A sensitive colorimetric and ratiometric fluorescent chemodosimeter for Hg2+ and its application for bioimaging†
Huie
Jiang
a,
Jie
Jiang
a,
Ju
Cheng
b,
Wei
Dou
a,
Xiaoliang
Tang
a,
Lizi
Yang
a,
Weisheng
Liu
*a and
Decheng
Bai
*b
aKey Laboratory of Nonferrous Metals Chemistry and Resources Utilization of Gansu Province and State Key Laboratory of Applied Organic Chemistry, College of Chemistry and Chemical Engineering, Lanzhou University, Lanzhou 730000, P. R. China. E-mail: liuws@lzu.edu.cn; Fax: +86-931-8912582; Tel: +86-931-8915151
bKey Lab of Preclinical Study for New Drugs of Gansu Province and Operative Surgery Institute, School of Basic Medical Sciences, Lanzhou University, Lanzhou 730000, P. R. China. E-mail: bdc@lzu.edu.cn
First published on 24th September 2013
Abstract
A colorimetric and ratiometric fluorescent chemodosimeter for Hg2+ has been developed based on mercury ion-promoted hydrolysis of a naphthalimide-derived aryl vinyl ether. The chemodosimeter displays a highly sensitive and selective response with significant changes in both color (from colorless to jade-yellow) and fluorescence (from blue to green) in PBS buffer solution (containing 1% CH3CN, pH = 7.4). The chemodosimeter exhibits a ratiometric fluorescent response towards Hg2+ with a very low detection limit (3.6 nM), which can be used to detect Hg2+ ions in drinking water. Furthermore, the chemodosimeter has been successfully applied to the imaging of Hg2+ ions in living cells with an emission change from blue to green.
Introduction
The widespread contamination of highly poisonous mercury species could jeopardize our ecosystem, posing a great threat to human health, because mercury can lead to dysfunction of the brain, kidney, stomach, and central nervous system due to its thiophilic nature for proteins and enzymes.1–4 As the most prevalent contaminant in biological and environmental samples, Hg2+ is widely distributed in air, water, and soil, which continues to be a major environmental and health concern. So, it is urgent to develop versatile tactics to monitor Hg2+ ions.In the past few years, many analytical methods, such as atomic absorption spectroscopy, inductively coupled plasma-mass spectrometry, high performance liquid chromatography, electrochemical sensing, etc., have been applied for the detection of Hg2+ concentrations.5–7 Though these techniques are accurate for Hg2+ ions, most of them are rather complicated, costly, and time consuming as well as inappropriate for on-line use or field monitoring, so the wide utilization of these methods is largely limited. Fluorometry, which has the favorable features of operational simplicity, cost-effectiveness, high sensitivity and selectivity, has attracted considerable attention.8–11 Thus, a number of fluorescent chemosensors for the selective detection of Hg2+ ions have been exploited.12–22 Most of these early fluorescent sensors are based on the coordination of heteroatom-based ligands to Hg2+, which usually show a fluorescence quenching response due to the spin–orbit coupling effect of the Hg2+ ion and incomplete selectivity over the competing metal ions.23–25 Since Czarnik and Chae reported the pioneering chemical reaction-based chemodosimeter for Hg2+,26 many chemodosimeters based on the mercury-triggered reaction have been reported gradually and have developed rapidly,27–40 which has provided superior selectivity towards Hg2+ with a large spectroscopic change and avoided Hg2+-induced fluorescence quenching.41,42 To the best of our knowledge, most of these chemodosimeters are based on the OFF–ON mechanism only with changes in the fluorescence intensity,43–45 which can be easy interfered with by the excitation power and detector sensitivity, strongly limiting the quantitative detection of Hg2+ ions.46,47 In contrast, ratiometric fluorescent chemodosimeter, which uses the ratio of two fluorescent bands rather than the absolute intensity of one band, can minimize the background signal and detect the analyte more accurately.48–51 However, only a handful of ratiometric fluorescent chemodosimeters for Hg2+ have been reported.28–30,34,36,38,40,48 Moreover, many of these chemodosimeters require a high proportion of organic solvent as the medium for analysis28,38,40,48 and only a few could be used to image Hg2+ ions in living cells.29,30,34,36 Thus, there remains urgency to develop more accurate and sensitive ratiometric chemodosimeters to detect Hg2+ ions in aqueous solution and living cells.
Recently we reported a 4-aminonaphthalimide-based probe for mercury species, which displayed a colorimetric and ratiometric response in a buffer solution via a mercury-promoted cleavage reaction, and detected CH3HgCl in living cells.52 Herein, 4-hydroxynaphthalimide with more desirable photophysical properties was adopted to obtain a much simpler chemodosimeter, (N-butyl-4-vinyl ether-1,8-naphthalimide) (1), which would work though the intramolecular charge transfer (ICT) process, owing to the stronger electron-donating ability of oxygen anions when the vinyl group is removed.53 As expected, in the presence of Hg2+, 1 could be hydrolysed readily and 2 released with a free hydroxy group in aqueous solution. This chemical transformation from vinyl ether to hydroxy turned on the ICT process from the oxygen anions to the fluorophore, resulting in ratiometric changes in both color and fluorescence. More importantly, biological application of 1 has been evaluated for the intracellular detection of Hg2+ with an emission change from blue to green.
Results and discussion
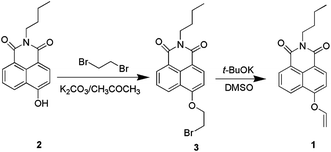
Synthesis of compound 1 is summarized in Scheme 1. N-Butyl-4-hydroxy-1,8-naphthalimide (compound 2) was synthesized according to the literature method.54 Then, chemodosimeter 1, N-butyl-4-vinyl ether-1,8-naphthalimide was prepared smoothly from 4-hydroxy-1,8-naphthalimide (2) through nucleophilic substitution and elimination steps in a satisfactory yield. The purity of 1 was fully confirmed by 1H, 13C NMR and ESI-MS analysis.We firstly assessed the UV-vis spectroscopic properties of 1 in PBS buffer solution (pH = 7.4, containing 1% CH3CN). 1 (5 μM) displayed a moderate UV-vis absorption around 368 nm. After being treated with Hg2+ (5 μM), the absorption band at 368 nm decreased and a new band at 447 nm appeared instantly with an isosbestic point at 400 nm. A marked color change from colorless to light yellow was observed, owing to the disappearance of vinyl ether and the formation of oxygen anions (Fig. 1). Other metal ions including Li+, Na+, K+, Ca2+, Mg2+, Ba2+, Cr3+, Mn2+, Fe3+, Fe2+, Co2+, Ni2+, Cu2+, Zn2+, Cd2+, Ag+, Au3+, Pd2+ and Pt2+ (5 μM) incubated in 1 (5 μM) for 30 min have no color change, so Hg2+ can be easily detected from other metal ions by the naked eye.
Then, the fluorescent spectroscopic properties of 1 were studied in PBS buffer solution. 1 (5 μM) exhibits a single emission band at 456 nm and strong fluorescence with a quantum yield (Φ) of ca. 0.285 in PBS buffer solution (pH = 7.4, containing 1% CH3CN). After being treated with Hg2+ (5 μM), a red-shift from 456 nm to 546 nm was also detected in the maximum emission spectrum, with an isosbestic point at 506 nm (Fig. 2). Other mercury compounds, such as Hg(ClO4)2 and Hg(OAc)2, gave similar results (Fig. S2, ESI†).55 This marked red shift is due to the ICT process between oxygen anions and the imide moieties in the fluorophore that was switched on via the Hg2+-promoted hydrolysis of aryl vinyl ether.
 | ||
| Fig. 2 Fluorescence spectral change of 1 (5 μM) upon treatment with HgCl2 (5 μM) in PBS buffer solutions (pH = 7.4, containing 1% CH3CN). λEx = 408 nm. Slit: 10.0 nm/10.0 nm. | ||
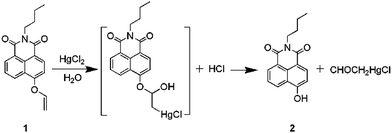
To gain insight into the sensing mechanism of 1 towards Hg2+, the reaction of 1 with HgCl2 was conducted under the same conditions as described above. Then, the green fluorescent reaction product was obtained and characterized to be 2 by electrospray ionization mass spectrum (ESI-MS), 1H NMR and 13C NMR (Fig. S13–S15, ESI†), which exhibits weak fluorescence with a quantum yield (Φ) of ca. 0.113 in PBS buffer solution (pH = 7.4, containing 1% CH3CN). Moreover, titration of 1 with HgCl2 (from 0 to 1.4 equiv.) showed saturated behavior at 1.0 equiv. of HgCl2 (Fig. 3), and the results obtained from Job's plots also show the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 stoichiometry for the reaction between 1 and Hg2+ (Fig. S3, ESI†),56–58 so the mechanism can be explained by the procedure in Scheme 2.
1 stoichiometry for the reaction between 1 and Hg2+ (Fig. S3, ESI†),56–58 so the mechanism can be explained by the procedure in Scheme 2.
When 1 equiv. of HgCl2 was added to 1 in PBS buffer, the fluorescence saturation took about 30 min (Fig. 4b). However, a linear relationship between the concentration of HgCl2 and the fluorescence intensity ratios at 550 nm and 506 nm (I550nm/I506nm) can be easily obtained at 15 min after the addition of HgCl2 (Fig. S4, ESI†). Therefore, it is not necessary to wait for the full recovery of fluorescence for quantification purposes in drinking water. When the amount of Hg2+ exceeds 5 μM in the sample, it can be quantified by diluting the sample, bringing the concentration within 5 μM.
Drinking water pretreated with different amounts of HgCl2 (0–2 μM), and then 1 (20 μL in CH3CN, [1]final = 5 μM) was added into the mixture. After 15 min, as shown in Fig. 5, a good linear relationship (R = 0.989) between the low concentration range of HgCl2 (0–2 μM) and the fluorescence intensity ratios (I550nm/I506nm) wasalso found. The detection limit is 3.6 nM, lower than many reported results34,36,45,52 and satisfies the U.S. Environmental Protection Agency (EPA) limit (10 nM) of Hg2+ detection in drinking water.59
The fluorescence spectrum of 1 was measured in the presence of other metal ions including Li+, Na+, K+, Ca2+, Mg2+, Ba2+, Cr3+, Mn2+, Fe3+, Fe2+, Co2+, Ni2+, Cu2+, Zn2+, Cd2+, Ag+, Au3+, Pd2+ and Pt2+ under identical conditions. After incubating 1 (5 μM) with metal ions (5 μM) individually for 30 min, all of these ions showed relatively little or no response to the emission of 1 (Fig. 6a), owing to little hydrolysis of the vinyl ether with these metals. To further explore the selectivity of Hg2+ in the presence of other metal ions, competition experiments were carried out. Fortunately, chemodosimeter 1 showed a full fluorescence response to mercury ions in the presence of 5 equiv. of all other metal ions, and even the good chelating agent, EDTA (Fig. 6b). The competition experiment showed that other metal species and EDTA have a negligible influence on the sensing of Hg2+. Moreover, the fluorescence spectrum of 1 was also measured in the presence of some anions, such as F−, Cl−, NO3−, ClO4−, AcO−, CO32− and SO42−. Both 1 and the 1–Hg2+ system showed little response to these anions tested (Fig. S5 and S6, ESI†). These results confirm that 1 shows a good sensitivity and selectivity towards Hg2+ over other competitive ions.
The effect of pH on the fluorescence of 1 and the 1–Hg2+ system have been studied. As seen from Fig. 7, 1 is pH insensitive, the emission intensity of 1 remaining unaffected from pH 1.60 to pH 12.12 after incubation for 30 min. Therefore, it reacted with Hg2+ within the biologically relevant pH range (1.60–12.12). Based on these findings, it is possible that 1 could be applied to image Hg2+ ions in living cells without interference from pH effects.
 | ||
| Fig. 7 The fluorescence intensity of 5 μM 1, 1–Hg2+ or 2 as a function of pH in aqueous solutions (containing 1% CH3CN). Each spectrum was acquired 30 min after 1, 1–Hg2+ or 2 addition. | ||
To further demonstrate the ability of 1 to image Hg2+ ions in living systems, we carried out experiments in live, human hepatocarcinoma SMMC-7721 cells. The cells incubated with 10 μM 1 in RPMI-1640 for 0.5 h at 37 °C exhibited blue fluorescence (Fig. 8b). However, when the cells were treated with 10 μM 1 and 20 μM HgCl2 they displayed green fluorescence (Fig. 8d). Obvious changes indicated that 1 can penetrate the cell membrane and enable ratiometric imaging of Hg2+ ions in the living cells. Brightfield imaging (Fig. 8a and 8c) confirmed cell viability during the imaging experiment.
Conclusion
In conclusion, we have developed a new and simple reactive fluorescent chemodosimeter for Hg2+ based on the mercury ion-promoted hydrolysis of aryl vinyl ether under mild conditions. The chemodosimeter showed specific response to Hg2+ and ratiometric fluorescence in PBS buffer solution (containing 1% CH3CN, pH = 7.4), with a marked color change and a low detection limit (3.6 nM). Besides, the chemodosimeter can enter live SMMC-7721 cells and indicate intracellular Hg2+ ions. Therefore, the chemodosimeter has potential applications for the study of the toxicity of Hg2+ and the detection of Hg2+ in living cells.Experimental section
Materials and general methods
All reagents and solvents were obtained commercially and used without further purification unless otherwise noted. 1H NMR and 13C NMR spectra were recorded using a Bruker DRX400 spectrometer and referenced to the solvent signals. Mass spectra (ESI) were performed using an LQC system (Finngan MAT, USA). The melting points were measured using an X-6 melting point apparatus without calibration (Beijing Fuka Keyi Science and Technology Co., LTD). All UV-visible spectra and fluorescence spectra were recorded using a Varian Cary 100 spectrophotometer and a Hitachi F-4500 luminescence spectrometer, respectively. Fluorescence quantum yields were determined by an absolute method using an integrating sphere on FLS920 of Edinburgh Instruments. All pH measurements were made with a pH-10C digital pH meter. All spectra were recorded at 25 °C. Fluorescence microscopy experiments were performed using an Olympus BX53 fluorescence microscope.Solutions of HgCl2, Hg(OAc)2, Hg(ClO4)2, LiClO4, NaCl, KCl, CaCl2, MgCl2, BaCl2, CrCl3, MnCl2, FeCl3, FeCl2, CoCl2, NiCl2, CuCl2, ZnCl2, CdCl2, and AgClO4 were prepared in acetonitrile with a concentration of 10 mM, respectively. All the anion solutions were prepared from NaF, NaCl, NaNO3, NaClO4, NaAcO, Na2SO4, and Na2CO3 in distilled water, with a concentration of 10 mM, respectively. PdCl2 was prepared in 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 brine–MeOH solution with a concentration of 10 mM. PtCl2 and AuCl3 were prepared in DMSO with a concentration of 10 mM.
3 brine–MeOH solution with a concentration of 10 mM. PtCl2 and AuCl3 were prepared in DMSO with a concentration of 10 mM.
Preparation of chemodosimeter
Cell incubation and imaging
The human cell line SMMC-7721 was maintained in RPMI-1640 medium supplemented with 10% heat-inactivated fetal calf serum, 100 units per mL penicillin, and 100 μg mL−1 streptomycin at 37 °C in a humidified atmosphere containing 5% CO2 for 24 h. Cells (5 × 108 L−1) were plated on 18 mm glass coverslips and allowed to adhere for 24 h. Then the cells were treated with the relative compound for fluorescence microscopic imaging on an Olympus BX53 fluorescence microscope.After SMMC-7721 cells were grown on a 12′′ orifice plate at 37 °C and in 5% CO2 atmosphere for 24 h, then treated with 1 (10 μM) and incubated for 0.5 h. Subsequently, the cells were treated with 20 μM HgCl2. Cells were incubated for 1.0 h and rinsed with PBS three times to remove free compound and ions before analysis. SMMC-7721 cells only incubated with 10 μM 1 for 1.5 h acted as a control. All fluorescence microscopic images were collected using an Olympus BX5 fluorescence microscope.
Acknowledgements
This study was supported by the NSFC (Grant 20931003, 91122007) and the Specialized Research Fund for the Doctoral Program of Higher Education (Grant No. 20110211130002).Notes and references
- R. von Burg, J. Appl. Toxicol., 1995, 15, 483–493 CrossRef CAS PubMed.
- M. Nendza, T. Herbst, C. Kussatz and A. Gies, Chemosphere, 1997, 35, 1875–1885 CrossRef CAS PubMed.
- H. H. Harris, I. J. Pickering and G. N. George, Science, 2003, 301, 1203 CrossRef CAS PubMed.
- I. Onyido, A. R. Norris and E. Buncel, Chem. Rev., 2004, 104, 5911–5930 CrossRef CAS PubMed.
- Y. Li, C. Chen, B. Li, J. Sun, J. Wang, Y. Gao, Y. Zhao and Z. Chai, J. Anal. At. Spectrom., 2006, 21, 94–96 RSC.
- O. T. Butler, J. M. Cook, C. F. Harrington, S. J. Hill, J. Rieuwerts and D. L. Miles, J. Anal. At. Spectrom., 2006, 21, 217–243 RSC.
- B. Vallant, R. Kadnar and W. Goessler, J. Anal. At. Spectrom., 2007, 22, 322–325 RSC.
- H. N. Kim, M. H. Lee, H. J. Kim, J. S. Kim and J. Yoon, Chem. Soc. Rev., 2008, 37, 1465–1472 RSC.
- E. M. Nolan and S. J. Lippard, Chem. Rev., 2008, 108, 3443–3480 CrossRef CAS PubMed.
- D.-G. Cho and J. L. Sessler, Chem. Soc. Rev., 2009, 38, 1647–1662 RSC.
- D. T. Quang and J. S. Kim, Chem. Rev., 2010, 110, 6280–6301 CrossRef CAS PubMed.
- Y. Zhao and Z. Zhong, J. Am. Chem. Soc., 2006, 128, 9988–9989 CrossRef CAS PubMed.
- C.-C. Huang, Z. Yang, K.-H. Lee and H.-T. Chang, Angew. Chem., Int. Ed., 2007, 119, 6948–6952 CrossRef.
- B. Nisar Ahamed, I. Ravikumar and P. Ghosh, New J. Chem., 2009, 33, 1825–1828 RSC.
- X. Peng, Y. Wang, X. Tang and W. Liu, Dyes Pigm., 2011, 91, 26–32 CrossRef CAS.
- M. Santra, B. Roy and K. H. Ahn, Org. Lett., 2011, 13, 3422–3425 CrossRef CAS PubMed.
- Y. Yang, Q. Zhao, W. Feng and F. Li, Chem. Rev., 2013, 113, 192–270 CrossRef CAS PubMed.
- K. Ghosh, T. Sarkar, A. Samadder and A. R. Khuda-Bukhsh, New J. Chem., 2012, 36, 2121–2127 RSC.
- R. Han, X. Yang, D. Zhang, M. Fan, Y. Ye and Y. Zhao, New J. Chem., 2012, 36, 1961–1965 RSC.
- G. Cui, Z. Ye, R. Zhang, G. Wang and J. Yuan, J. Fluoresc., 2012, 22, 261–267 CrossRef CAS PubMed.
- N. Zhang, G. Li, J. Zhao and T. Liu, New J. Chem., 2013, 37, 458–463 RSC.
- A. Hemamalini and T. Mohan Das, New J. Chem., 2013, 37, 2419–2425 RSC.
- J. S. Kim, M. G. Choi, K. C. Song, K. T. No, S. Ahn and S.-K. Chang, Org. Lett., 2007, 9, 1129–1132 CrossRef CAS PubMed.
- S. M. Park, M. H. Kim, J.-I. Choe, K. T. No and S.-K. Chang, J. Org. Chem., 2007, 72, 3550–3553 CrossRef CAS PubMed.
- K.-C. Song, M. H. Kim, H. J. Kim and S.-K. Chang, Tetrahedron Lett., 2007, 48, 7464–7468 CrossRef CAS.
- M. Y. Chae and A. W. Czarnik, J. Am. Chem. Soc., 1992, 114, 9704–9705 CrossRef CAS.
- M. G. Choi, D. H. Ryu, H. L. Jeon, S. Cha, J. Cho, H. H. Joo, K. S. Hong, C. Lee, S. Ahn and S.-K. Chang, Org. Lett., 2008, 10, 3717–3720 CrossRef CAS PubMed.
- G.-Q. Shang, X. Gao, M.-X. Chen, H. Zheng and J.-G. Xu, J. Fluoresc., 2008, 18, 1187–1192 CrossRef CAS PubMed.
- B. Tang, B. Ding, K. Xu and L. Tong, Chem.–Eur. J., 2009, 15, 3147–3151 CrossRef CAS PubMed.
- J. Du, J. Fan, X. Peng, P. Sun, J. Wang, H. Li and S. Sun, Org. Lett., 2010, 12, 476–479 CrossRef CAS PubMed.
- S. Ando and K. Koide, J. Am. Chem. Soc., 2011, 133, 2556–2566 CrossRef CAS PubMed.
- Z. Xing, H.-C. Wang, Y. Cheng, T. D. James and C. Zhu, Chem.–Asian J., 2011, 6, 3054–3058 CrossRef CAS PubMed.
- S. Yan, R. Huang, C. Wang, Y. Zhou, J. Wang, B. Fu, X. Weng and X. Zhou, Chem.–Asian J., 2012, 7, 2782–2785 CrossRef CAS PubMed.
- X. Ma, J. Wang, Q. Shan, Z. Tan, G. Wei, D. Wei and Y. Du, Org. Lett., 2012, 14, 820–823 CrossRef CAS PubMed.
- Y. Chen, C. Zhu, Z. Yang, J. Li, Y. Jiao, W. He, J. Chen and Z. Guo, Chem. Commun., 2012, 48, 5094–5096 RSC.
- W. Xuan, C. Chen, Y. Cao, W. He, W. Jiang, K. Liu and W. Wang, Chem. Commun., 2012, 48, 7292–7294 RSC.
- F. Wang, S.-W. Nam, Z. Guo, S. Park and J. Yoon, Sens. Actuators, B, 2012, 161, 948–953 CrossRef CAS.
- P. Srivastava, R. Ali, S. S. Razi, M. Shahid and A. Misra, Sens. Actuators, B, 2013, 181, 584–595 CrossRef CAS.
- X. Zhang, Y. Xu, P. Guo and X. Qian, New J. Chem., 2012, 36, 1621–1625 RSC.
- F. Lu, M. Yamamura and T. Nabeshima, Dalton Trans., 2013, 42, 12093–12100 RSC.
- J. C. Koziar and D. O. Cowan, Acc. Chem. Res., 1978, 11, 334–341 CrossRef CAS.
- C. N. Burress, M. I. Bodine, O. Elbjeirami, J. H. Reibenspies, M. A. Omary and F. P. Gabbaï, Inorg. Chem., 2007, 46, 1388–1395 CrossRef CAS PubMed.
- F. Song, S. Watanabe, P. E. Floreancig and K. Koide, J. Am. Chem. Soc., 2008, 130, 16460–16461 CrossRef CAS PubMed.
- M. Santra, D. Ryu, A. Chatterjee, S.-K. Ko, I. Shin and K. H. Ahn, Chem. Commun., 2009, 2115–2117 RSC.
- W. Jiang and W. Wang, Chem. Commun., 2009, 3913–3915 RSC.
- K. Komatsu, Y. Urano, H. Kojima and T. Nagano, J. Am. Chem. Soc., 2007, 129, 13447–13454 CrossRef CAS PubMed.
- D. Srikun, E. W. Miller, D. W. Domaille and C. J. Chang, J. Am. Chem. Soc., 2008, 130, 4596–4597 CrossRef CAS PubMed.
- B. Liu and H. Tian, Chem. Commun., 2005, 3156–3158 RSC.
- Z. Xu, K.-H. Baek, H. N. Kim, J. Cui, X. Qian, D. R. Spring, I. Shin and J. Yoon, J. Am. Chem. Soc., 2010, 132, 601–610 CrossRef CAS PubMed.
- M. Dong, Y.-W. Wang and Y. Peng, Org. Lett., 2010, 12, 5310–5313 CrossRef CAS PubMed.
- D. W. Domaille, L. Zeng and C. J. Chang, J. Am. Chem. Soc., 2010, 132, 1194–1195 CrossRef CAS PubMed.
- J. Jiang, W. Liu, J. Cheng, L. Yang, H. Jiang, D. Bai and W. Liu, Chem. Commun., 2012, 48, 8371–8373 RSC.
- R. M. Duke, E. B. Veale, F. M. Pfeffer, P. E. Kruger and T. Gunnlaugsson, Chem. Soc. Rev., 2010, 39, 3936–3953 RSC.
- J. Ren, Z. Wu, Y. Zhou, Y. Li and Z. Xu, Dyes Pigm., 2011, 91, 442–445 CrossRef CAS.
- There was no obvious change when 1 was tested with organic mercury. That is to say, 1 could distinguish inorganic mercury and organic mercury, which may be attributed to the suitable reactive ability between our vinyl ether and mercury.
- Y. Xie, Y. Ding, X. Li, C. Wang, J. P. Hill, K. Ariga, W. Zhang and W. Zhu, Chem. Commun., 2012, 48, 11513–11515 RSC.
- Y. Ding, X. Li, T. Li, W. Zhu and Y. Xie, J. Org. Chem., 2013, 78, 5328–5338 CrossRef CAS PubMed.
- H. Wang, G. Zhou, H. Gai and X. Chen, Chem. Commun., 2012, 48, 8341–8343 RSC.
- The U.S. Environmental Protection Agency (EPA) has set the limit of mercury in drinking water to be 10 nM.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental details, characterization of the compounds, and additional spectroscopic data, NMR and MS spectra. See DOI: 10.1039/c3nj00766a |
| This journal is © The Royal Society of Chemistry and the Centre National de la Recherche Scientifique 2014 |