Silver(I) coordination chemistry: from 1-D chains to molecular rectangles†
Mohammad Abul Haja, Christer B. Aakeröy*b and John Desperb
aDepartment of Chemistry and Chemical Technology, Al-Quds University, Palestine. E-mail: mabulhaj@science.alquds.edu; Fax: +972 2 2796960; Tel: +972 2 2799753
bDepartment of Chemistry, Kansas State University, Manhattan, Kansas 66506, USA. E-mail: aakeroy@ksu.edu; Fax: +1 785 532 6666; Tel: +1 785 532 6096
First published on 17th October 2012
Abstract
A series of silver(I) coordination compounds with the ligand 2-(4-pyridyl)imidazole 4-PyIm has been synthesized and crystallographically characterized in order to explore the role of counterions, solvents and ligand charge in the resulting supramolecular architectures. With perchlorate and hexafluorophosphate, two iso-structural 1-D coordination-polymers were obtained, but when changing the counterion to nitrate and using water as solvent, an unusual molecular rectangle composed of four ligands and four Ag(I) ions was obtained, and the assembly was controlled by hydrogen bonding via the anion and water molecules. Finally, when the ligand was deprotonated, the change in the number of binding sites (from two to three) was reflected in an increase in the coordination number for the silver ion.
Introduction
Interest in silver(I) in crystal engineering and supramolecular chemistry remains high due to the diverse structural topologies that this ion can generate,1 and the potential use of Ag(I)-complexes in a range of optical,2 electrical,3 nano-material,4 catalytic,5 and adsorptive applications.6In coordination chemistry, the silver ion has been widely used due to its soft acceptor characteristic as well as for its relatively flexible coordination sphere. The latter allows the ion to be coordinated by a variety of ligands possessing several geometries and heteroatoms resulting in an extensive array of networks and topologies. Although Ag(I) is frequently thought of as having a strong preference for linear coordination, it has been reported7 that out of 3319 solid-state structures of silver(I) complexes, 24% are two-coordinate, 23% are three-coordinate, 44% are four-coordinate, with the remaining 9% displaying coordination numbers greater than four. The flexibility of silver(I) coordination geometries can be traced back to the lack of stereochemical directionality that arises from a d10 configuration.8
As a result, it should be possible to control the coordination number and geometry of silver(I) complexes using non-covalent interactions due to the relatively weak nature of most silver(I)-ligand bonds.9 Factors that influence the supramolecular assembly of silver(I) complexes into extended structures include, in addition to the inherent properties of the metal ion and ligand, non-covalent interactions involving counterions and/or solvent molecules. The balance between these weak interactions determines the structural outcome of any preparation of silver-based coordination complexes, and it is essential to examine these closely, preferably one variable at a time, if we want to gain more control over the way in which silver complexes are assembled in the solid state.
Silver(I) complexes are mostly two, three, or four-coordinate, and for coordination numbers greater than two, the coordination sphere is usually completed by solvent molecules or counterions. In this study we are targeting a relatively simple ligand, 2-(4-pyridyl)imidazole, 4-PyIm, Scheme 1, with restricted conformational flexibility that can exist in a neutral or anionic form which allows us to explore the supramolecular roles that anions and solvent molecules may play in the assembly of silver(I) containing architectures. 4-PyIm contains two or three donor atoms (depending on charge) and the donor moieties are positioned at a 90° angle with respect to each other, Scheme 1.
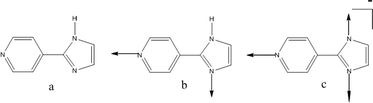 | ||
| Scheme 1 (a) 4-PyIm neutral, (b) donor atoms for neutral 4-PyIm, (c) donor atoms for anionic 4-PyIm. | ||
A survey of crystallographically characterized pyridine- and imidazole-based Ag(I) complexes shows a strong bias towards the low coordination number; 90% of all Ag(I)-py complexes (from 1130 hits) and 97% of all Ag(I)-Im complexes (from 250 hits) have a coordination number of either two or three, Table 1.
If 4-PyIm is deprotonated, the number of donor atoms increases from two to three and they are configured in a T-shaped manner, Scheme 1.
As silver(I) has no preference for a particular site on the ligand, we postulate that any of the following architectures may appear with neutral 4-PyIm and a coordination number of two: coordination polymers (a and b), cyclic closed structures (c), and helical 1-D coordination polymers (d), Scheme 2 for a neutral ligand and a coordination number of three: cyclic closed structure (h) as well as another coordination polymer (g), Scheme 4. For anionic 4-PyIm with coordination numbers two and three we can envision coordination polymers with repeating cyclic units (e–f), Scheme 3.
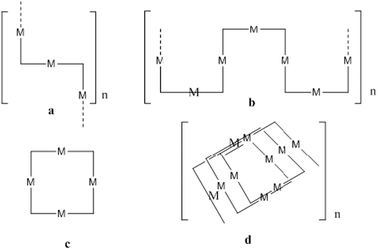 | ||
Scheme 2 Some possible Ag(I) networks, CN![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) 2 (a–d). 2 (a–d). | ||
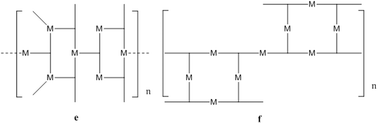 | ||
| Scheme 3 Some possible networks for silver(I) with 4-PyIm in its anionic form (e–f). | ||
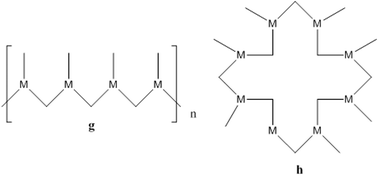 | ||
Scheme 4 Some possible structures for Ag(I) networks with 4-PyIm in its neutral form with CN![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) 3 (g–h). 3 (g–h). | ||
In order to identify and isolate the effects imparted by counterions or solvent molecules on the resulting structures of Ag:4-PyIm complexes, a synthetic approach was carried out using acetonitrile, nitromethane and water (acidic to be more soluble and basic to generate anion) as solvents; and nitrate, tetrafluoroborate, perchlorate, and hexafluorophosphate as counterions (summarized in Scheme 5).
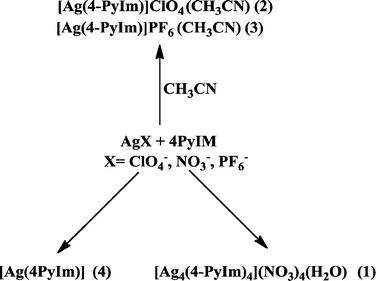 | ||
| Scheme 5 Synthetic plan. | ||
We now report the four crystal structures of 4-PyIm and its corresponding Ag(I) complexes that were obtained as a result of this synthetic effort and our analysis of the effect that each variable has on the eventual crystal structure.
Experimental
All chemicals, silver nitrate, silver tetrafluoroborate, silver perchlorate, and silver hexafluorophosphate, were purchased from Aldrich and used with no further purification. The synthesis of 2-(4-pyridyl)imidazole 4PyIm was carried out according to previously reported procedures. Melting points were determined on a Fisher-Johns melting point apparatus and are uncorrected. 1H NMR was recorded on a Varian Unity plus 400 MHz spectrometer in d6-DMSO. Infrared spectroscopy (IR) was done on a Nicolet 380 FT-IR.Synthesis
Crystallography: data collection and refinement
Datasets were collected on a Bruker SMART APEX II system at 120 K using APEX2 software.11 An Oxford Croystream 700 low-temperature device was used to control temperature. MoKα radiation was used. Initial cell constants were found by small widely separated “matrix” runs. Data collection strategies were determined using COSMO.12 Scan speeds and scan widths were chosen based on scattering power and peak rocking curves. Unit cell constants and orientation matrices were improved by least-squares refinement of reflections “thresholded” from the entire dataset. Integrations were performed with SAINT,13 using these improved unit cells as a starting point. Precise unit cell constants were calculated in SAINT from the final merged datasets. Lorenz and polarization corrections were applied. Absorption corrections were applied using SADABS.14 Datasets were reduced with SHELXTL.15 The structures were solved by direct methods without incident. Unless noted below, coordinates for all carboxylic acid, amine, ammonium, and phenol hydrogen atoms were allowed to refine. All other hydrogen atoms were assigned to idealized positions and were allowed to ride. Isotropic thermal parameters for the hydrogen atoms were constrained to be 1.5 × (methyl)/1.2 × (all other) that of the connected atom.1: Geometry of the solvent water molecules was idealized by using DFIX commands. Coordinates for the imidazole hydrogens H13 and H33 were allowed to refine. 2: The perchlorate anion was disordered and was modeled as two closely spaced molecules. The perchlorate oxygens were assigned to isotropic thermal parameters. Thermal parameters for the chlorines and oxygens for the two anions were pairwise restrained using EADP commands. Geometry was idealized using DFIX commands. Coordinates for the imidazole hydrogen H13 were allowed to refine. 3: Coordinates for the imidazole hydrogen H13 were allowed to refine.
Results
The structure of 2 is dominated by a 1-D zig-zag polymer, where the silver(I) ion coordinates to two ligands in a near-linear fashion (N–Ag–N; 171°), through one pyridyl nitrogen atom and one imidazole nitrogen atom, Fig. 1. Similar deviations from perfect linearity are commonplace as shown by an examination of relevant structures in the CSD.16 The 1-D zig-zag polymer corresponds to the motif postulated in Scheme 1a.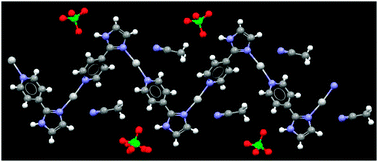 | ||
| Fig. 1 Part of the crystal structure of 2 showing the infinite 1-D polymer; coordination number for silver(I) is two. | ||
Adjacent chains in the structure of 2 are arranged in a parallel and in-phase fashion, Fig. 2, with counterions and solvent molecules filling the space in between. There is a weak hydrogen-bond involving the perchlorate ion and the imidazole moiety (C(H)⋯O; 2.84 Å, but there are no notable Ag⋯Ag or Ag⋯π contacts, Fig. 3.
 | ||
| Fig. 2 Adjacent chains in the crystal structure of 2. | ||
 | ||
| Fig. 3 Close-packing of 2-D layers in the crystal structure of 2. | ||
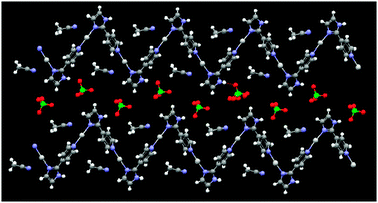
The crystal structure of 3 is identical to that of 2, and the main feature is a 1-D polymer with near-linear coordination around each silver(I) ion with solvent molecules and counterions simply occupying the space between chains, Fig. 4. Again, the coordination polymer can schematically be described as postulated in Scheme 1(a).
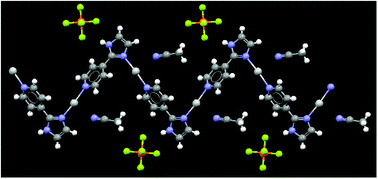 | ||
| Fig. 4 Part of the crystal structure of 3 showing the infinite 1-D polymer; coordination number for silver(I) is two. | ||
The adjacent chains in 3 are again parallel and in-phase, Fig. 5, with counterions and solvent molecules filling the space in between them. No notable inter-layer short contacts could be identified, Fig. 6.
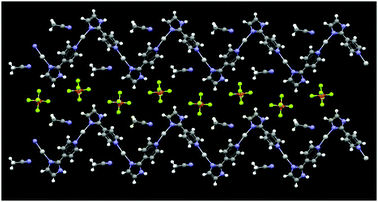 | ||
| Fig. 5 The 2-D crystal structure of 3 showing the adjacent 1-D polymers. | ||
 | ||
| Fig. 6 The antiparallel layers of the crystal structure of 3. | ||
Compound 1 comprises a nitrate ion which is well-known for participating in stronger and structurally more relevant interactions than do perchlorate and hexafluorophosphate ions. The structure of 1 is akin to what was postulated in Scheme 1(c), and demonstrates that the 1-D polymers of 2 and 3 have been abandoned in preference for a centrosymmetric molecular rectangle constructed from four silver(I) ions and four ligands, Fig. 7.
The anions and water molecules provide hydrogen-bonded bridges between adjacent rectangles, Fig. 8, and there are no obvious π⋯π interactions between these cationic species, Fig. 9.
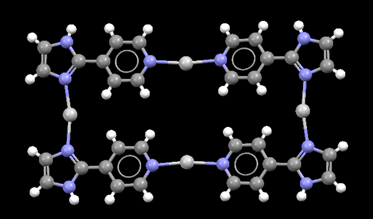 | ||
| Fig. 7 Molecular rectangle in the crystal structure of 1. | ||
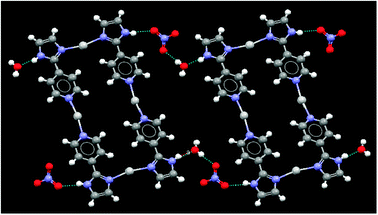 | ||
| Fig. 8 Non-covalent bridges between molecular squares in the crystal structure of 1. | ||
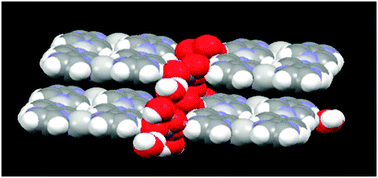 | ||
| Fig. 9 Packing in the crystal structure of 1. | ||
The crystal structure of 4 contains cyclic repeating units where the silver(I) ions have a coordination number of three, and form a distorted planar geometry, two of the imidazole nitrogen atoms coordinate to silver(I) in a near-linear mode while the third pyridine nitrogen atom is almost perpendicular to this linear motif, Fig. 10. The overall topology of this assembly is similar to what was postulated in Scheme 3(e). The ligand is anionic and contains three coordination sites, all of which are engaged with Ag(I) ions. These repeating units are further linked by metal–ligand coordinate covalent bond, Fig. 11.
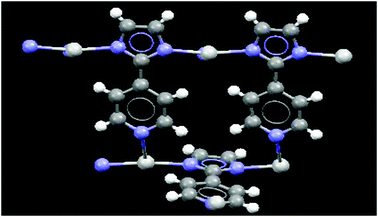 | ||
| Fig. 10 Cyclic closed unit: the crystal structure of 4. | ||
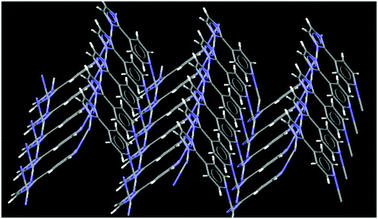 | ||
| Fig. 11 Extended network in the crystal structure of 4. | ||
Discussion
Four coordination complexes of silver(I) and 4PyIm have been characterized by single crystal X-ray diffraction, Table 2, in order to examine how counterions and solvent molecules may affect the assembly and coordination geometry of these complexes.| 1 | 2 | 3 | 4 | |
|---|---|---|---|---|
| Systematic name | Tetrakis(2-(4-pyridyl)imidazole) silver(I) nitrate tetrahydrate | [2-(4-Pyridyl)imidazole silver(I)] perchlorate acetonitrile | [2-(4-Pyridyl)imidazole silver(I)] hexafluorophosphate acetonitrile | 2-(4-Pyridyl)imidazole silver(I) |
| Formula moiety | (C8H7N3)4 (AgNO3)4 (H2O)3 | (C8H7N3) Ag(ClO4) (C2H3N) | (C8H7N3) (AgPF6) (C2H3N) | (C8H6N3) Ag |
| Empirical formula | C32 H34 Ag4 N16 O15 | C10H10AgClN4O4 | C10H10AgClN4O4 | C8H6AgN3 |
| Molecular weight | 1314.23 | 393.54 | 439.06 | 252.03 |
| Color, Habit | Colorless plate | Colorless needle | Colorless plate | Colorless needle |
| Crystal system | Triclinic | Monoclinic | Monoclinic | Monoclinic |
| Space group, Z | P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) , 1 , 1 | P21/c, 4 | P21/c, 4 | P21/n |
| a/Å | 6.9704(4) | 6.9519(6) | 6.7927(3) | 6.4496(5) |
| b/Å | 7.8319(5) | 14.9904(13) | 15.7016(7) | 13.5953(11) |
| c/Å | 19.4544(11) | 12.8623(13) | 13.1938(6) | 8.6168(6) |
| α (°) | 79.749(2) | 90.00 | 90.00 | 90.00 |
| β (°) | 85.086(2) | 103.276(6) | 102.723(2) | 94.244(3) |
| γ (°) | 81.023(2) | 90.00 | 90.00 | 90.00 |
| V/Å3 | 1030.46(11) | 1304.6(2) | 1372.65(11) | 753.49(10) |
| X-ray wavelength | 0.71073 | 0.71073 | 0.71073 | 0.71073 |
| μ mm−1 | 1.963 | 1.768 | 1.655 | 2.609 |
| Absorption corr | Multi scan | Multi scan | Multi scan | Multi scan |
| Trans min/max | 0.8913/0.5723 | 0.9326/0.6762 | 0.9072/0.6195 | 0.9028/0.5976 |
| Flections collected | 24![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 393 393 | 13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 548 548 | 16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 389 389 | 7600 |
| Independent | 7216 | 3745 | 4609 | 2502 |
| Observed | 6483 | 2271 | 3931 | 2115 |
| Threshold expression | >2sigma(I) | >2sigma(I) | >2sigma(I) | >2sigma(I) |
| R1 (observed) | 0.0261 | 0.0667 | 0.0278 | 0.0321 |
| wR2 (all) | 0.0596 | 0.1769 | 0.0648 | 0.0712 |
| GooF | 1.091 | 0.970 | 1.020 | 1.151 |
| Completeness | 0.919 | 0.944 | 0.857 | 0.902 |
| θmax | 33.140 | 30.500 | 33.460 | 32.720 |
IR spectroscopy was initially employed for characterizing the ligand and the four complexes, Table 3. An important feature in the spectrum of the free ligand is the peak at 1608 cm−1, which shifts toward higher wavenumbers upon formation of a coordination compound with a neutral ligand, while in the anionic complex the shift is toward lower wavenumbers. The presence of the ligand in the anionic form was confirmed by the absence of the N–H peak.
| 4-PyIm | (1) | (2) | (3) | (4) | |
|---|---|---|---|---|---|
| ν(cm−1) | 1608 | 1615 | 1621 | 1619 | 1601 |
The 1H NMR study of the ligand and the soluble complexes indicates the presence of a well-defined N–H peak in all the complexes with the neutral ligand, while in the 1H NMR spectra of the free ligand the peak is absent (see ESI†). No evidence could be found in the 1 H NMR data to indicate that the molecular square observed in the solid-state structure of 1 is retained in solution.
Structural analysis of 1–4 reveals the effect of the anion and the solvent on the structural outcome of the desired compounds. In the case of the compounds with perchlorate and hexafluorophosphate counterions and in the presence of acetonitrile as solvent we noticed that both structures are iso-structural and give the same 1-D zig-zag polymer, Fig. 12, with anions and the solvent molecules providing charge-balance and space-filling, respectively. An examination of the CSD for silver(I) complexes bearing a pyridine moiety as a coordinating ligand showed that changing the counterion from perchlorate to hexafluorophosphate produced iso-structural architectures in 80% of all occurrences (given similar experimental conditions). These two counterions have similar shape and are also relatively non-coordinating, which consequently means that most of the time, they will produce similar crystalline assemblies.
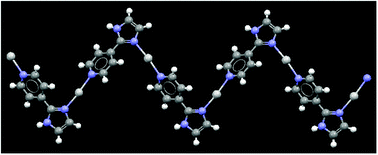 | ||
| Fig. 12 1-D polymer in compound (2) and (3). | ||
The case when using a nitrate ion as a counterion is markedly different. First of all the ion has smaller volume, Table 4, and secondly this counterion is frequently involved in strong and structure-directing hydrogen-bond interactions with most suitable hydrogen-bond donor molecules such as water, methanol, and ethanol. In this particular case, it seems that the nitrate ion is interacting with the initial polymer in such a way that the rotation around the C–C single bond between the two aromatic rings is slowed down which, in turn, facilitates the assembly of a closed molecular rectangle in preference to an infinite 1-D motif. The effect of the ion size on the formation of coordination polymers was examined by Kim et al.17 and they found that with a bent bipyridine ligand with C–C based conformational flexibility small-volume counterions gave rise to helical and cyclic structures whereas larger ions produced a 1-D zig-zag polymer. Similarly, Ag(I) complexes of bis(aminopyridine)18 change from cyclic to polymeric structures as the volume of the counterion increases.
| Ion | NO3− | ClO4− | PF6− |
|---|---|---|---|
| Volume (Å3) | 40 | 54 | 75 |
Formation of closed cyclic structures with rectangular geometry composed of two ligands and two silver(I) ions is relatively common in the literature,19 and there are a few examples of square metallocyclic composed of four ligands and four silver(I) ions.20 As far as we are aware, however, the structure of 1 contains the first metallocycle comprising four ligands and four silver(I) ions having a rectangular geometry.
The range of sizes of the counterions in our study is shown in Table 4, and it is important to note that there is a larger difference between ClO4− and PF6− than between ClO4− and NO3−, yet the former are iso-structural, whereas the nitrate containing complex is very different. Although a simple close-packing argument may occasionally help to explain structural differences in closely related systems, such behavior cannot be generalized to all systems21 and we need therefore to also examine other possible non-covalent interactions that may be induced or activated by the chemical nature of the counterion.22
An important distinction of the nitrate ion in the structure of 1 is related to its ability to form strong hydrogen bonds and this also means that it is likely to bring suitable hydrogen-bond donors along into the crystalline lattice thereby producing a hydrated complex. It seems that the molecular rectangles present in the crystal structure of 1 are quite stable, and the Ag(I) does not need to increase the coordination number beyond two. Instead, the nitrate ion (which is frequently coordinated to metal ions), simply acts as a bridge between adjacent rectangles using water molecules as appropriate hydrogen-bond donors. Again, an examination of the CSD demonstrates that for silver(I) complexes with pyridine-based ligands, similar structures were observed 80% of the time. However, when the solvent was altered from non-polar organic solvents to polar solvents (H2O and EtOH), capable of supporting strong hydrogen bonds, dramatic structural changes were obtained which also agrees with what we observed when changing from acetonitrile to water as a solvent.
The Cambridge Structural Database (CSD) supports the idea that ClO4− and PF6− are weaker coordinating anions, while NO3− is a stronger coordinating ion,23Table 5, and in several cases it competes with the ligand in coordination. The nitrate ion can form bonds with solvent molecules with great ease, and when combining an aqueous medium with nitrate ions there are considerable possibilities for incorporating water molecules within the lattice, especially if the nitrate ion is not ‘needed’ for metal-ion coordination. For example, more than 24% of all structures in the CSD containing nitrate ions also include one or more water molecules. The counterion may obviously play an important role in directing the final crystalline motif in the supramolecular array itself, but frequently it also changes the structure by virtue of bringing in solvent molecules capable of forming structure-directing hydrogen bonds.
| Percentage of the ion as coordinating ligand | NO3− | ClO4− | PF6− |
| 54 | 23 | 1 |
Moving from a charged complex with counterions (1–3) to an uncharged complex in the absence of counterions produces a dramatic change in coordination behavior; the anionic ligand has three available coordination sites, which are all consumed and, interestingly, this also increases the coordination number for silver(I) from two to three in this family of compounds.
Conclusions
Any systematic structural examination of the coordination geometry and supramolecular chemistry of metal-complexes requires a detailed analysis of the coordinating ability of counterions and solvent molecules as well as of their capacity for forming strong non-covalent interactions.24 In this study, we have focused on a system that allows us to change one variable at a time (solvent, counterion, and ligand charge), in order to try to elucidate cause and effect in the solid-state behavior in this family of compounds. Size is certainly less important than the chemical nature of the counterions in this series, and the introduction of the nitrate ion effectively ‘activated’ a new set of intermolecular interactions by virtue of bringing polar solvent molecules into the lattice. Although the unique molecular rectangle obtained in the crystal structure of 1 does not hold opportunities for host–guest chemistry or ion recognition behavior, we now have access to a set of guidelines that can inform the next step in this investigation, which will focus on the synthesis of larger metallocycles with rectangular geometry with potential applications as functional materials. The results and observations regarding the effect of counterions and solvents are important for controlling the synthesis of the desired target compound and also contribute to more refined and versatile guidelines for inorganic crystal engineering.Acknowledgements
We are grateful for a Fulbright Fellowship (to MAB) by the U.S. Department of State Bureau of Educational and Cultural Affairs and the Council for International Exchange of Scholars.Notes and references
- (a) C. Chen, B. Kang and C. Su, Aust. J. Chem., 2006, 59, 3 CrossRef CAS; (b) C. B. Aakeröy and A. M. Beatty, J. Mol. Struct., 1999, 474, 91 CrossRef CAS; (c) C. B. Aakeröy, A. M. Beatty and D. S. Leinen, CrystEngComm, 2002, 4, 310 RSC; (d) W. L. Leong and J. J. Vittal, Chem. Rev., 2011, 111, 688 CrossRef CAS.
- (a) N. Fang, H. Lee, C. Sun and X. Zhang, Science, 2005, 308, 534 CrossRef CAS; (b) C. M. Soukoulis, S. Linden and M. Wegener, Science, 2007, 315, 47 CrossRef CAS; (c) R. Jin, Y. Cao, C. A. Mirkin, K. L. Kelly, G. C. Schatz and J. G. Zheng, Science, 2001, 294, 1901 CrossRef CAS.
- (a) B. Y. Ahn, E. B. Buoss, M. J. Motala, X. Guo, S. Park, Y. Xiong, J. Yoon, R. G. Nuzzo, J. A. Rogers and J. A. Lewis, Science, 2009, 323, 1590 CrossRef CAS; (b) K. F. Hsu, S. Loo, F. Guo, W. Chen, J. S. Dyck, C. Uher, T. Hogan, E. K. Polychroniadis and M. G. Kanatzidis, Science, 2004, 303, 818 CrossRef CAS.
- (a) C. M. Jones and E. Hoek, J. Nanopart. Res., 2010, 12, 1513 Search PubMed; (b) A. K. Singh, D. Senapati, A. Neely, G. Kalowole, C. Hawker and P. C. Ray, Chem. Phys. Lett., 2009, 48, 94 Search PubMed; (c) M. Sowwan, M. Abul-Haj, M. Faroun, Z. Hawash, J. Ghabboun, A. Rida, A. Karmi, W. Sultan and G. A. Husseini, J. Nanomater., 2010, 2010, 1 Search PubMed.
- (a) K. Shimizu, Y. Miyamoto and A. Satsuma, J. Catal., 2010, 270, 86 CrossRef CAS; (b) G. J. Arsenault, C. M. Anderson and R. J. Puddephatt, Organometallics, 1988, 7, 2094 CrossRef CAS; (c) G. Zon and L. A. Paquette, J. Am. Chem. Soc., 1974, 96, 215 Search PubMed; (d) C. V. King, J. Am. Chem. Soc., 1927, 49, 2689 Search PubMed; (e) L. A. Paquette, Acc. Chem. Res., 1971, 4, 280 CrossRef CAS.
- (a) P. C. Lee and D. Meisel, J. Phys. Chem., 1982, 86, 3391 CrossRef CAS; (b) A. W. Czanderna, S. C. Chen and J. R. Biegen, J. Catal., 1972, 33, 163 Search PubMed; (c) H. A. Engelhardt and D. Menzel, Surf. Sci., 1976, 57, 591 CrossRef CAS; (d) M. Brust and C. J. Kiely, Colloids Surf., A, 2002, 202, 175 CrossRef CAS.
- A. Young and L. Hanton, Coord. Chem. Rev., 2008, 252, 1346 CrossRef CAS.
- D. Hutchinson, S. Cameron, L. Hanton and S. Moratti, Inorg. Chem., 2012, 51, 5070 CrossRef CAS.
- G. Swiegers and T. Malefetse, Chem. Rev., 2000, 100, 3483 CrossRef CAS.
- M. Voss, C. Beer, S. Mitchell, P. Blomgren and P. Zhichkin, Tetrahedron, 2008, 64, 645 CrossRef CAS.
- APEXII v2009. 5-1, ©, Bruker Analytical X-ray Systems, Madison, WI, 2009 Search PubMed.
- COSMO v1. 60, ©, Bruker Analytical X-ray Systems, Madison, WI, 1999–2009 Search PubMed.
- SAINT v7. 60a, ©, Bruker Analytical X-ray Systems, Madison, WI, 1997–2008 Search PubMed.
- SADABS v2008/1, ©, Bruker Analytical X-ray Systems, Madison, WI, 2008 Search PubMed.
- SHELXTL v2008/4, ©, Bruker Analytical X-ray Systems, Madison, WI, 2008 Search PubMed.
- (a) S. Muthu, J. H. Yip and J. J. Vittal, Dalton Trans., 2001, 3577 RSC; (b) J. Liu, X. Su, W. Wang, Z. Mao and R. Xie, Acta Crystallogr., Sect. E: Struct. Rep. Online, 2006, 62, m1173 Search PubMed; (c) L. Raehm, L. Mimassi, C. Guyard-Duhayon, H. Amouri and M. N. Rager, Inorg. Chem., 2003, 42, 5654 CrossRef CAS; (d) R. Wang, M. Hong, J. Luo, F. Jiang, L. Han, Z. Liu and R. Cao, Inorg. Chim. Acta, 2004, 357, 103 CrossRef CAS; (e) E. M. Zerbe, C. Wolper and P. G. Jones, Z. Naturforsch., B: Chem. Sci., 2011, 66, 449 Search PubMed; (f) J. Fau, W. Sun, T. Okamura, W. Tang and N. Ueyama, Inorg. Chim. Acta, 2004, 357, 2385 CrossRef CAS; (g) C. B. Aäkeroy, A. M. Beatty and D. S. Leinen, CrystEngComm, 2002, 4, 310 RSC; (h) X. Huang, D. Li and X. Cheu, CrystEngComm, 2006, 8, 351 RSC.
- H. Kim, W. Zin and M. Lee, J. Am. Chem. Soc., 2004, 126, 7009 CrossRef CAS.
- N. L. S. Yue, M. C. Jennings and R. J. Puddephatt, Dalton Trans., 2010, 39, 1273 RSC.
- (a) M. Oh, C. Stern and C. Mirkin, Inorg. Chem., 2005, 44, 2647 CrossRef CAS; (b) K. Kilpin, M. Growa, S. Telfor, G. Jameson and J. Crowley, Inorg. Chem., 2011, 50, 1123 CrossRef CAS; (c) H. Zhang, L. Chen, H. Sang and G. Zi, Inorg. Chim. Acta, 2011, 366, 320 CrossRef CAS; (d) P. Byrne, G. Lloyod, L. Applegarth, K. Anderson, N. Clarke and J. Steed, New J. Chem., 2010, 34, 2261 RSC; (e) L. Childs, M. Pascu, A. Clarke, N. Alcock and M. Hannon, Chem.–Eur. J., 2004, 10, 4291 CrossRef CAS.
- (a) I. Belosos, J. Casto, J. Vazquez, P. Lourido, J. Romero and A. Sousa, Inorg. Chem., 2005, 44, 336 CrossRef CAS; (b) L. Engelhaydt, G. Jacobsen, W. Patalinglug, B. Skelton, C. Rasta and A. White, Dalton Trans., 1991, 11, 2859 Search PubMed; (c) R. Feazell, C. Carson and K. Klausmeyer, Inorg. Chem., 2006, 45, 2627 CrossRef CAS; (d) O. Jung, Y. Lee, Yu. Kim and J. Hang, Cryst. Growth Des., 2002, 2, 497 CrossRef CAS.
- L. Raehm, L. Mimassi, C. Guyard, H. Amouri and M. N. Rager, Inorg. Chem., 2003, 42, 5654 CrossRef CAS.
- (a) D. Pocic, J. M. Planeix, N. Kyritsakas, A. Jouaiti and M. Hosseini, CrystEngComm, 2005, 7, 624 RSC; (b) A. Y. Robin, J. L. Sagué and K. M. Fromm, CrystEngComm, 2006, 8, 403 RSC; (c) D. Pogozhev, S. A. Baudron and M. Hosseini, Dalton Trans., 2011, 40, 437 RSC.
- M. Kato, T. Fujihara, D. Yanoc and A. Nagasawaa, CrystEngComm, 2008, 10, 1460 RSC.
- (a) C. B. Aakeröy, A. M. Beatty, J. Desper, M. O'Shea and J. Valdés-Martínez, Dalton Trans., 2003, 3956 RSC; (b) C. B. Aakeröy, J. Desper and N. S. Schultheiss, Inorg. Chem., 2005, 44, 4983 CrossRef; (c) A. Y. Robin, M. Meuwly, K. M. Fromm, H. Goesmann and G. Bernardinelli, CrystEngComm, 2004, 6, 336 RSC; (d) F. Gschwind and K. M. Fromm, CrystEngComm, 2012, 14, 4008 RSC.
Footnote |
| † Electronic supplementary information (ESI) available: Crystallographic data (cif files) and experimental details. CCDC 894290–894293. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c2nj40669a |
| This journal is © The Royal Society of Chemistry and the Centre National de la Recherche Scientifique 2013 |
