Pomegranate: a fruit that ameliorates metabolic syndrome
Svjetlana
Medjakovic
and
Alois
Jungbauer
*
Department of Biotechnology, Christian-Doppler-Laboratory of Receptor Biotechnology, University of Natural Resources and Life Sciences Vienna, Muthgasse 18, 1190 Vienna, Austria. E-mail: alois.jungbauer@boku.ac.at; Tel: 0043 1 476546226; Fax: 0043 1 476546675
First published on 14th June 2012
Abstract
Pomegranate is an ancient fruit that is still part of the diet in the Mediterranean area, the Middle East, and India. Health-promoting effects have long been attributed to this fruit. Modern research corroborates the use of pomegranate as a folk remedy for diabetes and metabolic syndrome, and is responsible for a new evaluation of nutritional and pharmaceutical aspects of pomegranate in the general public. In the last decade, industry and agricultural production have been adapted to meet higher market demands for pomegranate. In vivo and in vitro studies have demonstrated that pomegranate exerts hypoglycaemic effects, including increased insulin sensitivity, inhibition of α-glucosidase, and impact on glucose transporter type 4 function, but is also responsible for a reduction of total cholesterol, and the improvement of blood lipid profiles, as well as anti-inflammatory effects through the modulation of peroxisome proliferator-activated receptor pathways. These effects may also explain how pomegranate-derived compounds function in the amelioration of adverse health effects caused by metabolic syndrome. Pomegranate contains polyphenols such as ellagitannins and anthocyanins, as well as phenolic acids, fatty acids and a variety of volatile compounds. Ellagitannins are some of the most prevalent compounds present in pomegranate, and may be responsible for certain benevolent characteristics associated with pomegranate. A brief overview of rising health problems due to obesity will be provided, followed by characterisation of the biological activity, bioavailability, and safety of pomegranate and pomegranate-derived compounds. Although the fruit is consumed in many countries, epidemiological and clinical studies are unavailable. Additional research is necessary to corroborate the promise of current in vivo and in vitro findings.
 Svjetlana Medjakovic | After her master study in Food Sciences and Biotechnology, Svjetlana Medjakovic completed her doctoral study in Life Sciences, specialization in Biotechnology at the University of Natural Resources and Life Sciences, Vienna. Currently, she is a post-doc at the Christian Doppler Laboratory for Receptor Biotechnology in Vienna. Her research is focused on the isolation of bioactive compounds from edible food, herbs and spices and the establishment of structure–function-relationships for the development of drugs or remedies for the therapy of the metabolic syndrome, the overactive bladder syndrome and benign prostate hyperplasia. |
 Alois Jungbauer | Professor Alois Jungbauer received his PhD in Food Technology and Biotechnology from the University of Natural Resources and Life Sciences Vienna, Austria 1986. He serves since then as a professor at the Department of Biotechnology. He teaches Protein Technology and Downstream Processing and Biochemical Engineering. He is head of the laboratory for Protein technology and Downstream Processing and the Christian Doppler Laboratory of Receptor Biotechnology. He has expertise in the identification of bioactive compounds from edible plants and has developed several in vitro test systems for the screening of bioactive compounds. In the past he has focused on the steroid hormone receptor family and other nuclear receptors. |
1 Introduction
For centuries, pomegranate has been used in ethnomedicine for several applications. Conclusive evidence is now emerging to support the hypothesis that pomegranate is able to ameliorate metabolic syndrome. The results of numerous studies indicate that pomegranate may be effective in the treatment of obesity, inflammation, diabetes, and the regulation of blood lipid parameters, and hence metabolic syndrome.Recent events have led to the restricted use (and in some cases, the withdrawal from the market) of glitazones, drugs that have been used to treat type 2 diabetes. In the face of the rising incidence of obesity and diabetes, proactive measures are demanded. Substances are desired that have the same acting principle as glitazones, but lack its malevolent side effects. Plants that regulate peroxisome proliferator-activated receptor (PPAR) γ-linked processes may be an option. Active compounds from these plants could be used for standardised medications. Tenenbaum et al. reviewed that pan-PPAR activators (ligands that simultaneously activate PPAR α, PPARβ/δ, and PPARγ) are a good therapeutic approach to treat metabolic syndrome, as they reduce serum triglyceride (TG) and glucose levels, improve insulin sensitivity, raise the high density lipoprotein (HDL) level, and reduce the incidence of cardiovascular disease and type 2 diabetes.1 In this context, pomegranate is a promising plant. The use of plant-derived compounds (e.g., from pomegranate) to treat obese adolescents at risk of developing metabolic syndrome is also being discussed.2
Katz et al.3 discussed pomegranate in connection to diabetes. Other reviews have also included the effects of pomegranate on atherosclerosis, cardiovascular disease, and diabetes in their characterisations of pomegranate;4–6 however, much has happened in recent pomegranate research. In this review, new results are discussed with the objective of pomegranate characterisation, especially concerning possible applications in obesity and diabetes therapy. First we provide a brief overview of rising health problems due to obesity and diabetes. Next, we describe more closely the biological activity, bioavailability, and safety of potential compounds present in pomegranate. The work concludes with a discussion of the necessity of further research.
2 Obesity, diabetes, and metabolic syndrome: now and in the future
Obesity is a growing problem; according to the World Health Organization (WHO) global estimates from 2008, more than 10% of adults are obese worldwide.7 In Western countries, the prevalence of obesity is manifestly higher; among U.S. adults, the overall prevalence of obesity was 33.8% in 2007–2008, and the prevalence estimates for overweight and obesity combined were 68%.8 By now, overweight and obesity are linked to more deaths worldwide than underweight, and they are the fifth leading risk for global deaths.7How do we define obesity and overweight? According to the WHO definition,7 overweight is reached when an individual's body mass index (BMI) is ≥25 kg m−2, and obesity is defined by a BMI ≥30 kg m−2.
In an undeniable upward trend that shows no sign of abating, more people become obese each year. Since the 1980s, the prevalence for obesity has more than doubled. In the United States alone, it has nearly tripled in several states between 1990 and 2009. In 1990, no state had a prevalence >14%, and in 2009 the majority of U.S. states had a prevalence of >25% (in some states, the prevalence of obesity topped 30%).9
These numbers are alarming; especially because there is no doubt that obesity and metabolic syndrome are directly associated. The International Diabetes Federation10 definition of metabolic syndrome is detailed in Table 1.
| Central obesitya = circumference ≥94 cm for male European individuals; ≥90 cm for male South Asian, Chinese, and Japanese individuals; and ≥80 cm for female European, South Asian, Chinese, and Japanese individuals + any two of the following factors: | |
|---|---|
| a If an individual's BMI is >30 kg m−2, central obesity can be assumed and waist circumference does not need to be measured. | |
| Triglycerides ↑ | ≥150 mg dl−1 (1.7 mmol l−1), or specific treatment for this lipid abnormality |
| HDL cholesterol ↓ | <40 mg dl−1 (1.03 mmol l−1) in males and <50 mg dl−1 (1.29 mmol l−1) in females, or specific treatment for this lipid abnormality |
| Blood pressure ↑ | systolic blood pressure ≥130 mmHg or diastolic blood pressure ≥85 mmHg, or treatment of previously diagnosed hypertension |
| Fasting serum glucose ↑ | ≥100 mg dl−1 (5.6 mmol l−1), or previously diagnosed type 2 diabetes |
The incidence of diabetes is rising simultaneously with the incidence of obesity. In the United States alone, the prevalence of diagnosed diabetes increased from 0.9% in 1958 to 6.95% in 201011 (Fig. 1). This means that in 2010, 21.13 million people in the United States were diagnosed with diabetes, while in 1958 only 1.6 million cases were reported. This is not due to population growth; the U.S. population did not even double between 1958 and 2009.
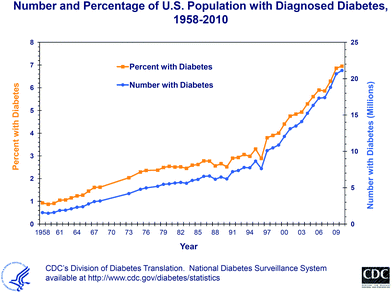 | ||
| Fig. 1 The trend in diabetes diagnosis in the United States from 1958 through 2010, as published by the Centers for Disease Control and Prevention in October 2011.11 | ||
There are several reasons for this growing health problem. We consume more calories than we burn. In addition, most of the food that is currently consumed is low in micronutrients, such as vitamins and minerals, but high in fat, salt, and sugars. This, together with the fact that physical activity has decreased dramatically due to an overall change in lifestyle (more sedentary work, escalators, elevators, urbanisation, etc.), leads to overweight and obesity, and subsequently to diabetes, hypertension, and cardiovascular diseases.
Without significant changes in lifestyle, we can assume that the situation will worsen. Predictions are difficult; however, if the trend is not at least halted (or better, reversed), the world is facing a serious pandemic problem. Wang et al.12 projected future overweight and obesity prevalence for U.S. adults, and concluded that if the current trend continues, 86.3% of American adults will be overweight or obese by 2030, and all American adults will be overweight or obese by 2048. A projection for diabetes concludes that over 44 million people in the United States will have diagnosed or undiagnosed diabetes by 2034.13 The slope of the trend for diabetes changed significantly around 2000 (Fig. 1). A comparison with previous projections for diabetes is alarming; in 2001, Boyle et al.14 projected, according to then-available data that the number of people in the United States with diagnosed diabetes would increase to almost 20 million in 2025. In fact, this number was already surpassed in 2009! The question arises why the trend changed so dramatically around the turn of the millennium. The timing of this trend shift may reflect the coming of age of the generation that was young in the 1970s and 1980s, which then reached an age at which the manifestation of lifestyle changes, such as changes in physical activity and the consumption of more convenience food, fast food, and high-sugar-containing soft drinks, became apparent.
Irrespective of a possible stabilisation of obesity and diabetes trends, there are more than 220 million individuals worldwide that currently have diabetes and need medication.15 Of these 220 million, 90% suffer from type 2 diabetes.15 Unlike type 1 diabetes, in which the pancreatic insulin-producing cells of the islets of Langerhans are lost due to an autoimmune attack, type 2 diabetes mellitus is characterised by insulin resistance, rather than by a lack of insulin production. Drugs that are used to treat type 2 diabetes increase pancreatic insulin secretion, increase insulin-sensitivity, or decrease glucose absorption. However, drugs that are currently used in diabetes therapy can have serious side effects. Glitazones are anti-diabetic drugs used to treat type 2 diabetes. They act as insulin-sensitizers through the activation of PPARγ, but do not initiate insulin production. Although medication with these compounds is necessary, it is not without risk. Pioglitazone has been linked to bladder tumors and has been therefore withdrawn in France from the market by the French Agency for the Safety of Health Products in June 2011. At the same time Germany's Federal Institute for Drugs and Medical Devices recommended to physicians a patient's medication without pioglitazone until further investigations have cleared health concerns. In the meantime, the U.S. Food and Drug Administration (FDA) has added a warning regarding an increased bladder cancer risk upon long-time use of pioglitazone to the drug label. Nevertheless pioglitazone is in many countries still on the market as active compound for diabetes therapy. Rosiglitazone was also widely used until 2010. Reports of serious side effects led to the restriction of its use in the United States; as part of a Risk Evaluation and Mitigation Strategy, the FDA has announced that healthcare providers and patients must enrol in a special program in order to prescribe and receive rosiglitazone-containing drugs.16 The FDA decided the restricted access to rosiglitazone in September 2010. In the European Union, the European Medicines Agency (EMA) recommended a total suspension of the marketing authorisations for the rosiglitazone-containing anti-diabetes medicines, also in September 2010.17 These decisions were based on studies that report an increased risk of ischemic heart disease associated with the use of rosiglitazone.18,19
3 Pomegranate in folk medicine and in diet
Pomegranate (Punica granatum L.) has been cultivated since ancient times. As one of the oldest edible fruits, it has been part of Middle Eastern and Mediterranean diet for a long time. The Romans called it Punic apple, and also used it for leather tanning because of its high polyphenol content. The pomegranate plant is indigenous to the region from Iran to the Himalayas in Northern India, but is now cultivated in the Middle East, Asia, Southern Europe, the United States, and the milder climatic regions of Africa. In addition to its economic relevance (currently largely for juice production), pomegranate is deeply rooted in cultural and religious aspects and is often used in ethnomedicine.The fruit is consumed fresh, or processed in the form of juice, jam, or syrup. The arils are also used as an ingredient in main dishes. Dried seeds are used (whole or powdered) as a spice for several main dishes in Indian cuisine. It is a sacred fruit for several religions, and is deeply rooted in the symbolism of mythology and legends, as well as in religious rites and traditions.
In ancient mythology, Hades the god of the underworld was able to convince Persephone to stay in the underworld by giving her a pomegranate. For the Israelites, pomegranate was a sign of the fertility of the land (Numbers 13:23). In medieval times, pomegranate fruits were a symbol of mercy. The Brothers of Mercy, a confraternity founded in Granada, Spain, took the pomegranate as their symbol.
The open pomegranate was considered a symbol of the mercy of god. As shown in Fig. 2, several very famous paintings depict the Madonna with child offering a pomegranate fruit, including “Maria, dem Kind einen Granatapfel reichend [Mary Offering a Pomegranate to the Child]” by Hans Holbein the Elder (approximately 1465), Botticelli's “Madonna della Melagrana [Madonna of the Pomegranate]” (1487), and the study “Virgin and Child with Pomegranate” by Raphael (1505). Another very famous painting, Lorenzo Di Credi's so-called “Madonna and Child with a Pomegranate (Dreyfus Madonna)” (which is displayed at the National Gallery in Washington D.C., USA), depicts a pomegranate offered to the child by Mary. The pomegranate was also a sign of power. Albrecht Duerer (1519) painted the emperor Maximilian I of Habsburg with a pomegranate.
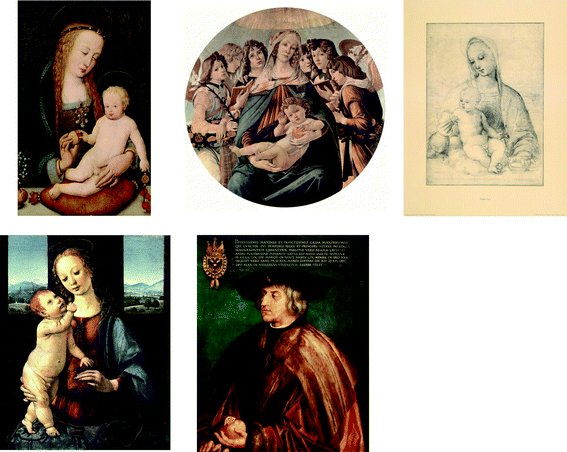 | ||
| Fig. 2 Works of art depicting pomegranate fruit, demonstrating the significance of this fruit in religion, culture, and society. First row, from left to right: “Maria, dem Kind einen Granatapfel reichend” by Hans Holbein the Elder, Boticelli's “Madonna of the Pomegranate” (1487), and Raphael's “Virgin and Child with Pomegranate”. Second row, from left to right: Lorenzo Di Credi's “Madonna and Child with a Pomegranate (Dreyfus Madonna)”, and Albrecht Duerer's “Maximilian I of Habsburg”. | ||
In ethnomedicine (e.g., Ayurveda, the traditional Indian medicine, or Unani, a traditional medicine from South Asia that is rooted in ancient Greek medicine), pomegranate seeds and flowers have reportedly been used for diabetes treatment.20–22 Tahraoui et al.23 list pomegranate in their ethnopharmacological survey study as a diabetes treatment in folk medicine in the Errachidia province in south eastern Morocco, where only 23% of the population uses Western medicine.
In the past ten years, pomegranate research has intensified, particularly regarding its possible anti-diabetic and anti-atherosclerotic effects. It remains to be seen whether the efficacy of various ethnomedicinal applications will be confirmed by scientific inquiry. This review aims to summarise recent scientific results, which will help to answer this question and to evaluate whether further studies are needed.
4 Description and chemical composition of pomegranate
Pomegranate plants grow in round bushes or as small trees and have round, orange-sized fruits with a prominent calyx at the top. It is one of the rare plants in which fruits and flowers are simultaneously present. The fruit is a false berry, with hundreds of seeds that are surrounded by a leathery skin and separated within the fruit by a white and spongy pericarp (rind). Every seed is packed in arils which contain the red juice that amounts to ∼30–40% of the whole fruit weight.24,25 A semi-schematic drawing is shown (Fig. 3).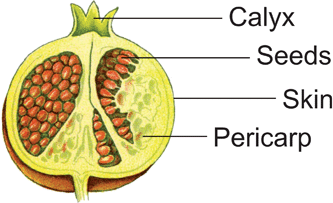 | ||
| Fig. 3 A semi-schematic drawing of a pomegranate fruit, adapted from Thomé.26 | ||
The juice is one of the main products of today's pomegranate cultivation. However, for extract preparation for medical use, other parts of the fruit, including the flowers, bark, roots, and leaves may also be of interest, as the entire fruit contains bioactive compounds. All pomegranate parts are used in ethnomedicine. Pomegranate is rich in flavonoids, anthocyanins, catechins, tannins, organic acids, and alkaloids. Other reviewers have summarised the occurrence of all of these compounds in pomegranate, including their distribution within the plant.27,28 An overview of compounds that are present in pomegranate is shown in Table 2, although we cannot claim that the list is complete.
| Compound class | Compounds | |
|---|---|---|
| Diverse polyphenols29–33 | Catechin | Gallocatechin |
| Ellagic acid | Kaempferol | |
| Epicatechin | Luteolin | |
| Epigallocatechin | Quercetin | |
| Ellagitannins29,30,32,34–38 | Brevifolin | Lagerstannin B |
| Brevifolin carboxylic acid | Pedunculagin | |
| Corilagin | Pomegranatate | |
| Castalagin | Punicafolin | |
| Casuarinin | Punicalin | |
| Gallagyldilactone | Punicalagin A | |
| Granatin A | Punicalagin B | |
| Granatin B | Tellimagranandin I | |
| Anthocyanins29,30,32,39–42 | Pelargonidin 3 O-glucoside | Cyanidin 3,5 O-diglucoside |
| Pelargonidin 3,5 O-diglucoside | Delphinidin 3 O-glucoside | |
| Cyanidin 3 O-glucoside | Delphinidin 3,5 O-diglucoside | |
| Volatile compounds43–47 | E-α-Bergamotene | trans-2-Hexenal |
| E-β-Bergamotene | cis-3-Hexenol | |
| Bisabolene | Heptanal | |
| Cadrene | Limonene | |
| Camphor | Menthol | |
| 3-Carene | β-Myrcene | |
| β-Caryophyllene | Nonanal | |
| p-Cumic aldehyde | Octanal | |
| p-Cymene | α-Phellandrene | |
| (Z,Z)-α-Farnesene | α-Pinene | |
| β-Farnesene | β-Pinene | |
| Fenchone | α-Terpinene | |
| Furfural | γ-Terpinene | |
| Hexanal | 4-Terpineol | |
| Hexanol | α-Terpineol | |
| cis-3-Hexenal | ||
| Organic & phenolic acids29,48–50 | Ascorbic acid | Gallic acid |
| Caffeic acid | Malic acid | |
| Chlorogenic acid | Oxalic acid | |
| Ctric acid | (-)-Quinic acid | |
| o-Coumaric acid | Sucinic acid | |
| p-Coumaric acid | Tartaric acid | |
| Ferulic acid | ||
| Fatty acids51–57 | Caproic acid (C6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0) 0) |
Linoleic acid (C18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) 2) |
Caprylic acid (C8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0) 0) |
Punicic acid (C18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3, 9-cis, 11-trans, 13-cis) 3, 9-cis, 11-trans, 13-cis) |
|
Capric acid (C10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0) 0) |
α-Eleostearic acid (C18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3, 9-cis, 11-trans, 13-trans) 3, 9-cis, 11-trans, 13-trans) |
|
Lauric acid (C12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0) 0) |
β-Eleostearic acid (C18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3, 9-trans, 11-trans, 13-trans) 3, 9-trans, 11-trans, 13-trans) |
|
Myristic acid (C14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0) 0) |
Catalpic acid (C18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3, 9-trans, 11-trans, 13-cis) 3, 9-trans, 11-trans, 13-cis) |
|
Myristoleic acid (C14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
Arachidic acid (C20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0) 0) |
|
Palmitic acid (C16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0) 0) |
Gadoleic acid (C20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
|
Palmitoleic acid (C16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
Lignoceric acid (C24![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0) 0) |
|
Stearic acid (C18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0) 0) |
Nervonic acid (C24![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 15-cis) 1, 15-cis) |
|
Oleic acid (C18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
||
| Lignans58 | Isolariciresinol | Pinoresinol |
| Matairesinol (only in wood knots) | Secoisolariciresinol | |
| Medioresinol | Syringaresinol | |
In fresh pomegranate juices, several phenolic acids, including gallic acid, chlorogenic acid, caffeic acid, ferulic acid, and coumaric acids, as well as flavonoids, such as catechin, phloridzin, and quercetin, can be identified.48 In addition to catechin, other flavan-3-ols such as epicatechin and epigallocatechin are present in pomegranate.31 These compounds are also prominent bioactive components of tea, and have been associated with its cancer-preventive effects (as reviewed by Yang and Wang59) (Fig. 4).
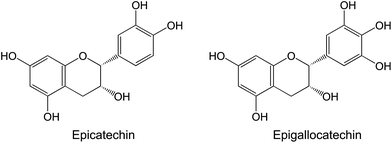 | ||
| Fig. 4 The principal catechins present in tea and pomegranate. | ||
Hydrolysable tannins, especially ellagitannins and gallotannins, form the most prevalent compound class in pomegranates. They are present in all plant parts. The bioactivity of pomegranate is largely due to these compounds; the main pomegranate ellagitannins are punicalagins and granatins (Fig. 5).
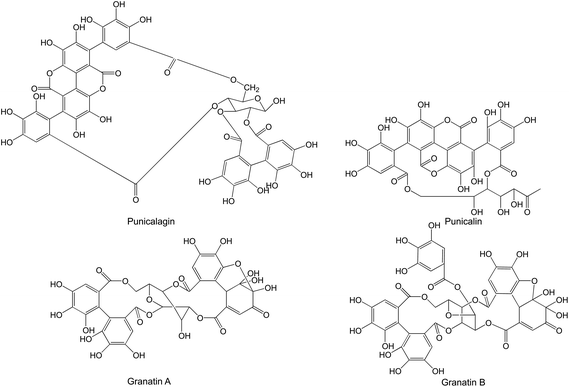 | ||
| Fig. 5 Punicalagins and granatins present in pomegranate. | ||
In commercially produced pure pomegranate juices, punicalagin A and punicalagin B have been observed at levels of 7.6–36.9 mg l−1 and 15.2–135.6 mg l−1, respectively, while granatin A and granatin B are present at levels of 27.2–108.9 and 8.8–38.4 mg l−1, respectively.30 Extremely high ellagitannin levels have also been reported in pomegranate juices; Cerda et al.60 made fresh pomegranate juice using a laboratory pilot press, and obtained a juice that contained 2.4 g l−1 punicalagin and 0.61 g l−1 free ellagic acid.
Punicalagin consists of ellagic acid and gallagic acid linked to a glucose molecule. Ellagic acid has been attributed several benevolent effects, which will be discussed in this review. As Wang et al.28 summarised, ellagic acid is present in pomegranate flowers, juice, leaves, and pericarp. The peel and mesocarp of pomegranate fruit contain much higher levels of ellagic acid, granatin B, and total ellagitannins.29 Pomegranate juice is made by pressing pomegranate arils; however, it is sometimes obtained by pressing the halved fruits, which results in the additional extraction of compounds from the peel and mesocarp. Ellagic acid levels as high as 214.5 mg l−1 have been reported, although a good portion of the juices had levels <20 mg l−1.29,49,50,61,62 A higher ellagic acid level of 172.8 mg l−1 has been reported in a commercially produced juice from concentrate, and ellagic acid levels of 29.0–155.6 mg l−1 have been observed in an analysis of commercially produced pure pomegranate juices.30
The characteristic red colour of the fruit is due to high anthocyanin content; pomegranate juice contains 3-O-glucoside and 3,5-O-diglucoside forms of delphinidin, cyanidin, and pelargonidin (Fig. 6).39,61,63 Mature pomegranates contain 50–100 μg anthocyanins per gram fresh aril weight.61,64
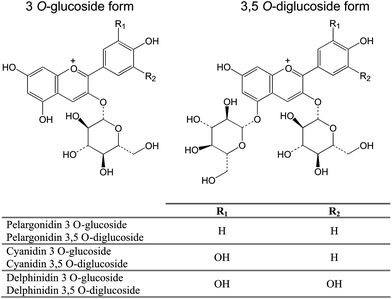 | ||
| Fig. 6 The main anthocyanins present in pomegranate, as 3-O-glucoside and 3,5-O-diglucoside forms. | ||
Pomegranates have a rather low concentration of volatile compounds,44,45 such as monoterpenes, aldehydes, esters, and alcohols. Calin-Sanchez et al.43 report a total concentration of volatile compounds ranging from 1.7 to 10.9 g kg−1. They were unable to find a clear relationship between the total concentration of volatile compounds and taste; however, a positive correlation was observed between consumer preference and the level of monoterpenes (α-pinene, β-pinene, β-myrcene, γ-terpenine, and limonene) present in pomegranate juice.
Several organic acids contribute to the taste and acidity of pomegranate juice. Among them are citric acid, malic acid, succinic acid, oxalic acid, ascorbic acid, gallic acid, coumaric acid, chlorogenic acid, caffeic acid, and ferulic acid (Fig. 7).29,48,49,65,66 Ascorbic acid content differs among cultivars, but range from ∼10–20 mg per 100 g juice.25 Hence, pomegranate is a good source of vitamin C.
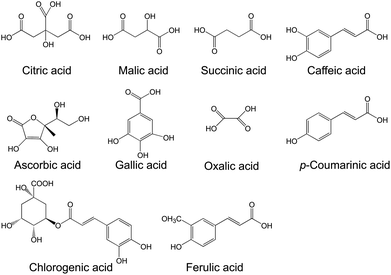 | ||
| Fig. 7 Organic and phenolic acids present in pomegranate. | ||
Fatty acids are more prevalent in pomegranate seeds. Total lipids range from 6–21.6%51 and consist of nearly 90% unsaturated fatty acids.51–53 Linolenic acid is the major fatty acid present, followed by linoleic acid, oleic acid, palmitic acid, stearic acid, gadoleic acid, lignoceric acid, arachidic acid, and myristic acid.51 The linolenic acid fraction consists of four different conjugated linolenic acid isomers:51,54 punicic acid (18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3: 9-cis, 11-trans, 13-cis) as the major isomer present,51,54 followed by α-eleostearic acid, catalpic acid, and β-eleostearic acid.51
3: 9-cis, 11-trans, 13-cis) as the major isomer present,51,54 followed by α-eleostearic acid, catalpic acid, and β-eleostearic acid.51
Kaufman and Wiesman54 report a quite high concentration of phytosterols in pomegranate seed oil, approximately 3–4 times higher than is observed in soybean oil. Major phytosterols are β-sitosterol, campesterol, and stigmasterol.
Lignans are also present in pomegranates. Bonzanini et al.58 report the detection of isolariciresinol, secoisolariciresinol, matairesinol, medioresinol, pinoresinol, and syringaresinol in commercial pomegranate juices. Lignan content was highest in seeds (36.1 mg kg−1), and somewhat lower in fruit pulp (11.2 mg kg−1) and in endocarp (3.3 mg kg−1). In commercially produced juices, they detected lignans at levels of approximately 0.4 to 4.4 mg kg−1.
5 Bioavailability and catabolism of ellagitannins
Although pomegranate contains several compounds that may contribute to the observed benevolent health effects, the ellagitannins appear to be the most promising candidates. Anthocyanins, which are also present in abundance in this plant, are not bioavailable. Anthocyanins, as well as metabolites, have not been detected in serum or urine after the consumption of pomegranate juice.67The bioavailability of ellagitannins has been studied in animals, as well as in humans. These compounds are large molecules and are mostly not absorbed as such in humans;60,67,68 however, in a single experiment with rats, which were fed a standard diet plus 6% punicalagin, the ellagitannin punicalagin was detected in the serum (30 μg ml−1) and 3–6% of ingested punicalagin was excreted in urine and faeces.69
Ellagic acid, which has been described as a highly bioactive compound in several studies, is a hydrolysate of ellagitannins such as punicalagin, which is present in pomegranate. After pomegranate consumption, as juice or extract, ellagic acid is released. The hydrolysis of ellagitannins and the resulting release of ellagic acid are not due to an enzymatic reaction but are pH-dependent; the hydrolysis of casuarictin (an ellagitannin of raspberry) and the release of ellagic acid were optimal at pH 8 and maximal release into cecal contents was observed after 1 h.70 The catabolism of punicalagin to form ellagic acid (representative of ellagitannin catabolism), as well as further metabolism of ellagic acid into a variety of urolithins in the large intestine, are depicted schematically in Fig. 8.
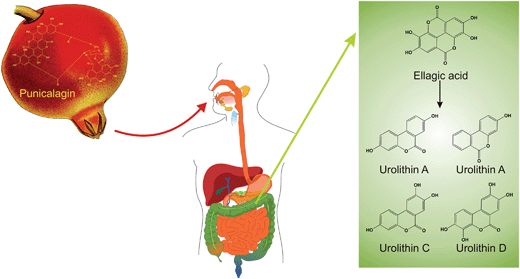 | ||
| Fig. 8 The catabolism of punicalagin. | ||
Ellagic acid is quickly absorbed; it is then converted into other metabolites after absorption or partially eliminated within 4–6 h.71,72In vitro anaerobic incubation of punicalagin and ellagic acid with human faecal suspensions from healthy participants revealed that ellagic acid is released immediately after the beginning of punicalagin incubation, and the concentration of ellagic acid declined 0–5 h afterwards, while formation of urolithins, the main metabolite of ellagic acid, began after 5 h and rose rapidly to be detected at their highest concentrations after 72 h. Notably, 80% of ellagic acid was metabolised to urolithins.73
Interestingly, in vitro ellagic acid was transported into cells after incubation with pomegranate leaf tannins, which also correlated with cholesterol alterations in the cells.74 Nevertheless, it is not quite clear whether these findings are relevant, considering the fact that ellagic acid is quickly metabolised and the benevolent health effects of pomegranate are mediated primarily by ellagic acid metabolites.
In a pharmacokinetic study of ellagic acid in rats that have been fed with pomegranate leaf extract, Lei et al.75 demonstrated that ellagic acid has poor absorption and rapid elimination; the maximum concentration (Cmax) of ellagic acid was 203 ng ml−1 after a time of maximum concentration (tmax) of 0.54 h.75 Cerda et al. observed no ellagic acid in serum or urine in their human studies.60,67 Meanwhile, others found ellagic acid in human serum with a mean Cmax and tmax of 33.8 ± 12.7 ng ml−1 at 1 h76 or 31.9 ± 2.4 ng ml−1 at 1 h72 after consumption of pomegranate extract or juice, respectively. In another study, Seeram et al.71 report a maximum concentration of 18.1 ng ml−1 at 0.98 h. In healthy humans, the consumption of ellagitannins from raspberry produced a similar pharmacokinetic result; maximum concentrations of ellagic acid in serum were observed after 1–2 h, and <1% of intake was absorbed and excreted in the urine.77 In a study of healthy subjects and patients with an ileostomy (an opening from the small bowel allowing faeces to leave the body without passing through the large intestine), urinary excretion of ellagic acid was <1% of intake.78 The same study also showed that in healthy subjects only (not in patients with an ileostomy), ellagitannins were further metabolised to form urolithins, which are the main metabolites. This strengthens the hypothesis that the large intestine is necessary for the breakdown of ellagic acids to form urolithins.
All mammals appear to produce urolithins. Gonzalez-Barrio et al.79 tested the production of urolithins and conjugates in different animals fed on ellagitannins, as well as in humans. All tested mammals including rats, mice, humans, pigs, squirrels, beavers, bull calves and sheep, metabolised ellagitannins to urolithins and derivates as main metabolites. While insects and birds only released ellagic acid and were unable to metabolise it further to form urolithins. The different mammalian species also had varying urolithin hydroxylation patterns due to differing microbiotas.
Urolithin A-glucuronide is the main metabolite of ellagitannins in humans, regardless of ellagitannin source,68 followed by urolithin B-glucuronide (3-hydroxy-6H-dibenzo[b,d]pyran-6-one glucuronide)60,67 and dimethylellagic acid glucuronide (DMEAG);68 urolithin C (trihydroxy-6H-dibenzo[b,d]pyran-6-one) is a minor metabolite.67 The formation of these metabolites is of microbial origin. Cerda et al.80 incubated ellagic acid and punicalagin with human faeces and urolithin A (3,8-dihydroxy-6H-dibenzo[b,d]pyran-6-one) was produced in all samples from different volunteers; although production rates and concentrations differed, which is explained by the large inter-individual variability of colonic microflora among the volunteers, and perhaps by nutrigenomic effects. High individual variability of metabolite formation has been observed in human studies,60,67,68,71,76 and is also true regarding the metabolism of ellagitannins from berries, walnuts, and wine. Cerda et al.81 reported high and low excreters of urolithin B and metabolites, and demonstrated that the amount of excreted urolithin B derivates was not proportionally related to the amount of ellagitannins consumed.
Due to active enterohepatic circulation, urolithins, such as urolithin A-glucuronide and urolithin B-glucuronide, remain for a long time in the body and can persist for up to 48 h after pomegranate juice consumption, while other metabolites, such as DMEAG, were detected in the urine only on the day of administration.71 Espin et al.82 elucidated the metabolism of ellagitannins intensively using a pig model, as the pig's digestive system is nearly identical to that of humans. It can be assumed that the observed metabolism of ellagitannins in the pig can be projected to humans. Their study showed that after ellagitannin ingestion, ellagic acid is released and directly absorbed in the first portion of the gastrointestinal tract. Next, ellagic acid is gradually metabolised in the intestine, beginning in the jejunum, where urolithin D (3,4,8,9-tetrahydroxy-6H-dibenzo[b,d]pyran-6-one) and urolithin C (and, in more distal parts of the intestine, urolithin A and urolithin B) are produced. The largest proportion of urolithin B is generated in the distal intestine. The absorption of urolithins increases as their lipophilicity increases. After absorption, the first glucuronidation takes place in the intestinal cells. In the liver, additional metabolism to diglucuronides and/or sulphates can be observed. These metabolites have also been observed in bile, while only derivates that are sufficiently metabolised, such as urolithin A glucuronide, urolithin B glucuronide, and DMEAG, have been observed in serum and urine. Urolithin C and urolithin D are absorbed earlier in the intestine, and are subject to enterohepatic circulation until their phenolic hydroxyl groups are decreased and the metabolites are able to enter serum and urine. Consistent with human studies, urolithin A and urolithin B were the major metabolites in serum and urine, while urolithin C was observed only as minor metabolite or in traces. The inter-individual variability of metabolism that has been reported in humans was also observed in pigs.
The metabolism of ellagic acid from pomegranate juice, liquid pomegranate extract, and powdered pomegranate extract is nearly the same.83 Ellagic acid release and clearing from the serum takes place within 6 h independent of the form of administration. Only pharmacokinetic parameters were delayed for the powdered pomegranate extract in comparison to liquid administrations. Also, in all cases urolithin A-glucuronide was observed at maximum concentrations up to 1 μg ml−1, persisting for 48 h after pomegranate consumption. The perdurance of urolithins was demonstrated in most participants in this study at 48 h after ingestion; urolithin A-glucuronide was not completely excreted in the urine.
Notably, urolithins, as metabolites caused by ellagitannin intake through the consumption of pomegranate or walnuts, are present in small amounts in prostate tissue. Urolithin A-glucuronide in particular was observed at concentrations of up to 2 ng g−1 tissue.68 The authors reasoned that the small amounts that were observed were due to a fasting period prior to surgery that led to nearly complete clearance. This hypothesis was supported by the results of an animal experiment within the same study, which revealed urolithin A-glucuronide in rat prostate tissue after feeding with pomegranate extract, but not when the rats were subjected to fasting prior to sacrifice. In mice, urolithin A and conjugates were concentrated at higher levels in prostate, colon, and intestinal tissues after the administration of synthesised urolithin A. This finding is of special interest, as the authors showed in the same study that pomegranate extract administration leads to a significant inhibition of prostate cancer cells in a xenograft model.84 In a study with pigs, no ellagitannin metabolites were observed in muscle, adipose, lung, liver, heart, or kidney tissues.82
Although ellagic acid is quickly released upon ellagitannin consumption, the bioavailability of this compound is rather low. However, the main metabolites of ellagic acid are highly bioavailable and persist for more than 48 h in the organism. The benevolent health effects that are observed due to ellagitannin consumption are in all likelihood mediated by these compounds. As the inter-individual variability of ellagitannin metabolism is high, it is possible that some individuals may experience greater benefits associated with pomegranate consumption than others.
6 Safety of pomegranate products
Given that pomegranates have been consumed for several millennia, the normal consumption of the fruit, raw or as an ingredient, can be regarded as absolutely safe. However the question arises whether this safety extends to extracts or pure compounds that may be used in a more concentrated form as dietary supplements.Sanchez-Lamar et al.85 report genotoxic effects of a whole pomegranate fruit extract in vitro and in vivo. However, this is the only study that reports adverse effects associated with pomegranate extract.
In rats and mice, the oral LD50 (lethal dose, required to kill 50% of a tested population) of pomegranate fruit extract (standardised to 30% punicalagins) was greater than 5 g kg−1 body weight (BW), while the intraperitoneal LD50 was 217 mg kg−1 BW for rats and 187 mg kg−1 BW for mice.86 In the same study, subchronic toxicity was evaluated in rats; however, up to an administration of 600 mg kg−1 BW/day for 90 days, no adverse effects could be observed. As this was the highest tested dosage, a no observed-adverse-effect level (NOAEL) of 600 mg kg−1 BW/day was defined.
Similar results were obtained using pomegranate seed oil and a whole fruit extract of pomegranate. Pomegranate seed oil showed no signs of mutagenicity in vitro (Ames test and chromosome aberration test), and in an acute oral toxicity study with rats no significant effects were observed at 2000 mg pomegranate seed oil per kg BW.87
Meerts et al.87 performed a subchronic toxicity study, with pomegranate seed oil administered for 28 days at concentrations from 0–150![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 ppm (resulting in a mean intake of 0–14
000 ppm (resulting in a mean intake of 0–14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 214 mg kg−1 BW), and observed increased hepatic enzyme activity and increased liver/body weight ratios only at the highest dose. However, this dosage is much higher than concentrations that have been demonstrated to have anti-diabetic and anti-inflammatory effects. Furthermore, the authors concluded that it is very likely that these adverse effects were due to exposure to abnormally high levels of fatty acids. The NOAEL for pomegranate seed oil that they determined was 4.3 g kg−1 BW (=50
214 mg kg−1 BW), and observed increased hepatic enzyme activity and increased liver/body weight ratios only at the highest dose. However, this dosage is much higher than concentrations that have been demonstrated to have anti-diabetic and anti-inflammatory effects. Furthermore, the authors concluded that it is very likely that these adverse effects were due to exposure to abnormally high levels of fatty acids. The NOAEL for pomegranate seed oil that they determined was 4.3 g kg−1 BW (=50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 ppm).
000 ppm).
A whole fruit hydroalcoholic pomegranate extract, which was produced for use in Cuban traditional medicine, was not toxic in an embryotoxicity study with chicken embryos at concentrations of <0.1 mg per embryo. The LD50 of this extract, as determined in an acute toxicity experiment with mice, was 731 mg kg−1 BW, and the repeated intraperitoneal administration of 0.4 and 1.2 mg kg−1 BW for 35 days resulted in no effects, while the administration of 7 mg kg−1 BW slightly increased serum creatinine and glucose levels. However, the subchronic exposure resulted in no toxic effects regarding behavioural or biochemical parameters, and had no effect on the weight of organs, including the brain, heart, spleen, fibrous muscular tissue, and nasal cartilaginous tissue.88
Although pomegranate root is documented as toxic,85 aqueous and hydroalcoholic extracts of pomegranate root have been observed to elicit rather high LD50s of 1858 and 2031 mg kg−1 BW in a toxicity test in mice, respectively.89 In the same study, Desta89 tested the effectiveness of the root extract as worm expellent for the parasite Taenia saginata L., as pomegranate is traditionally used as an herbal drug for this purpose in Ethiopia; they found the extract, which is administered in Ethiopian ethnomedicine as a hot water extract, effective at a median single dose of 12.6 g, which resulted in a worm expulsion time of 16.7 h. Hence, for this traditional purpose, pomegranate root extract is effective far below the LD50.
An ethanol extract of pomegranate bark had an LC50 of 22.4 mg l−1 in a toxicity test with snails (Lymnaea acuminate), but had no effect on a fish species (Colisa fasciatus) that shares the same habitat.90
Pomegranate husk extract is rich in punicalagin (final administration of 6% punicalagin) and is equivalent to a very high pomegranate juice intake. In rats, repeated oral administration of pomegranate husk extract for 37 days resulted in no adverse effects, and the histopathological analysis of kidney and liver revealed no signs of toxicity.91
During a 4-week human safety assessment study of 64 overweight subjects, the administration of a pomegranate preparation (ellagitannin-enriched and standardised to ≥90% pomegranate polyphenols) providing 710 or 1420 mg extract (1 or 2 capsules; equivalent to 435 or 870 mg of gallic acid equivalents, respectively) resulted in no adverse effects.92 The authors report in this manuscript (as unpublished data) an NOAEL of this preparation of 1500 mg kg−1 BW. Also, none of the 86 participants reported allergic reactions.
For pomegranate-derived compounds, data regarding safety and toxicity is rare. Tasaki et al.93 evaluated the safety of ellagic acid in a 90-day subchronic toxicity study in rats with doses up to 5% in powdered basal diet, and observed no signs of toxicity; furthermore, no mortality or treatment-related clinical signs and no histopathological effects were observed. The only effect was that body weight gain was significantly reduced in female rats. In this study, the no-observed-effect level (NOEL) and the NOAEL for male rats was estimated to be 5%; however, while the NOAEL was also 5% for females, the NOEL for females was <1.25% considering the effect on body weight. Meerts et al.87 provided a detailed composition of the diet administered with pomegranate seed oil, and it can be concluded that the 6% punicic acid content was safe in this toxicity study. In another study, ellagitannins from source other than pomegranates were tested; oak-flavored milk powder containing ellagitannins and ellagic acid was administered to rats for 96 days (total intake per rat in the entire experiment was 9.024 mg; this dosage was equivalent to 2.8 g extrapolated to a 70 kg person, which would result in a daily intake of 29.4 mg day−1 for humans); no effect was observed on blood parameters, growth rate, or food utility index, and the histopathological analysis revealed no tissue alterations or significant effects on haematological or serum biochemical parameters.94
Pomegranate extracts appear to be safe in concentrations that have been used in traditional ethnomedicine and that are currently available as dietary supplements. However, more studies that examine exposure over a long period of time would be helpful to exclude health risks completely. Moreover, additional studies that evaluate the toxicity of pure compounds would be desirable.
7 Agricultural use and importance of pomegranate
A global analysis of pomegranate production is not available. In 2008, the United States Agency for International Development (USAID) published an agribusiness program for Iraq in which they list Iran and India as the main pomegranate producers, producing 800![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 and 900
000 and 900![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 metric tons per year, respectively. Spain produces approximately one-tenth of the yield of Iran or India, but is the main supplier of fresh pomegranates in the European region.95 Pomegranate production has increased recently in the United States. In the last Census of Agriculture (2007), which is an evaluation of the United States Department of Agriculture taken every five years, pomegranate cultivation reportedly increased from 9535 acres (∼3859 ha) to 24
000 metric tons per year, respectively. Spain produces approximately one-tenth of the yield of Iran or India, but is the main supplier of fresh pomegranates in the European region.95 Pomegranate production has increased recently in the United States. In the last Census of Agriculture (2007), which is an evaluation of the United States Department of Agriculture taken every five years, pomegranate cultivation reportedly increased from 9535 acres (∼3859 ha) to 24![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 517 acres (∼9922 ha) in total. More than 99% of U.S. pomegranate acreage is in California.96
517 acres (∼9922 ha) in total. More than 99% of U.S. pomegranate acreage is in California.96
Pomegranates are cultivated in smaller quantities in several countries; however, only a fraction of the fruit yield is exported (e.g., Afghanistan produces approximately 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000–20
000–20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 metric tons, of which the majority is consumed in local markets and only 5% is exported).97
000 metric tons, of which the majority is consumed in local markets and only 5% is exported).97
The production of pomegranate is increasing worldwide98 due to higher demand as the newly gained popularity of the fruit for juice preparations and cosmetics has increased. This increased interest is linked to a rising estimation of the nutritional and pharmaceutical value of the fruit.
7.1 Compositional changes with respect to cultivar and habitat
Cultivar and habitat have a great impact on the chemical composition of plants. Plenty of cultivated pomegranate species are known. Important characteristics of pomegranate cultivars include fruit size, juice content, sweetness, acidity, and colouring (skin and arils). These parameters are essential for consumer preference, as well as for manufacturing processes. Some characteristics of several cultivars are summarised in Table 3; however, this is only a small selection, as there are several hundred cultivars worldwide.| Cultivar/variety | Area | Juice yield (g kg−1) | Titratable acidity (g citric acid kg−1) | pH | Total anthocyanins † (mg l−1 juice) ‡ (mg 100 g juice−1) | Ref. |
|---|---|---|---|---|---|---|
| Mollar de Eiche | Spain | 340.4–398.1 | 1.9–2.5 | 3.84–4.00 | 148.8–303.9† | 62 |
| Valenciana | Spain | 412.9–482.7 | 2.3–2.9 | 3.60–3.67 | 34.2–108.4† | 62 |
| Wonderful | Worldwide | 242.8–392.2 | 5.2–29.7 | 2.52–3.71 | 287.2–1074.6† | 62 |
| Agha Mandali Save | Iran | 465.5 | 4.9 | 3.46 | 9.56‡ | 25 |
| Alak Shirin Save | Iran | 289.4 | 4.0 | 4.02 | 6.37‡ | 25 |
| Bazmani Pust Nazok | Iran | 300.1 | 5.6 | 4.09 | 6.23‡ | 25 |
| Dom Ambaroti | Iran | 341.5 | 3.6 | 3.37 | 8.57‡ | 25 |
| Khazar Bajestani | Iran | 285.3 | 16.7 | 3.43 | 6.90‡ | 25 |
| Lili Post Koloft | Iran | 361.7 | 15.1 | 3.56 | 8.72‡ | 25 |
| Malas Pust Sorkh | Iran | 310.5 | 3.3 | 3.99 | 8.09‡ | 25 |
| Malas Save | Iran | 377.9 | 7.0 | 3.91 | 9.87‡ | 25 |
| Malas Yazdi | Iran | 382.5 | 7.8 | 3.78 | 8.99‡ | 25 |
| Pust Sefeed Dezfol | Iran | 444.2 | 10.7 | 3.56 | 9.05‡ | 25 |
| Save Pust Ghermez | Iran | 384.8 | 17.0 | 3.52 | 30.11‡ | 25 |
| Save Pust Sefeed | Iran | 336.9 | 7.9 | 3.65 | 11.85‡ | 25 |
| Shirin Dane Ghermez Ferdows | Iran | 390.4 | 10.8 | 3.37 | 6.44‡ | 25 |
| Shirin Dane Sefeed Ferdows | Iran | 443.6 | 10.9 | 3.57 | 7.41‡ | 25 |
| Shirin Pust Ghermez | Iran | 269.5 | 23.1 | 3.93 | 7.63‡ | 25 |
| Shirin Pust Sefeed | Iran | 407.0 | 9.6 | 3.83 | 5.72‡ | 25 |
| Shishe Kap | Iran | 393.7 | 10.2 | 3.73 | 10.07‡ | 25 |
| Torsh Shahvar Ferdows | Iran | 439.5 | 24.4 | 3.58 | 10.32‡ | 25 |
| Torsh Shahvar Kashmar | Iran | 423.3 | 10.0 | 3.74 | 5.56‡ | 25 |
| Zagh Yazdi | Iran | 304.0 | 12.8 | 3.16 | 7.20‡ | 25 |
| Aghaye | Iran | 530.8 | 9.9 | 3.37 | 27.7‡ | 99 |
| Faroogh | Iran | 635.2 | 11.2 | 3.06 | 21.2‡ | 99 |
| Rabbab-e-Fars | Iran | 480.2 | 13.5 | 3.23 | 24.4‡ | 99 |
| Shahvar | Iran | 604.9 | 5.8 | 3.70 | 7.9‡ | 99 |
| Shirin-e-Bihaste | Iran | 579.9 | 5.1 | 3.58 | 9.9‡ | 99 |
| Shirin-e-Mohali | Iran | 597.8 | 5.4 | 3.74 | 8.3‡ | 99 |
| Gabsi | Tunisia | n.s. | n.s. | n.s. | 11–178† | 100 |
| Tounsi | Tunisia | n.s. | n.s. | n.s. | 51–99† | 100 |
Fruit colour depends mainly on anthocyanin content and composition. The latter changes during fruit ripening,61,64 which is reflected in juice pigmentation. A very strong influence on anthocyanin accumulation and composition was observed in fruit arils due to climate conditions: low temperatures enhance accumulation, while higher temperatures reduce anthocyanin concentration.63 Dafny-Yalin et al.65 learned that fruits that ripen during a colder season not only have a higher level in anthocyanins and total soluble solids, which contributes to deeper red colouring of the arils, but were also more sour in taste due to high acidity, although sugar levels did not vary to a great extent.
Climate conditions, ripening, and storage also affect the content of total phenolics, sugars, and organic acids.66,101–103
The chemical composition of pomegranates differs depending on cultivar, area of cultivation, and associated climate and production conditions, as well as on ripening stage. It can be concluded that these characteristics also manifestly influence the bioactivity of pomegranate fruits or extracts. If a certain bioactivity must be guaranteed, then it is necessary to standardise these characteristics to achieve a defined amount of a known bioactive compound. In any case, regular pomegranate consumption is certainly a good way to benefit from the bioactive effects of fruit-derived compounds. Pomegranate should be recommended by nutritionists as part of a healthy diet.
8 Evidence of the beneficial effects of pomegranate on metabolic syndrome
For approximately ten years, increasing investigations have been published to support ethnomedical reports, or to find new nutritional or medical properties of pomegranate.8.1 Hypoglycaemic and insulin-sensitising effects
Insulin resistance is a risk factor for the development of type 2 diabetes. During the development of type 2 diabetes, cells become increasingly insensitive to insulin. Normal physiological insulin levels become less effective at lowering the blood sugar level by triggering the uptake of glucose into cells as an energy source. As this condition worsens, more and more insulin is needed to elicit the same effect. The pancreas obliges this request and enhances insulin production; however, in advanced stages insulin resistance cannot be overcome, and as an end result it is possible to have extremely elevated levels of circulating glucose and insulin concomitantly. Drugs (e.g., glitazones) that reverse insulin resistance and help sensitise cells to insulin are used as therapy against type 2 diabetes. Glitazones exhibit this action by activating PPARγ.Pomegranate has proven to be a modulator of PPARγ both in vitro104,105 and in vivo.106 Shiner et al.105 demonstrated that the PPARγ-mediated in vitro effect of pomegranate juice results in reduced oxidative stress in macrophages; this effect was abrogated by PPARγ inhibition, and was also observed after macrophages were incubated with the pomegranate polyphenols punicalagin and gallic acid. Pomegranate flower has been shown to transactivate PPARα in vitro107 and to enhance hepatic PPARα gene expression in Zucker diabetic rats,108 and has also been shown to suppress cardiac overexpression of PPARα mRNA in the same rat model.107 The latter effect prevents cardiac pathological conditions that are similar to cardiac changes due to diabetes.
Several parts of the pomegranate, including the peel, seed, and flower, have demonstrated hypoglycaemic effects in vivo, although their activity profiles differ slightly. Peel extract did not alter insulin levels in normoglycaemic healthy rats (Wistar albino male rats), but did lower serum glucose levels.109 Meanwhile, in single-dose alloxan-treated rats (Swiss albino male rats) that exhibited increased serum glucose levels and reduced serum insulin levels due to treatment, co-treatment with pomegranate peel extract normalised all alloxan-induced malfunctions.110 Interestingly, Khalil111 also demonstrated an increase in relative beta cell number due to pomegranate peel extract in alloxan-induced diabetic rats, and concomitantly demonstrated an augmentation in insulin levels and reduced blood glucose levels after a 4-week course of treatment. However, no effect on beta cells was observed in a shorter study (15 days) that used pomegranate seed to treat alloxan-induced diabetic rats.112
Jafri et al.113 reported that pomegranate flower had a blood glucose lowering effect in normal rats, in normal fasted rats, and in alloxan-induced diabetic rats. Similar to results obtained with pomegranate peel, Manoharan et al.114 report the normalisation of blood glucose levels in streptozotocin-induced diabetic rats after the administration of pomegranate flower extract. Li et al.115 showed that Zucker diabetic fatty rats have an innately higher glucose level than Zucker lean rats under fasting conditions, and that glucose level was also much higher in the non-fasting than in the fasting state. Under non-fasting conditions, pomegranate flower extract significantly reduced serum glucose levels to a great extent, but had little effect under fasting conditions. The authors concluded that a mechanism that enables pomegranate flower to improve postprandial hyperglycaemia is mediated by the inhibition of intestinal α-glucosidase activity, which they demonstrated in vitro. A reduction of α-glucosidase activity was also observed in the saliva of healthy humans after the consumption of pomegranate extract during an intervention study.116
Another mechanism that may contribute to the modulation of glucose levels is the modulation of glucose transporter expression; this mechanism was demonstrated for the insulin-dependent isoform GLUT4 in Zucker diabetic fatty rats, in which pomegranate flower extract restored the down-regulated GLUT4 mRNA expression.117
The results regarding pomegranate seeds are somewhat contradictory. Das et al.118 report hypoglycaemic activity in streptozotocin-induced diabetic rats due to treatment with pomegranate seed extract, while Jelodar et al.112 were unable to observe a reduction in blood glucose levels in alloxan-induced diabetic rats. The route of administration may be the reason for differing results: while Das et al.118 orally administered a methanol-extracted seed extract (aqueous solution), Jelodar et al.112 mixed ground seed powder into the normal diet, which could likely hinder the uptake of bioactive compounds. Punicic acid, a main ingredient of pomegranate seed oil, reduced fasting serum glucose levels in obese mice and improved glucose-normalising abilities.119 However, pomegranate seed oil had no observed effect on serum glucose concentration in mice.120,121
Still, pomegranate seed oil has been associated with improved insulin sensitivity in rodent models.117,120,121 Vroegrijk et al.121 specify that they observed improved peripheral insulin sensitivity, but no effect on liver insulin sensitivity. Pomegranate flower polyphenols improved insulin sensitivity in streptozotocin-induced diabetic rats.122 In a small clinical trial with obese humans, pomegranate juice did not modify insulin secretion or sensitivity after 1 month of administration in a fasted state; however, the natural regain of weight that was observed in the control group was halted in the treated group.123
Levels of resistin, an adipocyte-derived adipokine, have been linked to obesity, insulin resistance, and type 2 diabetes, as reviewed recently by Harwood.124 Pomegranate fruit extract, as well as ellagic acid, significantly reduced secretion and intracellular protein levels, but did not alter mRNA expression of this signal molecule;125 data from this study suggest that the degradation of resistin protein levels is promoted by pomegranate fruit extract and ellagic acid treatment. However, in the same study, no effect on adiponectin level (which is also associated with insulin sensitivity) was observed.
8.2 Effect on body weight
Pomegranate seed oil has a significant lowering effect on body weight in inbred121 and outbred wild-type120 mice fed with a high-fat diet. Interestingly, energy intake was not affected.120,121 Vroegrijk et al.121 observed a decrease in body fat mass but no alterations in lean mass in male C57BI/J6 mice, while McFarlin et al.120 did not observe significant differences in absolute fat mass of male wild-type CD-1 mice. An additional finding of McFarlin et al.,120 which was not measured but is based solely on observation, is that the mice in the treatment group tended to be more active in the later weeks of the study compared with mice in the control group, who were more sedentary. Pomegranate peel not only reduced body weight gain, but also reduced food consumption in male rats fed a high-cholesterol diet.126Results in calves are inconsistent; oral administration of pomegranate extract had no effect on food intake or body weight in the first month of age (this experiment started at 2 ± 1 days of age, with the day of birth designated as postnatal day 0); however, after the first month, both food intake and body weight decreased with pomegranate feeding compared with controls.127 In a smaller study with older calves (not yet 1 year old), pomegranate peel had no significant effect on body weight, although a slight positive tendency toward increased weight gain was observed by the authors among bull calves.128 As mentioned in section 8.1, pomegranate juice halted the regain of weight after a period of fasting in obese humans.123
Pomegranate leaf extract significantly decreased body weight and energy intake in an anti-obesity test with mice; the appetite of the obese mice that were fed a high-fat diet was significantly reduced by pomegranate leaf extract, although it had no effect on female and male CD-1 (ICR) mice fed a normal diet.129
8.3 Reduction of total cholesterol and improvement of lipid profiles
Most studies report that pomegranate extracts have a significant impact on blood lipids (Table 4). This effect has been shown regarding pomegranate seed oil, pomegranate juice, and extracts of pomegranate flower, peel, and leaf. In mice, pomegranate seed oil had no effect on total cholesterol120,130 or HDL levels.120 Findings regarding TG levels have been inconsistent; Yamasaki et al.130 reported an increase in TG levels after pomegranate seed oil administration, while McFarlin et al.120 observed no effect. In hypercholesterolaemic rabbits, no significant effect on serum lipid profile was detected after administration of up to 2% pomegranate fruit juice or seed oil.131 By contrast, in a human study employing pomegranate seed oil, serum TG, TG![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) HDL ratio, and cholesterol
HDL ratio, and cholesterol![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) HDL ratio were reduced after 4 weeks, while total cholesterol, low density lipoprotein (LDL), and LDL
HDL ratio were reduced after 4 weeks, while total cholesterol, low density lipoprotein (LDL), and LDL![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) HDL ratio did not differ between treatment and control groups.132
HDL ratio did not differ between treatment and control groups.132
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) HDL), total cholesterol (total chol), low density lipoprotein (LDL), LDL-to-HDL ratio (LDL
HDL), total cholesterol (total chol), low density lipoprotein (LDL), LDL-to-HDL ratio (LDL![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) HDL), and very low density lipoprotein (VLDL)]. ↓ symbolises a decreased effect, ↑ symbolises an increased effect, and – symbolises no significant effect
HDL), and very low density lipoprotein (VLDL)]. ↓ symbolises a decreased effect, ↑ symbolises an increased effect, and – symbolises no significant effect
| TG | TG![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) HDL HDL |
Chol![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) HDL HDL |
Total chol | LDL | LDL![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) HDL HDL |
VLDL | HDL | Subjects | |
|---|---|---|---|---|---|---|---|---|---|
| Seed oil | ↓ | ↓ | ↓ | Humans, hyperlipidaemic (n = 51, double-blind, placebo-controlled)132 | |||||
| ↑ | — | C57BL/6N mice (inbred strain), standard diet130 | |||||||
| — | — | — | CD-1 mice (wild-type), high-fat diet120 | ||||||
| Juice | ↓ | ↓ | ↓ | ↓ | Humans, diabetic, hyperlipidaemic (n = 26, pilot study)134 | ||||
| Flower | ↓ | ↓ | ↓ | ↓ | ↑ | Streptozotocin-induced diabetic rats (albino Wistar), standard diet133 | |||
| ↓ | ↓ | Zucker diabetic fatty rats, standard diet107 | |||||||
| ↓ | — | Zucker diabetic fatty rats, standard diet; only hepatic parameters evaluated108 | |||||||
| Peel | ↓ | ↓ | — | Hypercholesterolaemic male albino rats, hypercholesterolaemic diet126 | |||||
| Leaf | ↓ | ↓ | ↓ | ICR mice, high-fat diet129 |
A reduction in TG and total cholesterol levels has also been observed in various other studies employing pomegranate juice, flower extract, peel extract, and leaf extract.107,108,126,129,133,134 Reductions in cholesterol![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) HDL ratio,129,134 LDL,133,134 LDL
HDL ratio,129,134 LDL,133,134 LDL![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) HDL ratio,134 and very low density lipoprotein (VLDL)133 have been reported. The impact on HDL is not quite clear; while some studies have reported no effect,120,126 Bagri et al.133 reported an increase.
HDL ratio,134 and very low density lipoprotein (VLDL)133 have been reported. The impact on HDL is not quite clear; while some studies have reported no effect,120,126 Bagri et al.133 reported an increase.
Blood lipid parameters of diabetes type 2 patients were improved after a 3-month trial with a supplement containing pomegranate extract, green tea extract and ascorbic acid; showing significantly decreased LDL and increased HDL levels135 However, trials with a mixed supplementation are difficult to interpret, as it is not quite clear which supplement part is responsible for the observed effect.
8.4 Anti-atherosclerotic effects of pomegranate
Diabetes and atherosclerosis are closely linked. Diabetes patients are at high risk of developing cardiovascular disease. One reason for this link is that diabetic patients exhibit much lower activity of paraoxigenase 1 (PON1),136 an enzyme that is part of the HDL complex. PON1 protects LDL and HDL from oxidation and stimulates cholesterol efflux from macrophages. Physiological functions, anti-oxidative properties, and anti-inflammatory properties of the paraoxonase gene family have been recently reviewed by Precourt et al. in detail.137PON1 associated more efficiently with HDL isolated from diabetic patients after the consumption of pomegranate juice than before consumption.138 In a clinical study with diabetic patients, consumption of pomegranate juice increased PON1 activity and PON1 binding to HDL by 30%.139 Other studies of pomegranate juice among diabetic patients corroborate this effect on PON1 activity140,141 and also show a reduction of serum LDL oxidative status140 and reduced cellular uptake of oxidised LDL.141
An in vitro study by Khateeb et al.142 demonstrated in hepatocytes that pomegranate juice and its major compounds (punicalagin, gallic acid, and to a smaller extent ellagic acid) were able to increase PON1 protein and mRNA expression and PON1 gene promoter activation via a PPARγ-dependent pathway. For PON2, another member of the same family with similar anti-oxidative functions, an up-regulation in macrophages upon pomegranate juice treatment or incubation with punicalagin, gallic acid, or pelargonidin-3-o-β-glucopyranoside was also observed; ellagic acid did not change PON2 protein or mRNA expression, but increased PON2 enzyme activity in a PPARγ-dependent manner.105 Furthermore, PON2 activity was significantly increased by up to 50% in apolipoprotein E-deficient (E0) mice after consumption of pomegranate by-products (the whole fruit after juice preparation).143 In another experiment with E0 mice, pomegranate juice was able to induce serum PON activity, reduce oxidised LDL uptake and cholesterol esterification, and increase cholesterol efflux; levels obtained after the reduction of oxidised LDL uptake and cholesterol esterification were even lower than in unsupplemented controls.144
Oxidised LDL induces vascular component cells (e.g., macrophages), and activates pro-atherogenic processes, such as the expression of matrix metalloproteinases (MMPs). Ellagic acid was able to significantly inhibit the expression of MMP-1 and MMP-3 induced by oxidised LDL,145 which contributes to anti-atherosclerotic effects.
The anti-atherogenic properties of pomegranate in vivo were elucidated by Aviram et al.;146 they tested extracts of pomegranate peel, arils, seeds, and flowers and compared these extracts with pomegranate whole fruit juice in an apolipoprotein E-deficient mouse model that develops atherosclerotic lesions resembling those in humans. All preparations except for the seed extract were able to reduce atherosclerotic lesion areas by up to 70% compared with placebo-treated animals. The extracts also increased cellular PON1 and PON2 activity in this model. Pomegranate juice was as effective as the tested extracts, although total phenolics were much lower (Fig. 9). It can be concluded that while the composition of compounds is essential, the amount is not necessarily so.
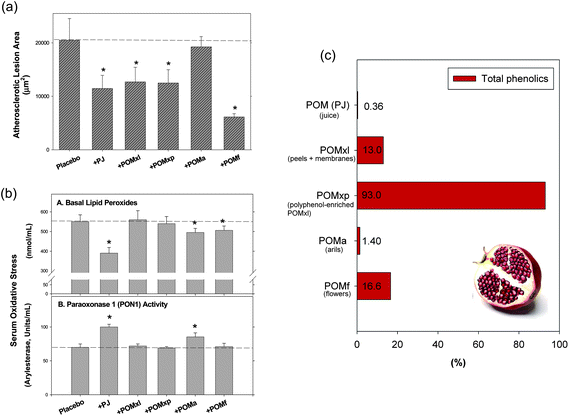 | ||
| Fig. 9 Effects of pomegranate extracts and juice on (a) atherosclerotic lesion size and (b) serum oxidative stress in apolipoprotein E-deficient mice.146 Panel (c) shows total phenolics (gallic acid equivalents) as published by Aviram et al.146 Abbreviations: POM (PJ), pomegranate whole fruit juice; POMa, pomegranate extract of arils; POMf, extract of ground pomegranate ground flowers; POMxl, pomegranate fruit liquid extract of peels and membranes; POMxp, pomegranate extract of polyphenol powder after polyphenol enrichment of POMxl. Reprinted (adapted) with permission from (M. Aviram, N. Volkova, R. Coleman, M. Dreher, M. K. Reddy, D. Ferreira and M. Rosenblat, J. Agric. Food Chem., 2008, 56, 1148–1157). Copyright (2008) American Chemical Society. | ||
Pomegranate fruit extract and pomegranate juice concentrate were also able to reverse pro-atherogenic effects of perturbed shear stress by reducing the expression of oxidation-sensitive response genes and enhancing eNOS expression in cultured human endothelial cells, as well as in atherosclerosis-prone areas of hypocholesterolaemic mice.147
Several of the anti-atherogenic effects are based on the anti-oxidative properties of pomegranate, including the ability to increase glutathione levels141,143 and the ability to elevate the enzymatic activities of superoxide dismutase, catalase, and glutathione reductase.148
8.5 Possible increase of reverse cholesterol transport via PPAR activation
Pomegranate-derived compounds are ligands of PPARα, PPARβ/δ, and PPARγ.21,105,119,142,145,149,150 These receptors mediate cholesterol efflux; this may be one mechanism that enables pomegranate and its constituents to modulate lipid parameters. Cholesterol efflux via receptor activation has been reported for PPARα151 and PPARγ.152 Experiments with PPARα knockout mice showed that these animals have a retarded serum HDL turnover rate and gain significantly more weight than wild type mice.153 PPARα and PPARγ regulate ATP-binding cassette transporter A1 (ABCA1) and ATP-binding cassette transporter G1 (ABCG1), enhancing cholesterol efflux and reducing intracellular lipid accumulation.154–156 ABCA1 and ABCG1 facilitate cholesterol efflux from cells to HDL; this is part of the reverse cholesterol transport pathway. The disruption of PPARγ gene functions in a knockout mouse model leads to a reduction in basal cholesterol efflux from cholesterol-loaded macrophages to HDL.157 Activation of PPARγ also leads to enhanced cholesterol efflux, despite increased uptake of oxidised LDL.158 Experiments with Zucker diabetic fatty rats suggest increased efficiency with PPARα and PPARγ ligands for the improvement of lipid metabolism through enhanced reverse cholesterol transport.159 The best treatment would be with a pan-activator of all PPARs, as PPARδ activation results in macrophage-to-faeces cholesterol transport but does not stimulate macrophage cholesterol efflux.160Park et al.161 demonstrated in murine macrophages that ellagic acid at concentrations ≥1 μM up-regulates PPARγ and ABCA1 in oxidised LDL-treated cells and promotes cholesterol efflux.
8.6 Anti-inflammatory effects
Inflammation is a natural process that maintains normal tissue function as part of the innate immune defence. It is a response to hazardous stimuli and (in normal physiological processes) leads to healing of the affected tissue. However, if the process is misled, it can manifest as chronic inflammation. This happens quite often, and is the basis for a variety of severe diseases, including cardiovascular disease, rheumatoid arthritis, atherosclerosis, and cancer.Less well-known is the fact that obesity is intimately connected with the inflammatory response;162 remodelled adipose tissue secretes increased amounts of pro-inflammatory signal molecules, such as cytokines [including interleukin (IL)-1, IL-6, IL-12, and IL-18], interferon (IFN)-γ, monocyte chemotactic protein-1 [MCP-1, also known as chemokine (C–C motif) ligand 2 (CCL2)] and tumour necrosis factors (TNFs, for example TNF-α). The persistent inflammatory response of obese adipose tissue is actually a chronic low-grade inflammation and leads to insulin resistance, type 2 diabetes, and metabolic syndrome.
Pomegranate juice, pomegranate extract (of various plant parts), and components of the pomegranate plant have been described as anti-inflammatory in various studies. The underlying mechanism is intricate and takes place during several stages of the inflammatory response (Fig. 10).
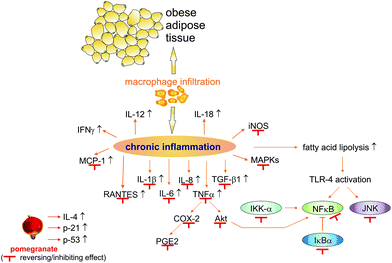 | ||
| Fig. 10 The effects of pomegranate on inflammation processes. Abbreviations: COX-2, cyclooxygenase 2; IFN, interferon; IKK-α, inhibitor of nuclear factor kappa-B kinase subunit alpha; IκBα, nuclear factor of kappa light polypeptide gene enhancer in B cells inhibitor alpha; IL, interleukin; iNOS, inducible nitric oxide synthase; JNK, c-Jun N-terminal kinases; MAPKs, mitogen-activated protein kinases; MCP-1, monocyte chemotactic protein-1; NF-κB, nuclear factor kappa-light-chain-enhancer of activated B cells; p21, cyclin-dependent kinase inhibitor 1; p53, tumour protein 53; PGE2, prostaglandin 2; RANTES, regulated on activation, normal T cell expressed and secreted; TGF-β1, transforming growth factor β1; TLR-4, toll-like receptor 4; TNFα, tumour necrosis factor α. | ||
In vitro, pomegranate juice reduced the levels of IL-6 and regulated on activation, normal T cell expressed and secreted (RANTES) in a prostate cancer cell model.163 RANTES is also known as chemokine (C–C motif) ligand 5 (CCL5), and is a chemotactic cytokine that recruits leukocytes into inflammatory sites. Pomegranate juice was also observed to suppress TNF-α-induced expression of cyclooxygenase (COX)-2,164 an enzyme that catalyses synthesis of prostaglandins, which are involved in pain and swelling during the inflammatory response. In the same study, pomegranate juice abolished TNFα-induced activation of Akt protein164 [also known as protein kinase B (PKB)], a serine/threonine protein kinase that is involved in proliferation, tumour cell survival, and invasiveness. Akt also activates nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB), which is a key player in the immune response and in cancer. Several in vitro studies demonstrate that pomegranate (extract or wine) acts as an NF-κB inhibitor.165–170 The inhibition of NF-κB and Akt pathways can be also mediated by estrogen pathways.171
Effects of TNF-α are also inhibited by pomegranate extract.166,172,173 As a consequence of the regulation of these important key players in the inflammatory process, pomegranate reduces the levels of pro-inflammatory cytokines, such as IL-1β, IL-6, and IL-8.163,165,168,169,172,174
Well-known pomegranate-derived compounds or metabolites have demonstrated similar effects in vitro; ellagic acid, urolithins, and punicalagin were able to reduce the production of pro-inflammatory interleukins, down-regulate COX-2, inhibit the production of prostaglandins such as PGE2, inhibit NF-κB and TNF-α activity, reduce Akt activation, and inhibit the activation of mitogen-activated protein kinase (MAPK) pathways;175–180MAPK proteins are also centrally involved in inflammation processes. Balwani et al.181 found a novel compound in pomegranate leaves, called 2-methyl-pyran-4-one-3-O-beta-D-glucopyranoside, which significantly blocked TNF-α-induced translocation and activation of NF-κB. Hence, the anti-inflammatory activity of pomegranate is due to the concerted action of various ingredients, which may be (at least in part) undiscovered and unknown.
More importantly, ellagic acid, urolithin, punicalagin, punicalin, punicic acid, and granatin B also exerted anti-inflammatory effects in animal experiments, mostly after oral administration.119,182–191 These compounds were able to decrease inflammation markers [COX-2, PGE2, inducible nitric oxide synthase (iNOS), NF-κB, TNF-α, IL-6, IL-1β, and MCP-1] and neutrophil infiltration, and to reduce activation of MAPKs [p38, c-Jun N-terminal kinases (JNK), and extracellular signal-regulated kinases (ERKs)] in vivo.119,182–191
Punicalagin treatment inhibited chemically induced chronic oedema in mice and decreased CD3+ T cell infiltration of inflamed tissue.192 Larrosa et al.182 investigated the effect of urolithin A on male Fisher rats for 25 days, and postulated that urolithin A is the most potent anti-inflammatory compound in pomegranate in healthy subjects, while anti-inflammatory effects in the context of colon inflammation are due to non-metabolised ellagitannins. Other groups have also demonstrated the beneficial anti-inflammatory effects of pomegranate-derived compounds in chemically induced colon inflammation and Crohn's disease, an inflammatory bowel disease.183,193 Induced paw inflammation in mice was also significantly reduced by ellagic acid,190 granatin B,192 punicalagin,191 and punicalin.191 At least some of the anti-inflammatory effects of pomegranate are due to PPARγ activation: Hontecillas et al.119 demonstrated in PPARγ null mice that the loss of the PPARγ gene was equivalent to the loss of the anti-inflammatory properties of punicic acid.
Pomegranate extract has also exhibited anti-inflammatory effects in vivo. As well as pomegranate-derived compounds exert benevolent effects on inflammatory bowel diseases, pomegranate extract significantly attenuated chemically induced colonic inflammation in mice.194 In an experiment using rats who were subjected to myringotomy, rats who received pomegranate extract after surgery had significantly fewer inflammatory cells and reduced acute inflammation in the tympanic membrane compared with control rats.195 In the same manner, chemically induced lung inflammation in mice was attenuated by pomegranate peel extract.196 Bagri et al.187 tested aqueous ethanol extracts of pomegranate fruit rind, flowers, and leaves for the ability to reduce chemically induced paw oedema in rats, and observed that all extracts were significantly anti-inflammatory and analgesic compared with control; fruit rind extract was the most effective.
As well as pomegranate-derived compounds, pomegranate extracts regulate inflammatory signal molecules in an anti-inflammatory manner in vivo. Reduced IL-1β, IL-6, and TNF-α levels were observed after feeding animals pomegranate extract in several rodent studies.197–199 However, in a short-term study involving dyslipidaemic patients with pomegranate seed oil, serum TNF-α was decreased but the effect was not significant.200
Other animal studies demonstrate the reduced expression of the inflammation marker transforming growth factor (TGF)-β1,201 the inhibition of NF-κB translocation,202,203 and inhibited activation of the NF-κB regulator inhibitor of nuclear factor kappa-B kinase subunit alpha (IKK-α),202,203 as well as inhibited degradation of the NF-κB inhibitor nuclear factor of kappa light polypeptide gene enhancer in B-cells inhibitor alpha (IκBα).202,203 Other animal studies have also demonstrated the inhibition of COX-2 and iNOS expression,203 COX-1 and COX-2 enzyme activity,204 and IL-1β-induced PGE2 production.204 Meanwhile, the level of anti-inflammatory cytokine IL-4 was increased in calves after pomegranate extract intake.127 Moreover, Afaq et al.202 observed that treatment with pomegranate fruit extract enhanced UVB-mediated increases in cyclin kinase inhibitor p21 and the tumour suppressor protein p53.
In a mouse model of rheumatoid arthritis, pomegranate extract-fed animals exhibited significantly lower severity of arthritis and reduced joint infiltration by inflammatory cells.197 The transferability of in vitro on in vivo data was demonstrated by Rettig et al.,205 who observed that pomegranate extract inhibits NF-κB and the cell viability of prostate cancer cells in a dose-dependent manner in vitro, and who were able to transfer these results into a xenograft mouse model. In the xenograft model, they observed a delayed onset of androgen-independent proliferation, and found that the NF-κB increase normally observed during the transition from androgen-dependent to androgen-independent cancer was abrogated by pomegranate extract. Given that the establishment of androgen-independence in cancer is partly due to NF-κB activity, pomegranate extract or an active lead compound may provide new therapeutic approaches.
9 Other transcription factors and the metabolic syndrome
PPARs can be considered as one of the major mediators of the anti-diabetic effects of pomegranate and its compounds. But several other transcription factors can contribute to these effects, such as the liver X receptor (LXR), the farnesoid X receptor (FXR) and the estrogen receptors (ERs). They are closely intertwined with PPAR pathways or are important metabolic regulators and have to be mentioned, although an in-depth discussion of all these receptors is beyond the scope of this review. Nevertheless, they have been also linked to hypoglycaemic, insulin sensitising, anti-inflammatory and anti-atherosclerotic effects. The cross-talk between LXR, FXR and PPARs is complex (reviewed by Nagao and Yanagita206) and makes a monocausal approach difficult. As for the estrogen receptors, we recommend a recently published review by Faulds et al., who excellently outlined the implication of estrogen signalling in the metabolic syndrome.207Polyphenols and compounds that are also constituents of pomegranate have been described as modulators of LXR, FXR and ER pathways. Procyanidins208 and epigallocatechin209 have been mentioned as FXR regulators, which in turn could enable reverse cholesterol transport and lipid homeostasis.210 FXR signalling is also involved in the regulation of PON-1 expression and enables anti-atherosclerotic effects via FXR antagonists.211,212
Ellagic acid up-regulates not only PPARγ and ABCA1, but also increases LXRα expression and transcription.161 Anthocyanins induce cholesterol efflux via a PPARγ/LXRα/ABCA1 signaling pathway.213 But not all effects of LXRα are connected to PPARγ; Wang et al.214 showed in their study that while the anthocyanin cyanidin-3-O-β-glucoside enhances the expression and transcriptional activities of both, PPARγ and LXRα, only an LXRα antagonist down-regulated anti-inflammatory effects via contradiction of the inhibitory effect of cyanidin-3-O-β-glucoside on iNOS and COX-2 expression. However, as the bioavailability of anthocyanins from pomegranate seems to be extremely low (see Chapter 5), the relevance of these in vitro data is not quite clear.
The reduction of LDL-serum levels by phytosterols and phytostanols have been attributed at least partly to the regulation of ABC transporters and LXR pathways.215–218 As these compound classes are present in pomegranate, a contribution to reverse cholesterol transport via these pathways can be assumed.
But the beneficial effects in regard to metabolic syndrome mediated by some pomegranate compounds can be assumed to function solely via PPARγ activation; punicic acid that has glucose-normalising abilities and anti-inflammatory properties, activates the PPARγ pathway but not LXR or other nuclear receptors such as the retinoid X receptor (RXR) or retinoic acid receptor (RAR).119
Potential anti-carcinogenic effects of pomegranate constituents have been also explained by inhibition of important key enzymes such as aromatase and 17-β-hydroxysteroid dehydrogenase via ER pathways (as reviewed by Sturgeon and Ronnenberg219).
10 Conclusion
There is strong in vivo and in vitro evidence that pomegranate exerts ameliorating effects on metabolic syndrome. When this evidence is considered together with information from bioavailability studies, one can conclude that pomegranate's bioactive compounds are absorbed in sufficient quantities to exert biological activity. Nevertheless, controlled human intervention studies and epidemiological studies are not available. The influence of cultivar and habitat on the synthesis of bioactive compounds is not fully understood, and would help plant breeders to improve nutritional quality because fruits from different cultivars and habitats may exert different biological properties. As the fruit contains so many different bioactive compounds, a better understanding of the concerted action of these compounds is definitely desired.There is sufficient scientific evidence to claim that whole pomegranate, parts of the fruit, and certain extracts have several benevolent health effects with respect to metabolic syndrome. The treatment of type 2 diabetes is an interesting and promising property of this fruit exerted through several biological properties, including hypoglycaemic and insulin sensitising effects mediated by the inhibition of α-glucosidase, regulation of glucose transporter type 4 function, regulation of adipokine levels, and the improvement of blood lipids (including a reduction of total cholesterol), among other mechanisms. Most, but not all effects, are mediated through the activation of PPAR pathways. Finally, the remarkable anti-inflammatory properties of pomegranate also help to explain why pomegranate-derived compounds have effects on many chronic diseases.
References
- A. Tenenbaum, M. Motro and E. Z. Fisman, Cardiovasc. Diabetol., 2005, 4, 14 Search PubMed
.
- M. Hashemi, R. Kelishadi, M. Hashemipour, A. Zakerameli, N. Khavarian, S. Ghatrehsamani and P. Poursafa, Cardiol. Young, 2010, 20, 73–77 Search PubMed
.
- S. R. Katz, R. A. Newman and E. P. Lansky, J. Med. Food, 2007, 10, 213–217 Search PubMed
.
- A. Basu and K. Penugonda, Nutr. Rev., 2009, 67, 49–56 Search PubMed
.
- J. S. Jurenka, Altern. Med. Rev., 2008, 13, 128–144 Search PubMed
.
- S. Mandel, L. Packer, M. B. Youdim and O. Weinreb, J. Nutr. Biochem., 2005, 16, 513–520 Search PubMed
.
- W. H. O. (WHO), Obesity and overweight – Fact sheet no. 311, http://www.who.int/mediacentre/factsheets/fs311/en/index.html, accessed 8th July 2011.
- K. M. Flegal, M. D. Carroll, C. L. Ogden and L. R. Curtin, JAMA, J. Am. Med. Assoc., 2010, 303, 235–241 CrossRef CAS
.
- Centers for Disease Control and Prevention, U.S. Obesity Trends, http://www.cdc.gov/obesity/data/trends.html, accessed 8th July 2011 Search PubMed.
- International Diabetes Federation, The IDF consensus worldwide definition of the metabolic syndrome, http://www.idf.org/webdata/docs/IDF_Meta_def_final.pdf, accessed 8th July 2011.
- Centers for Disease Control and Prevention, Long-term Trends in Diabetes, http://www.cdc.gov/diabetes/statistics/slides/long_term_trends.pdf, accessed 16th May 2012.
- Y. Wang, M. A. Beydoun, L. Liang, B. Caballero and S. K. Kumanyika, Obesity, 2008, 16, 2323–2330 Search PubMed
.
- E. S. Huang, A. Basu, M. O'Grady and J. C. Capretta, Diabetes Care, 2009, 32, 2225–2229 Search PubMed
.
- J. P. Boyle, A. A. Honeycutt, K. M. Narayan, T. J. Hoerger, L. S. Geiss, H. Chen and T. J. Thompson, Diabetes Care, 2001, 24, 1936–1940 Search PubMed
.
- World Health Organization (WHO), Diabetes – Fact sheet no. 312, http://www.who.int/mediacentre/factsheets/fs312/en/, accessed 13th July 2011.
- U.S. Food and Drug Administration (FDA), FDA Drug Safety Communication: Updated Risk Evaluation and Mitigation Strategy (REMS) to Restrict Access to Rosiglitazone-containing Medicines including Avandia, Avandamet, and Avandaryl, http://www.fda.gov/Drugs/DrugSafety/ucm255005.htm, accessed 12th July 2011.
- European Medicines Agency (EMA), European Medicines Agency recommends suspension of Avandia, Avandamet and Avaglim, http://www.ema.europa.eu/docs/en_GB/document_library/Press_release/2010/09/WC500096996.pdf, accessed 12th July 2010.
- S. E. Nissen and K. Wolski, N. Engl. J. Med., 2007, 356, 2457–2471 Search PubMed
.
- D. J. Graham, R. Ouellet-Hellstrom, T. E. MaCurdy, F. Ali, C. Sholley, C. Worrall and J. A. Kelman, Jama, 2010, 304, 411–418 Search PubMed
.
- A. Saxena and N. K. Vikram, J. Altern. Complement. Med., 2004, 10, 369–378 Search PubMed
.
- Y. Li, Y. Qi, T. H. Huang, J. Yamahara and B. D. Roufogalis, Diabetes, Obes. Metab., 2008, 10, 10–17 CAS
.
- A. G. Pirbalouti, S. Azizi, A. Koohpayeh and B. Hamedi, Acta Pol. Pharm., 2010, 67, 511–516 Search PubMed
.
- A. Tahraoui, J. El-Hilaly, Z. H. Israili and B. Lyoussi, J. Ethnopharmacol., 2007, 110, 105–117 Search PubMed
.
- S. Gozlekci, O. Saracoglu, E. Onursal and M. Ozgen, Pharmacogn. Mag., 2011, 7, 161–164 Search PubMed
.
- A. Tehranifar, M. Zarei, Z. Nemati, B. Esfandiyari and M. R. Vazifeshenas, Sci. Hortic., 2010, 126, 180–185 Search PubMed
.
-
O. W. Thomé, Flora von Deutschland, Österreich und der Schweiz, Friedrich von Zezschwitz, Gera-Untermhaus, 1885 Search PubMed
.
- E. P. Lansky and R. A. Newman, J. Ethnopharmacol., 2007, 109, 177–206 CrossRef CAS
.
- R. Wang, Y. Ding, R. Liu, L. Xiang and L. Du, Fruit, Veg. Cereal Sci. Biotechnol., 2010, 4, 77–87 Search PubMed
.
- U. A. Fischer, R. Carle and D. R. Kammerer, Food Chem., 2011, 127, 807–821 CrossRef CAS
.
- G. Borges, W. Mullen and A. Crozier, Food Funct., 2010, 1, 73–83 RSC
.
- S. de Pascual-Teresa, C. Santos-Buelga and J. C. Rivas-Gonzalo, J. Agric. Food Chem., 2000, 48, 5331–5337 CrossRef
.
- M. I. Gil, F. A. Tomas-Barberan, B. Hess-Pierce, D. M. Holcroft and A. A. Kader, J. Agric. Food Chem., 2000, 48, 4581–4589 CrossRef CAS
.
- D. A. van Elswijk, U. P. Schobel, E. P. Lansky, H. Irth and J. van der Greef, Phytochemistry, 2004, 65, 233–241 Search PubMed
.
- H. Satomi, K. Umemura, A. Ueno, T. Hatano, T. Okuda and T. Noro, Biol. Pharm. Bull., 1993, 16, 787–790 Search PubMed
.
- M. A. M. Nawwar, S. A. M. Hussein and I. Merfort, Phytochemistry, 1994, 36, 793–798 Search PubMed
.
- T. Tanaka, G. I. Nonaka and I. Nishioka, Chem. Pharm. Bull., 1986, 34, 650–655 CAS
.
- T. Tanaka, G. I. Nonaka and I. Nishioka, Phytochemistry, 1985, 24, 2075–2078 Search PubMed
.
- R. Wang, W. Wei, L. Wang, R. Liu, D. Yi and L. Du, Fitoterapia, 2006, 77, 534–537 Search PubMed
.
- H. Alighourchi, M. Barzegar and S. Abbasi, Eur. Food Res. Technol., 2008, 227, 881–887 Search PubMed
.
- L. Zhang, Q. Fu and Y. Zhang, Food Chem., 2011, 127, 1444–1449 Search PubMed
.
- C. Fanali, L. Dugo, G. D'Orazio, M. Lirangi, M. Dacha, P. Dugo and L. Mondello, J. Sep. Sci., 2011, 34, 150–159 Search PubMed
.
- G. Mousavinejad, Z. Emam-Djomeh, K. Rezaei and M. H. H. Khodaparast, Food Chem., 2009, 115, 1274–1278 Search PubMed
.
- A. Calin-Sanchez, J. J. Martinez, L. Vazquez-Araujo, F. Burlo, P. Melgarejo and A. A. Carbonell-Barrachina, J. Sci. Food Agric., 2011, 91, 586–592 Search PubMed
.
- L. Vazquez-Araujo, E. Chambers, K. Adhikari and A. A. Carbonell-Barrachina, J. Food Sci., 2010, 75, S398–S404 Search PubMed
.
- P. Melgarejo, A. Calin-Sanchez, L. Vazquez-Araujo, F. Hernandez, J. J. Martinez, P. Legua and A. A. Carbonell-Barrachina, J. Food Sci., 2011, 76, S114–120 Search PubMed
.
- L. Vazquez-Araujo, E. Chambers, K. Adhikari and A. A. Carbonell-Barrachina, LWT–Food Sci. Technol., 2011, 44, 2119–2125 Search PubMed
.
- L. Vazquez-Araujo, K. Koppel, E. Chambers Iv, K. Adhikari and A. A. Carbonell-Barrachina, Flavour Fragrance J., 2011, 26, 129–138 Search PubMed
.
- E. Poyrazoglu, V. Gökmen and N. Artik, J. Food Compos. Anal., 2002, 15, 567–575 Search PubMed
.
- Y. Amakura, M. Okada, S. Tsuji and Y. Tonogai, J. Chromatogr., A, 2000, 891, 183–188 CrossRef CAS
.
- N. Alper, P. Onsekizoglu and J. Acar, J.Food Process. Preserv., 2011, 35, 313–319 Search PubMed
.
- W. Elfalleh, M. Ying, N. Nasri, H. Sheng-Hua, F. Guasmi and A. Ferchichi, Int. J. Food Sci. Nutr., 2011, 62, 200–206 Search PubMed
.
- A. Fadavi, M. Barzegar and M. Hossein Azizi, J. Food Compos. Anal., 2006, 19, 676–680 CrossRef CAS
.
- G. Pande and C. C. Akoh, J. Agric. Food Chem., 2009, 57, 9427–9436 Search PubMed
.
- M. Kaufman and Z. Wiesman, J. Agric. Food Chem., 2007, 55, 10405–10413 Search PubMed
.
- S. E. El-Nemr, I. A. Ismail and M. Ragab, Nahrung, 1990, 34, 601–606 Search PubMed
.
- M. Kyralan, M. Gölükcü and H. Tokgöz, J. Am. Oil Chem. Soc., 2009, 86, 985–990 Search PubMed
.
- A. Parashar, Int. J. Fruit Sci., 2010, 10, 425–430 Search PubMed
.
- F. Bonzanini, R. Bruni, G. Palla, N. Serlataite and A. Caligiani, Food Chem., 2009, 117, 745–749 Search PubMed
.
- C. S. Yang and X. Wang, Nutr. Cancer, 2010, 62, 931–937 Search PubMed
.
- B. Cerda, C. Soto, M. D. Albaladejo, P. Martinez, F. Sanchez-Gascon, F. Tomas-Barberan and J. C. Espin, Eur. J. Clin. Nutr., 2006, 60, 245–253 Search PubMed
.
- M. I. Gil, C. Garcia-Viguera, F. Artes and F. A. Tomas-Barberan, J. Sci. Food Agric., 1995, 68, 77–81
.
- P. Mena, C. Garcia-Viguera, J. Navarro-Rico, D. A. Moreno, J. Bartual, D. Saura and N. Marti, J. Sci. Food Agric., 2010, 91, 1893–1906 Search PubMed
.
- H. Borochov-Neori, S. Judeinstein, M. Harari, I. Bar-Ya'akov, B. S. Patil, S. Lurie and D. Holland, J. Agric. Food Chem., 2011, 59, 5325–5334 Search PubMed
.
- F. Hernandez, P. Melgarejo, F. A. Tomas-Barberan and F. Artes, Eur. Food Res. Technol., 1999, 210, 39–42 CrossRef CAS
.
- M. Dafny-Yalin, I. Glazer, I. Bar-Ilan, Z. Kerem, D. Holland and R. Amir, J. Agric. Food Chem., 2010, 58, 4342–4352 Search PubMed
.
- E. Schwartz, R. Tzulker, I. Glazer, I. Bar-Ya'akov, Z. Wiesman, E. Tripler, I. Bar-Ilan, H. Fromm, H. Borochov-Neori, D. Holland and R. Amir, J. Agric. Food Chem., 2009, 57, 9197–9209 Search PubMed
.
- B. Cerda, J. C. Espin, S. Parra, P. Martinez and F. A. Tomas-Barberan, Eur. J. Nutr., 2004, 43, 205–220 CAS
.
- A. Gonzalez-Sarrias, J. A. Gimenez-Bastida, M. T. Garcia-Conesa, M. B. Gomez-Sanchez, N. V. Garcia-Talavera, A. Gil-Izquierdo, C. Sanchez-Alvarez, L. O. Fontana-Compiano, J. P. Morga-Egea, F. A. Pastor-Quirante, F. Martinez-Diaz, F. A. Tomas-Barberan and J. C. Espin, Mol. Nutr. Food Res., 2010, 54, 311–322 Search PubMed
.
- B. Cerda, R. Llorach, J. J. Ceron, J. C. Espin and F. A. Tomas-Barberan, Eur. J. Nutr., 2003, 42, 18–28 Search PubMed
.
- E. M. Daniel, S. Ratnayake, T. Kinstle and G. D. Stoner, J. Nat. Prod., 1991, 54, 946–952 Search PubMed
.
- N. P. Seeram, S. M. Henning, Y. Zhang, M. Suchard, Z. Li and D. Heber, J Nutr, 2006, 136, 2481–2485 CAS
.
- N. P. Seeram, R. Lee and D. Heber, Clin. Chim. Acta, 2004, 348, 63–68 Search PubMed
.
- R. Gonzalez-Barrio, E. Christine and A. Crozier, Drug Metab. Dispos., 2011 Search PubMed
.
- J. Lan, F. Lei, L. Hua, Y. Wang, D. Xing and L. Du, Biomed. Chromatogr., 2009, 23, 531–536 CrossRef CAS
.
- F. Lei, D. M. Xing, L. Xiang, Y. N. Zhao, W. Wang, L. J. Zhang and L. J. Du, J. Chromatogr., B: Anal. Technol. Biomed. Life Sci., 2003, 796, 189–194 CrossRef CAS
.
- S. U. Mertens-Talcott, P. Jilma-Stohlawetz, J. Rios, L. Hingorani and H. Derendorf, J. Agric. Food Chem., 2006, 54, 8956–8961 CrossRef CAS
.
- G. D. Stoner, C. Sardo, G. Apseloff, D. Mullet, W. Wargo, V. Pound, A. Singh, J. Sanders, R. Aziz, B. Casto and X. Sun, J. Clin. Pharmacol., 2005, 45, 1153–1164 Search PubMed
.
- R. Gonzalez-Barrio, G. Borges, W. Mullen and A. Crozier, J. Agric. Food Chem., 2010, 58, 3933–3939 CrossRef
.
- R. Gonzalez-Barrio, P. Truchado, H. Ito, J. C. Espin and F. A. Tomas-Barberan, J. Agric. Food Chem., 2011, 59, 1152–1162 Search PubMed
.
- B. Cerda, P. Periago, J. C. Espin and F. A. Tomas-Barberan, J. Agric. Food Chem., 2005, 53, 5571–5576 CrossRef CAS
.
- B. Cerda, F. A. Tomas-Barberan and J. C. Espin, J. Agric. Food Chem., 2005, 53, 227–235 CrossRef CAS
.
- J. C. Espin, R. Gonzalez-Barrio, B. Cerda, C. Lopez-Bote, A. I. Rey and F. A. Tomas-Barberan, J. Agric. Food Chem., 2007, 55, 10476–10485 CrossRef CAS
.
- N. P. Seeram, Y. Zhang, R. McKeever, S. M. Henning, R. P. Lee, M. A. Suchard, Z. Li, S. Chen, G. Thames, A. Zerlin, M. Nguyen, D. Wang, M. Dreher and D. Heber, J. Med. Food, 2008, 11, 390–394 CrossRef CAS
.
- N. P. Seeram, W. J. Aronson, Y. Zhang, S. M. Henning, A. Moro, R. P. Lee, M. Sartippour, D. M. Harris, M. Rettig, M. A. Suchard, A. J. Pantuck, A. Belldegrun and D. Heber, J. Agric. Food Chem., 2007, 55, 7732–7737 Search PubMed
.
- A. Sanchez-Lamar, G. Fonseca, J. L. Fuentes, R. Cozzi, E. Cundari, M. Fiore, R. Ricordy, P. Perticone, F. Degrassi and R. De Salvia, J. Ethnopharmacol., 2008, 115, 416–422 Search PubMed
.
- C. Patel, P. Dadhaniya, L. Hingorani and M. G. Soni, Food Chem. Toxicol., 2008, 46, 2728–2735 Search PubMed
.
- I. A. T. M. Meerts, C. M. Verspeek-Rip, C. A. F. Buskens, H. G. Keizer, J. Bassaganya-Riera, Z. E. Jouni, A. H. B. M. van Huygevoort, F. M. van Otterdijk and E. J. van de Waart, Food Chem. Toxicol., 2009, 47, 1085–1092 Search PubMed
.
- A. Vidal, A. Fallarero, B. R. Pena, M. E. Medina, B. Gra, F. Rivera, Y. Gutierrez and P. M. Vuorela, J. Ethnopharmacol., 2003, 89, 295–300 Search PubMed
.
- B. Desta, J. Ethnopharmacol., 1995, 45, 27–33 Search PubMed
.
- S. M. Tripathi and D. K. Singh, Braz. J. Med. Biol. Res., 2000, 33, 1351–1355 Search PubMed
.
- B. Cerda, J. J. Ceron, F. A. Tomas-Barberan and J. C. Espin, J. Agric. Food Chem., 2003, 51, 3493–3501 Search PubMed
.
- D. Heber, N. P. Seeram, H. Wyatt, S. M. Henning, Y. Zhang, L. G. Ogden, M. Dreher and J. O. Hill, J. Agric. Food Chem., 2007, 55, 10050–10054 Search PubMed
.
- M. Tasaki, T. Umemura, M. Maeda, Y. Ishii, T. Okamura, T. Inoue, Y. Kuroiwa, M. Hirose and A. Nishikawa, Food Chem. Toxicol., 2008, 46, 1119–1124 Search PubMed
.
- M. Azorin-Ortuno, C. Urban, J. J. Ceron, F. Tecles, A. Gil-Izquierdo, F. J. Pallares, F. A. Tomas-Barberan and J. C. Espin, J. Agric. Food Chem., 2008, 56, 2857–2865 Search PubMed
.
- United States Agency for International Development (USAID), Iraq – A Strategy for Pomegranates, https://www.inma-iraq.com/?pname=open&f=doc100224_TR_pomegranate_strategy_april08.pdf&id=224&type=file, accessed 8th August 2011.
- United States Department of Agriculture (USDA), 2007 Census Report, http://www.agcensus.usda.gov/Publications/2007/Full_Report/Volume_1,_chapter_2_US_State_level/st99_2_032_032.pdf, accessed 8th August 2011.
- G. A. Finetto, Acta Hortic., 2011, 890, 45–54 Search PubMed
.
- D. Rymon, Acta Hortic., 2011, 890, 599–602 Search PubMed
.
- M. Zarei, M. Azizi and Z. Bashiri-Sadr, J. Food Technol., 2010, 8, 112–117 Search PubMed
.
- C. El Kar, A. Ferchichi, F. Attia and J. Bouajila, J. Food Sci., 2011, 76, C795–C800 Search PubMed
.
- A. Aarabi, M. Barzegar and M. H. Azizi, ASEAN Food J., 2008, 15, 45–55 Search PubMed
.
- V. Akbarpour, K. Hemmati and M. Sharifani, Am.–Eurasian J. Agric. Environ. Sci., 2009, 6, 411–416 Search PubMed
.
- E. Shwartz, I. Glazer, I. Bar-Ya'akov, I. Matityahu, I. Bar-Ilan, D. Holland and R. Amir, Food Chem., 2009, 115, 965–973 CrossRef CAS
.
- M. Mueller and A. Jungbauer, Food Chem., 2009, 117, 660–667 Search PubMed
.
- M. Shiner, B. Fuhrman and M. Aviram, Atherosclerosis, 2007, 195, 313–321 Search PubMed
.
- H. Kohno, R. Suzuki, Y. Yasui, M. Hosokawa, K. Miyashita and T. Tanaka, Cancer Sci., 2004, 95, 481–486 Search PubMed
.
- T. H. Huang, G. Peng, B. P. Kota, G. Q. Li, J. Yamahara, B. D. Roufogalis and Y. Li, Br. J.
Pharmacol., 2005, 145, 767–774 CrossRef CAS
.
- K. Z. Y. Xu, C. Zhu, M. S. Kim, J. Yamahara and Y. Li, J. Ethnopharmacol., 2009, 123, 280–287 Search PubMed
.
- H. S. Parmar and A. Kar, J. Med. Food, 2008, 11, 376–381 Search PubMed
.
- H. S. Parmar and A. Kar, BioFactors, 2007, 31, 17–24 Search PubMed
.
- E. A. M. Khalil, Egypt J. Hosp. Med., 2004, 16, 92–99 Search PubMed
.
- G. Jelodar, M. Mohsen and S. Shahram, Afr. J. Tradit., Complementary Altern. Med., 2007, 4, 299–305 Search PubMed
.
- M. A. Jafri, M. Aslam, K. Javed and S. Singh, J. Ethnopharmacol., 2000, 70, 309–314 Search PubMed
.
- S. Manoharan, P. Ranezab Anishkumar, K. Panjamurthy, L. Vellaichamy and A. Linsa Mary, World Heart J., 2008, 1, 325–331 Search PubMed
.
- Y. Li, S. Wen, B. P. Kota, G. Peng, G. Q. Li, J. Yamahara and B. D. Roufogalis, J. Ethnopharmacol., 2005, 99, 239–244 Search PubMed
.
- R. A. DiSilvestro, D. J. DiSilvestro and D. J. DiSilvestro, Phytother. Res., 2009, 23, 1123–1127 Search PubMed
.
- T. H. Huang, G. Peng, B. P. Kota, G. Q. Li, J. Yamahara, B. D. Roufogalis and Y. Li, Toxicol. Appl. Pharmacol., 2005, 207, 160–169 CrossRef CAS
.
- A. K. Das, S. C. Mandal, S. K. Banerjee, S. Sinha, B. P. Saha and M. Pal, Phytother. Res., 2001, 15, 628–629 Search PubMed
.
- R. Hontecillas, M. O'Shea, A. Einerhand, M. Diguardo and J. Bassaganya-Riera, J. Am. Coll. Nutr., 2009, 28, 184–195 Search PubMed
.
- B. K. McFarlin, K. A. Strohacker and M. L. Kueht, Br. J. Nutr., 2009, 102, 54–59 Search PubMed
.
- I. O. Vroegrijk, J. A. van Diepen, S. van den Berg, I. Westbroek, H. Keizer, L. Gambelli, R. Hontecillas, J. Bassaganya-Riera, G. C. Zondag, J. A. Romijn, L. M. Havekes and P. J. Voshol, Food Chem. Toxicol., 2011, 49, 1426–1430 Search PubMed
.
- Q. Dou, Y. Y. Wei, Y. Li, D. Yan, Adili, T. Yuan, Y. Kurexi and K. Parhat, Chin. Pharmacol. Bull., 2010, 26, 794–797 Search PubMed
.
- M. Gonzalez-Ortiz, E. Martinez-Abundis, M. C. Espinel-Bermudez and K. G. Perez-Rubio, Ann. Nutr. Metab., 2011, 58, 220–223 Search PubMed
.
- H. J. Harwood, Jr, Neuropharmacology, 2012, 63, 57–75 Search PubMed
.
- Y. Makino-Wakagi, Y. Yoshimura, Y. Uzawa, N. Zaima, T. Moriyama and Y. Kawamura, Biochem. Biophys. Res. Commun., 2012, 417, 880–885 Search PubMed
.
- F. L. A. Hossin, Pak. J. Nutr., 2009, 8, 1251–1257 Search PubMed
.
- R. A. Oliveira, C. D. Narciso, R. S. Bisinotto, M. C. Perdomo, M. A. Ballou, M. Dreher and J. E. Santos, J. Dairy Sci., 2010, 93, 4280–4291 Search PubMed
.
- A. Shabtay, H. Eitam, Y. Tadmor, A. Orlov, A. Meir, P. Weinberg, Z. G. Weinberg, Y. Chen, A. Brosh, I. Izhaki and Z. Kerem, J. Agric. Food Chem., 2008, 56, 10063–10070 Search PubMed
.
- F. Lei, X. N. Zhang, W. Wang, D. M. Xing, W. D. Xie, H. Su and L. J. Du, Int. J. Obes., 2007, 31, 1023–1029 Search PubMed
.
- M. Yamasaki, T. Kitagawa, N. Koyanagi, H. Chujo, H. Maeda, J. Kohno-Murase, J. Imamura, H. Tachibana and K. Yamada, Nutrition, 2006, 22, 54–59 Search PubMed
.
- T. Radjabian, H. Fallah Husseini, M. Karami, I. Rasooli and S. Faghihzadeh, J. Med. Plants Res., 2008, 7, 93–104 Search PubMed
.
- P. Mirmiran, M. R. Fazeli, G. Asghari, A. Shafiee and F. Azizi, Br. J. Nutr., 2010, 104, 402–406 Search PubMed
.
- P. Bagri, M. Ali, V. Aeri, M. Bhowmik and S. Sultana, Food Chem. Toxicol., 2009, 47, 50–54 CrossRef CAS
.
- A. Esmaillzadeh, F. Tahbaz, I. Gaieni, H. Alavi-Majd and L. Azadbakht, Int. J. Vitam. Nutr. Res., 2006, 76, 147–151 Search PubMed
.
- A. K. Fenercioglu, T. Saler, E. Genc, H. Sabuncu and Y. Altuntas, J. Endocrinol. Invest., 2010, 33, 118–124 Search PubMed
.
- M. R. Lakshman, C. S. Gottipati, S. J. Narasimhan, J. Munoz, P. Marmillot and E. S. Nylen, Metab., Clin. Exp., 2006, 55, 1201–1206 Search PubMed
.
- L. P. Precourt, D. Amre, M. C. Denis, J. C. Lavoie, E. Delvin, E. Seidman and E. Levy, Atherosclerosis, 2011, 214, 20–36 Search PubMed
.
- B. Fuhrman, N. Volkova and M. Aviram, Nutrition, 2010, 26, 359–366 CrossRef CAS
.
- W. Rock, M. Rosenblat, R. Miller-Lotan, A. P. Levy, M. Elias and M. Aviram, J. Agric. Food Chem., 2008, 56, 8704–8713 CrossRef CAS
.
- M. Aviram, M. Rosenblat, D. Gaitini, S. Nitecki, A. Hoffman, L. Dornfeld, N. Volkova, D. Presser, J. Attias, H. Liker and T. Hayek, Clin. Nutr., 2004, 23, 423–433 CrossRef CAS
.
- M. Rosenblat, T. Hayek and M. Aviram, Atherosclerosis, 2006, 187, 363–371 CrossRef CAS
.
- J. Khateeb, A. Gantman, A. J. Kreitenberg, M. Aviram and B. Fuhrman, Atherosclerosis, 2010, 208, 119–125 CrossRef CAS
.
- M. Rosenblat, N. Volkova, R. Coleman and M. Aviram, J. Agric. Food Chem., 2006, 54, 1928–1935 CrossRef CAS
.
- M. Kaplan, T. Hayek, A. Raz, R. Coleman, L. Dornfeld, J. Vaya and M. Aviram, J. Nutr., 2001, 131, 2082–2089 Search PubMed
.
- M. Y. Kuo, H. C. Ou, W. J. Lee, W. W. Kuo, L. L. Hwang, T. Y. Song, C. Y. Huang, T. H. Chiu, K. L. Tsai, C. S. Tsai and W. H. H. Sheu, J. Agric. Food Chem., 2011, 59, 5100–5108 Search PubMed
.
- M. Aviram, N. Volkova, R. Coleman, M. Dreher, M. K. Reddy, D. Ferreira and M. Rosenblat, J. Agric. Food Chem., 2008, 56, 1148–1157 CrossRef CAS
.
- F. de Nigris, S. Williams-Ignarro, V. Sica, L. O. Lerman, F. P. D'Armiento, R. E. Byrns, A. Casamassimi, D. Carpentiero, C. Schiano, D. Sumi, C. Fiorito, L. J. Ignarro and C. Napoli, Cardiovasc. Res., 2007, 73, 414–423 Search PubMed
.
- H. Waghulde, M. Mohan, S. Kasture and R. Balaraman, Int. J. Pharm. Tech. Res., 2010, 2, 60–67 Search PubMed
.
- J. Bassaganya-Riera, M. DiGuardo, M. Climent, C. Vives, A. Carbo, Z. E. Jouni, A. W. Einerhand, M. O'Shea and R. Hontecillas, Br. J. Nutr., 2011, 106, 878–886 Search PubMed
.
- A. Zoechling, F. Liebner and A. Jungbauer, Food Funct., 2011, 2, 28–38 RSC
.
- K. Nakaya, J. Tohyama, S. U. Naik, H. Tanigawa, C. MacPhee, J. T. Billheimer and D. J. Rader, Arterioscler., Thromb., Vasc. Biol., 2011, 31, 1276–1282 Search PubMed
.
- A. D. Baker, A. Malur, B. P. Barna, M. S. Kavuru, A. G. Malur and M. J. Thomassen, Biochem. Biophys. Res. Commun., 2010, 393, 682–687 Search PubMed
.
- N. Akita, M. Tsujita, T. Yokota, F. J. Gonzalez, N. Ohte, G. Kimura and S. Yokoyama, J. Atheroscler. Thromb., 2010, 17, 1149–1159 Search PubMed
.
- Y. H. Wang, Y. F. Chen, S. R. Chen, X. Chen, J. W. Chen, X. Y. Shen, Y. G. Mou and P. Q. Liu, Pharmacology, 2010, 86, 320–326 Search PubMed
.
- H. Li, Y. Zhao, S. Zhou and C. K. Heng, Biochemistry, 2010, 49, 9508–9517 Search PubMed
.
- A. Malur, A. D. Baker, A. J. McCoy, G. Wells, B. P. Barna, M. S. Kavuru, A. G. Malur and M. J. Thomassen, Am. J. Physiol.: Lung Cell. Mol. Phys., 2011, 300, L73–80 Search PubMed
.
- T. E. Akiyama, S. Sakai, G. Lambert, C. J. Nicol, K. Matsusue, S. Pimprale, Y. H. Lee, M. Ricote, C. K. Glass, H. B. Brewer, Jr and F. J. Gonzalez, Mol. Cell. Biol., 2002, 22, 2607–2619 Search PubMed
.
- C. A. Argmann, C. G. Sawyez, C. J. McNeil, R. A. Hegele and M. W. Huff, Arterioscler., Thromb., Vasc. Biol., 2003, 23, 475–482 Search PubMed
.
- J. Tanabe, N. Tamasawa, M. Yamashita, K. Matsuki, H. Murakami, J. Matsui, K. Sugimoto, M. Yasujima and T. Suda, Diabetes, Obes. Metab., 2008, 10, 772–779 Search PubMed
.
- F. Briand, S. U. Naik, I. Fuki, J. S. Millar, C. Macphee, M. Walker, J. Billheimer, G. Rothblat and D. J. Rader, Clin. Transl. Sci., 2009, 2, 127–133 Search PubMed
.
- S. H. Park, J. L. Kim, E. S. Lee, S. Y. Han, J.
H. Gong, M. K. Kang and Y. H. Kang, J. Nutr., 2011, 141, 1931–1937 Search PubMed
.
- A. Jungbauer and S. Medjakovic, Maturitas, 2012, 71, 227–239 Search PubMed
.
- L. Wang, A. Alcon, H. Yuan, J. Ho, Q. J. Li and M. Martins-Green, Integr. Biol., 2011, 3, 742–754 RSC
.
- L. S. Adams, N. P. Seeram, B. B. Aggarwal, Y. Takada, D. Sand and D. Heber, J. Agric. Food Chem., 2006, 54, 980–985 Search PubMed
.
- S. Ahmed, N. Wang, B. B. Hafeez, V. K. Cheruvu and T. M. Haqqi, J. Nutr., 2005, 135, 2096–2102 Search PubMed
.
- K. H. Jung, M. J. Kim, E. Ha, Y. K. Uhm, H. K. Kim, J. H. Chung and S. V. Yim, Biol. Pharm. Bull., 2006, 29, 1258–1261 Search PubMed
.
- G. N. Khan, M. A. Gorin, D. Rosenthal, Q. Pan, L. W. Bao, Z. F. Wu, R. A. Newman, A. D. Pawlus, P. Yang, E. P. Lansky and S. D. Merajver, Integr. Cancer Ther., 2009, 8, 242–253 Search PubMed
.
- Z. Rasheed, N. Akhtar, A. N. Anbazhagan, S. Ramamurthy, M. Shukla and T. M. Haqqi, J. Inflammation (London, U. K.), 2009, 6 DOI:10.1186/1476-9255-6-1
.
- B. Romier-Crouzet, J. Van De Walle, A. During, A. Joly, C. Rousseau, O. Henry, Y. Larondelle and Y. J. Schneider, Food Chem. Toxicol., 2009, 47, 1221–1230 CrossRef CAS
.
- S. Y. Schubert, I. Neeman and N. Resnick, FASEB J., 2002, 16, 1931–1933 Search PubMed
.
- J. S. Lewis-Wambi and V. C. Jordan, Breast Cancer Res., 2009, 11, 206 Search PubMed
.
- Y. H. Kim and E. M. Choi, Phytother. Res., 2009, 23, 737–739 Search PubMed
.
- Y. Xie, T. Morikawa, K. Ninomiya, K. Imura, O. Muraoka, D. Yuan and M. Yoshikawa, Chem. Pharm. Bull., 2008, 56, 1628–1631 CrossRef CAS
.
- Z. Rasheed, N. Akhtar and T. M. Haqqi, Arthritis Res. Ther., 2010, 12, R195 Search PubMed
.
- J. Y. Bae, J. S. Choi, S. W. Kang, Y. J. Lee, J. Park and Y. H. Kang, Exp. Dermatol., 2010, 19, e182–190 CrossRef
.
- A. Gonzalez-Sarrias, M. Larrosa, F. A. Tomas-Barberan, P. Dolara and J. C. Espin, Br. J. Nutr., 2010, 104, 503–512 Search PubMed
.
- M. Dell'agli, G. V. Galli, M. Bulgari, N. Basilico, S. Romeo, D. Bhattacharya, D. Taramelli and E. Bosisio, Malar. J., 2010, 9, 208 Search PubMed
.
- B. Gauliard, D. Grieve, R. Wilson, A. Crozier, C. Jenkins, W. D. Mullen and M. Lean, J. Med. Food, 2008, 11, 382–384 Search PubMed
.
- H. C. Ou, W. J. Lee, S. D. Lee, C. Y. Huang, T. H. Chiu, K. L. Tsai, W. C. Hsu and W. H. Sheu, Toxicol. Appl. Pharmacol., 2010, 248, 134–143 Search PubMed
.
- S. Karlsson, E. Nanberg, C. Fjaeraa and J. Wijkander, Br. J. Nutr., 2010, 103, 1102–1109 Search PubMed
.
- S. Balwani, D. Nandi, P. Jaisankar and B. Ghosh, Biochimie, 2011, 93, 921–930 Search PubMed
.
- M. Larrosa, A. Gonzalez-Sarrias, M. J. Yanez-Gascon, M. V. Selma, M. Azorin-Ortuno, S. Toti, F. Tomas-Barberan, P. Dolara and J. C. Espin, J. Nutr. Biochem., 2010, 21, 717–725 CrossRef CAS
.
- M. A. Rosillo, M. Sanchez-Hidalgo, A. Cardeno and C. A. de la Lastra, Biochem. Pharmacol., 2011, 82, 737–745 CrossRef CAS
.
- S. I. Lee, B. S. Kim, K. S. Kim, S. Lee, K. S. Shin and J. S. Lim, Biochem. Biophys. Res. Commun., 2008, 371, 799–803 CrossRef CAS
.
- S. Umesalma and G. Sudhandiran, Basic Clin. Pharmacol. Toxicol., 2010, 107, 650–655 Search PubMed
.
- Y. H. Choi and G. H. Yan, Biol. Pharm. Bull., 2009, 32, 1118–1121 Search PubMed
.
- P. Bagri, M. Ali, V. Aeri, S. Sultana and M. Bhowmik, Int. J. Drug Dev. Res., 2010, 2, 698–702 Search PubMed
.
- C. Y. Chao, M. C. Mong, K. C. Chan and M. C. Yin, Mol. Nutr. Food Res., 2010, 54, 388–395 CrossRef CAS
.
- P. C. Chao, C. C. Hsu and M. C. Yin, Nutr. Metab., 2009, 6 DOI:10.1186/1743-7075-6-33
.
- S. Corbett, J. Daniel, R. Drayton, M. Field, R. Steinhardt and N. Garrett, J. Perianesth. Nurs., 2010, 25, 214–220 Search PubMed
.
- C. C. Lin, Y. F. Hsu and T. C. Lin, Am. J. Chin. Med., 1999, 27, 371–376 Search PubMed
.
- C. J. Lee, L. G. Chen, W. L. Liang and C. C. Wang, Food Chem., 2010, 118, 315–322 Search PubMed
.
- T. Boussetta, H. Raad, P. Letteron, M. A. Gougerot-Pocidalo, J. C. Marie, F. Driss and J. El-Benna, PLoS One, 2009, 4, e6458 Search PubMed
.
- K. Singh, A. S. Jaggi and N. Singh, Phytother. Res., 2009, 23, 1565–1574 CrossRef CAS
.
- V. Kahya, A. Meric, M. Yazici, M. Yuksel, A. Midi and O. Gedikli, J. Laryngol. Otol., 2011, 125, 370–375 Search PubMed
.
- R. Bachoual, W. Talmoudi, T. Boussetta, F. Braut and J. El-Benna, Food Chem. Toxicol., 2011, 49, 1224–1228 Search PubMed
.
- M. Shukla, K. Gupta, Z. Rasheed, K. A. Khan and T. M. Haqqi, Nutrition, 2008, 24, 733–743 CrossRef CAS
.
- H. Z. Toklu, M. U. Dumlu, O. Sehirli, F. Ercan, N. Gedik, V. Gokmen and G. Sener, J. Pharm. Pharmacol., 2007, 59, 1287–1295 Search PubMed
.
- H. Z. Toklu, O. Sehirli, H. Özyurt, A. A. Mayadagli, E. Eksioglu-Demiralp, S. Cetinel, H. Sahin, B. C. Yegen, M. Ulusoylu Dumlu, V. Gökmen and G. Sener, J. Radiat. Res., 2009, 50, 345–353 Search PubMed
.
- G. Asghari, S. Sheikholeslami, P. Mirmiran, A. Chary, M. Hedayati, A. Shafiee and F. Azizi, Int. J. Food Sci. Nutr., 2011, 63, 368–371 Search PubMed
.
- F. de Nigris, M. L. Balestrieri, S. Williams-Ignarro, F. P. D'Armiento, C. Fiorito, L. J. Ignarro and C. Napoli, Nitric Oxide, 2007, 17, 50–54 Search PubMed
.
- F. Afaq, N. Khan, D. N. Syed and H. Mukhtar, Photochem. Photobiol., 2010, 86, 1318–1326 Search PubMed
.
- N. Khan, D. N. Syed, H. C. Pal, H. Mukhtar and F. Afaq, Photochem. Photobiol., 2012 DOI:10.1111/j.1751-1097.2011.01063.x
.
- M. Shukla, K. Gupta, Z. Rasheed, K. A. Khan and T. M. Haqqi, J. Inflammation, 2008, 5, 9 Search PubMed
.
- M. B. Rettig, D. Heber, J. An, N. P. Seeram, J. Y. Rao, H. Liu, T. Klatte, A. Belldegrun, A. Moro, S. M. Henning, D. Mo, W. J. Aronson and A. Pantuck, Mol. Cancer Ther., 2008, 7, 2662–2671 CrossRef CAS
.
- K. Nagao and T. Yanagita, Prog. Lipid Res., 2008, 47, 127–146 CrossRef CAS
.
- M. H. Faulds, C. Zhao, K. Dahlman-Wright and J. A. Gustafsson, J. Endocrinol., 2012, 212, 3–12 Search PubMed
.
- J. M. Del Bas, M. L. Ricketts, M. Vaque, E. Sala, H. Quesada, A. Ardevol, M. J. Salvado, M. Blay, L. Arola, D. D. Moore, G. Pujadas, J. Fernandez-Larrea and C. Blade, Mol. Nutr. Food Res., 2009, 53, 805–814 Search PubMed
.
- G. Li, W. Lin, J. J. Araya, T. Chen, B. N. Timmermann and G. L. Guo, Toxicol. Appl. Pharmacol., 2012, 258, 268–274 Search PubMed
.
- M. K. Hansen and T. M. Connolly, Curr. Opin. Invest. Drugs, 2008, 9, 247–255 Search PubMed
.
- D. M. Shih, H. R. Kast-Woelbern, J. Wong, Y. R. Xia, P. A. Edwards and A. J. Lusis, J. Lipid Res., 2006, 47, 384–392 Search PubMed
.
- A. Gutierrez, E. P. Ratliff, A. M. Andres, X. Huang, W. L. McKeehan and R. A. Davis, Arterioscler., Thromb., Vasc. Biol., 2006, 26, 301–306 Search PubMed
.
- M. Xia, M. Hou, H. Zhu, J. Ma, Z. Tang, Q. Wang, Y. Li, D. Chi, X. Yu, T. Zhao, P. Han, X. Xia and W. Ling, J. Biol. Chem., 2005, 280, 36792–36801 Search PubMed
.
- Q. Wang, M. Xia, C. Liu, H. Guo, Q. Ye, Y. Hu, Y. Zhang, M. Hou, H. Zhu, J. Ma and W. Ling, Life Sci., 2008, 83, 176–184 Search PubMed
.
- C. Yang, L. Yu, W. Li, F. Xu, J. C. Cohen and H. H. Hobbs, J. Clin. Invest., 2004, 114, 813–822 Search PubMed
.
- J. Plat, J. A. Nichols and R. P. Mensink, J. Lipid Res., 2005, 46, 2468–2476 CAS
.
- E. Kaneko, M. Matsuda, Y. Yamada, Y. Tachibana, I. Shimomura and M. Makishima, J. Biol. Chem., 2003, 278, 36091–36098 Search PubMed
.
- L. Calpe-Berdiel, J. C. Escola-Gil and F. Blanco-Vaca, Atherosclerosis, 2009, 203, 18–31 CrossRef CAS
.
- S. R. Sturgeon and A. G. Ronnenberg, Nutr. Rev., 2010, 68, 122–128 Search PubMed
.
| This journal is © The Royal Society of Chemistry 2013 |
