Chronic L-arginine treatment improves metabolic, cardiovascular and liver complications in diet-induced obesity in rats
Md. Ashraful Alama, Kathleen Kauterb, Kerry Withersb, Conrad Serniaa and Lindsay Brown*b
aSchool of Biomedical Sciences, The University of Queensland, Brisbane, QLD 4072, Australia
bDepartment of Biological and Physical Sciences, University of Southern Queensland, Toowoomba, QLD 4350, Australia. E-mail: Lindsay.Brown@usq.edu.au; Fax: +61 746311530; Tel: +61 746311319
First published on 4th September 2012
Abstract
L-Arginine is an important dietary amino acid in both health and disease, especially of the cardiovascular system. This study has determined whether dietary supplementation with L-arginine attenuates cardiovascular, metabolic, pancreatic and liver changes in a rat model of the human metabolic syndrome. Male Wistar rats (8–9 weeks old) were divided into four groups. Two groups of rats were fed a corn starch-rich diet (C) whereas the other two groups were given a high carbohydrate, high fat diet (H) with 25% fructose in the drinking water, for 16 weeks. One group fed each diet was supplemented with 5% L-arginine in the food for the final 8 weeks of this protocol. The corn starch diet (C) contained ∼68% carbohydrates mainly as polysaccharides, while the high-carbohydrate, high-fat diet contained ∼68% carbohydrates mainly as fructose and sucrose together with 24% fat mainly as saturated and monounsaturated fats from beef tallow. The high-carbohydrate, high-fat diet-fed rats showed the symptoms of metabolic syndrome including obesity and hypertension with heart and liver damage. Supplementation with L-arginine attenuated impairment in left ventricular and liver structure and function, glucose tolerance, and decreased blood pressure, abdominal fat pads, inflammatory cell infiltration, pancreatic cell hypertrophy and oxidative stress. This study indicates that oral supplementation with L-arginine attenuated or normalised obesity-related changes in the heart, liver and pancreas by reducing inflammation and oxidative stress associated with high carbohydrate, high fat feeding in rats.
1. Introduction
L-Arginine is an important amino acid in nutrition as well as in health and disease.1,2 Metabolic pathways with L-arginine produce nitric oxide (NO), polyamines, proline, glutamate, creatine and agmatine.3 NO plays key physiological roles such as maintenance of vascular tone and platelet function, regulation of blood pressure4,5 and regulation of metabolism of glucose and fatty acids.6L-Arginine reduced blood pressure and fibrosis in rat models of hypertension7 and reduced adiposity and improved insulin signalling in rat models of obesity.8 Dietary supplementation with L-arginine reduced white fat mass in Zucker diabetic fatty rats9 and diet-induced obese rats.10 Together, obesity, insulin resistance and hypertension define the metabolic syndrome that increases the risk of cardiovascular disease and diabetes; non-alcoholic fatty liver disease (NAFLD) is also increased in metabolic syndrome.11,12 The role of L-arginine in the treatment of fatty liver is unknown, but increased endothelial NO may limit obesity-induced inflammation and insulin resistance in hepatocytes.13 These reports suggest that L-arginine may be useful in reducing the multi-organ damage of the metabolic syndrome.This study has defined the effect of L-arginine on the components of metabolic syndrome including cardiovascular remodeling, metabolic changes and fatty liver in a rat model of diet-induced metabolic syndrome.14 Rats were fed with either corn starch (C) or high carbohydrate, high fat diets (H) for 16 weeks with L-arginine supplementation for the last 8 weeks of the protocol. At the end of 16 weeks, metabolic parameters and structure and function of the heart and the liver were measured. We have previously reported that cardiovascular, metabolic and liver symptoms in these high carbohydrate, high fat diet-fed rats can be reversed by intervention with natural products such as olive leaf,15 chia seeds,16 purple carrots,17 rutin18 and coffee.19
2. Materials and methods
2.1 Rats and diets
36 Male Wistar rats (weight 328 ± 3 g; 9–10 weeks old) were purchased from The University of Queensland Biological Resources facility and individually caged at the Animal House Facility at the University of Southern Queensland. The Animal Ethics Committee of the University of Southern Queensland and The University of Queensland approved all experimental protocols, under the guidelines of the National Health and Medical Research Council of Australia. The rats were randomly divided into 4 groups: corn starch diet-fed rats (C; n = 9), C + L-arginine (CA; n = 9), high carbohydrate, high fat diet-fed rats (H, n = 9), and H + L-arginine (HA, n = 9). L-Arginine was obtained from Sigma Aldrich, Sydney, Australia. C and H rats were fed their respective diets for the entire 16 weeks (Table 1). C rats were provided with normal tap water while drinking water in the H-fed rats was augmented with 25% fructose. C and H diets have been previously described.14–19L-Arginine was incorporated as 5% of diet in both CA and HA diets starting 8 weeks after initiation of the high carbohydrate, high fat diet for a further 8 weeks.| Parameters | C | CA | H | HA | P value | ||
|---|---|---|---|---|---|---|---|
| DIET | A | DIET × A | |||||
| a Normalised to tibial length.b Relative beta cell mass was calculated by using the formula: relative beta cell mass = (average beta cell cross sectional area/total cross sectional area of pancreas) × pancreatic wet weight. Data are presented as mean ± SEM, n = 8–9 unless otherwise specified.c Data from C, CA, H and HA groups were tested by two-way ANOVA. When interactions of the main effects were significant, means were compared using Newman–Keuls multiple-comparison post hoc test. Statistical significance was considered as p < 0.05. C, corn starch-rich diet-fed rats; CA, corn starch-rich diet-fed rats treated with L-arginine; H, high carbohydrate, high fat diet-fed rats; HA, high carbohydrate, high fat diet-fed rats treated with L-arginine. d,e Means without a common letter in a row differ, P < 0.05. | |||||||
| Initial body weight, g | 332 ± 5 | 330 ± 2 | 328 ± 3 | 327 ± 2 | 0.3358 | 0.5457 | 0.8796 |
| Final body weight, g | 471 ± 15 | 407 ± 6 | 524 ± 6 | 471 ± 12 | <0.0001 | <0.0001 | 0.5809 |
| % of weight gain from week 9 to week 16 | 13 ± 2 | 10 ± 2 | 21 ± 4 | 11 ± 1 | 0.1068 | 0.0114 | 0.1445 |
| Food intake (g day−1) at week 16 | 33.8 ± 0.3 | 31.2 ± 0.6 | 28.0 ± 0.7 | 26.4 ± 0.3 | <0.0001 | 0.0002 | 0.3319 |
| Water intake (mL day−1) at week 16 | 31.9 ± 0.5d | 32.7 ± 0.4d | 23.0 ± 0.4e | 27.3 ± 0.2d | <0.0001 | <0.0001 | <0.0001 |
| Drug intake mg kg−1 per day | 0 | 4450 ± 40 | 0 | 2970 ± 40 | — | — | — |
| Energy intake (kJ day−1) at week 16 | 411 ± 4 | 379 ± 6 | 592 ± 13 | 580 ± 6 | <0.0001 | 0.0072 | 0.2082 |
| Kidney weighta (mg mm−1) | 46.1 ± 1.7 | 48.8 ± 1.5 | 55.5 ± 3.6 | 54.7 ± 2.0 | 0.0027 | 0.6888 | 0.4620 |
| Spleena (mg mm−1) | 16.7 ± 0.7 | 15.6 ± 0.6 | 20.8 ± 2.9 | 16.6 ± 1.2 | 0.1288 | 0.1150 | 0.3504 |
| Liver wet weighta (mg mm−1) | 239.0 ± 6.8d | 221.5 ± 5d | 339.0 ± 17.3e | 264.1 ± 18.3d | <0.0001 | 0.0015 | 0.0383 |
| Abdominal fat padsa weight (mg mm−1) | 443.9 ± 51.6 | 296.3 ± 17.9 | 726.9 ± 47.1 | 525.0 ± 58.3 | 0.0007 | <0.0001 | 0.5623 |
| Retroperitoneal fata (mg mm−1) | 265.2 ± 29.7 | 138.9 ± 13.2 | 423.0 ± 25.0 | 277.8 ± 33.9 | <0.0001 | <0.0001 | 0.7247 |
| Epididymal fata (mg mm−1) | 95.0 ± 17.9 | 87.8 ± 7.3 | 171.0 ± 21.0 | 124.9 ± 13.0 | 0.0989 | 0.0010 | 0.2239 |
| Omental fata (mg mm−1) | 83.6 ± 0.5 | 69.7 ± 8.4 | 132.9 ± 17.0 | 122.3 ± 17 | 0.0007 | 0.3717 | 0.9036 |
| Pancreas wet weighta (mg mm−1) | 44.5 ± 2.2 | 35.1 ± 2.7 | 60.8 ± 3.6 | 39.2 ± 3.7 | 0.0025 | <0.0001 | 0.0589 |
| % Islet area (n = 6) | 11.5 ± 0.7d | 13.1 ± 1.4d | 21.3 ± 1.3e | 17.3 ± 1.2d | <0.0001 | 0.3419 | 0.0255 |
| Relative beta cell massb | 25.3 ± 1.7 | 27.1 ± 2.5 | 47.8 ± 2.7 | 40.6 ± 3.5 | <0.0001 | 0.3394 | 0.1142 |
| Abdominal circumference at 16 weeks (cm) | 21.9 ± 0.3 | 18.5 ± 0.2 | 23.7 ± 0.3 | 21.2 ± 0.4 | <0.0001 | <0.0001 | 0.1272 |
Organ weights, metabolic measurements (energy intake, blood glucose, insulin and lipid concentrations after overnight fasting, abdominal circumference and abdominal fat pads), cardiovascular evaluations (systolic blood pressure, echocardiography,20 left ventricular stiffness in the Langendorff heart, thoracic aortic contractility, collagen deposition and inflammatory cell infiltration) and liver measurements (plasma enzymes, liver structure) were determined as described previously.14–19,21,22
Nitrite and nitrate (NOx) concentrations were determined in plasma and liver homogenate by the Griess method.23 Malondialdehyde concentrations were measured as thiobarbituric acid reactive substances by spectrophotometry.24
2.2 Gelatin zymography
To detect MMP-9 and MMP-2 activity, tissue protein was collected and assayed for protein concentration by protein assay kit. 150 μg of protein was electrophorised on zymogram gelatin gels. Gelatin zymography was performed on a 10% sodium dodecyl sulphate acrylamide gel containing 0.1% gelatin, rinsed in double-distilled water followed by incubation with 300 mL of re-naturation buffer (2.7%Triton X-100 in dd-H2O) at room temperature for one hour with gentle shaking. The enzyme activity was developed in 50 mM Tris pH 7.5, 0.2 M NaCl, 5 mM CaCl2 at 37°C for 24 hours and stained with Coomassie blue with images obtained with a digital photo imager.252.3 Mitochondrial preparations
Mitochondria were isolated from liver of control rats and prepared in a medium containing (in mM) 200 sucrose, 1 EGTA and 10 Tris–HCl (pH 7.2). Liver mitochondria were prepared according to standard differential centrifugation procedures.26 Mitochondrial protein content was determined by the bicinconinic acid protein assay method, with BSA as standard. Mitochondria were incubated at a final concentration of 0.35 mg mL−1 in a Clark-type electrode chamber for oxygen consumption measurements. All experiments were performed at 37 °C in the following buffer (in mM): 125 KCl, 1 EGTA, 1 KH2PO4, and 10 Tris–HCl (pH 7.2). The control (nonphosphorylating) state of respiration was initiated by the addition of 5 mM succinate and 5 μM rotenone. State 3 (phosphorylating respiration) was obtained after the addition of 1 mM ADP. State 4 respiration was considered as the respiratory state in the absence of ADP. The efficiency of the mitochondrial oxidative phosphorylation was assessed by the state 3 to state 4 ratio, the respiratory control ratio (RCR).2.4 Histology
Immediately after removal of the heart, liver and pancreas, tissues were blotted, weighed, cut into 3–4 mm slices and fixed in 10% buffered formalin for 3 days. Formalin solution was changed every day to remove traces of tissue debris from the tissue. The samples were then dehydrated and embedded in paraffin wax. Thin sections (5 μm) were cut and stained with haematoxylin and eosin stain for determination of inflammatory cell infiltration and general architecture of the tissues. Collagen distribution in left ventricle of the heart was measured following picrosirius red staining and analysed by laser confocal microscopy (Zeiss LSM 510 upright Confocal Microscope).14–19 Collagen distribution in liver was defined with Milligan's trichrome stain. Collagen deposition was quantified by colour intensity using NIH-ImageJ free software. The insulin-positive area in the pancreatic islets of Langerhans was stained using aldehyde fuchsin followed by orange G counter-stain.2.5 Statistical analysis
All data sets are represented as mean ±SEM. Data from C, CA, H and HA groups were tested by 2-way ANOVA. When interactions of the main effects were significant, means were compared using Newman–Keuls multiple-comparison post hoc test. The oral glucose tolerance test (OGTT) was assessed by calculating the area under the curve (AUC). OGTT and blood pressure values were also individually analysed by one-way ANOVA repetitive measures. All statistical analyses were performed using Graph Pad Prism version 5.00 for Windows.3. Results
3.1 Body weight, food and water intakes
H and HA rats showed reduced food consumption compared with the C and CA groups. Food intake was higher in C and CA rats compared to H and HA rats although energy intake was higher in H rats; this was associated with an increased final body weight and increased abdominal fat deposition compared with C rats (Table 1). L-Arginine supplementation did not alter food and energy intake, but reduced body weight gain in both CA and HA rats (Table 1).3.2 Metabolic changes, oxidative stress and inflammation
Plasma total cholesterol, triglyceride and NEFA concentrations were higher in H rats compared to C rats (Table 2). HA rats showed lower plasma total cholesterol but not triglyceride concentrations (Table 2). H rats had a near doubling of abdominal fat deposition and greater abdominal circumference compared with the C rats after 16 weeks. L-Arginine supplementation reduced the abdominal fat deposition in both the CA and HA rats and attenuated the increased abdominal circumference. Fasting plasma glucose concentrations were higher in H rats compared to C rats, and glucose intolerance was shown by higher area under the curve (AUC) with higher plasma glucose concentrations two hours after glucose loading (Fig. 1, Table 2). Plasma insulin concentrations were higher in H rats compared to C rats; L-arginine treatment normalised insulin concentrations both in HA and CA rats (Table 2). H rats showed increased pancreatic wet weight and relative beta cell mass (Table 1), and increased the islets of Langerhans size compared with C rats (Fig. 2). L-Arginine supplementation normalised islet size and insulin-positive area in both the CA and HA rats. Plasma and liver malondialdehyde concentrations and plasma C reactive protein concentrations were higher in H rats than in C rats but lower in HA rats (Table 2).| Parameters | C | CA | H | HA | P value | ||
|---|---|---|---|---|---|---|---|
| DIET | A | DIET × A | |||||
| a AUC was calculated with x-axis as the baseline.b LDH, lactate dehydrogenase, NEFA, non-esterified fatty acid, ALP, alkaline phosphatase; ALT, alanine transaminase; AST, aspartate transaminase, NOx, nitrate + nitrite. Data are presented as mean ± SEM, n = 8–9 unless otherwise specified.c Data from C, CA, H and HA groups were tested by two-way ANOVA. When interactions of the main effects were significant, means were compared using Newman–Keuls multiple-comparison post hoc test. Statistical significance was considered as p < 0.05. C, corn starch-rich diet-fed rats; CA, corn starch-rich diet-fed rats treated with L-arginine; H, high carbohydrate, high fat diet-fed rats; HA, high carbohydrate, high fat diet-fed rats treated with L-arginine. d,e Means without a common letter in a row differ, P < 0.05. | |||||||
| Liver parameters | |||||||
| ALT (U L−1) | 24.0 ± 1.7d | 30.4 ± 1.6d | 38.7 ± 2.4e | 29.1 ± 1.0d | 0.0008 | 0.3808 | 0.0001 |
| AST (U L−1) | 76.7 ± 5.4 | 69.5 ± 2.8 | 94.7 ± 4.2 | 73.1 ± 4.7 | 0.0216 | 0.0030 | 0.1166 |
| ALP (U L−1) | 127.9 ± 11.1d | 136.9 ± 8.1d | 205.4 ± 19.2e | 136.7 ± 8.8d | 0.0091 | 0.0391 | 0.0088 |
| LDH (U L−1) | 235.5 ± 33.4 | 264.7 ± 62.9 | 419.2 ± 54.5 | 276.7 ± 20.4 | 0.0420 | 0.2283 | 0.0721 |
| Bilirubin (mmol L−1) | 1.6 ± 0.3 | 2.0 ± 0.3 | 2.2 ± 0.1 | 1.8 ± 0.2 | 0.1327 | 1.0000 | 0.4433 |
| Triglycerides (mmol L−1) | 0.7 ± 0.1 | 0.8 ± 0.1 | 1.3 ± 0.2 | 0.9 ± 0.2 | 0.0352 | 0.3521 | 0.1257 |
| Total cholesterol (mmol L−1) | 1.3 ± 0.1d | 1.5 ± 0.1d | 1.9 ± 0.1d | 1.6 ± 0.1e | 0.0015 | 0.6220 | 0.0185 |
| NEFA (mmol L−1) | 2.2 ± 0.3 | 2.6 ± 0.2 | 2.6 ± 0.5 | 3.7 ± 0.4 | 0.0061 | 0.0477 | 0.7700 |
| Kidney parameters | |||||||
| Creatinine (mmol L−1) | 44.9 ± 1.6d | 42.5 ± 2.2d | 50.7 ± 2.4e | 3.7d | 0.9688 | 0.0033 | 0.0322 |
| Uric acid (mmol L−1) | 49.7 ± 5.6 | 44.7 ± 2.3 | 58.3 ± 7.4 | 37.3 ± 3.9 | 0.9177 | 0.0318 | 0.1752 |
| Urea (mmol L−1) | 3.6 ± 0.2 | 2.5 ± 0.2 | 5.1 ± 0.6 | 3.1 ± 0.2 | 0.0069 | 0.0002 | 0.2233 |
| Na+ (mmol L−1) | 146.0 ± 0.4 | 142.8 ± 0.3 | 147.0 ± 0.8 | 141.9 ± 0.4 | 0.9256 | <0.0001 | 0.0836 |
| K+ (mmol L−1) | 4.2 ± 0.1 | 4.7 ± 0.1 | 4.4 ± 0.4 | 3.8 ± 0.2 | 0.0312 | 0.8386 | 0.1606 |
| TBARS (μmol L−1) | |||||||
| Plasma | 20.4 ± 2.7 | 18.4 ± 1.8 | 29.6 ± 2.6 | 22.0 ± 0.7 | 0.0064 | 0.0344 | 0.2014 |
| Liver | 29.6 ± 3.0d | 26.9 ± 1.5d | 73.4 ± 1.3e | 5 2.3d | <0.0001 | 0.0004 | 0.0081 |
| Liver NOx (μmol L−1) | 35.2 ± 2.8 | 50.5 ± 4.7 | 17.0 ± 2.0 | 44.8 ± 2.5 | 0.0012 | <0.0001 | 0.0642 |
| C-Reactive protein (μmol L−1) | 25.3 ± 1.5d | 30.6 ± 0.9d | 53 ± 2.7e | 43.2 ± 1.3d | <0.0001 | 0.2014 | 0.0002 |
| Plasma insulin concentration (pmol mL−1) | 1.9 ± 0.3 | 1.5 ± 0.1 | 4.0 ± 1.0 | 2.1 ± 0.2 | 0.0246 | 0.0525 | 0.1966 |
| OGTT (mmol L−1min)a | |||||||
| AUC at 0 week | 681 ± 23 | 662 ± 24 | 666 ± 11 | 676 ± 29 | 0.9878 | 0.8392 | 0.5467 |
| AUC at 8 week | 678 ± 11 | 680 ± 18 | 802 ± 19 | 774 ± 11 | <0.0001 | 0.4087 | 0.3237 |
| AUC at 16 week | 699 ± 11d | 673 ± 32d | 854 ± 18e | 680 ± 32d | 0.0032 | 0.0004 | 0.0065 |
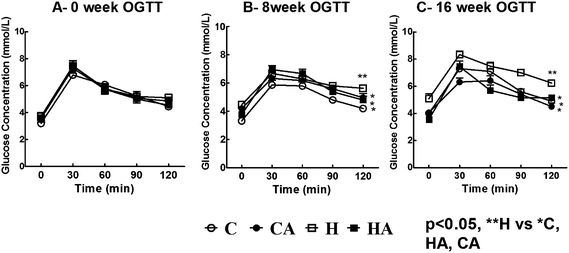 | ||
| Fig. 1 Oral glucose tolerance test (OGTT) for H rats compared to C rats, with reduction in CA and HA rats (A – 0 week OGTT; B – 8 week OGTT and C – 16 week OGTT); one way ANOVA with repeated measures, statistical significance was considered as p < 0.05. C, corn starch-rich diet-fed rats; CA, corn starch-rich diet-fed rats treated with L-arginine; H, high carbohydrate, high fat diet-fed rats; HA, high carbohydrate, high fat diet-fed rats treated with L-arginine. ** significantly different from *. | ||
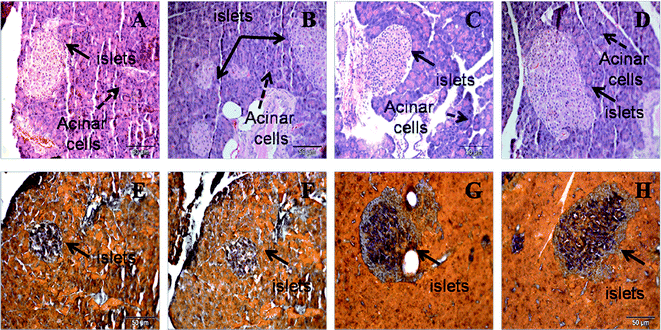 | ||
| Fig. 2 Haematoxylin and eosin staining (upper panel) of pancreas (×20) showing hypertrophied islets of Langerhans in H (B) rats compared to C (A) rats, with reduction in CA (C) and HA (D) rats. Aldehyde fuchsin stain (lower panel) for islets showing insulin-positive area as dark red inside the islets of Langerhans in H (F) rats compared to C (E) rats, with reduction in CA (G) and HA (H) rats. C, corn starch-rich diet-fed rats; CA, corn starch-rich diet-fed rats treated with L-arginine; H, high carbohydrate, high fat diet-fed rats; HA, high carbohydrate, high fat diet-fed rats treated with L-arginine. | ||
3.3 Cardiovascular changes
Systolic blood pressure was higher in H rats compared to C rats; L-arginine supplementation in HA rats reduced the systolic blood pressure to near normal at 16 week (Fig. 3). Echocardiographic assessment of H rats showed larger internal diameter (LVIDd), reduced systolic function (fractional shortening and ejection fraction), greater relative wall thickness, higher estimated left ventricular mass, reduced mitral flow (E:A) ratio, and decreased mitral closing–opening duration compared with C rats (Table 3). Compared with H rats, HA rats showed reduced internal diameter and a greater fractional shortening (Table 3). H rats showed higher ventricular stiffness than C rats; in HA rats, ventricular stiffness was not different to C rats (Table 3). H rats had higher left ventricular wet weight compared with the C, CA and HA rats (Table 3). H rats showed greater left ventricular inflammatory cell infiltration and interstitial collagen deposition than C, CA and HA rats (Fig. 4; Table 3). MMP-2 activities were not significantly changed in any group, while MMP-9 activity decreased with L-arginine supplementation (Table 3). H rats also showed cardiomyocyte hypertrophy (Fig. 4). H rats showed diminished vascular responses in isolated thoracic aortic rings to noradrenaline, sodium nitroprusside and acetylcholine compared with C rats; acetylcholine-induced relaxation was greater in HA rats than in H rats (Fig. 5).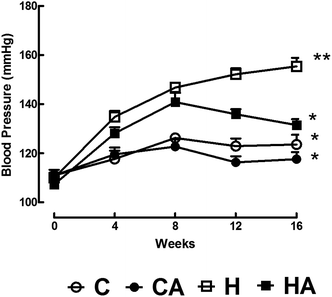 | ||
| Fig. 3 L-Arginine effect on systolic blood pressure over 16 weeks in C, CA, H and HA rats. Values are mean ± SEM, n = 8; one way ANOVA with repeated measures, statistical significance was considered as p < 0.05. C, corn starch-rich diet-fed rats; CA, corn starch-rich diet-fed rats treated with L-arginine; H, high carbohydrate, high fat diet-fed rats; HA, high carbohydrate, high fat diet-fed rats treated with L-arginine. ** significantly different from *. | ||
| Parameters | C | CA | H | HA | P value | ||
|---|---|---|---|---|---|---|---|
| DIET | A | DIET × A | |||||
| a Normalised to tibial length.b Data are presented as mean ± SEM, n = 8–9 unless otherwise specified. Data from C, CA, H and HA groups were tested by two-way ANOVA. When interactions of the main effects were significant, means were compared using Newman–Keuls multiple-comparison post hoc test. Statistical significance was considered as p < 0.05. C, corn starch-rich diet-fed rats; CA, corn starch-rich diet-fed rats treated with L-arginine; H, high carbohydrate, high fat diet-fed rats; HA, high carbohydrate, high fat diet-fed rats treated with L-arginine. c,d Means without a common letter in a row differ, P < 0.05. | |||||||
| Heart rate (bpm) | 286 ± 21 | 274 ± 25 | 272 ± 31 | 267 ± 16 | 0.6614 | 0.7227 | 0.8798 |
| LVIDd, mm | 0.72 ± 0.02c | 0.77 ± 0.0c | 0.82 ± 0.03d | 0.76 ± 0.02c | 0.0429 | 0.8154 | 0.0150 |
| LVPWd, mm | 0.18 ± 0.00c | 0.17 ± 0.01c | 0.22 ± 0.01d | 0.17 ± 0.01c | 0.0285 | 0.0017 | 0.0285 |
| Fractional shortening, % | 47.7 ± 1.2 | 47.4 ± 2.2 | 39.0 ± 3.4 | 48.3 ± 2.8 | 0.1300 | 0.0842 | 0.0671 |
| Ejection fraction, % | 90.9 ± 1.0c | 94.1 ± 1.2c | 95.2 ± 1.3c | 92.4 ± 1.0c | 0.2631 | 0.8397 | 0.0141 |
| Relative wall thickness | 0.48 ± 0.01 | 0.45 ± 0.01 | 0.49 ± 0.01 | 0.46 ± 0.01 | 0.3259 | 0.0056 | 1.0000 |
| Ascending aorta diameter, mm | 0.85 ± 0.02 | 0.79 ± 0.05 | 1.04 ± 0.06 | 0.97 ± 0.04 | 0.0003 | 0.1597 | 0.9123 |
| Descending aorta diameter, mm | 0.79 ± 0.0.5 | 0.94 ± 0.06 | 1.02 ± 0.09 | 0.91 ± 0.6 | 0.1450 | 0.7665 | 0.0614 |
| Ejection time, ms | 85.5 ± 2.2 | 85.9 ± 3.7 | 76.4 ± 2.5 | 86.1 ± 2.4 | 0.1182 | 0.0763 | 0.0997 |
| Deceleration time, ms | 50.8 ± 1.9 | 50 ± 2.2 | 45.4 ± 2.9 | 42.7 ± 3.1 | 0.0238 | 0.4997 | 0.7174 |
| E/A ratio | 1.9 ± 0.1 | 2.2 ± 0.1 | 1.4 ± 0.2 | 1.7 ± 0.1 | 0.0285 | 0.0003 | 0.8311 |
| MC-MO | 114.8 ± 1.0c | 115.6 ± 2.0c | 108.0 ± 0.6d | 118.0 ± 2.6c | 0.0081 | 0.2365 | 0.0200 |
| Estimated LV mass, g | 0.84 ± 0.03c | 0.90 ± 0.03c | 1.21 ± 0.0c | 0.9 ± 0.05c | 0.0020 | 0.0399 | 0.0031 |
| Heart wet weight (mg mm−1)a | 25.9 ± 0.8 | 24.1 ± 0.8 | 30.1 ± 1.7 | 26.3 ± 1.2 | 0.0109 | 0.0243 | 0.4047 |
| LV wet weight (mg mm−1)a | 20.1 ± 0.4 | 17.9 ± 0.6 | 22.7 ± 0.9 | 19.5 ± 0.7 | 0.0039 | 0.0003 | 0.4639 |
| RV wet weight (mg mm−1)a | 4.6 ± 0.2 | 4.4 ± 0.3 | 4.6 ± 0.3 | 5.0 ± 0.3 | 0.2893 | 0.7218 | 0.2893 |
| % LV collagen (n = 3) | 10.7 ± 0.5 | 8.3 ± 1.3 | 21.7 ± 1.2 | 15.8 ± 0.6 | <0.0001 | 0.0026 | 0.1079 |
| LV stiffness constant | 23.1 ± 0.5c | 23.3 ± 0.6c | 28.4 ± 0.7d | 22.9 ± 0.7c | 0.0010 | 0.0006 | 0.0002 |
| LVEDP-V at 0 mmHg, μL | 123.8 ± 27.8 | 160 ± 24.5 | 136.3 ± 21.9 | 172.5 ± 22.3 | 0.6100 | 0.1458 | 1.0000 |
| LVEDP-V at 30 mmHg, μL | 276.2 ± 22.9 | 310 ± 23.3 | 261.3 ± 16.7 | 352.5 ± 26.9 | 0.5510 | 0.0105 | 0.2173 |
| Volume needed to increase EDP from 0 to 30 mmHg, μL | 152.5 ± 15.0 | 150 ± 13.6 | 125.0 ± 13.4 | 180 ± 22.0 | 0.9398 | 0.1210 | 0.0908 |
| MMP activity (proportion of control) | |||||||
| MMP-9 | 1.0 ± 0.0 | 0.8 ± 0.1 | 1.3 ± 0.1 | 1.0 ± 0.2 | 0.0561 | 0.0406 | 0.7724 |
| MMP-2 | 1.0 ± 0.0 | 0.9 ± 0.2 | 1.4 ± 0.2 | 1.04 ± 0.1 | 0.1179 | 0.0676 | 0.3148 |
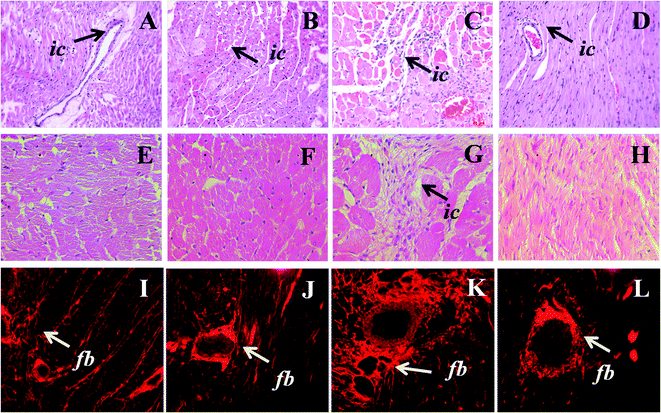 | ||
| Fig. 4 Upper panel shows haematoxylin and eosin staining of heart (×20) for inflammatory cell infiltration (arrow head) in C (A), CA (B), H (C) and HA (D) rats. Middle panel shows higher magnification (40×) of heart section of C (E), CA (F), H (G) and HA (H) rats; lower panel shows picrosirius red staining of perivascular and interstitial collagen deposition in C (I), CA (J), H (K), and HA (L) rats. ic-Inflammatory cells, fb-fibrosis; C, corn starch-rich diet-fed rats; CA, corn starch-rich diet-fed rats treated with L-arginine; H, high carbohydrate, high fat diet-fed rats; HA, high carbohydrate, high fat diet-fed rats treated with L-arginine. | ||
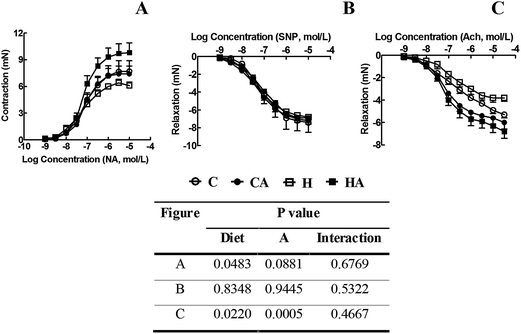 | ||
| Fig. 5 Cumulative concentration–response curves for noradrenaline (A), sodium nitroprusside (B) and acetylcholine (C) in thoracic aortic rings derived from C, CA, H and HA rats. Data are shown as mean ± SEM, n = 8; two way ANOVA, statistical significance was considered as p < 0.05. C, corn starch-rich diet-fed rats; CA, corn starch-rich diet-fed rats treated with L-arginine; H, high carbohydrate, high fat diet-fed rats; HA, high carbohydrate, high fat diet-fed rats treated with L-arginine. | ||
3.4 Liver structure and function
The wet weights of livers and the plasma activities of liver enzymes (AST, ALT and ALP) were higher in H rats compared to C rats. Liver weights and plasma liver enzyme activities were lower in HA rats than in H rats (Table 2). H rats showed increased infiltration of inflammatory cells and increased deposition of collagen around the hepatic triad region compared to C rats (Fig. 6). Apart from this, H rats showed increased deposition of fat droplets in liver, which was rarely visible in livers from C and CA rats (Fig. 6). L-Arginine supplemented rats displayed reduced macrovesicular steatosis, portal inflammation, and fibrosis (Fig. 6H). Liver concentrations of NOx were decreased in H rats and increased in both CA and HA rats compared to C rats (Table 2).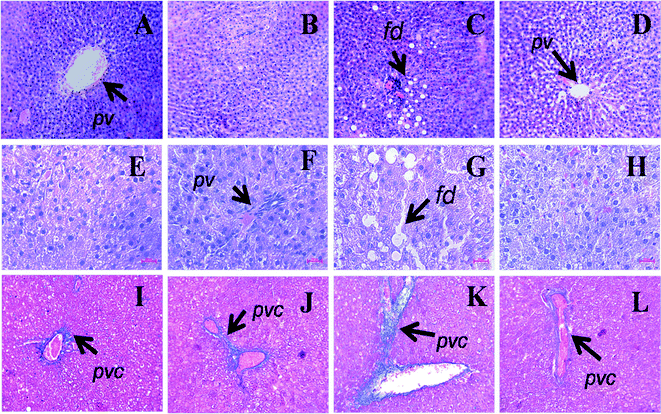 | ||
| Fig. 6 Haematoxylin and eosin staining of liver (×20) showing portal vein (pv), fat droplets (fd, arrow head) in C (A), CA (B), H (C) and HA (D) rats (upper panel). Middle panel shows higher magnification (40×) of liver section of C (E), CA (F), H (G) and HA (H) rats. Milligan's trichrome staining of the hepatic tissue (×20) showing collagen deposition as darker blue region in C (I), CA (J), H (K) and HA (L) rats (lower panel). pv – Portal vein, pvc – portal vein collagen, fd – fat droplet; C, corn starch-rich diet-fed rats; CA, corn starch-rich diet-fed rats treated with L-arginine; H, high carbohydrate, high fat diet-fed rats; HA, high carbohydrate, high fat diet-fed rats treated with L-arginine. | ||
3.5 Isolated mitochondrial respiration
L-Arginine dose-dependently inhibited ADP-dependent respiration in isolated mitochondria from normal liver (Fig. 7; Table 4). State 4 was affected only at higher concentrations. Respiratory control ratio was relatively unaffected under all experimental conditions.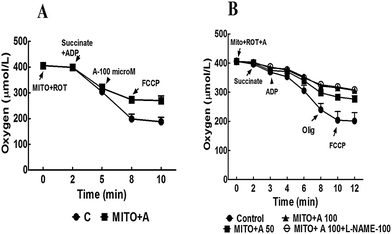 | ||
| Fig. 7 (A) Oxygen consumption of isolated liver mitochondria (0.35 mg mitochondrial protein) incubated in respiratory buffer. Addition of 100 μM L-arginine inhibited ADP-dependent (state 3) oxygen consumption that is not altered in presence of FCCP. (B) Oxygen consumption rate was inhibited by L-arginine in dose-dependent manner which is not altered in presence of 100 μM L-NAME (a NO synthase inhibitor). Rot, rotenone; Succ, succinate; Mito, mitochondrial suspension; ADP, adenosine diphosphate; FCCP, carbonylcyanide p-(trifluoromethoxy)phenylhydrazone; Olig, oligomycin; L-NAME, L-NGω-nitroarginine methyl ester. | ||
| Group | Oxygen consumption rate (nmol O2 consumed per min per mg protein) | RCRa | |
|---|---|---|---|
| State 3 | State 4 | ||
| a RCR = respiratory control ratio; data represented as mean ± SEM, n = 4. One way ANOVA was done for the comparison among the groups with Newman–Keuls multiple-comparison post hoc test. Statistical significance was considered as p < 0.05. b,c Means without a common letter in a column differ, P < 0.05. | |||
| Control (without L-arginine) | 70.6 ± 8.1b | 15.2 ± 2.2b | 4.7 ± 0.3b |
| Mitochondria + L-arginine (50 μM) | 41.1 ± 5.8c | 7.7 ± 0.8c | 5.5 ± 1.0b |
| Mitochondria + L-arginine (100 μM) | 34.3 ± 4.1c | 5.0 ± 0.7c | 7.1 ± 1.0b |
4. Discussion
Obesity, hypertension and diabetes, as the major signs of the metabolic syndrome, cause illness and death throughout the world; thus, effective but readily available treatments are necessary. L-Arginine plays important roles in many body functions, including wound healing, immune function and cardiovascular control; most of the body's requirements are produced by synthesis from L-citrulline. This study indicates that L-arginine, also available in the diet from many sources including dairy products, meat, nuts and wheat germ, may provide a dietary alternative to improve metabolic, cardiovascular and liver changes in the metabolic syndrome. Intervention with L-arginine in high carbohydrate, high fat fed rats reduced abdominal fat deposition, improved glucose tolerance, decreased plasma lipid profile and accumulation of lipid in liver, and improved cardiovascular structure and function. These responses are similar to those with other food-derived compounds tested in the same model, such as purple carrots,17 chia seeds,16 rutin,18 quercetin,21 ellagic acid,27 coffee19 and caffeine.22Intervention with L-arginine usually improves cardiovascular responses in rats and rabbits but human studies often show minimal if any benefits. In deoxycorticosterone acetate (DOCA)-salt hypertensive rats, L-arginine decreased systolic blood pressure and collagen deposition, improving cardiac stiffness and function.7 In ageing SHR, administration of L-arginine reduced systolic blood pressure, left ventricular mass and collagen deposition and improved coronary haemodynamics.28 The area of infarction was reduced by L-arginine treatment in SHR and hypercholesterolaemic rats.29L-Arginine reduced atheromatous lesions and improved endothelium-induced vasorelaxation in cholesterol-fed rabbits;30L-arginine decreased lesion frequency in mature rabbits, but not in immature rabbits.31 However, L-arginine treatment in patients with an acute myocardial infarction did not improve cardiovascular indices and may have increased mortality;32 further, no improvement in coronary artery disease mortality was found in elderly men.33L-Arginine improved endothelial function in hypercholesterolaemic males34 but not in young type 1 diabetic males.35
In comparison with the cardiovascular responses, changes with L-arginine treatment in metabolic disorders are more consistent between animal models and humans.8L-Arginine promoted the oxidation of glucose and long-chain fatty acids while decreasing de novo synthesis of glucose and triacylglycerols in diet-induced obese Sprague-Dawley rats.1,10 Plasma triglycerides and lipid concentrations were lowered in diabetic Sprague-Dawley rats with arginine supplementation.9,36 In rats fed a high fat diet, L-arginine showed markedly reduced white fat, lower serum concentrations of glucose, leptin, triglycerides and urea, and improved glucose tolerance.10L-Arginine stimulated both β-cell insulin secretion,37,38 and antioxidant and protective responses, enabling increased functional integrity of β-cells and islets in the presence of pro-inflammatory cytokines.37 Insulin sensitivity was improved in type 2 diabetic patients with chronic L-arginine treatment.39 Further, L-arginine treatment for 30 days increased the responses to a combined hypocaloric diet and exercise in obese type 2 diabetic patients with insulin resistance.40 In addition, L-arginine promoted fat reduction and spared lean mass during weight loss.40 Possible mechanisms include stimulation of mitochondrial biogenesis and brown fat development through multiple cell signalling molecules and increased expression of genes promoting fat and glucose metabolism.8
The development of fatty liver is characteristic of patients with the metabolic syndrome.41L-Arginine protected the liver from ischaemia-reperfusion injury with NO as the major mediator of these responses.42,43 Increased endothelial NO may limit obesity-induced inflammation and insulin resistance in hepatocytes.13 In diet-induced fatty liver, L-arginine improved and L-NAME reduced hepatic arterial and portal blood flows as well as the microcirculation.44 Currently, no literature is available on improvement of hepatic lipid accumulation and steatosis with L-arginine supplementation. L-Arginine may reduce liver damage by reducing advanced glycation end-products and the interactions with their receptor.45 Our study confirmed this reduction in liver damage by showing decreased plasma liver enzyme activity and improved histology. Mitochondrial function measured as antioxidant and respiratory marker enzymes was protected by L-arginine during ischaemia.46 Inhibition of ADP-dependent respiration, as shown in this study, should reduce oxygen demand in steatotic hepatocytes, thus increasing cellular survival.47
L-Arginine showed pronounced anti-inflammatory responses as decreased inflammatory cell infiltration in both heart and liver, together with decreased fibrosis, despite an unchanged high carbohydrate, high fat diet. These actions may be mediated by NO removal of the reactive superoxide molecules, giving antioxidant responses as shown by reduced plasma and liver malondialdehyde concentrations.
Translating the dose used in our study in rats to humans48 would give daily doses of approximately 35 g L-arginine. The doses used in this study did not produce signs of toxicity, but they are 3–4 fold higher than doses reported in human studies such as 3 g three times a day.40 The Third National Health and Nutrition Examination Survey reported mean arginine intakes for the US adult population of 4.4 g per day.49 Reported adverse effects of L-arginine dosage include abdominal discomfort, nausea and vomiting although these are reduced with divided daily doses.
In summary, L-arginine improved most of the symptoms of the metabolic syndrome by attenuating cardiovascular, metabolic and liver changes in rats fed a chronic high carbohydrate, high fat diet. Both anti-inflammatory and antioxidant actions of L-arginine, probably via NO, are likely to be responsible for these changes. Clinical trials with chronic administration of L-arginine, possibly at higher doses than currently used, may show the reduction of the multi-organ changes in diet-induced metabolic syndrome.
Acknowledgements
MAA was supported by a Merit Development Scholarship from the Islamic Development Bank, Jeddah, Saudi Arabia and UQIRTA from The University of Queensland. We thank Mr Brian Bynon, School of Veterinary Science, and Mr Paul Addison, School of Biomedical Sciences, both at The University of Queensland, for their help with plasma biochemical analyses and histology, respectively. We also thank Mr Jason Brightwell, The Prince Charles Hospital, Brisbane, for his assistance with echocardiography. All the authors have nothing to disclose.References
- G. Wu, F. W. Bazer, T. A. Davis, S. W. Kim, P. Li, J. Marc Rhoads, M. Carey Satterfield, S. B. Smith, T. E. Spencer and Y. Yin, Amino Acids, 2009, 37, 153–168 CrossRef CAS.
- L. Ezeanyika and A. Egbuonu, J. Med. Med. Sci., 2011, 2, 657–662 Search PubMed.
- G. Wu and S. M. Morris, Jr, Biochem J., 1998, 336(Pt 1), 1–17 CAS.
- L. J. Ignarro, G. Cirino, A. Casini and C. Napoli, J. Cardiovasc. Pharmacol., 1999, 34, 879–886 CrossRef CAS.
- G. Wu and C. J. Meininger, J. Nutr., 2000, 130, 2626–2629 CAS.
- W. S. Jobgen, S. K. Fried, W. J. Fu, C. J. Meininger and G. Wu, J. Nutr. Biochem., 2006, 17, 571–588 CrossRef CAS.
- A. Fenning, G. Harrison, R. Rose'meyer, A. Hoey and L. Brown, Am. J. Physiol.: Heart Circ. Physiol., 2005, 289, H1408–1416 CrossRef CAS.
- J. R. McKnight, M. C. Satterfield, W. S. Jobgen, S. B. Smith, T. E. Spencer, C. J. Meininger, C. J. McNeal and G. Wu, Amino Acids, 2010, 39, 349–357 CrossRef CAS.
- W. J. Fu, T. E. Haynes, R. Kohli, J. Hu, W. Shi, T. E. Spencer, R. J. Carroll, C. J. Meininger and G. Wu, J. Nutr., 2005, 135, 714–721 CAS.
- W. Jobgen, C. J. Meininger, S. C. Jobgen, P. Li, M. J. Lee, S. B. Smith, T. E. Spencer, S. K. Fried and G. Wu, J. Nutr., 2009, 139, 230–237 CrossRef CAS.
- H. Cortez-Pinto, M. E. Camilo, A. Baptista, A. G. De Oliveira and M. C. De Moura, Clin. Nutr., 1999, 18, 353–358 CrossRef CAS.
- V. T. Samuel, Z.-X. Liu, X. Qu, B. D. Elder, S. Bilz, D. Befroy, A. J. Romanelli and G. I. Shulman, J. Biol. Chem., 2004, 279, 32345–32353 CrossRef CAS.
- S. Tateya, N. O. Rizzo, P. Handa, A. M. Cheng, V. Morgan-Stevenson, G. Daum, A. W. Clowes, G. J. Morton, M. W. Schwartz and F. Kim, Diabetes, 2011, 60, 2792–2801 CrossRef CAS.
- S. K. Panchal, H. Poudyal, A. Iyer, R. Nazer, A. Alam, V. Diwan, K. Kauter, C. Sernia, F. Campbell, L. Ward, G. Gobe, A. Fenning and L. Brown, J. Cardiovasc. Pharmacol., 2011, 57, 611–624 CrossRef.
- H. Poudyal, F. Campbell and L. Brown, J. Nutr., 2010, 140, 946–953 CrossRef CAS.
- H. Poudyal, S. K. Panchal, J. Waanders, L. Ward and L. Brown, J. Nutr. Biochem., 2012, 23, 153–162 CrossRef CAS.
- H. Poudyal, S. Panchal and L. Brown, Br. J. Nutr., 2010, 104, 1322–1332 CrossRef CAS.
- S. K. Panchal, H. Poudyal, T. V. Arumugam and L. Brown, J. Nutr., 2011, 141, 1062–1069 CrossRef CAS.
- S. K. Panchal, H. Poudyal, J. Waanders and L. Brown, J. Nutr., 2012, 142, 690–697 CrossRef CAS.
- L. Brown, A. Fenning, V. Chan, D. Loch, K. Wilson, B. Anderson and D. Burstow, Heart, Lung and Circulation, 2002, 11, 167–173 CrossRef.
- S. K. Panchal, W. Y. Wong, K. Kauter, L. C. Ward and L. Brown, Nutrition, 2012, 28, 1055–1062 CrossRef CAS.
- S. K. Panchal, H. Poudyal and L. Brown, J. Nutr., 2012, 142, 1026–1032 CrossRef CAS.
- K. Miranda, M. Espey and D. Wink, Nitric Oxide: Biology and Chemistry, 2001, vol. 5, pp. 62–71 Search PubMed.
- W. Niehius and D. Samuelsson, Eur. J. Biochem., 1968, 6, 126–130 CrossRef.
- A. K. M. T. Zaman, S. Fujii, D. Goto, T. Furumoto, T. Mishima, Y. Nakai, J. Dong, S. Imagawa, B. E. Sobel and A. Kitabatake, J. Mol. Cell. Cardiol., 2004, 37, 525–535 CrossRef CAS.
- C. Frezza, S. Cipolat and L. Scorrano, Nat. Protoc., 2007, 2, 287–295 CrossRef CAS.
- S. K. Panchal and L. Brown, Eur. J. Nutr., 2012 DOI:10.1007/s00394-012-0358-9.
- D. Susic, A. Francischetti and E. D. Frohlich, Hypertension, 1999, 33, 451–455 CrossRef CAS.
- V. Pineiro, A. Ortiz-Moreno, R. Mora-Escobedo, M. D. Hernandez-Navarro, G. Ceballos-Reyes and G. Chamorro-Cevallos, Plant Foods Hum. Nutr., 2010, 65, 31–37 CrossRef CAS.
- T. Hayashi, P. A. Juliet, H. Matsui-Hirai, A. Miyazaki, A. Fukatsu, J. Funami, A. Iguchi and L. J. Ignarro, Proc. Natl. Acad. Sci. U. S. A., 2005, 102, 13681–13686 CrossRef CAS.
- S. G. Cremers, S. J. Wolffram and P. D. Weinberg, Br. J. Nutr., 2011, 105, 1439–1447 CrossRef CAS.
- S. P. Schulman, L. C. Becker, D. A. Kass, H. C. Champion, M. L. Terrin, S. Forman, K. V. Ernst, M. D. Kelemen, S. N. Townsend, A. Capriotti, J. M. Hare and G. Gerstenblith, JAMA, J. Am. Med. Assoc., 2006, 295, 58–64 CrossRef CAS.
- C. M. Oomen, M. J. van Erk, E. J. Feskens, F. J. Kok and D. Kromhout, Arterioscler., Thromb., Vasc. Biol., 2000, 20, 2134–2139 CrossRef CAS.
- H. Kawano, T. Motoyama, N. Hirai, K. Kugiyama, H. Yasue and H. Ogawa, Atherosclerosis, 2002, 161, 375–380 CrossRef CAS.
- M. J. Mullen, D. Wright, A. E. Donald, S. Thorne, H. Thomson and J. E. Deanfield, J. Am. Coll. Cardiol., 2000, 36, 410–416 CrossRef CAS.
- J. D. Mendez and F. Balderas, Biochimie, 2001, 83, 453–458 CrossRef CAS.
- M. S. Krause, N. H. McClenaghan, P. R. Flatt, P. I. Homem de Bittencourt, C. Murphy and P. Newsholme, J. Endocrinol., 2011, 211, 87–97 CrossRef CAS.
- S. Lenzen, Am. J. Physiol., 1979, 236, E391–400 CAS.
- P. M. Piatti, L. D. Monti, G. Valsecchi, F. Magni, E. Setola, F. Marchesi, M. Galli-Kienle, G. Pozza and K. G. Alberti, Diabetes Care, 2001, 24, 875–880 CrossRef CAS.
- P. Lucotti, E. Setola, L. D. Monti, E. Galluccio, S. Costa, E. P. Sandoli, I. Fermo, G. Rabaiotti, R. Gatti and P. Piatti, Am. J. Physiol.: Endocrinol. Metab., 2006, 291, E906–912 CrossRef CAS.
- E. Fabbrini, S. Sullivan and S. Klein, Hepatology, 2010, 51, 679–689 CrossRef CAS.
- M. Abu-Amara, S. Y. Yang, A. Seifalian, B. Davidson and B. Fuller, Liver Int., 2012, 32, 531–543 CrossRef CAS.
- G. Jeyabalan, J. R. Klune, A. Nakao, N. Martik, G. Wu, A. Tsung and D. A. Geller, Nitric Oxide, 2008, 19, 29–35 CrossRef CAS.
- S. Ijaz, W. Yang, M. C. Winslet and A. M. Seifalian, Microvasc. Res., 2005, 70, 129–136 CrossRef CAS.
- M. H. Pai, K. H. Huang, C. H. Wu and S. L. Yeh, Br. J. Nutr., 2010, 104, 686–692 CrossRef CAS.
- P. Chattopadhyay, G. Shukla, A. Verma and A. K. Wahi, BioFactors, 2007, 31, 99–106 CrossRef CAS.
- S. K. Mantena, D. P. Vaughn, K. K. Andringa, H. B. Eccleston, A. L. King, G. A. Abrams, J. E. Doeller, D. W. Kraus, V. M. Darley-Usmar and S. M. Bailey, Biochem. J., 2009, 417, 183–193 CrossRef CAS.
- S. Reagan-Shaw, M. Nihal and N. Ahmad, FASEB J., 2008, 22, 659–661 CrossRef CAS.
- D. E. King, A. G. Mainous, III and M. E. Geesey, Nutr. Res., 2008, 28, 21–24 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2013 |
