 10−5 M) was spread over an aqueous dispersion of a saponite clay (10 mg L−1). After 30 min, the surface was compressed at a rate of 10 cm2 min−1 until the surface pressure reached 10 mN m−1. Keeping the surface pressure at 10 mN m−1 for 30 minutes, the film was transferred onto a hydrophilic quartz plate (1 cm × 2 cm) by the vertical deposition method at a dipping rate of 10 mm min−1. Double- or triple-layered films with different layer sequences were prepared by the layer-by-layer method. In this procedure, the quartz plates were dried under vacuum for 30 minutes after depositing each layer.Emission measurements
For the measurements of emission spectra from a deposited film, the quartz substrate was placed at 45° with respect to the incident light. The emitted light was detected at 90° with respect to the incident light. The emission was measured either under vacuum (<0.1 kPa) or under an oxygen atmosphere at room temperature.Acknowledgements
We thank late Mr Yasuhiko Nakatani (Ehime University) for his preparation of the LB films and Dr Kazuya Morimoto (Ehime University) for measuring AFM. Thanks are also due to Prof. Akihiko Yamagishi (Toho University) for his valuable suggestions. We appreciate the Integrated Center for Science, Ehime University for the mass spectral measurements. This work has been financially supported by the MEXT KAKENHI Grant-Aid-for Scientific Research (B) Number 23350069 of Japan, a Grant-in-Aid for Exploratory Research Number 23655200 and the Nippon Sheet Glass Foundation of Materials and Science and Engineering of Japan, JST A-STEP Number AS2421179M.Notes and references
- V. Balzani, A. Juris, M. Venturi, S. Campagna and S. Serroni, Chem. Rev., 1996, 96, 759 CrossRef CAS PubMed.
- W.-Y. Wong and C.-L. Ho, Coord. Chem. Rev., 2009, 253, 1709 CrossRef CAS PubMed; Y. Hasegawa, T. Nakagawa and T. Kawai, Coord. Chem. Rev., 2010, 254, 2643 CrossRef PubMed.
- Y. Chi and P.-T. Chou, Chem. Soc. Rev., 2010, 39, 638 RSC.
- H. Sato and A. Yamagishi, J. Photochem. Photobiol., C, 2007, 8, 67 CrossRef CAS PubMed.
- M. Schäferling, Angew. Chem., Int. Ed., 2012, 51, 35322 CrossRef CAS PubMed; K. A. McGee and K. R. Mann, J. Am. Chem. Soc., 2009, 131, 1896 CrossRef PubMed.
- Y. Amao, Microchim. Acta, 2003, 143, 1 CrossRef CAS.
- B. Happ, A. Winter, M. D. Hager and U. S. Schubert, Chem. Soc. Rev., 2012, 41, 2222 RSC; K. K.-W. Lo, S. P.-Y. Li and K. Y. Zhang, New J. Chem., 2011, 35, 265 RSC.
- J. Zhao, S. Ji, W. Wu, H. Guo, J. Sun, H. Sum, Y. Liu, Q. Li and L. Huang, RSC Adv., 2012, 2, 1712 RSC.
- G. Zhou, W. Y. Wong and X. Yang, Chem.–Asian. J., 2011, 6, 1706 CrossRef CAS PubMed.
- C. Rothe, C.-J. Chiang, V. Jankus, K. Abdullah, X. Zeng, R. Jitchati, A. Batsanov, M. R. Bryce and A. P. Monkman, Adv. Funct. Mater., 2009, 19, 2038 CrossRef CAS.
- C. Ulbricht, B. Beyer, C. Friebe, A. Winter and U. S. Schubert, Adv. Mater., 2009, 21, 4418 CrossRef CAS.
- V. Guerchais and J.-L. Fillaut, Coord. Chem. Rev., 2011, 255, 2448 CrossRef CAS PubMed.
- M. S. Lowry and S. Bernhard, Chem.–Eur. J., 2006, 12, 7970 CrossRef CAS PubMed.
- N. Tian, D. Lenkeit, S. Pelz, L. H. Fischer, D. Escudero, R. Schiewek, D. Klink, O. J. Schmitz, L. Gomzález, L. M. Schäferling and E. Holder, Eur. J. Inorg. Chem., 2010, 4875 CrossRef CAS.
- Z. Xie, L. Ma, K. E. de Krafft Jin and W. Lin, J. Am. Chem. Soc., 2010, 132, 922 CrossRef CAS PubMed.
- K. A. King, P. J. Spellane and R. J. Watts, J. Am. Chem. Soc., 1985, 107, 1431 CrossRef CAS.
- A. Tsuboyama, H. Iwawaki, M. Furugori, T. Mukaide, J. Kamatani, S. Igawa, T. Moriyama, S. Miura, T. Takiguchi, S. Okada, H. Hoshino and K. Ueno, J. Am. Chem. Soc., 2003, 125, 12971 CrossRef CAS PubMed.
- A. B. Tamayo, B. D. Alleyne, P. I. Djurovich, S. Lamansky, I. Tsyba, N. N. Ho, R. Bau and M. E. Thompson, J. Am. Chem. Soc., 2003, 125, 7377 CrossRef CAS PubMed.
- A. B. Tamayo, S. Garon, S. T. Sajoto, P. I. Djurovich, I. M. Tsyba, R. Bau and M. E. Thompson, Inorg. Chem., 2005, 44, 8723 CrossRef CAS PubMed.
- T. Sajoto, P. I. Djurovich, A. B. Tamayo, J. Oxgaard, W. A. Goddard III and M. E. Thompson, J. Am. Chem. Soc., 2009, 131, 9813 CrossRef CAS PubMed.
- M. C. DeRosa, P. J. Mosher, G. P. A. Yap, K.-S. Focsaneanu, R. J. Crutchley and C. E. B. Evans, Inorg. Chem., 2003, 42, 4864 CrossRef CAS PubMed.
- M. C. DeRosa, D. J. Hodgson, G. D. Enright, B. Dawson, C. E. B. Evans and R. J. Crutchley, J. Am. Chem. Soc., 2004, 126, 7619 CrossRef CAS PubMed.
- S. Ladouceur, D. Fortin and E. Zysman-Colman, Inorg. Chem., 2010, 49, 5625 CrossRef CAS PubMed.
- M. Clemente-León, E. Coronado, Á. López-Muñoz, D. Repetto, T. Ito, T. Konya, T. Yamase, E. C. Constable, C. E. Housecroft, K. Doyle and S. Graber, Langmuir, 2010, 26, 1316 CrossRef PubMed.
- H. J. Bolink, E. Baranoff, M. Clemente-León, E. Coronado, N. Lardiés, A. López-Muñoz, D. Repetto and M. K. Nazeeruddin, Langmuir, 2010, 26, 11461 CrossRef CAS PubMed.
- C. Roldán-Carmona, A. M. González-Delgado, A. Guerrero-Martínez, L. De Cola, J. J. Giner-Casares, M. Pérez-Morales, M. T. Martín-Romero and L. Camacho, Phys. Chem. Chem. Phys., 2011, 13, 2834 RSC.
- H. Sato, K. Tamura, M. Taniguchi and A. Yamagishi, New J. Chem., 2010, 34, 617 RSC.
- H. Sato, K. Tamura, K. Ohara, S. Nagaoka and A. Yamagishi, New J. Chem., 2011, 35, 394 RSC.
- S. M. Borisov and I. Klimant, Anal. Chem., 2007, 79, 7501 CrossRef CAS PubMed.
- C. S. K. Mak, D. Pentlehner, M. Stich, O. S. Wolfbeis, W. K. Chan and H. Yersin, Chem. Mater., 2009, 21, 2173 CrossRef CAS.
- M. M.-S. Toro, J. F. Ferández-Sánchez, E. Baranoff, M. K. Nazeeruddin, M. Graetzela and A. Ferandez-Gutierrez, Talanta, 2010, 82, 620 CrossRef PubMed.
- A. L. Medina-Castillo, J. F. Ferández-Sánchez, C. Klein, M. K. Nazeeruddin, A. Segura-Carretero, A. Fernández-Gutiérrez, M. Graetzel and U. E. Spichinger-Keller, Analyst, 2007, 132, 929 RSC.
- M. Marín-Suárez, B. F. E. Curchod, I. Tavernelli, U. Rothlisberger, R. Scopelliti, I. Jung, D. Di Censo, M. Grätzel, J. F. Fernández-Sánchez, A. Fernándex-Gutiérrez, Md. K. Nazeeruddin and E. Baranoff, Chem. Mater., 2012, 24, 2330 CrossRef.
- L. H. Fischer, M. I. J. Stich, O. S. Wolfbeis, N. Tian, E. Holder and M. Scäferling, Chem.–Eur. J., 2009, 15, 10857 CrossRef CAS PubMed.
- D. Aiello, A. M. Talarico, F. Teocoli, E. I. Szerb, I. Aiello, F. Testa and M. Ghedini, New J. Chem., 2011, 35, 141 RSC.
- K. Mori, M. Tottori, K. Watanabe, M. Che and H. Yamashita, J. Phys. Chem. C, 2011, 115, 21358 CAS.
- M. Waki, N. Mizoshita, T. Tani and S. Inagaki, Angew. Chem., Int. Ed., 2011, 50, 11667 CrossRef CAS PubMed.
- H. Sato, K. Tamura, M. Taniguchi and A. Yamagishi, Chem. Lett., 2009, 14 CrossRef CAS; H. Sato, K. Tamura, R. Aoki, M. Kato and A. Yamagishi, Chem. Lett., 2011, 63 CrossRef.
- S. A. Hussain, S. Chakraborty, D. Bhattacharjee and R. A. Schoonheydt, Spectrochim. Acta, Part A, 2010, 75, 664 CrossRef PubMed.
- T. Shichi and K. Takagi, J. Photochem. Photobiol., C, 2000, 1, 113 CrossRef CAS.
- M. Ogawa and K. Kuroda, Chem. Rev., 1995, 95, 399 CrossRef CAS.
- H. Sato, Y. Hiroe, K. Tamura and A. Yamagishi, J. Phys. Chem. B, 2005, 109, 18935 CrossRef CAS PubMed.
- R. Sasai, T. Itoh, W. Ohmori, H. Itoh and M. Kusunoki, J. Phys. Chem. C, 2009, 113, 415 CAS.
- Y. Umemura, A. Yamagishi, R. A. Schoonhedyt, A. Persoons and F. De Schryver, J. Am. Chem. Soc., 2002, 124, 992 CrossRef CAS PubMed; Y. Umemura and E. Shinohara, Langmuir, 2005, 21, 4520 CrossRef; T. Shimada, H. Yamada and Y. Umemura, J. Phys. Chem. B, 2012, 116, 4484 CrossRef PubMed.
- Y. Ishida, T. Shimada, D. Masui, H. Tachibana, H. Inoue and S. Takagi, J. Am. Chem. Soc., 2011, 133, 14280 CrossRef CAS PubMed; Y. Ishida, D. Masui, H. Tachibana, H. Inoue, T. Shimada and S. Takagi, ACS Appl. Mater. Interfaces, 2012, 4, 811 Search PubMed; Y. Ishida, D. Masui, T. Shimada, H. Tachibana, H. Inoue and S. Takagi, J. Phys. Chem. C, 2012, 116, 7879 Search PubMed; Y. Ishida, T. Shimada, H. Tachibana, H. Inoue and S. Takagi, J. Phys. Chem. A, 2012, 116, 12065 CrossRef PubMed.
- S. Takagi, D. A. Tryk and H. Inoue, J. Phys. Chem. B, 2002, 106, 5455 CrossRef CAS; S. Takagi, M. Eguchi, D. A. Tryk and H. Inoue, Langmuir, 2006, 22, 1406 CrossRef PubMed.
- K. Morimoto, T. Nakae, K. Ohara, K. Tamura, S. Nagaoka and H. Sato, New J. Chem., 2012, 36, 2467 RSC.
- K. Okamoto, K. Tamura, M. Takahashi and A. Yamagishi, Colloids Surf., A, 2000, 169, 241 CrossRef CAS.
Footnote |
† Electronic supplementary information (ESI) available: 1H NMR and mass spectroscopy data for iridium(III) complexes; the electronic absorption spectra of iridium(III) complexes in methanol; the AFM images of the hybrid films deposited onto glass substrates; the UV-vis spectra of single-, double- and triple-layered hybrid films; the emission spectra of a single-layered hybrid film containing a mixture of DFPPY, PPY and PIQ at a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio (denoted {DFPPY, PPY, PIQ/SAP}); emission spectra of heterogeneous double-layered films; quantitative analysis of energy transfer in heterogeneous double-layered hybrid film under vacuum; the reconstruction of emission spectra of the triple-layered heterogeneous hybrid films according to eqn (1) in the text; and the Stern–Volmer plots for quenching by oxygen molecules for the hybrid single- and double-layered films. See DOI: 10.1039/c3nj00879g 1 ratio (denoted {DFPPY, PPY, PIQ/SAP}); emission spectra of heterogeneous double-layered films; quantitative analysis of energy transfer in heterogeneous double-layered hybrid film under vacuum; the reconstruction of emission spectra of the triple-layered heterogeneous hybrid films according to eqn (1) in the text; and the Stern–Volmer plots for quenching by oxygen molecules for the hybrid single- and double-layered films. See DOI: 10.1039/c3nj00879g |
| This journal is © The Royal Society of Chemistry and the Centre National de la Recherche Scientifique 2014 |